Abstract
Background
Access to the liver transplant waitlist for patients with hepatocellular carcinoma (HCC) depends on tumour presentation, biology, and response to treatments. The Milan Criteria (MC) represent the benchmark for expanded criteria that incorporate additional prognostic factors. The purpose of this study was to determine the added value of skeletal muscle index (SMI) in HCC patients beyond the MC.
Method
Patients with HCC that were transplanted beyond the MC were included in this retrospective multicentre study. SMI was quantified using the Computed Tomography (CT) within 3 months prior to transplantation. Cox regression models were used to identify predictors of overall survival (OS). The discriminative performance of SMI extended Metroticket 2.0 and AFP models was also assessed.
Results
Out of 889 patients transplanted outside the MC, 528 had a CT scan within 3 months prior to liver transplantation (LT), of whom 176 (33%) were classified as sarcopenic. The median time between assessment of the SMI and LT was 1.8 months (IQR: 0.77–2.67). The median follow‐up period was 5.1 95% CI [4.7–5.5] years, with a total of 177 recorded deaths from any cause. In a linear regression model with SMI as the dependent variable, only male gender (8.55 95% CI [6.51–10.59], P < 0.001) and body mass index (0.74 95% CI [0.59–0.89], P < 0.001) were significant. Univariable survival analysis of patients with sarcopenia versus patients without sarcopenia showed a significant difference in OS (HR 1.44 95% CI [1.07 − 1.94], P = 0.018). Also the SMI was significant (HR 0.98 95% CI [0.96–0.99], P = 0.014). The survival difference between the lowest SMI quartile versus the highest SMI quartile was significant (log‐rank: P = 0.005) with 5 year OS of 57% and 71%, respectively. Data from 423 patients, describing 139 deaths, was used for multivariate analysis. Both sarcopenia (HR 1.45 95% CI [1.02 − 2.05], P = 0.036) and SMI were (HR 0.98 95% CI [0.95–0.99], P = 0.035) significant. On the survival scale this translates to a 5 year OS difference of 11% between sarcopenia and no sarcopenia. Whereas for SMI, this translates to a survival difference of 8% between first and third quartiles for both genders.
Conclusions
Overall, we can conclude that higher muscle mass contributes to a better long‐term survival. However, for individual patients, low muscle mass should not be considered an absolute contra‐indication for LT as its discriminatory performance was limited.
Keywords: Hepatocellular carcinoma, Liver transplantation, Skeletal muscle mass, Sarcopenia, Survival
Introduction
Liver transplantation (LT) is currently considered as the best treatment for selected patients with hepatocellular carcinoma (HCC) with 5 year survival rates of 60–80%. 1 , 2 Worldwide, various models have been developed to select patients and predict a successful outcome in order to legitimize the allocation of scarce donor livers. The Milan criteria (MC) has been widely adopted within European as well as non‐European transplant centres to guide selection of candidates with HCC. 3 , 4 Yet several modifications and expansions of the MC have been developed because a number of patients outside the MC attain good long‐term survival and benefit from LT. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 As the search for the optimal selection criteria continuous, previously unrecognized characteristics of patients with HCC should be investigated to improve patient selection and prognostication in this setting.
We have hypothesized that, in addition to the tumour characteristics already included in the MC, a patient's general health may reflect the aggressiveness of the malignant process as a result of changes in the metabolism. Even though a patient's general health is important in all clinical examinations, it has rarely been scrutinized as a theoretical driver of long‐term post‐transplant survival, presumably because it is often measured subjectively. However, over the past years, sarcopenia (i.e. low muscle mass) has gained attention as an indirect measurement of general health. In fact, muscle mass can be measured objectively on a single axial slice or volumetrically on computed tomography (CT). This can be used as a surrogate marker for a patient's general health 15 , 16 , 17 , 18 , 19 and as a significant prognostic marker in various malignant and non‐malignant diseases. 20 , 21 , 22
Few studies have been published on the association between preoperative muscle mass and survival after LT for HCC, and convincing evidence that muscle mass is a useful predictor in this population is lacking. Moreover, the results of these studies are conflicting, and inference is hindered by the heterogeneity of the study populations. 23 , 24 , 25 , 26 , 27 , 28 , 29 In addition, none of the studies investigated patients transplanted beyond the MC. Empirical proof that muscle mass is a useful predictor in this specific population is, however, needed before changes in the selection policy could be advised. Therefore, we aimed to rigorously determine the impact of muscle mass on post‐transplant survival in patients transplanted for HCC beyond the MC.
