Abstract
Toxoplasma gondii was isolated in mice from different tissues of a captive black-and-gold howler monkey (Alouatta caraya) kept in a colony at the Primatology Center of Rio de Janeiro State, Brazil, and it was genotypically characterized based on using PCR-RFLP and Microsatellite Analysis (MS), later on. T. gondii was successfully isolated from inocula deriving from heart, liver and tissue pool (heart, liver, lungs, axillary lymph nodes and cerebellum) samples. The isolate was named TgBgHmBrRJ1. The high virulence of the aforementioned strain was observed in infected mice. Non-archetypal genotype (ToxoDB PCR-RFLP #206) was obtained through PCR-RFLP. This genotype had been previously described in 12 isolates from different hosts, also in Southeastern Brazil, a fact that indicates likely high circulation of this genotype in this region. The isolate was also classified as non-archetypal, based on MS genotyping, as well as presented genotypic identity close to that of strains isolated from free-range non-symptomatic chickens (TgCkBr244,245,278,279) in Espírito Santo State. It is worth emphasizing that despite the large number of reports about clinical toxoplasmosis in neotropical primates in Brazil, this is just the second isolate of this parasite ever reported in this group of animals.
Keywords: Neotropical primates. toxoplasmosis. bioassays. genotyping
Graphical abstract
Highlights
-
•
Toxoplasma gondii was isolated in a murine model from Alouatta caraya in captivity.
-
•
An intermediate virulence of the isolated in the infected murine model was verified.
-
•
A non-archetypal genotype (ToxoDB PCR-RFLP #206) was obtained by PCR-RFLP.
-
•
This is the second isolation of T. gondii from a non-human primate in Brazil.
-
•
For the first time, this genotype was associated with clinical disease in a host.
1. Introduction
Brazil holds the greatest neotropical non-human primate (NHP) biodiversity in the world (Rylands and Mittermeier, 2009). Among these primates, howler monkeys (Alouatta spp.) are classified as threatened species in major Brazilian ecosystems (IUCN, 2020). Several parasites can infect neotropical non-human primates (NHP) and lead to symptomatic infections with different prognoses; Toxoplasma gondii stands out among these biological agents (Catão-Dias et al., 2013).
Toxoplasmosis is a zoonotic infection caused by T. gondii that affects mammals and birds worldwide (Dubey, 2010). Cats and wild felids are definitive hosts of this protozoan, because they can shed resistant oocysts in the environment through their feces. Domestic and wild mammals, as well as birds, are intermediate hosts that can develop cysts in their tissues in chronic infections (Dubey et al., 2020). Clinical toxoplasmosis is associated with host factors, such as immune status and genetic profile, as well as with parasite factors, such as dose, stage and strain (Dubey, 2010). Neotropical NHPs are one of the mammalian groups mostly susceptible to T. gondii infection and they often develop fatal disease (Epiphanio et al., 2003; Catão-Dias et al., 2013; Dubey et al., 2021). Reports of acute toxoplasmosis in captive neotropical NHPs were found in Brazil (Santos et al., 2013; Paula et al., 2020; Santana et al., 2021).
Toxoplasma gondii presents high genetic diversity in Central and South America, where almost 200 genotypes were already identified, whereas only few types of it, mainly classical types II and III, circulate in North America, Europe and Asia, (Shwab et al., 2014). In total, 177 PCR-RFLP genotypes were identified in Brazil among almost 720 samples genotyped from domestic and wild animals, as well as from humans, in dozens of published studies - BrI, BrII and BrIII are the most prevalent clonal Brazilian lineage types (Pena et al., 2008).
Given the vulnerability of howler monkeys, good sanitary management practices, such as taking preventive measures against different pathogens, are of paramount importance to enable the conservation and maintenance of these animals in captivity. Despite the high genetic diversity of T. gondii strains in Brazil, reports on its isolation from neotropical primates remain scarce. The aim of the current report was to describe T. gondii isolation in mice bioassays, as well as the genotypic strain characterization of this protozoan in a captive howler monkey from Rio de Janeiro State, Brazil.
