Abstract
Cu-containing dissimilatory nitrite reductase (CuNiR) was purified from denitrifying cells of a halophilic archaeon, Haloarcula marismortui. The purified CuNiR appeared blue in the oxidized state, possessing absorption peaks at 600 and 465 nm in the visible region. Electron paramagnetic resonance spectroscopy suggested the presence of type 1 Cu (gII = 2.232; AII = 4.4 mT) and type 2 Cu centers (gII = 2.304; AII = 13.3 mT) in the enzyme. The enzyme contained two subunits, whose apparent molecular masses were 46 and 42 kDa, according to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. N-terminal amino acid sequence analysis indicated that the two subunits were identical, except that the 46-kDa subunit was 16 amino acid residues longer than the 42-kDa subunit in the N-terminal region. A nirK gene encoding the CuNiR was cloned and sequenced, and the deduced amino acid sequence with a residual length of 361 amino acids was homologous (30 to 41%) with bacterial counterparts. Cu-liganding residues His-133, Cys-174, His-182, and Met-187 (for type 1 Cu) and His-138, His-173, and His-332 (for type 2 Cu) were conserved in the enzyme. As generally observed in the halobacterial enzymes, the enzymatic activity of the purified CuNiR was enhanced during increasing salt concentration and reached its maximum in the presence of 2 M NaCl with the value of 960 μM NO2− · min−1 · mg−1.
Denitrification, a biological reduction of nitrate that generates N2 or N2O gases, is an important process that contributes to the global nitrogen cycle of the Earth (41). Microorganisms capable of denitrification are distributed not only among bacteria but also among eukaryotic fungi and archaea (41). Denitrifying archaea have been reported to be present in some extreme halophiles (36, 39) and in a hyperthermophile, Pyrobaculum aerophilum (2, 38). Because the archaea are predominant microbial populations in extreme environments like saltwater lakes and hot springs, denitrifying archaea sustain the nitrogen cycle under such hypersaline or hot conditions.
The biochemistry of denitrification has been well investigated in some bacterial denitrifiers (4, 41); nitrate is converted to N2 through nitrite, NO, and N2O by successive reductions catalyzed by dissimilatory nitrate reductase (NaR), nitrite reductase (NiR), NO reductase, and N2O reductase, respectively. Reduction of the nitrogen compounds in the denitrifiers has been confirmed to couple with ATP synthesis, which was inhibited by uncoupling reagents (24), indicating that denitrification is an anaerobic respiratory system in which nitrate is used as a terminal electron acceptor instead of O2. Additionally, because bacterial denitrification has been considered the ancestor of aerobic respiration (32, 37), biochemical investigation of denitrification in archaea is, therefore, interesting from the evolutionary aspect.
NiR, which catalyzes the reduction of nitrite and produces NO, is a key enzyme in denitrification. Two types of NiRs with distinct molecular structures, Cu-containing dissimilatory NiR (CuNiR) and “cytochrome cd1” containing hemes c and d1 as the prosthetic cofactors, have been reported in the denitrifying bacteria (41). CuNiR is a homotrimer with a characteristic triangular structure that has been resolved by X-ray diffraction analysis of the crystal (11, 16). The enzyme contains two Cu atoms, which are distinguished from each other by their optical and electron paramagnetic resonance (EPR) spectroscopic properties (blue or green type 1 Cu center and colorless type 2 Cu center), per subunit molecule. In the eukaryotic microorganisms with denitrifying capability, CuNiR has been reported from the fungus Fusarium oxysporum (23). CuNiR has also been reported from the denitrifying halophilic archaeon Haloferax denitrificans (18). However, the gene structures of the fungal and the archaeal CuNiR have not been reported yet.
In the present study, we purified CuNiR from denitrifying cells of an extreme halophilic archaeon, Haloarcula marismortui, and characterized its molecular and enzymatic properties. Further, the nirK gene encoding the CuNiR was sequenced and phylogenetic analysis was performed. This is the first report of the genetic analysis on the archaeal denitrifying enzymes.
MATERIALS AND METHODS
Cultivation. Anaerobic cultivation of H. marismortui
ATCC 43049 was used in the presence of nitrate as described previously (40). The growth medium contained 1.0 g of peptone (Difco, Detroit, Mich.), 1.0 g of yeast extract (Difco), 5.0 g of K2SO4, 5.1 g of NaNO3, 125 g of NaCl, 160 g of MgCl2 · 6H2O, 0.13 g of CaCl2, 1.0 mg of MnSO4, 0.83 mg of Fe2(SO4)3, 10 μg of CuSO4 · 5H2O, and 10 μg of (NH4)6Mo7O24 · 4H2O per liter. The pH of the medium was adjusted to 7.0. After it was autoclaved, Na ascorbate was added to the medium to remove dissolved O2. Cultivation was performed at 40°C for about 3 days. Cells at the late exponential growth stage were harvested by centrifugation and stored at −20°C until experimental use.
Physical measurements.
Protein concentration was determined by a modified Lowry method (12) using bovine serum albumin as the standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out by the method of Schägger and von Jagow (34). Spectroscopic measurements in the UV-visible regions were performed using a 220A Spectrophotometer (Hitachi Ltd., Tokyo, Japan) with a 1-cm-long lightpath cuvette. The EPR spectrum was measured with a JEOL RE-1X X-band spectrometer (JEOL Ltd., Tokyo, Japan) at 77 K. The magnetic field was calibrated with diphenylpicrylhydrazine and MnO. The molecular weight of the purified enzyme was measured by size exclusion chromatography using a Sephacryl S-300 (Pharmacia, Uppsala, Sweden) column (2.0 by 100 cm) equilibrated with 10 mM Tris-HCl buffer (pH 8.0) containing 2 M NaCl. Hemoglobin (molecular mass, 67 kDa), yeast alcohol hydrogenase (150 kDa), aldolase (156 kDa), and apoferritin (450 kDa) were used as molecular weight standards.