Method
The study was approved by the Medical Ethics Committee of Erasmus MC, Erasmus University Medical Centre, Rotterdam, the Netherlands (MEC‐2016‐277) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The reporting of this multicentre, retrospective observational cohort study adheres to the STROBE guidelines (Supporting Information, Table S1). 30
Population
In this retrospective multicentre international study, patients were included if they had received LT for HCC beyond the MC in the period between January 2000 and December 2019 at one of the 18 participating centres. Patients were considered beyond the MC if their tumour number or tumour size exceeded the criteria at radiological and/or histopathological examination. Patients were excluded if the diagnosis of HCC was not confirmed upon pathology, or if no preoperative CT scan was available within 3 months prior to the transplantation. The CT scan had to be contrast enhanced and had to enable analysis for muscle mass at the level of the third lumbar vertebra (L3). Lastly, patients were excluded if data concerning height, weight, or survival were missing.
All participating centres used a standardized template for data extraction that encompassed: patient demographics, aetiology of liver disease, liver function (Child–Pugh and MELD score), cancer stage at diagnosis, alpha‐fetoprotein (AFP), bridging therapies, operative findings, complications, cancer stage at the histological examination of the liver specimen, date of recurrence, and date of death. The preoperative CT scans were centrally collected and assessed by the same author (B. B.).
Skeletal muscle mass
Skeletal muscle mass area was measured on CT scans. These scans were part of the preoperative diagnosis and work‐up for each patient. The total cross‐sectional skeletal muscle area (cm2) was measured at the L3 level on a slice that showed both transversal processes. Using a validated software package FatSeg v4 developed by the Biomedical Imaging Group Rotterdam. The psoas, the paraspinal, transverse abdominal, external oblique, internal oblique and rectus abdominis were manually outlined using Hounsfield Units (HU) thresholds (i.e. −30 to +150 HU). 31 , 32 This area was then normalized by the patient's squared height (m2) resulting in the L3 skeletal muscle mass index (SMI; cm2/m2). Sarcopenia was defined based on the current study population. Patients were stratified into four strata based on their gender and whether their body mass index (BMI) was above or below 25 kg/m2. For each stratum the patients in the lowest tertial of the SMI distribution were classified as being sarcopenic. This resulted in the following cut‐off values: 37 cm2/m2 for women with a BMI < 25 kg/m2, 42 cm2/m2 for women with a BMI ≥ 25 kg/m2, 45 cm2/m2 for men with a BMI < 25 kg/m2, and 51 cm2/m2 for men with a BMI ≥ 25 kg/m2. The mean skeletal muscle mass radiation attenuation (SMRA, in HU), a measure for intramuscular adipose tissue infiltration, of the selected skeletal muscle tissue was also recorded. Furthermore, we measured the total subcutaneous adipose tissue area (SAT, cm2), and visceral adipose tissue area (VAT, cm2), including the renal adipose tissue and excluding the intestinal content. Lastly, also the maximum splenic length, as a measure for portal hypertension, was assessed.
Outcome parameters
The primary outcome was overall survival (OS), defined as the time in days between the date of LT and the date of death or last follow‐up.
Statistical analysis
Univariate survival analysis was performed stratified with regard to the subsets of the MC (i.e. Beyond MC at radiology only, at pathology only, or at both assessments). If no significant survival difference between these subsets was found, then the diagnostic modality is not a confounder in this study. After this preliminary analysis, the full set was analysed jointly to improve the statistical power. For the descriptive statistics, discrete data was represented in absolute numbers and percentages. Continuous data was represented using the mean, the standard deviation (SD), the first, second and third quartiles, and the range. Descriptive statistics were compared between included and excluded patients. In addition, Kaplan–Meier analysis was used to determine if patients with a CT scan within 3 months prior to LT had a survival advantage compared with those with a scan outside that window. For the included data, the characteristics were compared between the sarcopenic and non‐sarcopenic groups. Differences were tested using the Chi‐squared or Mann–Whitney U test where appropriate. Additionally, for each centre, the distributions of SMI, BMI, MELD‐score, AFP, tumour number and size at radiology were graphically displayed using boxplots. Univariable and multivariable association with SMI was researched by means of a Pearson correlation and a linear regression model.
