Abstract
We investigated the associations of insulin resistance and β-cell secretion with bone mineral density (BMD) and osteoporosis using data from the National Health and Nutrition Examination Survey. Data on BMD assessed using dual-energy x-ray absorptiometry from 5292 participants were analyzed. Insulin resistance and β-cell secretion were assessed using the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) and β-cell function (HOMA-β), respectively. We divided the study population into four groups according to HOMA-IR (<2 vs. ≥ 2) and HOMA-β (<100 vs. ≥ 100). BMD and T score at the lumbar spine, hip joint, and femur were used for analyses. Osteoporosis was defined as a T score ≤ -2.5. Logistic regression analyses were conducted to examine the associations of HOMA-IR and HOMA-β with osteoporosis, and the joint effects of HOMA-IR and HOMA-β on osteoporosis. We found a positive association between HOMA-IR and osteoporosis in participants with a HOMA-β ≥ 100 (OR 8.773, 95% CI 2.160-35.637, p=0.002 at the femoral neck). A negative association between HOMA-β and osteoporosis was noted in those with a HOMA-IR <2 (OR 0.183, 95% CI 0.038-0.882, p=0.034 at the femoral neck). Compared with participants who had HOMA-IR <2 and HOMA-β <100, those with HOMA-IR <2 and HOMA-β ≥ 100 had a lower risk of osteoporosis (OR 0.126, 95% CI 0.020-0.805, p=0.032 at the femoral neck). In conclusion, the association between HOMA-β and BMD/osteoporosis changed as HOMA-IR increased. HOMA-β was negatively associated with osteoporosis when HOMA-IR <2. The association was not significant when HOMA-IR ≥ 2.
Keywords: bone mineral density, insulin, insulinemia, insulin resistance, osteoporosis
Introduction
Osteoporosis is characterized by low bone mass and abnormal microstructure, leading to bone fragility and susceptibility to fracture (1). The diagnosis of osteoporosis is based on an assessment of bone mineral density (BMD), which can be conducted using a modality such as dual-energy x-ray absorptiometry (DXA) (2, 3). Osteoporosis is defined as a BMD T score of ≤ -2.5 (2, 3). The likelihood of osteoporosis tends to increase as a function of age, and therefore the incidence of osteoporotic fractures has increased markedly worldwide (4, 5). It has been predicted that the number of people at high risk of osteoporotic fracture in 2040 will be doubled the number of people at high risk in 2010 (5). This may lead to a substantial health care burden and even an excess risk of mortality (5–7).
Although BMD has been used to diagnose osteoporosis, its clinical use might be limited as most fragility fractures occur in people with a BMD T score > -2.5 (8, 9). For example, patients with type 2 diabetes are at an increased risk of fracture (10, 11); however, they have a higher BMD than those without the disease (12, 13). This may be partly explained by the association of bone fragility with the pathogenesis of diabetes (14). Moreover, insulinemia and insulin resistance (IR) might have conflicting effects on bone mass. Insulinemia has been associated with a higher BMD (15, 16), while IR has been inversely associated with bone mass (17, 18). Findings in previous studies are not consistent (15–20).
The complex effects of insulinemia and IR on bone mass are not yet clear. In this study, we aimed to investigate the associations of IR and pancreatic β-cell secretion with BMD and osteoporosis using cross-sectional data from the National Health and Nutrition Examination Survey (NHANES).
Materials and methods
This study was conducted using publicly available data from the NHANES (https://www.cdc.gov/nchs/nhanes/index.htm), which is a multistage, cross-sectional, nationwide evaluation of health parameters in the U.S. conducted by the National Center for Health Statistics. Data on BMD assessed using DXA were available, and thus we investigated the associations of IR and pancreatic β-cell secretion with BMD and osteoporosis in this population. Our study protocol was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan (approval number: CE18312A). We conducted this study in accordance with the Declaration of Helsinki, and all the NHANES participants provided informed consent.