Activity assay.
Nitrite-reducing activity was measured as follows: enzyme preparations were mixed with 1 ml of 10 mM Na phosphate buffer (pH 7.0) containing 2 M NaCl and 1 mM NaNO2, and then the reaction was started by adding 10 μl of 1 M Na2S2O4. After an appropriate reaction time, the concentration of nitrite that remained in the solution was assayed by a diazocoupling method (28).
Purification.
Cultured archaeal cells (30 g [wet weight]) were suspended in 10 mM Na phosphate buffer (pH 7.0) containing 2.0 M NaCl, and then the cells were disrupted by treatment with an ultrasonic oscillating device. The resulting solution was centrifuged at 10,000 × g for 30 min to precipitate intact cells. The cell-free supernatant thus obtained was further centrifuged at 70,000 × g for 60 min. The resulting supernatant was used as the starting material for subsequent purification of NiR. The supernatant was dialyzed for 12 h against a 20-fold volume of 50%-saturated (NH4)2SO4 that had been neutralized by NH3. The resulting solution was centrifuged at 10,000 × g for 15 min, and then the precipitate was removed. The supernatant was combined with (NH4)2SO4 granules to produce a 60%-saturated solution, which was mixed with about 100 ml (bed volume) of Butyl-Toyopearl 650M (Tosoh Co., Tokyo, Japan) that had been equilibrated with neutralized 60%-saturated (NH4)2SO4. The suspension was loaded onto the empty column; then the resulting column was washed with the equilibrating buffer until the eluted solution became clear. The NiR that was adsorbed hydrophobically onto the column was eluted by loading 10 mM Tris-HCl buffer (pH 8.0) containing 2 M NaCl, and then the eluate was pooled for further purification. The preparation thus obtained was mixed with a 1.5-fold volume of saturated (NH4)2SO4 and was loaded onto the Butyl-Toyopearl column (2.5 by 20 cm) that had been equilibrated with 10 mM Tris-HCl buffer (pH 8.0) containing 60%-saturated (NH4)2SO4. The enzyme adsorbed was eluted by the linear gradient generated from 10 mM Tris-HCl buffer (pH 8.0) containing 60%-saturated (NH4)2SO4 and from the buffer containing 2 M NaCl. The eluate showing nitrite-reducing activity was collected, mixed with the same volume of saturated (NH4)2SO4, and then loaded onto the Sepharose CL-6B (Bio-Rad, Richmond, Calif.) column (2.5 by 20 cm) that had been equilibrated with 10 mM Tris-HCl buffer (pH 8.0) containing 50%-saturated (NH4)2SO4. The enzyme adsorbed onto the column was eluted by the linear gradient generated from 10 mM Tris-HCl buffer (pH 8.0) containing 50%-saturated (NH4)2SO4 and from the buffer containing 2 M NaCl. The preparation involving nitrite-reducing activity was mixed with the same volume of a saturated (NH4)2SO4 solution and was charged onto a small Butyl-Toyopearl column (0.5 by 2.0 cm) to concentrate the preparation. The sample that was eluted by 10 mM Tris-HCl buffer (pH 8.0) containing 2 M NaCl was then subjected to a Sephacryl S-300 (Pharmacia) gel filtration column (2.0 by 100 cm) equilibrated with the same solution. The enzyme thus eluted was mixed with the same volume of a saturated (NH4)2SO4 solution and was then subjected to hydrophobic chromatography on an Octyl-Sepharose (Pharmacia) column (2.0 by 10 cm) that had been equilibrated with 10 mM Tris-HCl buffer (pH 8.0) containing 50%-saturated (NH4)2SO4. The enzyme adsorbed was eluted with a linear gradient generated from 100 ml each of 10 mM Tris-HCl buffer (pH 8.0) containing 50%-saturated (NH4)2SO4 and of the buffer containing 2 M NaCl. The eluate with nitrite-reducing activity was collected and dialyzed against 10 mM morpholineethanesulfonic acid (MES)-NaOH buffer (pH 6.5) containing 2 M NaCl for 12 h. The resulting sample was subjected to a hydroxyapatite (Wako Pure Chemicals Industries, Ltd., Osaka, Japan) column (1.0 by 10 cm) that had been equilibrated with the same buffer that was used for the dialysis. After washing of the column with the same buffer, the NiR adsorbed was eluted with a linear gradient generated from 50 ml each of the same buffer and 10 mM Na phosphate buffer (pH 6.5) containing 2 M NaCl. The eluate that appeared blue was collected, concentrated using a Centricon 25 (Amicon Inc., Beverly, Mass.), and then used as the purified enzyme for the experiments. All the purification procedures were performed at 4°C except the hydroxyapatite chromatography, which was done at room temperature.
Protein sequencing.
The purified NiR was subjected to SDS-PAGE, and the protein bands were electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore Co., Bedford, Mass.) using an electroblotting apparatus (Atto Co., Tokyo, Japan). The Coomassie brilliant blue-stained protein bands on the membrane were cut out separately and placed on the glass block of a protein sequencer (model PPSQ-21) (Shimadzu Co., Kyoto, Japan) to analyze their N-terminal sequences.
DNA manipulation and sequencing.