The reliability of the total cross‐sectional skeletal muscle area measurements was assessed using a two‐way mixed agreement, single‐measure intraclass correlation (ICC). 33 , 34 For the intra‐rater reliability (B. B.), 100 randomly selected records were re‐assessed; for the inter‐rater reliability (B. B. and J. V.), 50 records were re‐assessed. ICC values less than 0.59 were considered poor, values between 0.60 and 0.74 good, and values between 0.75 and 1.0 were considered excellent. 35 In this research, both the intra‐rater and inter‐rater reliability were scored as excellent (intra: ICC: 0.92 95% CI [0.88–0.95], inter: 0.93 95% CI [0.89–0.97]).
For the primary outcome, we performed a univariable survival analysis by means of the Kaplan–Meier method and the Cox proportional hazards model. As heterogeneity between centres is likely, a per‐centre analysis with meta‐analysis using inverse variance pooling was performed for the centres including more than 10 patients. 36 Furthermore, multivariable regression was used to distil the independent effect of the SMI and sarcopenia on survival. The variables male gender, age, and BMI were added in the multivariable regression to satisfy the assumption that all observations are independent and identically distributed (i.i.d.). Furthermore, we accounted for confounders with the Child–Pugh score, MELD score, and ALBI grade describing the liver function. Additionally, preoperative AFP, tumour number and size at pathology, and vascular invasion were used to model cancer stage (Supporting Information, Figure S1). 37 , 38 , 39
Furthermore, to determine the relationship between body composition compartments, a multivariate regression was used to determine the impact of changes in SMI, SMRA, VAT and SAT on OS. Nonlinear relationship of the SMI with OS was investigated using polynomial terms up to the third degree. Also, effect modification of SMI by the tumour burden was investigated by stratifying patients in to four categories. The categories were based on whether the patients SMI was above or below the median value and whether the sum of tumour number and tumour size was above or below seven. Hereafter, Kaplan–Meier analysis was performed to inspect if the spread between high and low values of SMI differed. Furthermore, the effect modification of the SMI and sarcopenia with age, male gender, BMI, tumour number, tumour size, AFP, vascular invasion, MELD score, Child–Pugh score and ALBI score was investigated using interaction terms in a cox regression model.
Lastly, we explored if predictions regarding overall survival from the AFP‐model and Metroticket 2.0 could be improved by adding information about SMI or sarcopenia. These models use tumour size, tumour number and AFP to predict survival. 13 , 40 The predictive performance was evaluated by means of the optimism corrected C‐index. The Likelihood Ratio test was used to compare the benchmark models against univariable and multivariable extensions with SMI or sarcopenia.
In all analyses, full case analysis was performed without imputation of missing data. Additionally, two‐sided P‐values <0.05 were considered significant. All analyses were performed using the R Project for Statistical Computing.
Results
Data were collected from the participating centres regarding 1,040 patients transplanted between 2000 and 2019. In 889 out of 1,040 patients, extension beyond the MC was confirmed either radiologically or on histopathological examination. After exclusion of patients without a proper CT scan within 3 months prior to their LT, missing data on height, weight, or survival data, 528 patients were selected for further analysis (Supporting Information, Figure S2, Table S2). Baseline characteristics between included and excluded patients largely overlapped. Only small differences with regard to spleen size (mean [SD]; included 14.2 [2.7] vs. excluded 13.5 [2.9], P‐value = 0.03), the use of TACE as pre‐treatment (n (%); included 262 (50) vs. excluded 135 (37), P‐value = 0 .001), SMI (mean [SD]; included 54 [10] vs. excluded 51 [9], P‐value ≤ 0.001) and SAT (mean [SD]; included 178 [105] vs. excluded 189 [97], P‐value = 0.046) were found (Supporting Information, Table S3). Furthermore, patients with a preoperative scan within 3‐months prior to LT had no survival advantage compared with patients with a preoperative scan outside that window (P‐value = 0.863).