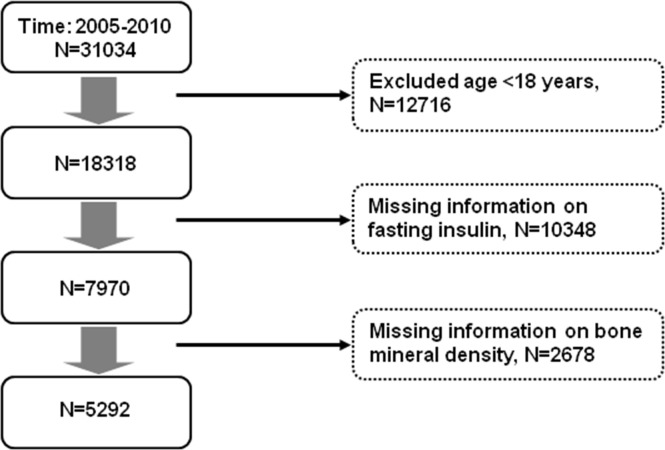
Figure 1 shows selection of the study population. Among the 31034 participants in the NHANES from 2005 to 2010, we excluded those aged <18 years or had missing data on fasting insulin or BMD. Finally, we had 5292 participants for analyses. We assessed IR and β-cell secretion of the study population using the Homeostatic Model Assessment (21) for Insulin Resistance (HOMA-IR) and β-cell function (HOMA-β), respectively. HOMA-IR = fasting insulin [μU/l] * fasting glucose [mmol/l]/22.5. HOMA-β = 20 * fasting insulin [μU/l]/(fasting glucose [mmol/l] - 3.5). We divided the study population into four groups according to HOMA-IR (<2 vs. ≥ 2) and HOMA-β (<100 vs. ≥ 100). The cut-off values were decided according to previous studies. Insulin resistance (assessed using HOMA-IR) was associated with low bone mass in men (22) and women (19). The mean HOMA-IR in the high insulin resistance group was 2.0-2.2 (19, 20). In a multi-ethnic study (23) investigating insulin resistance and pancreatic β-cell function, the median HOMA-IR was 1.8-2.2 while the median HOMA-β was 100-120. Hence, we used the cut-off values of HOMA-IR and HOMA-β as 2 and 100, respectively. We used the Chronic Kidney Disease Epidemiology Collaboration equation (24) to determine renal function (estimated glomerular filtration rate, eGFR). An eGFR < 60 ml/min/1.73 m2 was considered to be chronic kidney disease. BMD and T score at the lumbar spine, hip joint, and various parts of the femur (femoral neck, greater trochanter, and femoral intertrochanter) were used for analyses. Osteoporosis was defined as a T score ≤ -2.5 (2, 3).
Figure 1.
Selection of study participants for analyses.
All analyses were conducted using the Statistical Analysis System survey procedures (SAS version 9.4, 2013, Cary, NC, USA). Analysis of Variance (ANOVA) and Chi-square test were used to examine the differences in baseline characteristics, BMD, and T score across the four groups (HOMA-IR <2 and HOMA-β <100, HOMA-IR <2 and HOMA-β ≥ 100, HOMA-IR ≥ 2 and HOMA-β <100, HOMA-IR ≥ 2 and HOMA-β ≥ 100). We used the SAS SURVEYREG procedure to perform the sample-weighted analysis of variance test according to the user’s guide of the analysis program. To examine the associations of HOMA-IR and HOMA-β with osteoporosis, logistic regression analyses were conducted using osteoporosis (T-score ≤ -2.5) as the dependent variable with adjustment for confounding factors (age, sex, race, body mass index, and chronic kidney disease). To examine the joint effects of HOMA-IR and HOMA-β on osteoporosis, logistic regression analyses were conducted using participants with HOMA-IR <2 and HOMA-β <100 as the reference group. Logistic regression was adequately weighted using the SURVEYLOGISTIC procedure. Due to the complex survey design of NHANES, we calculated the weighted data according to analytic guidelines [National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2014 and 2015–2016 (https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx)]. A two-sided p value less than 0.05 was considered to be statistically significant in all of the statistical analyses.
Results
Table 1 shows the characteristics of the study population according to HOMA-IR and HOMA-β. Participants who had a HOMA-IR ≥ 2 were older and more likely to be male, had a higher body mass index, a higher systolic and diastolic blood pressure, and a higher proportion of having diabetes, compared with those who had a HOMA-IR <2. The former group also had worse metabolic profiles (lower high-density lipoprotein cholesterol, higher triglycerides, fasting plasma glucose, and HbA1c) than the latter.
Table 1.
Characteristics of the study participants according to HOMA-IR and HOMA-β.