2. Methods
2.1. Sample collection and T. gondii isolation
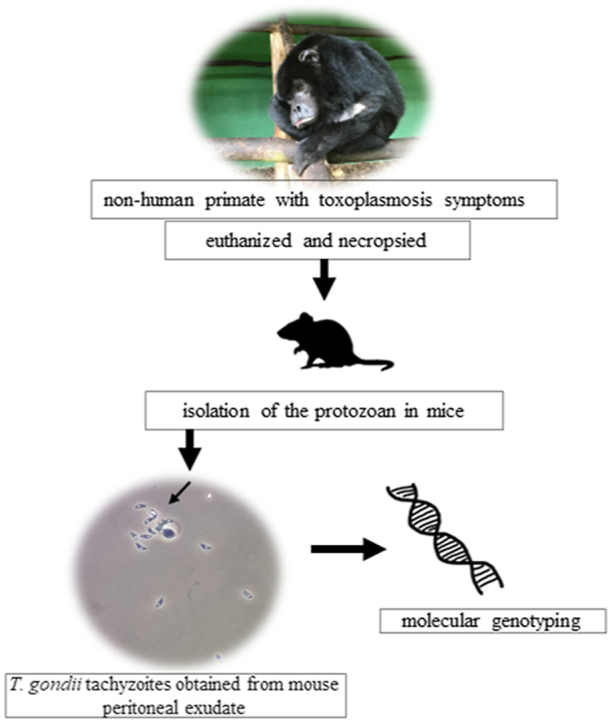
Samples were collected from a 10-year-old male black-and-gold howler monkey (Alouatta caraya) kept at the Primatology Center of Rio de Janeiro State, Brazil. The aforementioned primate presented fever, prostration, inappetence, abdominal distension and pain, intestinal hypomotility and weight loss, and it was under medical treatment for suspected toxoplasmosis. It was euthanized after 36 days of treatment, when it did not show significant clinical improvement. All descriptions comprising symptoms, anti-T. gondii serology, treatment, disease evolution and post-mortem macroscopic and microscopic lesions were previously reported by Moreira et al. (2022). T. gondii isolation attempts were performed based on using 33g of liver, 19.8g of heart, 13.6g of lungs, 2g of axillary lymph nodes and 1.7g of cerebellum, due to suspected toxoplasmosis in the herein investigated primate. Tissue samples were digested in acid pepsin solution, according to Dubey (1998). Digested samples of each tissue, in separate, and a pooled tissue homogenate (comprising all tissues mentioned above) were subcutaneously inoculated in fourteen female Swiss Webster mice in the age group 25-to-30 days, who were divided into six different groups, as follows: cerebellum and heart (3 mice, each); and liver, lungs, lymph nodes and pooled tissues (2 mice, each). Animals showing clinical signs compatible to acute toxoplasmosis (ruffled coat, hunched behavior, ascites and inactivity) were euthanized for peritoneal washing in order to investigate the presence of tachyzoites. Isolate's virulence profile was defined based on Pena et al. (2008). Asymptomatic mice were euthanized 60 days after inoculation in order to investigate the presence of cysts in brain macerates. All procedures involving the animals used in the herein described bioassay were approved by the Ethics Committee on the Use of Animals, IOC/Fiocruz, under license L-041/2019.
2.2. Genetic characterization
Toxoplasma gondii DNA was extracted from peritoneal exudates deriving from mice, based on using Dneasy® Blood & Tissue commercial kit (Qiagen® Inc., USA), by following the manufacturer's protocols. Then, PCR amplification was performed in T. gondii, as described by Homan et al. (2000), based on using the 529-bp repeat element (REP529) fragment as target; DNA from T. gondii RH reference strain was used as positive control. The amplified DNA was visualized through electrophoresis on 2% agarose gels stained in SYBR® Safe DNA gel stain (Invitrogen™, USA).
T. gondii isolate genotyping was achieved first based on using multilocus PCR-Restriction Fragment Length Polymorphism (RFLP); then, it was compared to, and classified based on, other previously characterized Brazilian T. gondii strains available in the ToxoDB database (http://toxodb.org/toxo/) and in recent publications.
PCR-RFLP was performed as described by Su et al. (2010), based on using the following genetic markers: SAG1, SAG2 (3′5′SAG2 e alt. SAG2), SAG3, BTUB, GRA6, C22–8, C29–2, L358, PK1, Apico and CS3 (Pena et al., 2008). Reference archetypal strains, such as RH (Type I), PTG (Type II) and CTG (Type III), as well as non-archetypal T. gondii strains (TgCgCa1, MAS and TgCatBr5), were used as positive controls in all reactions.