Of the included patients, 349 out of 528 (66%) were from 13 centres in Europe, 99 (19%) from two centres in North America, 72 (14%) from two centres in Asia, and eight (2%) from one centre in Australia. Furthermore, 81 (15%) patients were beyond MC at radiology only, 212 (40%) patients were beyond MC at pathology only, and 235 (45%) patients were beyond the MC at radiology and pathology. Subsequent analyses were aggregated as the 5 year OS between these groups was similar with 67% 95% CI [60–74] for the pathology only group, 68% 95% CI [57–81] for the radiology only group, and 64% 95% CI [57–71] for patients beyond MC at radiology and pathology (P = 0.41) (Figure 1).
Figure 1.
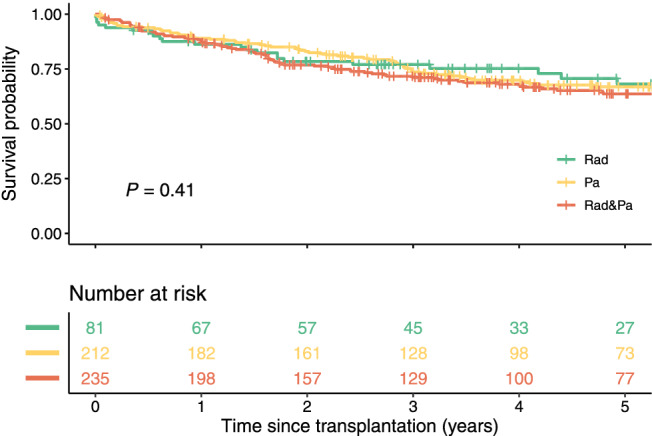
Survival curves of the subsets beyond the Milan criteria. Survival curves of patients beyond the Milan Criteria based upon radiology (Rad), pathology (Pa) or upon both radiology and pathology (Rad & Pa). Created in R.
The descriptive statistics, stratified by sarcopenia status, showed significant differences between BMI (mean [SD]; No sarcopenia 27 [5] vs. Sarcopenia 26 [5], P‐value = 0.008) and SMRA (mean [SD]; No sarcopenia 40 [8] vs. Sarcopenia 37 [9], P‐value < 0.001) (Table 1, Supporting Information, Table S4). More than 78% of the patients had undergone LT between 2011 and 2019. The incidence rate of Sarcopenia did not change over the last 20 years (P = 0.454). Overall, the median time between the last preoperative CT scan and LT was 1.8 months (IQR: 0.77–2.67). In total, 176 (33%) patients were considered sarcopenic before transplantation. The SMI values ranged from 25 to 75 cm2/m2 with an IQR of eight and SD of 8.6. The median [95% CI] follow‐up period was 5.1 [4.7–5.5] years, with a total of 177 recorded deaths from any cause. The important clinicopathological characteristics per centre are provided in Supporting Information, Figure S3.
Table 1.