| Variables | HOMA-IR <2, HOMA-β <100 | HOMA-IR <2, HOMA-β ≥100 | HOMA-IR ≥2, HOMA-β <100 | HOMA-IR ≥2, HOMA-β ≥100 | P-value |
|---|---|---|---|---|---|
| N | 1856 | 288 | 961 | 2187 | |
| Age, year | 43.7 (42.6-44.7) | 34.9 (33.4-36.4) | 50.2 (48.7-51.6) | 42.2 (41.1-43.3) | <0.001 |
| Male, n (%) | 993 (53.5) | 86 (29.9) | 569 (59.2) | 1103 (50.4) | <0.001 |
| Race/ethnicity, n (%) | <0.001 | ||||
| Non-Hispanic white | 1000 (53.9) | 108 (37.5) | 420 (43.7) | 859 (39.3) | <0.001 |
| Non-Hispanic black | 351 (18.9) | 74 (25.7) | 165 (17.2) | 457 (20.9) | <0.001 |
| Mexican American/others | 505 (27.2) | 106 (36.8) | 376 (39.1) | 871 (39.8) | <0.001 |
| Body mass index, kg/m2 | 24.5 (24.3-24.7) | 25.9 (25.2-26.6) | 28.7 (28.3-29.1) | 30.6 (30.2-31.0) | <0.001 |
| Systolic blood pressure, mm Hg | 117.8 (117.0-118.7) | 113.0 (111.0-115.0) | 125.6 (124.3-126.9) | 121.2 (120.1-122.3) | <0.001 |
| Diastolic blood pressure, mm Hg | 68.5 (67.7-69.4) | 65.9 (64.2-67.7) | 71.0 (69.8-72.2) | 71.0 (70.2-71.9) | <0.001 |
| Smoking, n (%) | 846 (45.6) | 76 (26.4) | 455 (47.3) | 861 (39.4) | <0.001 |
| Chronic kidney disease, n (%)a | 37 (2.0) | 2 (0.7) | 55 (5.7) | 51 (2.3) | <0.001 |
| Hypertension, n (%) | 377 (20.3) | 37 (12.8) | 426 (44.3) | 630 (28.8) | <0.001 |
| Diabetes, n (%) | 99 (5.3) | 4 (1.4) | 354 (36.8) | 170 (7.8) | <0.001 |
| Total cholesterol, mg/dl | 195.4 (192.9-197.8) | 190.7 (185.6-195.9) | 199.2 (195.7-202.7) | 195.6 (193.2-197.9) | <0.001 |
| HDL cholesterol, mg/dl | 61.6 (60.8-62.4) | 57.2 (55.1-59.3) | 51.0 (49.5-52.5) | 48.0 (47.4-48.6) | <0.001 |
| Triglycerides, mg/dl | 94.1 (89.9-98.4) | 96.0 (88.7-103.4) | 152.3 (139.1-165.5) | 151.4 (144.6-158.1) | <0.001 |
| Fasting plasma glucose, mg/dl | 90.9 (89.5-92.2) | 79.3 (78.2-80.4) | 122.1 (118.4-125.9) | 95.5 (94.8-96.2) | <0.001 |
| HbA1c, % | 5.3 (5.3-5.4) | 5.2 (5.1-5.2) | 6.1 (6.0-6.2) | 5.4 (5.4-5.5) | <0.001 |
| eGFR, mL/min/1.73 m2 | 97.7 (96.4-98.9) | 105.0 (102.4-107.6) | 93.4 (91.6-95.3) | 100.0 (98.5-101.5) | <0.001 |
| HOMA-IR | 1.2 (1.2-1.2) | 1.6 (1.5-1.6) | 3.8 (3.5-4.0) | 5.0 (4.8-5.2) | <0.001 |
| HOMA-β | 57.6 (56.4-58.9) | 156.6 (128.4-184.7) | 72.5 (70.8-74.1) | 189.9 (182.6-197.1) | <0.001 |
Data are presented as mean (95% CI) or n (%). eGFR, estimated glomerular filtration rate. HbA1c, glycated hemoglobin. HDL, high-density lipoprotein. HOMA-IR, homeostasis model assessment-insulin resistance. aeGFR< 60 mL/min/1.73 m2.
Table 2 shows the BMD and T score at various sites (L-spine, hip, and femur) according to HOMA-IR and HOMA-β. Participants with a HOMA-IR ≥ 2 and a HOMA-β ≥ 100 had the highest BMD at L-spine, hip, and femur (all p<0.001) among the four groups. However, they had the lowest BMD at all sites after adjustment for age, sex, race, body mass index, and chronic kidney disease. In contrast, participants with a HOMA-IR <2 and a HOMA-β ≥ 100 had the highest BMD at L-spine, hip, and trochanter and intertrochanter of the femur after adjustment for the confounders. Similar findings were noted regarding the T score ( Table 2 ).
Table 2.
BMD and T score of the study participants according to HOMA-IR and HOMA-β.