Extraction of genomic DNA from cultivated H. marismortui cells was performed as previously described (7). Plasmid isolation was performed by the alkaline lysis method (6). Standard methods were used for restriction digestions, agarose gel electrophoresis, and ligations. Taq polymerase (Takara Shuzo Co. Ltd., Kyoto, Japan) was used for PCR, and then the products were cloned into a pT7 Blue T vector (Novagen Inc., Madison, Wis.). DNA labeling with alkaline phosphatase was done with the materials and protocols of a chemiluminescence detection kit (Amersham Pharmacia Biotech, Little Chalfont, England) for Southern hybridization. Digested DNA fragments that were cloned into the pUC118 cloning vector were sequenced by the dideoxy chain termination method (8) using a model 4200 DNA sequencer (LI-COR Co., Lincoln, Nebr.). Two degenerate oligonucleotides (5′-GCNATYGARCAGGCNACCVGARGCNGARAC-3′ and 5′-GCBGTBGGRTCNGCNGCRATNCGRTC-3′; R, Y, V, B, and N represent A+G, C+T, A+C+G, C+G+T, and A+C+G+T, respectively) were synthesized based on the N-terminal amino acid sequences of the subunits of the purified NiR and were used as primers for the amplification with the archaeal genomic DNA as the template. After cloning and sequencing to confirm its identity, the amplified 93-bp fragment was labeled with alkaline phosphatase and was then used as the DNA probe for Southern hybridization.
Nucleotide sequence accession number.
The nirK gene from H. marismortui has been assigned accession number AJ278286 in the European Molecular Biology Laboratory (EMBL) nucleotide sequence database.
RESULTS
Purification of NiR.
When the NiR preparation was transferred to an asaline condition, its activity gradually decreased to about 70% after 1 h. Purification of the enzyme was, therefore, usually performed in coexistence with NaCl or (NH4)2SO4, the concentrations of which were at least 2 M. Most (96%) of the NiR activity in the cell extract was recovered in the soluble fraction. The blue enzyme was purified 833-fold to gain the final recovery of 17.8% through six preparative steps, including hydrophobic, hydrogen-bound, gel filtration, and hydroxyapatite chromatographies as summarized in Table 1. Enhancement of the nitrite-reducing activity of the enzyme during the gel filtration step was usually observed in the three individual purification procedures, raising the possibility of the presence of an inhibitor against the enzyme in the soluble fraction, as formerly suggested in the purification of the CuNiRs from the denitrifying bacteria Achromobacter cycloclastes (26) and Rhodobacter sphaeroides (33).
TABLE 1.
Purification procedure of H. marismortui NiR
| Purification step | Vol (ml) | Total protein (mg) | Total activity (mmol of NO2− · min−1) | Sp act (μmol of NO2− · min−1 · mg−1) | Yield (%) |
|---|---|---|---|---|---|
| Soluble fraction | 238 | 6,710 | 7.74 | 1.14 | 100 |
| 1st hydrophobic (Butyl-Toyopearl) | 340 | 2,700 | 5.77 | 2.16 | 74.6 |
| 2nd hydrophobic (Butyl-Toyopearl) | 71.0 | 292 | 2.74 | 9.36 | 35.3 |
| Hydrogen-bound (Sepharose CL-6B) | 41.0 | 86.9 | 2.05 | 23.6 | 26.5 |
| Gel filtration (Sephacryl S-300) | 42.0 | 52.9 | 5.29 | 100 | 68.4 |
| 3rd hydrophobic (Octyl-Sepharose) | 24.0 | 19.9 | 2.77 | 139 | 35.7 |
| Hydroxyapatite | 15.9 | 1.43 | 1.37 | 960 | 17.8 |
Subunit structure.
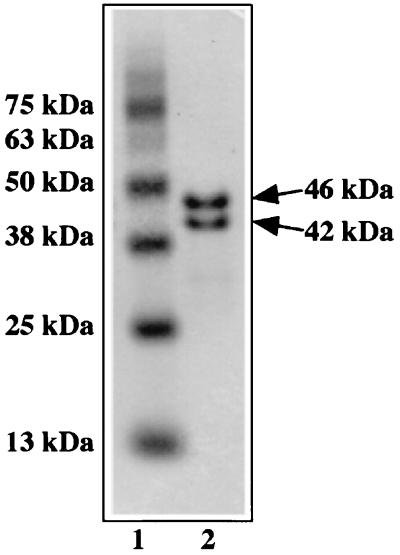
SDS-PAGE of the purified NiR gave two protein bands whose molecular masses appeared to be 46 and 42 kDa with an approximately 1:1 stoichiometry, as shown in Fig. 1. N-terminal amino acid sequences of the 46- and 42-kDa subunits were determined to be A(I/K)EQATEAETTPQE(P)AMNAAQQTDV and MNAAQQTDVDRIAADPTAIDD, respectively. The sequence of the 46-kDa subunit after the 17th amino acid was identical to the N-terminal sequence of the 42-kDa subunit, indicating that the NiR was composed of identical subunits, except for the 16-amino-acid difference. The 42-kDa subunit of the enzyme is probably generated by a secondary proteolytic reaction of the 46-kDa subunit. The molecular mass of the enzyme in a mature state was estimated to be 167 kDa by gel filtration, suggesting that the purified enzyme is a multicomplex of an identical subunit.
FIG. 1.
Subunit composition of the purified H. marismortui NiR. SDS-PAGE analysis of the purified NiR was performed, and then the gel was stained with Coomassie brilliant blue. The purified NiR (lane 2) and standard proteins (lane 1) were contained with their molecular masses shown beside the gel.
Structure of gene encoding NiR.
Based on the N-terminal sequences of the 46- and 42-kDa subunits of the purified enzyme, the DNA fragment encoding the N-terminal region of the enzyme was amplified and used as a probe for Southern hybridization. Hybridization upon HindIII-, KpnI-, PstI-, and SmaI-digested gene fragments showed a single positive band corresponding to 5.2, 2.5, 2.8, and 2.4 kbp, respectively, suggesting that only one copy of the gene would be present in the archaeal genomic DNA (data not shown). The nucleotide sequence of the KpnI-digested fragment was determined using standard DNA manipulation methods. The 2.5-kbp DNA fragment of the H. marismortui genome included two complete open reading frames, nirK and pcn, which encoded the present NiR with 361 amino acids and a proliferating cell-nuclear antigen with 256 amino acids, respectively. The N-terminal amino acid sequences of the 46- and 42-kDa subunits of the purified enzyme matched completely with parts of the sequence of the nirK product. Molecular weights of the 46- and 42-kDa subunits were calculated to be 35,821 and 34,138, respectively, from the sequence. The calculated molecular weights were quite small compared with those estimated from the SDS-PAGE analysis. Nucleotide and amino acid sequence data are available under accession number AJ278286 in the EMBL database, the number given at the end of Materials and Methods.