Descriptive statistics stratified by sarcopenia
| No sarcopenia | Sarcopenia | P‐value | ||
|---|---|---|---|---|
| n | 352 | 176 | ||
| Period, n (%) | [2000, 2005] | 16 (5) | 5 (3) | 0.454 |
| (2005, 2010] | 58 (16) | 38 (22) | ||
| (2010, 2015] | 197 (56) | 95 (54) | ||
| (2015, 2020] | 81 (23) | 38 (22) | ||
| Male, n (%) | Missing | 0 (0) | 0 (0) | |
| n (%) | 302 (86) | 151 (86) | 1.000 | |
| Age (years) | Missing (%) | 1 (0) | 0 (0) | |
| Mean (SD) | 57 (8) | 56 (10) | 0.611 | |
| Q1|Q2|Q3 | 53|58|62 | 52|58|62 | ||
| Range | 20; 74 | 14; 76 | ||
| BMI (kg/m2) | Missing (%) | 0 (0) | 0 (0) | |
| Mean (SD) | 27 (5) | 26 (5) | 0.008* | |
| Q1|Q2|Q3 | 24|26|30 | 22|26|28 | ||
| Range | 5; 42 | 14; 49 | ||
| Meld score | Missing (%) | 40 (11) | 19 (11) | |
| Mean (SD) | 12 (6) | 13 (7) | 0.054 | |
| Q1|Q2|Q3 | 8|10|15 | 9|11|15 | ||
| Range | 2; 40 | 2; 40 | ||
| Spleen size (cm) | Missing (%) | 144 (41) | 74 (42) | |
| Mean (SD) | 14 (2.7) | 14.5 (2.9) | 0.292 | |
| Q1|Q2|Q3 | 12.3|13.9|15.3 | 12.4|14.1|16.4 | ||
| Range | 4.4; 23.3 | 7.2; 21.9 | ||
| Child‐Pugh class n (%) | Missing (%) | 20 (6) | 7 (4) | |
| A | 177 (50) | 87 (49) | 0.591 | |
| B | 122 (35) | 60 (34) | ||
| C | 33 (9) | 22 (12) | ||
| ALBI score | Missing (%) | 12 (3) | 8 (5) | |
| Mean (SD) | −2 (0.7) | −1.9 (0.7) | 0.200 | |
| Q1|Q2|Q3 | −2.6|−2|−1.5 | −2.5|−1.9|−1.4 | ||
| Range | −3.8; 0 | −3.6; −0.1 | ||
| Ascites | Missing (%) | 25 (7) | 15 (9) | |
| n (%) | 102 (30) | 62 (36) | 0.173 | |
| Encephalopathy | Missing (%) | 26 (7) | 16 (9) | |
| n (%) | 49 (14) | 32 (19) | 0.249 | |
| Pre‐treatment n (%) | Missing (%) | 46 (13) | 13 (7) | |
| n (%) | 238 () | 122 () | 0.766 | |
| TACE | 174 (49) | 88 (50) | 0.975 | |
| Ablation | 40 (11) | 19 (11) | 0.692 | |
| Ethanol | 17 (5) | 4 (2) | 0.238 | |
| Resection | 9 (3) | 3 (2) | 0.757 | |
| Tumour number | Missing (%) | 5 (1) | 3 (2) | |
| Mean (SD) | 3 (2) | 3 (3) | 0.781 | |
| Q1|Q2|Q3 | 2|3|5 | 2|3|5 | ||
| Range | 1; 105 | 0; 100 | ||
| Tumour size (mm) | Missing (%) | 3 (1) | 2 (1) | |
| Mean (SD) | 35 (21) | 38 (31) | 0.720 | |
| Q1|Q2|Q3 | 25|37|50 | 24|40|50 | ||
| Range | 1; 260 | 0; 470 | ||
| AFP (log10 (ng/mL)) | Missing (%) | 21 (6) | 18 (10) | |
| Mean (SD) | 1.4 (0.9) | 1.6 (1.1) | 0.344 | |
| Q1|Q2|Q3 | 0.7|1.1|2 | 0.7|1.2|2.3 | ||
| Range | −0.3; 4.9 | −0.2; 4.9 | ||
| Vascular invasion | Missing (%) | 14 (4) | 4 (2) | |
| n (%) | 139 (41) | 81 (47) | 0.180 | |
| SMI (cm2/m2) | Mean (SD) | 54 (7) | 43 (5) | |
| Q1|Q2|Q3 | 50|54|59 | 39|43|47 | ||
| Range | 37; 75 | 25; 51 | ||
| SMRA (HU) | Missing (%) | 0 (0) | 1 (1) | |
| Mean (SD) | 40 (8) | 37 (9) | <0.001* | |
| Q1|Q2|Q3 | 36|41|46 | 32|37|43 | ||
| Range | 11; 58 | 13; 64 | ||
| VAT (cm2) | Missing (%) | 1 (0) | 1 (1) | |
| Mean (SD) | 147 (85) | 142 (88) | 0.459 | |
| Q1|Q2|Q3 | 82|139|195 | 85|119|192 | ||
| Range | 5; 449 | 4; 481 | ||
| SAT (cm2) | Missing (%) | 1 (0) | 1 (1) | |
| Mean (SD) | 183 (102) | 167 (111) | 0.057 | |
| Q1|Q2|Q3 | 110|167|234 | 87|154|216 | ||
| Range | 4; 532 | 3; 785 | ||
| Recurrence n (%) | 96 (27) | 50 (28) | 0.863 | |
| Death n (%) | 105 (30) | 72 (41) | 0.015 | |
| Median follow‐up [95% CI] (years) | 4.9 [4.4–5.4] | 5.6 [5–6.3] | 0.256 | |
| Median overall survival [95% CI] (years) | 12.6 [10.7–NA] | 8.1 [6–NA] | 0.017 | |
| 5 year overall survival [95% CI] | 0.69 [0.64–0.75] | 0.58 [0.5–0.66] | 0.565 | |
Main characteristics stratified by sarcopenia. Tumour number and size measured at pathology. Meld score, ALBI score and AFP are the last measurement prior to liver transplantation. Testing for SMI was omitted as it is different over the two groups by construction. AFP, alpha fetoprotein; BMI, body mass index; SMI, L3 skeletal muscle mass index; SMRA, mean skeletal muscle radiation attenuation; VAT, visceral adipose tissue area; SAT, subcutaneous adipose tissue area.