| HOMA-IR <2, HOMA-β <100 | HOMA-IR <2, HOMA-β ≥100 | HOMA-IR ≥2, HOMA-β <100 | HOMA-IR ≥2, HOMA-β ≥100 | P-value | |
|---|---|---|---|---|---|
| BMD | |||||
| Lumbar spine | 1.020 (1.012, 1.027) | 1.050 (1.032, 1.068) | 1.045 (1.032, 1.057) | 1.051 (1.043, 1.059) | <0.001 |
| Total hip | 0.951 (0.943, 0.958) | 0.986 (0.967, 1.006) | 0.989 (0.975, 1.004) | 1.019 (1.012, 1.027) | <0.001 |
| Femoral neck | 0.823 (0.815, 0.832) | 0.869 (0.852, 0.887) | 0.846 (0.833, 0.859) | 0.884 (0.876, 0.892) | <0.001 |
| Greater trochanter | 0.720 (0.713, 0.727) | 0.742 (0.726, 0.759) | 0.750 (0.737, 0.762) | 0.765 (0.757, 0.772) | <0.001 |
| Femoral intertrochanter | 1.119 (1.111, 1.127) | 1.160 (1.138, 1.183) | 1.167 (1.150, 1.183) | 1.200 (1.192, 1.209) | <0.001 |
| Adjusted BMDa | |||||
| Lumbar spine | 1.041 (1.028, 1.054)c | 1.055 (1.036, 1.075)c | 1.047 (1.032, 1.062)c | 1.029 (1.014, 1.044) | 0.005 |
| Total hip | 0.979 (0.969, 0.989) | 0.994 (0.976, 1.012)c | 0.983 (0.970, 0.997) | 0.974 (0.962, 0.985) | 0.072 |
| Femoral neck | 0.854 (0.845, 0.862) | 0.859 (0.844, 0.875) | 0.860 (0.848, 0.872) | 0.848 (0.838, 0.859) | 0.211 |
| Greater trochanter | 0.735 (0.725, 0.744)c | 0.746 (0.730, 0.762)c | 0.738 (0.726, 0.751) | 0.726 (0.715, 0.737) | 0.008 |
| Femoral intertrochanter | 1.155 (1.142, 1.168) | 1.175 (1.151, 1.198)bc | 1.160 (1.143, 1.177) | 1.150 (1.137, 1.164) | 0.090 |
| T score | |||||
| Lumbar spine | -1.503 (-1.565, -1.440) | -1.180 (-1.325, -1.034) | -1.327 (-1.432, -1.221) | -1.248 (-1.315, -1.182) | <0.001 |
| Total hip | -0.306 (-0.353, -0.259) | 0.129 (-0.000, 0.258) | -0.083 (-0.182, 0.016) | 0.202 (0.144, 0.261) | <0.001 |
| Femoral neck | -0.564 (-0.623, -0.505) | -0.090 (-0.214, 0.035) | -0.432 (-0.531, -0.334) | -0.098 (-0.162, -0.034) | <0.001 |
| Greater trochanter | -0.246 (-0.302, -0.191) | 0.103 (-0.030, 0.236) | -0.034 (-0.138, 0.070) | 0.162 (0.093, 0.230) | <0.001 |
| Femoral intertrochanter | -0.207 (-0.252, -0.163) | 0.236 (0.106, 0.366) | 0.026 (-0.073, 0.124) | 0.304 (0.250, 0.358) | <0.001 |
| Adjusted T scorea | |||||
| Lumbar spine | -1.323 (-1.430, -1.216)c | -1.205 (-1.369, -1.041)c | -1.277 (-1.401, -1.153)c | -1.423 (-1.548, -1.298) | 0.005 |
| Total hip | -0.101 (-0.176, -0.025) | 0.020 (-0.115, 0.155)bc | -0.060 (-0.162, 0.043) | -0.129 (-0.216, -0.042) | 0.071 |
| Femoral neck | -0.333 (-0.399, -0.267) | -0.280 (-0.399, -0.161) | -0.278 (-0.369, -0.186) | -0.368 (-0.452, -0.284) | 0.191 |
| Greater trochanter | -0.122 (-0.210, -0.033) | -0.011 (-0.161, 0.138)c | -0.078 (-0.192, 0.035) | -0.187 (-0.288, -0.087) | 0.014 |
| Femoral intertrochanter | 0.017 (-0.064, 0.098) | 0.154 (0.001, 0.306)bc | 0.057 (-0.053, 0.168) | -0.002 (-0.090, 0.087) | 0.084 |
Data are presented as mean (95% CI). BMD, bone mineral density. HOMA-IR, homeostasis model assessment-insulin resistance. aAdjusted for age, sex, race, body mass index, and chronic kidney disease. bp<0.05 vs. HOMA-IR <2, HOMA-β <100. cp<0.05 vs. HOMA-IR ≥2, HOMA-β ≥100.
The association between HOMA-IR and osteoporosis is shown in Table 3 . Overall, there was no significant association between HOMA-IR and osteoporosis. The findings were similar in participants with a HOMA-β <100. Nevertheless, we observed a positive association between HOMA-IR and osteoporosis in participants who had a HOMA-β ≥ 100, especially at the femoral neck (OR 8.773, 95% CI 2.160-35.637, p=0.002, p interaction 0.010, ( Table 3 ).
Table 3.