The isolate was also genotyping by microsatellite analysis (MS) with eight typing markers (TUB2, W35, TgMA, B18, B17, M33, IV.1 and XI.1) and seven fingerprinting markers (N60, n82, AA, N61, N83, M48 and M102), as previously described (Ajzenberg et al., 2010). Results were analyzed in GeneMapper 4.1 software (Applied Biosystems). The PTG reference strain (Type II) was used as positive control.
3. Results
Toxoplasma gondii was isolated from heart, liver and tissue pool homogenate samples. The new isolate was named TgBgHmBrRJ1 (Tg = T. gondii; Bg = Black-and-gold; Hm = Howler monkey; Br=Brazil; RJ1 = first isolate of this species in Rio de Janeiro State). All seven mice inoculated with the aforementioned samples were infected with T. gondii and died of acute toxoplasmosis, a fact that indicated T. gondii's high virulence. Clinical signs, such as ascites, ruffled coat (moderate to severe) and inactivity, were identified from the 7th (to the 12th post-inoculation day p.i.d), when the sick mice were followed up and, subsequently, euthanized in compliance with animal welfare guidelines. The remaining mice survived until the 60th p.i.d.; however, no cysts were observed in brain macerates of mice inoculated with digested lung, lymph node and cerebellum samples.
A non-archetypal genotype (ToxoDB PCR-RFLP #206) was obtained through PCR-RFLP. This isolate was also classified as non-archetypal, based on MS genotyping, and it presented genotypic identity close to that previously identified in isolates deriving from four free-range asymptomatic chickens (TgCkBr244, 245, 278, 279) in Espírito Santo State (Beltrame et al., 2012; HFJP, personal communication) (Table 1).
Table 1.
Microsatellite genotyping of Toxoplasma gondii from a black-and-gold howler monkey (A. caraya) from Rio de Janeiro state, Brazil, which died with acute toxoplasmosis and comparison with other genotypically close T. gondii isolates.
| Host | Strain designation* | MS Type | Microsatellite markers** |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUB2 | W35 | TgM-A | B18 | B17 | M33 | IV.1 | XI.1 | M48 | M102 | N60 | N82 | AA | N61 | N83 | ||||
| Black-and-gold howler monkey (A. caraya) | TgBgHmBrRJ1 (this study) | Non- archetypal | 291 | 242 | 207 | 162 | 342 | 165 | 278 | 358 | 243 | 164 | 172 | 107 | 297 | 93 | 308 | |
| Chicken | TgCkBr244 | Non- archetypal | 291 | 242 | 207 | 162 | 342 | 165 | 278 | 358 | 229 | 164 | 168 | 107 | 297 | 93 | 308 | |
| Chicken | TgCkBr245 | Non- archetypal | 291 | 242 | 207 | 162 | 342 | 165 | 278 | 358 | 231 | 164 | 164 | 107 | 295 | 97 | 308 | |
| Chicken | TgCkBr278 | Non- archetypal | 291 | 242 | 207 | 162 | 342 | 165 | 278 | 358 | 231 | 164 | 164 | 107 | 295 | 93 | 308 | |
| Chicken | TgCkBr279 | Non- archetypal | 291 | 242 | 207 | 162 | 342 | 165 | 278 | 358 | 231 | 164 | 178 | 107 | 299 | 95 | 308 | |
| Reference | GT1 | Type I | 291 | 248 | 209 | 160 | 342 | 169 | 274 | 358 | 209 | 166 | 145 | 119 | 267 | 87 | 306 | |
| Reference | ME49 | Type II | 289 | 242 | 207 | 158 | 336 | 169 | 274 | 356 | 215 | 174 | 142 | 111 | 265 | 91 | 310 | |
| Reference | NED | Type III | 289 | 242 | 205 | 160 | 336 | 165 | 278 | 356 | 209 | 190 | 147 | 111 | 267 | 91 | 312 | |
4. Discussion
Toxoplasma gondii isolation was performed based on using digested heart, liver and pooled tissue samples from a black-and-gold howler monkey. However, there are only four T. gondii isolates from neotropical NHP reported in the literature (Dubey et al., 2021). Pena et al. (2011) have isolated this parasite from tissue homogenate (heart and brain) from a captive red-handed howler monkey (Alouatta belzebul) who died of toxoplasmosis in Brazil. This protozoan was also isolated from squirrel monkeys in Argentina, China and Japan (Pardini et al., 2015; Huang et al., 2018; Nishimura et al., 2019)
Moreira et al. (2022) had previously reported clinical disease features, such as lesions in the liver, lungs, lymph nodes and spleen, in A. caraya - the same individual the herein described isolate was collected from. Acute presentation of toxoplasmosis in neotropical NHPs may be followed by systemic protozoan dissemination in animals' tissues. Therefore, it is recommended applying T. gondii isolation methods in suspected fatal cases of toxoplasmosis in Neotropical NHPs, based on using tissues deriving from these animals' necropsy, mainly heart tissues. It is essential isolating this protozoan to enable the subsequent assessment of factors, such as parasite's virulence and genotypic profile, as shown in the current study.