For the univariable correlations with SMI, only the variables male gender, BMI, and log10(AFP) were significant (Supporting Information, Table S5). With regard to the linear regression model for SMI, only male gender (8.55 95% CI [6.51–10.59], P < 0.001) and BMI (0.74 95% CI [0.59–0.89], P < 0.001) remained significant (Supporting Information, Table S6). Inspection of the distribution stratified for gender and BMI revealed markedly different distributions for these subgroups (Supporting Information, Figure S4, Table S7).
Univariable survival analysis comparing patients with sarcopenia versus patients without sarcopenia showed a significant difference in OS with HR 1.44 95% CI [1.07–1.94] (P = 0.018). The continuous variable SMI was significant with HR 0.98 95% CI [0.96–0.99] (P = 0.014) (Supporting Information, Table S8). Furthermore, there was a significant survival difference between the lowest SMI quartile versus the highest SMI quartile (i.e. those patients with SMI ≤ 45 cm2/m2 vs. SMI ≥ 56 cm2/m2) with 5 year OS of 57% and 71%, respectively (log‐rank: P = 0.005; Figure 2). In the per‐centre analysis and meta‐analysis, a significant impact of sarcopenia on post‐transplant survival was not found when considering centres individually (Supporting Information, Figure S5). For SMI, only the dataset of centre E showed a significant result (HR 0.95 95% CI [0.90–0.99], P = 0.035). Results from meta‐analysis for SMI did not achieve significance with HR 0.99 95% CI [0.97–1.00].
Figure 2.
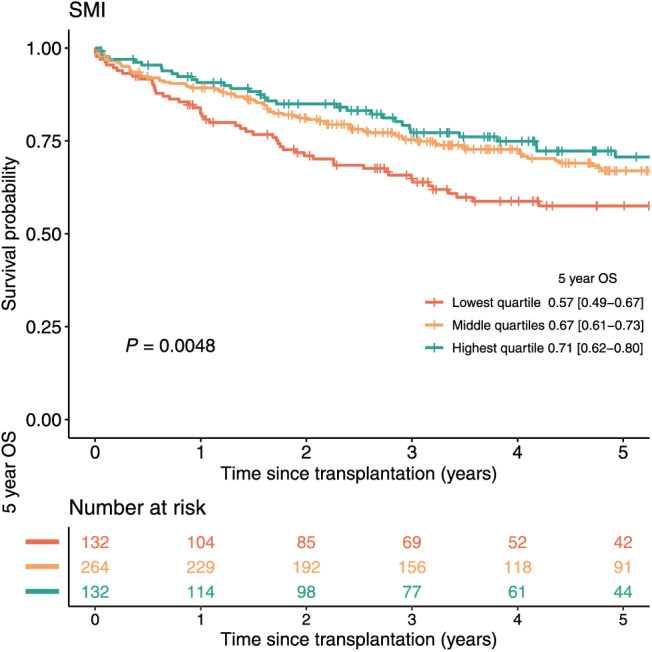
Survival curves highest and lowest quartile SMI. Survival curves of patients in the highest, middle, and lowest quartile of the SMI. The 95% confidence interval of the 5 year overall survival (OS) estimate is displayed between the square brackets. Abbreviations: L3 Skeletal muscle mass index (SMI). Created in R.