Association of HOMA-IR with osteoporosisa.
| Overall | HOMA-β <100 | HOMA-β ≥100 | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI)b | P | OR (95% CI)b | P | OR (95% CI)b | P | P for interaction | |
| Lumbar spine | 1.109 (0.903-1.362) | 0.325 | 1.047 (0.781-1.403) | 0.761 | 1.248 (0.785-1.984) | 0.349 | 0.176 |
| Total hip | 0.788 (0.396-1.568) | 0.498 | 0.553 (0.227-1.347) | 0.192 | 2.601 (0.780-8.670) | 0.120 | 0.188 |
| Femoral neck | 1.270 (0.834-1.934) | 0.265 | 1.072 (0.669-1.717) | 0.774 | 8.773 (2.160-35.637) | 0.002 | 0.010 |
| Greater trochanter | 1.230 (0.584-2.589) | 0.586 | 0.935 (0.396-2.203) | 0.877 | 3.016 (0.439-20.726) | 0.262 | 0.238 |
| Femoral intertrochanter | 0.654 (0.298-1.437) | 0.291 | 0.492 (0.167-1.454) | 0.200 | 2.851 (0.212-38.264) | 0.429 | 0.089 |
HOMA-IR, homeostasis model assessment-insulin resistance. aT score ≤ -2.5. bAdjusted for age, sex, race, body mass index, and chronic kidney disease.
Table 4 shows the association between HOMA-β and osteoporosis. There was no significant association between HOMA-β and osteoporosis in the overall population. In contrast to the non-significant association in participants who had a HOMA-IR ≥ 2, we observed a negative association between HOMA-β and osteoporosis in participants with a HOMA-IR <2 (especially at the femoral neck, OR 0.183, 95% CI 0.038-0.882, p=0.034, p interaction 0.010, Table 4 ).
Table 4.
Association of HOMA-β with osteoporosisa.
| Overall | HOMA-IR <2 | HOMA-IR ≥2 | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI)b | P | OR (95% CI)b | P | OR (95% CI)b | P | P for interaction | |
| Lumbar spine | 1.107 (0.887-1.382) | 0.369 | 0.876 (0.495-1.552) | 0.650 | 1.128 (0.873-1.457) | 0.358 | 0.176 |
| Total hip | 0.959 (0.474-1.939) | 0.906 | 0.554 (0.130-2.350) | 0.423 | 1.208 (0.603-2.420) | 0.594 | 0.188 |
| Femoral neck | 1.128 (0.605-2.105) | 0.704 | 0.183 (0.038-0.882) | 0.034 | 1.118 (0.505-2.474) | 0.783 | 0.010 |
| Greater trochanter | 1.123 (0.608-2.076) | 0.711 | 0.473 (0.130-1.728) | 0.258 | 1.197 (0.555-2.581) | 0.647 | 0.238 |
| Femoral intertrochanter | 0.865 (0.447-1.673) | 0.667 | 0.250 (0.033-1.888) | 0.179 | 1.386 (0.650-2.957) | 0.398 | 0.089 |
HOMA-IR, homeostasis model assessment-insulin resistance. aT score ≤ -2.5. bAdjusted for age, sex, race, body mass index, and chronic kidney disease.
Table 5 shows the joint effect of HOMA-IR and HOMA-β on osteoporosis. Compared with participants who had HOMA-IR <2 and HOMA-β <100, those with HOMA-IR <2 and HOMA-β ≥ 100 had a lower risk of osteoporosis (OR 0.126, 95% CI 0.020-0.805, p=0.032 at the femoral neck). This was not the case in participants with HOMA-IR ≥ 2. With regard to osteoporosis at the femoral neck, HOMA-IR ≥ 2 was associated with a higher risk compared with HOMA-IR <2 and HOMA-β ≥ 100. The risk was even higher in participants with HOMA-IR ≥ 2 and HOMA-β ≥ 100 ( Table 5 ).
Table 5.
Joint effect of HOMA-IR and HOMA-β on osteoporosisa.
| HOMA-IR <2, HOMA-β <100 | HOMA-IR <2, HOMA-β ≥100 | HOMA-IR ≥2, HOMA-β <100 | HOMA-IR ≥2, HOMA-β ≥100 | |
|---|---|---|---|---|
| Lumbar spine | Ref | 0.826 (0.457-1.493) | 0.986 (0.755-1.287) | 1.145 (0.897-1.460) |
| Total hip | Ref | 0.433 (0.091-2.053) | 0.599 (0.259-1.389) | 0.766 (0.344-1.706) |
| Femoral neck | Ref | 0.126 (0.020-0.805) | 1.070 (0.656-1.744)b | 1.256 (0.625-2.526)b |
| Greater trochanter | Ref | 0.427 (0.103-1.778) | 1.036 (0.438-2.448) | 1.278 (0.554-2.945) |
| Femoral intertrochanter | Ref | 0.225 (0.028-1.796) | 0.493 (0.175-1.392) | 0.827 (0.381-1.796) |
Data are presented as OR (95% CI), adjusted for age, sex, race, body mass index, smoking, hypertension, and chronic kidney disease. HOMA-IR, homeostasis model assessment-insulin resistance. aT score ≤ -2.5. bp<0.05 vs. HOMA-IR <2, HOMA-β ≥100.