Sequence alignment.
In Fig. 2, the deduced amino acid sequence of the H. marismortui NiR is shown and aligned with those of CuNiRs from four denitrifying bacteria, Neisseria gonorrhoeae, R. sphaeroides, Alcaligenes xylosoxidans, and A. cycloclastes. Putative Cu-liganding residues His-133, Cys-174, His-182, and Met-187 for type 1 Cu and His-138, His-173, and His-332 for type 2 Cu were completely retained in the H. marismortui enzyme. Sequential coincidence of the archaeal NiR with the bacterial enzymes was observed in the overall region, especially with the gonorrheal pathogen N. gonorrhoeae, having in common the presence of two short deletions (boxed regions of the sequence in Fig. 2).
FIG. 2.
Amino acid sequence alignment of the H. marismortui NiR with bacterial CuNiRs. Shading highlights amino acid residues of bacterial CuNiRs that are identical with those in the H. marismortui enzyme. Amino acids are numbered at the right margin. Signal sequences are shown by lowercase letters. Putative Cu-binding residues for type 1 and type 2 Cu are indicated by I and II, respectively. Accession numbers of the sequences shown here are as follows: N. gonorrhoeae (Ng) (GenBank M97926), R. sphaeroides (Rs) (GenBank U62291), A. xylosoxidans (Ax) (EMBL AF051831), and A. cycloclastes (Ac) (GenBank Z48635).
Optical absorption and EPR spectra.
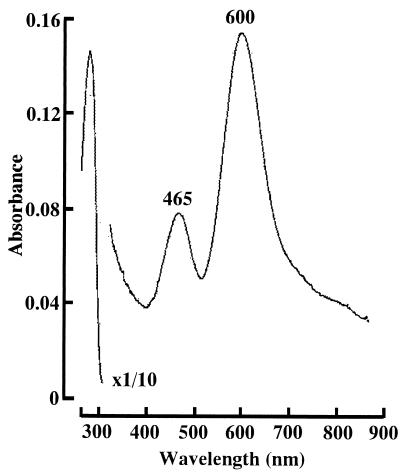
The purified enzyme appeared blue in an oxidized state, and the color disappeared on reduction with Na2S2O4. The absorption spectrum of the enzyme exhibited absorption maxima at 465 and 600 nm with a small shoulder around 820 nm in the visible region (Fig. 3). The extinction coefficient at 600 nm of NiR was estimated to be 2.88 mM−1 · cm−1 when the molecular weight of the subunit was considered to be 35,000, as an average of those of the 46- and 42-kDa subunits.
FIG. 3.
Absorption spectrum of the H. marismortui NiR. Purified NiR (1.91 mg/ml) was dissolved in 10 mM Tris-HCl buffer (pH 8.0) containing 2 M NaCl. The absorbance of the enzyme at around 280 nm was reduced to a scale of 1/10 the natural intensity.
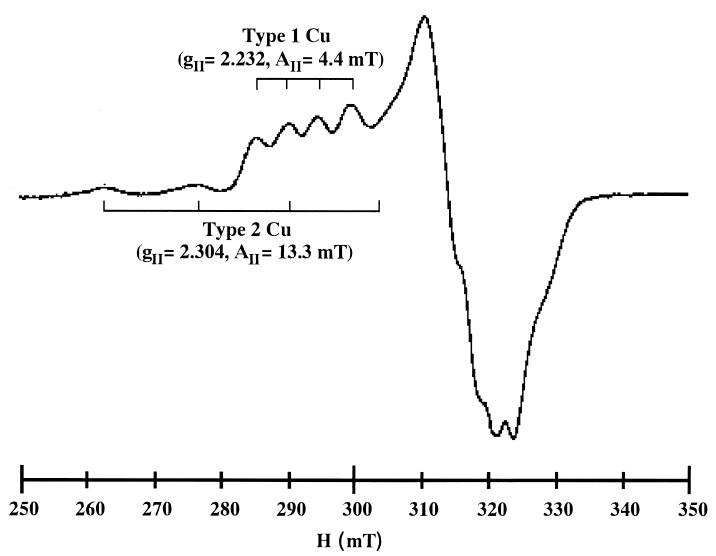
EPR measurement also revealed the presence of Cu in the purified enzyme. The spectrum of the oxidized enzyme showed signals characteristic of the type 1 Cu center with narrow, sharp, hyperfine splitting (gII = 2.232; AII = 4.4 mT) and characteristic of the type 2 Cu center with a broader split (gII = 2.304; AII = 13.3 mT), as shown in Fig. 4. Overall, spectroscopic features of the purified enzyme resembled those of CuNiRs isolated from denitrifying bacteria. Composition of Cu atoms in the purified enzyme was estimated to be 1.5 mol · mol−1 of the subunit by double integration of the EPR spectrum, suggesting that two prosthetic Cu atoms are present in a subunit molecule. These EPR spectroscopic analyses provided evidence that one molecule each of type 1 and type 2 Cu centers is present in a subunit of the archaeal NiR.
FIG. 4.
EPR spectrum of H. marismortui NiR at 77 K. The purified NiR (19.1 mg/ml) was dissolved in 10 mM Tris-HCl buffer (pH 8.0) containing 2 M NaCl. Conditions of the EPR run were microwave frequency, 9.21 GHz; microwave power, 3.13 mW; modulation amplitude, 1.0 mT; sweep time, 8 min; time constant, 0.1 s.
Catalytic properties.