In the multivariable analysis, data from 423 patients was analysed, including 139 deaths. The coefficient for sarcopenia was estimated to be 0.371 (HR 1.45 95% CI [1.03–2.05], P = 0.034); the coefficient for SMI was −0.025 (HR 0.98 95% CI [0.95–0.99], P = 0.035) (Table 2). These correspond to a 5 year OS difference of 11% between sarcopenia and no sarcopenia (Supporting Information, Figure S6). Whereas the SMI coefficient translated to a survival difference of 8% between first and third quartiles for both genders (Figure 3).
Table 2.
Multivariable
| Sarcopenia | SMI | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Coeff | HR [95% CI] | Wald | P‐value | Coeff | HR [95% CI] | Wald | P‐value |
| Sarcopenia | 0.375 | 1.45 [1.03–2.05] | 4.52 | 0.034* | ‐ | ‐ | ‐ | ‐ |
| SMI | ‐ | ‐ | ‐ | ‐ | −0.025 | 0.98 [0.95–0.99] | 4.42 | 0.035 a |
| Age | 0.028 | 1.03 [1.01–1.05] | 5.83 | 0.016* | 0.027 | 1.03 [1.00–1.05] | 5.44 | 0.020 a |
| BMI | −0.006 | 0.99 [0.96–1.03] | 0.11 | 0.742 | 0.008 | 1.01 [0.97–1.05] | 0.14 | 0.709 |
| Male | −0.046 | 0.96 [0.58–1.57] | 0.03 | 0.857 | 0.141 | 1.15 [0.68–1.95] | 0.27 | 0.602 |
| Tumour nr (pa) | 0.019 | 1.02 [1.01–1.03] | 7.57 | 0.006* | 0.019 | 1.02 [1.01–1.03] | 7.19 | 0.007 a |
| Tumour size (pa) | 0.010 | 1.01 [1.01–1.01] | 28.74 | <0.001* | 0.010 | 1.01 [1.01–1.01] | 28.62 | <0.001 a |
| log10(AFP) | 0.442 | 1.56 [1.32–1.83] | 28.82 | <0.001* | 0.451 | 1.57 [1.34–1.84] | 30.00 | <0.001 a |
| Vascular invasion | −0.111 | 0.90 [0.62–1.28] | 0.36 | 0.547 | −0.092 | 0.91 [0.64–1.31] | 0.25 | 0.617 |
| MELD (last) | 0.019 | 1.02 [0.99–1.05] | 1.29 | 0.256 | 0.019 | 1.02 [0.99–1.05] | 1.29 | 0.257 |
| Child–Pugh score | 0.020 | 1.02 [0.89–1.16] | 0.09 | 0.770 | 0.017 | 1.02 [0.89–1.16] | 0.07 | 0.796 |
| ALBI score (last) | −0.322 | 0.72 [0.52–1.00] | 3.85 | 0.050 | −0.318 | 0.73 [0.53–1.01] | 3.67 | 0.055 |
Multivariable cox regression showing the risk of death related to a unit increase in SMI. pa, pathology; AFP, alpha fetoprotein; Coeff, regression coefficient; HR, hazard ratio; CI, confidence interval; SMI, L3 skeletal muscle mass index.
Significance at alpha 0.05.
Figure 3.