Discussion
In this cross-sectional study using data from the NHANES, we demonstrated that HOMA-β ≥ 100 with HOMA-IR <2 was associated with a higher BMD and T score, as well as a lower risk of osteoporosis (vs. HOMA-β <100 with HOMA-IR <2). In contrast, HOMA-β ≥ 100 with HOMA-IR ≥ 2 was associated with lower BMD and T scores (vs. HOMA-β ≥ 100 with HOMA-IR <2). Our findings suggest that the association between insulinemia and BMD/T score might be different depending on an individual’s IR status.
Previous studies revealed that insulin plays an important role in the anabolic effect on bone mass and trabecular bone microarchitecture (15, 16, 25, 26). Increased proliferation and collagen synthesis in response to insulin treatment were noted in in vitro studies using cultured osteoblasts (27–29). Moreover, insulin may exert synergistic effects with insulin-like growth factor 1 and parathyroid hormone (30, 31), both of which have anabolic effects on bone cells. These findings are in line with previous studies using insulin-deficient animal models in which reduced bone formation was noted (32, 33). Furthermore, insulin treatment might reverse the deficiency (34, 35). The aforementioned results are supported by the findings of decreased bone mass in patients with type 1 diabetes (36–38).
Nevertheless, there are conflicting findings in type 2 diabetes. People with type 2 diabetes have a higher BMD, but a higher risk of fracture, than those without the disease (39–41). This phenomenon suggests that IR may influence the effects of insulinemia on bone mass. Shin D, et al. (22) reported different associations between insulinemia and BMD at various levels of HOMA-IR in men. Consistent with our findings, fasting insulin level was positively associated with BMD at low HOMA-IR. However, a negative association was observed at a higher IR state. Similarly, fasting insulin was positively associated with BMD in adolescents in a cross-sectional study (42). Nevertheless, an inverse association was noted after adjustment for fat mass. Hence, IR may affect the association between insulinemia and bone mass, and investigations on different populations may yield inconsistent results (15–20). We examined the joint effect of HOMA-IR and HOMA-β on osteoporosis ( Table 5 ). Our findings suggest that HOMA-β ≥ 100 with HOMA-IR <2 was associated with a lower risk of osteoporosis. In contrast, the risk increased with HOMA-β ≥ 100 and HOMA-IR ≥ 2.
It is interesting to note that the associations among HOMA-β, HOMA-IR, and BMD/osteoporosis were more apparent at the femoral neck ( Table 3 – 5 ). IR has been negatively associated with cortical bone volume and bone strength at the femoral neck in postmenopausal women (18). Similar findings were noted in a general population (43), although the negative association between IR and BMD at the femoral neck became non-significant after adjustment for body mass index. The associations among HOMA-IR, HOMA-β, and BMD/osteoporosis at different anatomic sites merit further investigation (44).
Our study had several limitations. First, we investigated the associations among HOMA-IR, HOMA-β, and BMD/osteoporosis using cross-sectional data. Thus, the causal relationships could not be confirmed. Second, some relevant factors (such as diet and exercise) were not analyzed. This might have confounded our results. Third, we did not have data on fracture events. We could not confirm that the associations among HOMA-IR, HOMA-β, and BMD/osteoporosis were linked to risk of fracture. Despite these limitations, we demonstrated a joint effect of HOMA-IR and HOMA-β on osteoporosis which may help explain the inconsistent findings in populations with high variations in IR and pancreatic β-cell function (15–20, 45).
In conclusion, the association between insulinemia (HOMA-β) and BMD/osteoporosis changed as IR (HOMA-IR) increased. HOMA-β was negatively associated with osteoporosis at a low level of HOMA-IR (<2). The association was not significant when HOMA-IR ≥ 2. The mechanisms by which IR affects the association between insulinemia and osteoporosis merit further investigation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Dietary&CycleBeginYear=2005.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan. The patients/participants provided their written informed consent to participate in this study.