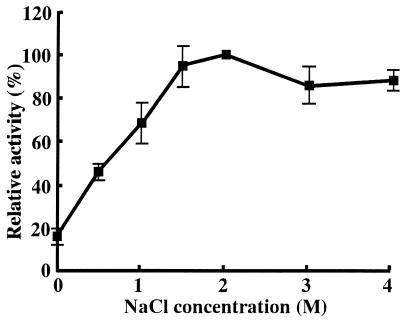
As generally observed in the halobacterial enzymes, the purified CuNiR was activated in the presence of high salt concentrations. As shown in Fig. 5, nitrite-reducing activity of the enzyme reached its maximum at concentrations of NaCl higher than 2 M. Using Na2S2O4 as the electron donor, enzymatic activity at 2 M NaCl was estimated to be 960 μM NO2− · min−1 · mg−1. Relative activities of the enzyme measured in the presence of 2 M KCl, LiCl, NH4Cl, and NaNO3 against the activity in 2 M NaCl were 87, 69, 84, and 65%, respectively, suggesting that the activity of CuNiR is not affected by specific ion species but requires only high ionic strength.
FIG. 5.
Effect of NaCl concentration on the enzymatic activity of H. marismortui CuNiR. Nitrite-reducing activity was measured in 10 mM Tris-HCl buffer (pH 8.0) containing each concentration of NaCl. Maximum activity (100%), which was measured at 2 M NaCl, was 960 μM NO2− · min−1 · mg−1. Each point is the mean value of three individual measurements.
The purified enzyme was found to accept electrons only from Na2S2O4. The physiological electron donor has remained unclear; potent physiological electron donors of the bacterial enzyme, low-molecular-weight blue Cu protein and cytochrome c, were not present in the denitrifying cells of H. marismortui according to optical spectroscopic observation. Artificial reductants, such as methyl viologen, phenazine methosulfate, and N,N,N′,N′-tetramethyl-p-phenylenediamine, showed no electron-donating ability to the purified enzyme. Treatment with 1 mM diethyldithiocarbamate or cyanide completely inhibited the activity of the purified enzyme, as is also true in the bacterial CuNiRs. Incubation with 1 mM EDTA for 1 h decreased the nitrite-reducing activity to below 30%, while the reduced activity was not restored by treatment with 50 μM CuSO4.
DISCUSSION
In the bacterial and fungal CuNiRs, blue and green enzymes have been reported (41). The blue CuNiR purified from A. xylosoxidans (1, 26), Pseudomonas aureofaciens (42) and the fungus F. oxysporum (23) showed a maximum peak at a wavelength of 580 to 600 nm that was accompanied by a small shoulder around 460 nm, while the green enzyme from A. cycloclastes (20), Alcaligenes faecalis (22), and R. sphaeroides (33) possessed dual peaks at around 590 and 460 nm with comparable absorptional intensities. The green enzyme has also been reported in another halophilic archaeon, H. denitrificans (18). Molecular and enzymatic properties of the CuNiRs from archaea (H. marismortui and H. denitrificans), bacteria (A. xylosoxidans and A. cycloclastes), and fungi (F. oxysporum) are summarized in Table 2.
TABLE 2.
Properties of CuNiRs
| Species (enzyme) | Molecular mass(es) (kDa) | Subunit mass (kDa) | Composition | Absorption peaksf (nm) | ɛmM at maximum peak (mM−1 · cm−1 of subunit) | Cu atom composition (mol · mol−1 of subunit) |
|---|---|---|---|---|---|---|
| H. marismortui (blue) | 167a | 46c (35.8)d + 42c (34.1)d | (α/α′)3 × 2a | 465, 600, 820 | 2.88 | 1.5 |
| H. denitrificans (green) | 127a | 64c | α2a | 462, 594, 682 | 1.8 | NDh |
| A. xylosoxidans (blue) | 70a, 103b | 36.5d | α3e | 460, 593, 770 | 3.7 | 1.8 |
| A. cycloclastes (green) | 69a, 105b | 37d | α3e | 400, 464, 590, 700 | 2.1 | 1.6 |
| F. oxysporum (blue) | 83.3a | 41.8c | α2a | 470, 595, 782 | 5.4 | 1.9 |
| EPR parameters for:
|
Electron donor
|
Activity (μM NO2− · min−1 · mg−1) | Inhibitor(s) | Source or reference(s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 Cu
|
Type 2 Cu
|
Cu protein | Cytochrome c | Methyl viologen- Na2S2O4 | Phenazine methosulfate-ascorbate | |||||
| gII | AII (mT) | gII | AII (mT) | |||||||
| 2.232 | 4.4 | 2.304 | 13.3 | −g | −g | + (Na2S2O4) | − | 960 | DDCi, CN−, EDTA | This study |
| ND | ND | ND | ND | ND | ND | ND | + | 25 | DDC | 18 |
| 2.208 | 6.3 | 2.298 | 14.2 | + (azurin) | + (cytochrome c553) | + | + | 240 | ND | 1, 10, 11, 26 |
| 2.195 | 7.3 | 2.262 | 17.5 | + (pseudoazurin) | ND | + | 260 | DDC, CN−, CO | 16, 20, 21, 25 | |
| 2.22 | 6.82 | 2.32 | ND | + (azurin) | + (cytochrome c549) | + | + | 447 | DDC, CN−, CO | 23 |
Determined by gel filtration.
Reflects sedimentation equilibrium.
Determined by SDS-PAGE.
Calculated from sequence.
Crystal structure.
Maximum peaks are given in boldface.
Blue Cu protein and cytochrome c are not present (−) (see text).
ND, not determined.
DDC, diethyldithiocarbamate.