A non-archetypal genotype (ToxoDB PCR-RFLP #206) was obtained through PCR-RFLP. This previously described genotype is characterized by the combination of typical alleles I, II and III to a unique allele (u-1) at SAG1 marker. In total, 12 T. gondii isolates were already characterized with this same genotype in Brazil: seven from free-range asymptomatic chickens (Pena et al., 2013; Ferreira et al., 2018; Silva et al., 2014); one, from an ostrich (Struthio camelus) in a slaughterhouse (da Silva and Langoni, 2016), and four, from humans affected by congenital toxoplasmosis (Carneiro et al., 2013; Silva et al., 2014). Similar to the present study, all these cases were reported in Brazilian Southeastern States; this finding indicates that this genotype may have high circulation in this region. Moreover, the non-virulent isolate deriving from the ostrich appears to be a variant strain, since it carries allele u-1 at the CS3 marker, whereas the other isolates carry allele II. All isolates #206 with allele II at the CS3 marker were referred to as virulent (as in the present study) or as having intermediate virulence in mice. Alleles I and II at the CS3 marker appeared to be linked to virulence in mice (Pena et al., 2008).
MS-based genotyping analysis of T. gondii strains is not often adopted in Brazil. The MS-based analysis of approximately 300 Brazilian strains (HFJP, personal communication) enabled finding that the TgBgHmBrRJ1 isolate is genotypically close to four isolates deriving from free-range asymptomatic chickens (TgCkBr244, 245, 278, 279) in Espírito Santo State (Beltrame et al., 2012). All five isolates have the same typing markers, but they are independent isolates, rather than clones, as indicated by their fingerprinting markers, a fact that corroborates the significant diversity of this parasite in Brazil.
The present study has contributed to the range of hosts with T. gondii infections associated with RFLP genotype #206. It was the first time a parasite strain with this genotype was associated with clinical disease in a neotropical NHP.
Declaration of competing interest
I declare that the authors of the article have no conflict of interest vis-a-vis the participants or any other direct or indirect contributor, in the development of the article hereby submitted “lsolation and genetic characterization of Toxoplasma gondii from captive black-and-gold howler monkey (Alouatta caraya Humboldt, 1812) in Brazil”, whose researchers involved are: “Maria Regina Reis Amendoeira, lgor Falco Arruda, Silvia Bahadian Moreira, Daniel Guimarães Ubiali, Alynne da Silva Barbosa, Hilda Fátima Jesus Pena, Asheley Henrique Barbosa Pereira, Clarissa Nascimento da Silveira, Thamires Francisco Bonifácio, Yara Souza Clemes, Thalita de Abreu Pissinatti, André Felipe Andrade dos Santos, Alcides Pissinatti".
Acknowledgment
The authors would like to thank Rio de Janeiro Primatology Center and the Environment State Institute for their collaboration and biological material supply. The authors are also grateful for the laboratory support provided by the Toxoplasmosis and other Protozoan Diseases Laboratory, Oswaldo Cruz Institute - IOC/Fiocruz, as well as to the Coordination for the Improvement of Higher Education Personnel (CAPES), and to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP -2018/26071–5) for their financial support.