Author contributions
C-LL and J-SW contributed to conception and design of the study. Y-HF and W-JL organized the database. W-JL and C-LL performed the statistical analysis. Y-HF and J-SW wrote the first draft of the manuscript. W-JL and C-LL reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Taichung Veterans General Hospital [grant number TCVGH-1093504C, TCVGH-1097316C, TCVGH-1097327D, TCVGH-1103502C, and TCVGH-1113502C]. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Acknowledgments
We would like to thank the participants in the NHANES, and the members of the National Center for Health Statistics for collecting the data and making it publicly available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3 [DOI] [PubMed] [Google Scholar]
- 2. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med (1993) 94(6):646–50. doi: 10.1016/0002-9343(93)90218-E [DOI] [PubMed] [Google Scholar]
- 3. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet (2011) 377:1276–87. doi: 10.1016/S0140-6736(10)62349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Odén A, McCloskey EV, Johansson H, Kanis JA. Assessing the impact of osteoporosis on the burden of hip fractures. Calcif Tissue Int (2013) 92:42–9. doi: 10.1007/s00223-012-9666-6 [DOI] [PubMed] [Google Scholar]
- 5. Odén A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int (2015) 26:2243–8. doi: 10.1007/s00198-015-3154-6 [DOI] [PubMed] [Google Scholar]
- 6. Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet (1999) 353:878–82. doi: 10.1016/S0140-6736(98)09075-8 [DOI] [PubMed] [Google Scholar]
- 7. Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA (2009) 301:513–21. doi: 10.1001/jama.2009.50 [DOI] [PubMed] [Google Scholar]
- 8. Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone (2004) 34:195–202. doi: 10.1016/j.bone.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 9. Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med (2004) 164:1108–12. doi: 10.1001/archinte.164.10.1108 [DOI] [PubMed] [Google Scholar]
- 10. Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res (2008) 23:1334–42. doi: 10.1359/jbmr.080323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol (2007) 166:495–505. doi: 10.1093/aje/kwm106 [DOI] [PubMed] [Google Scholar]
- 12. van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A, et al. Bone density in non-insulin-dependent diabetes mellitus. Rotterdam Study Ann Intern Med (1995) 122:409–14. doi: 10.7326/0003-4819-122-6-199503150-00002 [DOI] [PubMed] [Google Scholar]
- 13. Hanley DA, Brown JP, Tenenhouse A, Olszynski WP, Ioannidis G, Berger C, et al. Canadian Multicentre osteoporosis study research group. associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: Cross-sectional results from the Canadian multicentre osteoporosis study. J Bone Miner Res (2003) 18:784–90. doi: 10.1359/jbmr.2003.18.4.784 [DOI] [PubMed] [Google Scholar]
- 14. Hofbauer LC, Busse B, Eastell R, Ferrari S, Frost M, Müller R, et al. Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol (2022) 10:207–20. doi: 10.1016/S2213-8587(21)00347-8 [DOI] [PubMed] [Google Scholar]
- 15. Barrett-Connor E, Kritz-Silverstein D. Does hyperinsulinemia preserve bone? Diabetes Care (1996) 19:1388–92. doi: 10.2337/diacare.19.12.1388 [DOI] [PubMed] [Google Scholar]
- 16. Stolk RP, Van Daele PL, Pols HA, Burger H, Hofman A, Birkenhäger JC, et al. Hyperinsulinemia and bone mineral density in an elderly population: The Rotterdam study. Bone (1996) 18:545–9. doi: 10.1016/8756-3282(96)00079-8 [DOI] [PubMed] [Google Scholar]
- 17. Choo MS, Choi SR, Han JH, Lee SH, Shim YS. Association of insulin resistance with near peak bone mass in the femur and lumbar spine of Korean adults aged 25-35: The Korean national health and nutrition examination survey 2008-2010. PloS One (2017) 12:e0177311. doi: 10.1371/journal.pone.0177311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang J, Hong N, Shim JS, Rhee Y, Kim HC. Association of insulin resistance with lower bone volume and strength index of the proximal femur in nondiabetic postmenopausal women. J Bone Metab (2018) 25:123–32. doi: 10.11005/jbm.2018.25.2.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW. Association between insulin resistance and bone structure in nondiabetic postmenopausal women. J Clin Endocrinol Metab (2016) 101:3114–22. doi: 10.1210/jc.2016-1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Napoli N, Conte C, Pedone C, Strotmeyer ES, Barbour KE, Black DM, et al. Effect of insulin resistance on BMD and fracture risk in older adults. J Clin Endocrinol Metab (2019) 104:3303–10. doi: 10.1210/jc.2018-02539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28:412–9. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 22. Shin D, Kim S, Kim KH, Lee K, Park SM. Association between insulin resistance and bone mass in men. J Clin Endocrinol Metab (2014) 99:988–95. doi: 10.1210/jc.2013-3338 [DOI] [PubMed] [Google Scholar]