In spite of the clear difference in the optical absorption spectroscopic features, EPR signal parameters of the blue CuNiRs, including the present archaeal enzyme, are quite similar and are hardly distinguishable from that of the green enzyme in Table 2. Crystal structures of the blue CuNiR from A. xylosoxidans (11) and of the green enzymes from A. cycloclastes (16) and A. faecalis (27) have already been determined with high resolution, and comparison of the Cu-liganding structures in the enzymes demonstrates that a small difference in the orientation of the Sδ atom of Met in the tetrahedral geometry around the type 1 Cu center causes variation in optical spectroscopic properties (19). CuNiR purified from H. marismortui in this study appeared blue and possessed a maximum absorption peak at 600 nm in the visible region, while it was accompanied by a peak at 465 nm whose intensity was about half that at 600 nm. The spectrum looks like a combination of the blue and green enzymes. Spectroscopic properties of the H. marismortui enzyme will be elucidated by future investigation of the crystal structure of the enzyme.
The amino acid sequence of the archaeal CuNiR showed homology with those of enzymes from four denitrifying bacteria, N. gonorrhoeae, R. sphaeroides, A. xylosoxidans, and A. cycloclastes, in the overall region of the sequence (Fig. 2). X-ray crystal structure analyses of CuNiRs from A. xylosoxidans (11) and A. cycloclastes (16) have demonstrated the characteristic triangular structure composed of three identical subunits. The similarity of the amino acid sequences suggests that the minimum functional unit of the archaeal enzyme is a trimeric complex of the identical subunits containing one molecule each of type 1 and type 2 coppers in a subunit molecule. Further, preliminary X-ray analysis of the archaeal CuNiR crystal also suggests that the enzyme is a hexamer, probably a dimer of a trimeric complex, of the subunit molecule in the mature state (H. Ichiki et al., unpublished results).
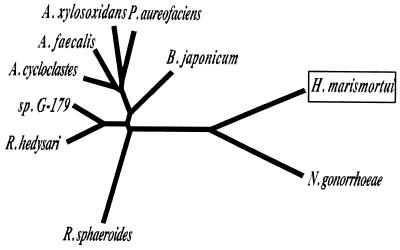
Interestingly, phylogenic analysis of the amino acid sequences indicated that the archaeal CuNiR is in a quite close relationship with the enzyme from the gonorrheal pathogen N. gonorrhoeae, as revealed in the unrooted phylogenetic tree shown in Fig. 6. The ratio of amino acid residues, identical to that for the N. gonorrhoeae CuNiR (41%), is remarkably high compared with those for the other enzymes (30 to 32%). Moreover, short deletions in the same two positions (boxed regions of the sequences in Fig. 2) were commonly present in the two enzymes. The structural similarity suggests the lateral transfer of the nirK gene between the halophilic archaea and the pathogenic proteobacteria. Lateral gene transfer between the halophilic archaea and the cyanobacteria has been proposed in the [2Fe-2S] type ferredoxin of Halobacterium salinarum (30).
FIG. 6.
Phylogenetic relationship of the H. marismortui CuNiR with bacterial CuNiRs based on the sequence alignment. Construction of an unrooted phylogenetic tree is inferred from the alignment of the amino acid sequences based on the ClustalW program (http://clustalw.genome.ad.jp/). Accession numbers for the sequences shown here (see Fig. 2 legend for other accession numbers) are A. faecalis (GenBank D13155), Bradyrhizobium japonicum (EMBL AJ002516), P. aureofaciens (GenBank Z21945), Pseudomonas sp. strain G-179 (GenBank M97294), and Rhizobium hedysari (GenBank U65658).
In the N terminus of the CuNiR precursor, the positively charged (-RRR-) and subsequent hydrophobic (-ALGVGTAALAG-) regions, which are characteristic to the bacterial signal peptide (29), were present (Fig. 2). By using the SignalP program (http://www.cbs.dtu.dk/services/SignalP/), a possible cleavage site of the archaeal CuNiR precursor by the signal peptidase was predicted to be between the 32nd and 33rd (-PGA/KEQ-, gram-positive mode) positions or between the 29th and 30th (-ASA/PGA-, gram-negative mode) positions, which are close enough to the cleavage site (-APG/AKE-) to give the 46-kDa subunit of CuNiR. The results suggest that the archaeal CuNiR is a periplasmic protein, as was previously revealed of the bacterial NiRs (9). However, a spheroplasting experiment for resolving the intracellular localization of the enzyme has not succeeded until now, because the denitrifying cells of H. marismortui are easily disrupted only by the precipitation of the cells by centrifugation.
The physiological electron donor to the CuNiR remains unclear. Azurin- or pseudoazurin-like blue Cu proteins (10, 23, 25) and soluble c-type cytochromes (23, 26) have been reported as the direct electron donors to the CuNiRs in some denitrifying bacteria. Although a pseudoazurin-like blue Cu protein named “halocyanin” has been reported from a haloalkaliphilic archaeon (35), no blue protein except the present enzyme was observed in H. marismortui during the fractionation of the proteins. Further, cytochromes c with low-spin hemes are detected in neither soluble nor membrane fractions of the archaeon from their difference spectra ([reduced] − [oxidized]), as formerly reported of the other halophilic archaeon, H. salinarum (14).
Enzymes from halophilic archaea generally require extreme salinity for their stabilization and/or enzymatic activity. It is known that the halophilic proteins contain an excess of negatively charged residues compared to their nonhalophilic homologs (15). Recent X-ray crystallographic analysis of some halobacterial enzymes showed the presence of characteristic salt bridge structures composed of acidic residues and solvent cations and the presence of an excess of bound water molecules on the surface of the protein molecule (13, 31). Denaturation of the halobacterial enzymes in the nonhalophilic condition is explained by the presence of clusters of the negative- charged residues that lead to instability of the protein molecule from the electrostatic repulsion among the clusters in a condition of low ionic strength. The H. marismortui CuNiR purified in this study is a typical halophilic enzyme, showing maximum activity at above 2 M NaCl while being denatured in the absence of salinity. However, the ratio of negatively charged residues in the archaeal enzyme (13.3%) was only a little higher than those in the nine bacterial enzymes (9.4 to 11.8%) that are shown in Fig. 6. Understanding the structural implication to a “haloadaptation” of CuNiR requires X-ray crystal structure analysis in the future.