References
- Ajzenberg D., Collinet F., Mercier A., Vignoles P., Dardé M.L. Genotyping of Toxoplasma gondii isolates with 15 microsatellite markers in a single multiplex PCR assay. J. Clin. Microbiol. 2010;48(12):4641–4645. doi: 10.1128/JCM.01152-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrame M.A., Pena H.F., Ton N.C., Lino A.J., Gennari S.M., Dubey J.P., Pereira F.E. Seroprevalence and isolation of Toxoplasma gondii from free-range chickens from Espírito Santo state, southeastern Brazil. Vet. Parasitol. 2012;188(3–4):225–230. doi: 10.1016/j.vetpar.2012.03.053. [DOI] [PubMed] [Google Scholar]
- Carneiro A.C.A.V., Andrade G.M., Costa J.G.L., Pinheiro B.V., Vasconcelos-Santos D.V., Ferreira A.M., Su C., Januário J.N., Vitor R.W.A. Genetic characterization of Toxoplasma gondii revealed highly diverse genotypes for isolates from newborns with congenital toxoplasmosis in southeastern Brazil. J. Clin. Microbiol. 2013;51(3):901–907. doi: 10.1128/JCM.02502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catão-Dias J.L., Epiphanio S., Kierulff M.C.M. In: Primates, Pathogens, and Evolution. Developments in Primatology: Progress and Prospects. Brinkworth J., Pechenkina K., editors. Springer; New York, NY: 2013. Neotropical primates and their susceptibility to Toxoplasma gondii: new insights for an old problem; pp. 253–289. [DOI] [Google Scholar]
- da Silva R.C., Langoni H. Risk factors and molecular typing of Toxoplasma gondii isolated from ostriches (Struthio camelus) from a Brazilian slaughterhouse. Vet. Parasitol. 2016;225:73–80. doi: 10.1016/j.vetpar.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. 2nd Ed. CRC Press; Maryland: 2010. Toxoplasmosis of Animals and Humans. [Google Scholar]
- Dubey J.P. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet. Parasitol. 1998;74(1):75–77. doi: 10.1016/S0304-4017(97)00135-0. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Cerqueira-Cézar C.K., Murata F.H.A., Kwok O.C.H., Yang Y.R., Su C. All about toxoplasmosis in cats: the last decade. Vet. Parasitol. 2020;283 doi: 10.1016/j.vetpar.2020.109145. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Murata F.H.A., Cerqueira-Cézar C.K., Kwok O.C.H., Yang Y., Su C. Recent epidemiologic, clinical, and genetic diversity of Toxoplasma gondii infections in non-human primates. Res. Vet. Sci. 2021;136:631–641. doi: 10.1016/j.rvsc.2021.04.017. [DOI] [PubMed] [Google Scholar]
- Epiphanio S., Sinhorini I.L., Catão-Dias J.L. Pathology of toxoplasmosis in captive new world primates. J. Comp. Pathol. 2003;129(2–3):196–204. doi: 10.1016/S0021-9975(03)00035-5. [DOI] [PubMed] [Google Scholar]
- Ferreira T.C.R., Buery J.C., Moreira N.I.B., Santos C.B., Costa J.G.L., Pinto L.V., Baraviera R.C.A., Vitor R.W.A., Fux B. Toxoplasma gondii: isolation, biological and molecular characterisation of samples from free-range Gallus gallus domesticus from countryside Southeast Brazil. Rev. Bras. Parasitol. Vet. 2018;27(3):384–389. doi: 10.1590/S1984-296120180028. [DOI] [PubMed] [Google Scholar]
- Homan W.L., Vercammen M., Braekeleer J., Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000;30:69–75. doi: 10.1016/S0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Huang M, Li GF, Zhi GL, Zhao CY, Liu KX, Zuo KJ, Lan H, Chen XJ, Yuan ZG. Isolation and identification of Toxoplasma gondii from black-crown squirrel monkey (Saimiri vanzolinii) of South China. Chin. Vet. Sci. 2018;48(3):316–322. https://caod.oriprobe.com/articles/53248907/Isolation_and_identification_of_Toxoplasma_gondii_.htm [Google Scholar]
- International union for conservation nature. 2020. https://www.iucnredlist.org/search?query=Alouatta&searchType=species Available in:
- Moreira S.B., Pereira A.H.B., Pissinatti T.A., Arruda I.F., de Azevedo R.R.M., Schiffler F.B., Workgroup Outbreak, Amendoeira M.R.R., Dos Santos A.F.A., Pissinatti A., Ubiali D.G. Subacute multisystemic toxoplasmosis in a captive black-and-gold howler monkey (Alouatta caraya) indicates therapy challenging. J. Med. Primatol. 2022;7 doi: 10.1111/jmp.12600. Jun. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Goyama T, Tomikwa S, Fereig RM, El-Alfy ESN, Nagamune K, Kobayashi Y, Nishikawa Y. Outbreak of toxoplasmosis in four squirrel monkeys (Saimiri sciureus) in Japan. Parasitol. Int. 2019;68(1):79–86. doi: 10.1016/j.parint.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Pardini L, Dellarupe A, Bacigalupe D, Quiroga MA, Moré G, Rambeaud M, Basso W, Unzaga JM, Schares G, Venturini MC. Isolation and molecular characterization of Toxoplasma gondii in a colony of captive black-capped squirrel monkeys (Saimiri boliviensis) Parasitol. Int. 2015;64(6):587–590. doi: 10.1016/j.parint.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Paula N.F., Dutra K.S., Oliveira A.R., Santos D.O.D., Rocha C.E.V., Vitor R.W.A., Tinoco H.P., Costa M.E.L.T.D., Paixão T.A.D., Santos R.L. Host range and susceptibility to Toxoplasma gondii infection in captive neotropical and Old-world primates. J. Med. Primatol. 2020;49(4):202–210. doi: 10.1111/jmp.12470. [DOI] [PubMed] [Google Scholar]
- Pena H.F., Marvulo M.F., Horta M.C., Silva M.A., Silva J.C., Siqueira D.B., Lima P.A., Vitaliano S.N., Gennari S.M. Isolation and genetic characterisation of Toxoplasma gondii from a red-handed howler monkey (Alouatta belzebul), a jaguarundi (Puma yagouaroundi), and a black-eared opossum (Didelphis aurita) from Brazil. Vet. Parasitol. 2011;175:377–381. doi: 10.1016/j.vetpar.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Pena H.F., Vitaliano S.N., Beltrame M.A., Pereira F.E., Gennari S.M., Soares R.M. PCR-RFLP genotyping of Toxoplasma gondii from chickens from Espírito Santo state, Southeast region, Brazil: new genotypes and a new SAG3 marker allele. Vet. Parasitol. 2013;192(1–3):111–117. doi: 10.1016/j.vetpar.2012.10.004. 18. [DOI] [PubMed] [Google Scholar]
- Pena H.F.J., Gennari S.M., Dubey J.P., Su C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol. 2008;38:561–569. doi: 10.1016/j.ijpara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Rylands A.B., Mittermeier R.A. In: South American Primates. Developments in Primatology: Progress and Prospects. Garber P.A., Estrada A., Bicca-Marques J.C., Heymann E.W., Strier K.B., editors. Springer; New York, NY: 2009. The diversity of the new world primates (Platyrrhini): an annotated taxonomy; pp. 23–54. [DOI] [Google Scholar]
- Santana C.H., de Oliveira A.R., dos Santos D.O., Pimentel S.P., de Souza L.D.R., Moreira L.G.A., Braz H.M.B., de Carvalho T.P., Lopes C.E.B., Oliveira J.B.S., de Paula N.F., de Carvalho M.P.N., Alves B.F., Pena H.F.J., Santos R.L. Genotyping of Toxoplasma gondii in a lethal toxoplasmosis outbreak affecting captive howler monkeys (Alouatta sp.) J. Med. Primatol. 2021;50(2):99–107. doi: 10.1111/jmp.12506. [DOI] [PubMed] [Google Scholar]
- Santos SV, Strefezzi RF, Pissinatti A, Kanamura CT, Takakura CFH, Duarte MIS, Catão-Dias JL. Detection of Toxoplasma gondii in two southern Wooly spider monkeys (Brachyteles arachnoides-Geoffroy, 1806) from the Rio de Janeiro primate center, Brazil. J. Med. Primatol. 2013;43(2):125–129. doi: 10.1111/jmp.12093. [DOI] [PubMed] [Google Scholar]
- Shwab E.K., Zhu X.Q., Majumdar D., Pena H.F.J., Gennari S.M., Dubey J.P., Su C. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. 2014;141(4):453–461. doi: 10.1017/S0031182013001844. [DOI] [PubMed] [Google Scholar]
- Silva L.A., Andrade R.O., Carneiro A.C., Vitor R.W. Overlapping Toxoplasma gondii genotypes circulating in domestic animals and humans in Southeastern Brazil. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0090237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C., Shwab E.K., Zhou P., Zhu X.Q., Dubey J.P. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137(1):1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]