- 23. Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJ, et al. Understanding the high prevalence of diabetes in U.S. south asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care (2014) 37:1621–8. doi: 10.2337/dc13-2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. CKD-EPI (Chronic kidney disease epidemiology collaboration). a new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khosla S, Samakkarnthai P, Monroe DG, Farr JN. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat Rev Endocrinol (2021) 17:685–97. doi: 10.1038/s41574-021-00555-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samakkarnthai P, Sfeir JG, Atkinson EJ, Achenbach SJ, Wennberg PW, Dyck PJ, et al. Determinants of bone material strength and cortical porosity in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab (2020) 105:e3718–29. doi: 10.1210/clinem/dgaa388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hashizume M, Yamaguchi M. Stimulatory effect of beta-alanyl-L-histidinato zinc on cell proliferation is dependent on protein synthesis in osteoblastic MC3T3-E1 cells. Mol Cell Biochem (1993) 122:59–64. doi: 10.1007/BF00925737 [DOI] [PubMed] [Google Scholar]
- 28. Canalis EM, Dietrich JW, Maina DM, Raisz LG. Hormonal control of bone collagen synthesis in vitro. Effects Insulin Glucagon Endocrinol (1977) 100:668–74. doi: 10.1210/endo-100-3-668 [DOI] [PubMed] [Google Scholar]
- 29. Rosen DM, Luben RA. Multiple hormonal mechanisms for the control of collagen synthesis in an osteoblast-like cell line, MMB-1. Endocrinology (1983) 112:992–9. doi: 10.1210/endo-112-3-992 [DOI] [PubMed] [Google Scholar]
- 30. Conover CA, Lee PD, Riggs BL, Powell DR. Insulin-like growth factor-binding protein-1 expression in cultured human bone cells: regulation by insulin and glucocorticoid. Endocrinology (1996) 137:3295–301. doi: 10.1210/endo.137.8.8754754 [DOI] [PubMed] [Google Scholar]
- 31. Suzuki K, Miyakoshi N, Tsuchida T, Kasukawa Y, Sato K, Itoi E. Effects of combined treatment of insulin and human parathyroid hormone(1-34) on cancellous bone mass and structure in streptozotocin-induced diabetic rats. Bone (2003) 33:108–14. doi: 10.1016/S8756-3282(03)00169-8 [DOI] [PubMed] [Google Scholar]
- 32. Verhaeghe J, van Herck E, Visser WJ, Suiker AM, Thomasset M, Einhorn TA, et al. Bone and mineral metabolism in BB rats with long-term diabetes. Decreased Bone Turnover Osteoporosis Diabetes (1990) 39:477–82. doi: 10.2337/diab.39.4.477 [DOI] [PubMed] [Google Scholar]
- 33. Verhaeghe J, Suiker AM, Visser WJ, Van Herck E, Van Bree R, Bouillon R. The effects of systemic insulin, insulin-like growth factor-I and growth hormone on bone growth and turnover in spontaneously diabetic BB rats. J Endocrinol (1992) 134:485–92. doi: 10.1677/joe.0.1340485 [DOI] [PubMed] [Google Scholar]
- 34. Hou JC, Zernicke RF, Barnard RJ. Effects of severe diabetes and insulin on the femoral neck of the immature rat. J Orthop Res (1993) 11:263–71. doi: 10.1002/jor.1100110214 [DOI] [PubMed] [Google Scholar]
- 35. Follak N, Klöting I, Merk H. Influence of diabetic metabolic state on fracture healing in spontaneously diabetic rats. Diabetes Metab Res Rev (2005) 21:288–96. doi: 10.1002/dmrr.537 [DOI] [PubMed] [Google Scholar]
- 36. Mathiassen B, Nielsen S, Johansen JS, Hartwell D, Ditzel J, Rødbro P, et al. Long-term bone loss in insulin-dependent diabetic patients with microvascular complications. J Diabetes Complications (1990) 4:145–9. doi: 10.1016/0891-6632(90)90012-T [DOI] [PubMed] [Google Scholar]
- 37. Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest (2000) 23:295–303. doi: 10.1007/BF03343726 [DOI] [PubMed] [Google Scholar]
- 38. Tuominen JT, Impivaara O, Puukka P, Rönnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care (1999) 22:1196–200. doi: 10.2337/diacare.22.7.1196 [DOI] [PubMed] [Google Scholar]
- 39. de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam study. Osteoporos Int (2005) 16:1713–20. doi: 10.1007/s00198-005-1909-1 [DOI] [PubMed] [Google Scholar]
- 40. Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med (2005) 165:1612–7. doi: 10.1001/archinte.165.14.1612 [DOI] [PubMed] [Google Scholar]
- 41. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int (2007) 18:427–44. doi: 10.1007/s00198-006-0253-4 [DOI] [PubMed] [Google Scholar]
- 42. Lawlor DA, Sattar N, Sayers A, Tobias JH. The association of fasting insulin, glucose, and lipids with bone mass in adolescents: findings from a cross-sectional study. J Clin Endocrinol Metab (2012) 97:2068–76. doi: 10.1210/jc.2011-2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Srikanthan P, Crandall CJ, Miller-Martinez D, Seeman TE, Greendale GA, Binkley N, et al. Insulin resistance and bone strength: findings from the study of midlife in the united states. J Bone Miner Res (2014) 29:796–803. doi: 10.1002/jbmr.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen C, Chen Q, Nie B, Zhang H, Zhai H, Zhao L, et al. Trends in bone mineral density, osteoporosis, and osteopenia among U.S. adults with prediabetes, 2005-2014. Diabetes Care (2020) 43:1008–15. doi: 10.2337/dc19-1807 [DOI] [PubMed] [Google Scholar]
- 45. Thrailkill KM, Lumpkin CK, Jr, Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab (2005) 289:E735–45. doi: 10.1152/ajpendo.00159.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Dietary&CycleBeginYear=2005.