Recent investigations of dissimilatory nitrate reduction in the archaea have indicated that the archaeal NaR resembles the bacterial membrane-bound NaR in terms of enzymatic properties (2, 3, 5, 17, 40), subunit composition (5, 17, 40), and DNA sequence (EMBL database accession no. AJ277440). In this study, we purified CuNiR from the denitrifying archaeon H. marismortui and cloned the nirK gene encoding the enzyme. In spite of the similarity of the sequence, the purified enzyme showed some peculiarities in the structural, spectroscopic, and catalytic properties compared with those of the bacterial CuNiRs. The biochemistry of the NO reducing system in archaeal denitrification, therefore, will be the focus of our next study.
ACKNOWLEDGMENTS
We thank H. Taguchi (Research Laboratory of Resources Utilization, Tokyo Institute of Technology, Tokyo, Japan) for the protein sequence analysis. We also thank T. Kohzuma (Faculty of Science, Ibaraki University, Mito-Shi, Japan) for valuable discussions.
H. Ichiki and Y. Tanaka contributed equally to this work.
This work was supported in part by grant-in-aid 12793001 from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Abraham Z H, Lowe D J, Smith B E. Purification and characterization of the dissimilatory nitrite reductase from Alcaligenes xylosoxidans subsp. xylosoxidans (NCIMB 11015): evidence for the presence of both type 1 and type 2 copper centres. Biochem J. 1993;295:587–593. doi: 10.1042/bj2950587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshar S, Kim C, Monbouquette H G, Schroder I. Effect of tungstate on nitrate reduction by the hyperthermophilic archaeon Pyrobaculum aerophilum. Appl Environ Microbiol. 1998;64:3004–3008. doi: 10.1128/aem.64.8.3004-3008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Ossorio M C, Muriana F J G, de la Rosa F F, Relimpio A M. Purification and characterization of nitrate reductase from the halophilic archaebacterium Haloferax mediterranei. Z Naturforsch Teil C. 1992;47:670–676. [Google Scholar]
- 4.Berks B C, Ferguson S J, Moir J W B, Richardson D J. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim Biophys Acta. 1995;1232:97–173. doi: 10.1016/0005-2728(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 5.Bickel-Sandkötter S, Ufer M. Properties of a dissimilatory nitrate reductase from the halophilic archaeon Haloferax volcanii. Z Naturforsch Teil C. 1995;50:365–372. [Google Scholar]
- 6.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blin N, Stafford D W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen E Y, Seeburg P H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985;4:165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- 9.Coyne M S, Arunakumari A, Pankratz H S, Tiedje J M. Localization of the cytochrome cd1 and copper nitrite reductases in denitrifying bacteria. J Bacteriol. 1990;172:2558–2562. doi: 10.1128/jb.172.5.2558-2562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd F E, Hasnain S S, Hunter W N, Abraham Z H L, Debenham M, Kanzler H, Eldridge M, Eady R R, Ambler R P, Smith B E. Evidence for two distinct azurins in Alcaligenes xylosoxidans (NCIMB 11015): potential electron donors to nitrite reductase. Biochemistry. 1995;34:10180–10186. doi: 10.1021/bi00032a011. [DOI] [PubMed] [Google Scholar]
- 11.Dodd F E, van Beeumen J, Eady R R, Hasnain S S. X-ray structure of a blue-copper nitrite reductase in two crystal forms. The nature of the copper sites, mode of substrate binding and recognition by redox partner. J Mol Biol. 1998;282:369–382. doi: 10.1006/jmbi.1998.2007. [DOI] [PubMed] [Google Scholar]
- 12.Dulley J R, Grieve P A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975;64:136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- 13.Frolow F, Harel M, Sussman J L, Mevarech M, Shoham M. Insights into protein adaptation to a saturated salt environment from the crystal structure of a halophilic 2Fe-2S ferredoxin. Nat Struct Biol. 1996;3:452–458. doi: 10.1038/nsb0596-452. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara T, Fukumori Y, Yamanaka T. aa3-type cytochrome c oxidase occurs in Halobacterium halobium and its activity is inhibited by higher concentration of salts. Plant Cell Physiol. 1987;28:29–36. [Google Scholar]
- 15.Fukumori Y, Fujiwara T, Okada-Takahashi Y, Mukohata Y, Yamanaka T. Purification and properties of a peroxidase from Halobacterium halobium L-33. J Biochem. 1985;93:1055–1061. doi: 10.1093/oxfordjournals.jbchem.a135352. [DOI] [PubMed] [Google Scholar]
- 16.Godden J W, Turley S, Teller D C, Adman E T, Liu M Y, Payne W J, LeGall J. The 2.3 angstrom X-ray structure of nitrite reductase from Achromobacter cycloclastes. Science. 1991;253:438–442. doi: 10.1126/science.1862344. [DOI] [PubMed] [Google Scholar]
- 17.Hochstein L I, Lang F. Purification and properties of a dissimilatory nitrate reductase from Haloferax denitrificans. Arch Biochem Biophys. 1991;288:380–385. doi: 10.1016/0003-9861(91)90210-a. [DOI] [PubMed] [Google Scholar]
- 18.Inatomi K, Hochstein L I. The purification and properties of a copper nitrite reductase from Haloferax denitrificans. Curr Microbiol. 1996;32:72–76. [Google Scholar]
- 19.Inoue T, Gotowda M, Deligeer, Kataoka K, Yamaguchi K, Suzuki S, Watanabe H, Gohow M, Kai Y. Type 1 Cu structure of blue nitrite reductase from Alcaligenes xylosoxidans GIFU 1051 at 2.05 A resolution: comparison of blue and green nitrite reductases. J Biochem. 1998;124:876–879. doi: 10.1093/oxfordjournals.jbchem.a022201. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki H, Matsubara T. A nitrite reductase from Achromobacter cycloclastes. J Biochem. 1972;71:645–652. [PubMed] [Google Scholar]
- 21.Iwasaki H, Noji S, Shidara S. Achromobacter cycloclastes nitrite reductase. The function of copper, amino acid composition, and ESR spectra. J Biochem. 1975;78:355–361. doi: 10.1093/oxfordjournals.jbchem.a130915. [DOI] [PubMed] [Google Scholar]
- 22.Kakutani T, Watanabe H, Arima K, Beppu T. Purification and properties of a copper-containing nitrite reductase from a denitrifying bacterium Alcaligenes faecalis strain S-6. J Biochem. 1981;89:453–461. doi: 10.1093/oxfordjournals.jbchem.a133220. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Shoun H. The copper-containing dissimilatory nitrite reductase involved in the denitrifying system of the fungus Fusarium oxysporum. J Biol Chem. 1995;270:4146–4151. doi: 10.1074/jbc.270.8.4146. [DOI] [PubMed] [Google Scholar]
- 24.Koike I, Hattori A. Energy yield of denitrification: an estimate from growth yield in continuous cultures of Pseudomonas denitrificans under nitrate-, nitrite- and oxide-limited conditions. J Gen Microbiol. 1975;88:11–19. doi: 10.1099/00221287-88-1-11. [DOI] [PubMed] [Google Scholar]
- 25.Liu M-Y, Liu M-C, Payne J, Legall J. Properties and electron transfer specificity of copper proteins from the denitrifier “Achromobacter cycloclastes.”. J Bacteriol. 1986;166:604–608. doi: 10.1128/jb.166.2.604-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuko M, Iwasaki H, Sakurai T, Suzuki S, Nakahara A. Characterization of nitrite reductase from a denitrifier, Alcaligenes sp. NCIB 11015. A novel copper protein. J Biochem. 1984;96:447–454. doi: 10.1093/oxfordjournals.jbchem.a134856. [DOI] [PubMed] [Google Scholar]
- 27.Murphy M E P, Turley S, Adman E T. Structure of nitrite bound to copper-containing nitrite reductase from Alcaligenes faecalis. Mechanistic implications. J Biol Chem. 1997;272:28455–28460. doi: 10.1074/jbc.272.45.28455. [DOI] [PubMed] [Google Scholar]
- 28.Nicholas D J D, Mason A. Determination of nitrate and nitrite. Methods Enzymol. 1957;3:981–984. [Google Scholar]
- 29.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifer F, Griffig J, Oesterhelt D. The fdx gene encoding the [2Fe-2S] ferredoxin of Halobacterium salinarium (H. halobium) Mol Gen Genet. 1993;239:66–71. doi: 10.1007/BF00281602. [DOI] [PubMed] [Google Scholar]
- 31.Richard S B, Madern D, Garcin E, Zaccai G. Halophilic adaptation: novel solvent protein interactions observed in the 2.9 and 2.6 Å resolution structures of the wild type and a mutant of malate dehydrogenase from Haloarcula marismortui. Biochemistry. 2000;39:992–1000. doi: 10.1021/bi991001a. [DOI] [PubMed] [Google Scholar]
- 32.Saraste M, Castresana J. Cytochrome oxidase evolved by tinkering with denitrification enzymes. FEBS Lett. 1994;341:1–4. doi: 10.1016/0014-5793(94)80228-9. [DOI] [PubMed] [Google Scholar]
- 33.Sawada E, Satoh T, Kitamura H. Purification and properties of a dissimilatory nitrite reductase of a denitrifying phototrophic bacterium. Plant Cell Physiol. 1978;19:1339–1351. [Google Scholar]
- 34.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 35.Scharf B, Engelhard M. Halocyanin, an archaebacterial blue copper protein (type 1) from Natronobacterium pharaonis. Biochemistry. 1993;32:12894–12900. doi: 10.1021/bi00210a043. [DOI] [PubMed] [Google Scholar]
- 36.Tomlinson G A, Jahnke L L, Hochstein L I. Halobacterium denitrificans sp. nov., an extreme halophilic denitrifying bacterium. Int J Syst Bacteriol. 1986;36:66–70. doi: 10.1099/00207713-36-1-66. [DOI] [PubMed] [Google Scholar]
- 37.van der Oost J, de Boer A P N, de Gier J W L, Zumft W G, Stouthamer A H, van Spanning R J B. The heme-copper oxidase family consists of three distinct types of terminal oxidases and is related to nitric oxide reductase. FEMS Microbiol Lett. 1994;121:1–10. doi: 10.1111/j.1574-6968.1994.tb07067.x. [DOI] [PubMed] [Google Scholar]
- 38.Völkl P, Huber R, Drobner E, Rachel R, Burggraf S, Trincone A, Stetter K O. Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl Environ Microbiol. 1993;59:2918–2926. doi: 10.1128/aem.59.9.2918-2926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werber M M, Mevarech M. Induction of a dissimilatory reduction pathway of nitrate in Halobacterium of the Dead Sea. A possible role for the 2 Fe-ferredoxin isolated from this organism. Arch Biochem Biophys. 1978;186:60–65. doi: 10.1016/0003-9861(78)90463-0. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimatsu K, Sakurai T, Fujiwara T. Purification and characterization of dissimilatory nitrate reductase from a denitrifying halophilic archaeon, Haloarcula marismortui. FEBS Lett. 2000;470:216–220. doi: 10.1016/s0014-5793(00)01321-1. [DOI] [PubMed] [Google Scholar]
- 41.Zumft W G. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zumft W G, Gotzmann D J, Kroneck P M H. Type 1, blue copper proteins constitute a respiratory nitrite-reducing system in Pseudomonas aureofaciens. Eur J Biochem. 1987;168:301–307. doi: 10.1111/j.1432-1033.1987.tb13421.x. [DOI] [PubMed] [Google Scholar]