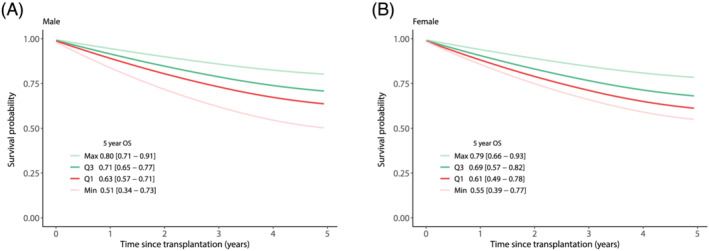
SMI multivariable. Expression of the adjusted coefficient for SMI in terms of survival. The curves are evaluated at the minimum value (min), first quartile (Q1), third quartile (Q3) and maximum (Max) for SMI. Survival was stratified for gender whereas other covariates were set at their mean value. For men that is for SMI: min = 29 cm2/m2, Q1 = 46 cm2/m2, Q3 = 57 cm2/m2, max = 75 cm2/m2. With average age = 56.6 years, BMI = 26.6 kg/m2, Tumour number = 5.4, Tumour size = 42 mm, AFP = 1.4 log10(ng/mL), vascular invasion = 0.4, Meld = 12.8, Child–Pugh points = 6.9, ALBI score = −1.98. For women that is for SMI: min = 29 cm2/m2, Q1 = 37 cm2/m2, Q3 = 47 cm2/m2, max = 65 cm2/m2. With average age = 56.3 years, BMI = 25.7 kg/m2, Tumour number = 7.3, Tumour size = 39.6 mm, APF = 1.5 log10(ng/mL), vascular invasion = 0.4, Meld = 10.6, Child–Pugh points = 6.6, ALBI score = −2.03. The 95% confidence interval of the 5 year overall survival (OS) estimate is displayed between the square brackets. OS, overall survival; SMI, L3 skeletal muscle mass index. Created in R.
Other body composition compartments did not change the impact of sarcopenia and SMI on survival. Contrary to sarcopenia and SMI, the variables SMRA, VAT and SAT did not attain statistical significance with all P‐values > 0.1 (Supporting Information, Table S9). In addition, we did not find a non‐linear relationship between SMI and survival (Supporting Information, Table S10). Also, we could not detect significant effect modification of SMI or Sarcopenia by liver function or cancer stage (Supporting Information, Figure S7, Table S11). With regard to predictive performance, the increase was of limited magnitude (optimism corrected C‐index +0.01), for both the AFP model and the Metroticket 2.0 model when information regarding muscle mass was added (Supporting Information, Table S12).
Discussion
To the best of our knowledge, this is the first study investigating muscle mass in HCC patients transplanted beyond the Milan Criteria. In this large multicentre study, we have demonstrated that Sarcopenia and SMI are significantly associated with post‐transplant survival on univariable and multivariable analyses. Furthermore, between the first and third quartiles of the SMI, overall survival was estimated to differ by 8% at 5 years after LT (63 vs. 71%) after adjusting for confounders. However, for individual prediction the performance of the Metroticket 2.0 and AFP‐model did not markedly improve when information regarding muscle mass was added.
With regard to the main outcome, there is no consensus in the currently available literature on whether low muscle mass is a contributing factor to poorer long‐term post‐transplant survival. 41 The study of Englesbe et al. in 2010 was the first to investigate muscle mass and mortality after LT in patients with cirrhosis. 23 They measured the total psoas area and found a large effect on the 3 year overall survival (26.4% for the first quartile versus 77.2% for the third quartile). Lee et al. in 2014 re‐analysed largely the same cohort from Michigan and confirmed the findings for the areas of the dorsal muscle group. 25 However, these cohorts included patients who had a CT scan in a 90‐day peri‐operative rather than a pre‐operative window. These studies also included patients who had a CT scan after LT. Potentially, this has introduced selection bias, as CT scans were not part of the standard postoperative protocol; this group presumably had indications for imaging. Nevertheless, Hamaguchi et al. confirmed the results by reporting a significant association between low muscle mass and post‐transplant survival after measuring the psoas muscle mass index in 235 living donor liver transplantation patients in Tokyo. 26 Lastly, Meza‐Junco published in 2012 a study describing the SMI of 116 transplantation patients with HCC and cirrhosis from Alberta. 42 They found a HR of 2.27 [1.29 − 3.96] (P = 0.004) for sarcopenia and for SMI a HR of 0.96 95% CI [0.94 − 0.99] (P = 0.02) at univariable Cox survival analysis for men and HR of 0.83 [0.70 − 0.98] (P = 0.03) for women. However, in an updated cohort from Alberta in 2013 including 240 patients, no univariable association between sarcopenia or SMI and risk of death was found. 28 Giusto et al. also studied SMI in 139 patients that were eligible for LT and reported no association with mortality. 29 Lastly, Valero concluded, based on 96 patients that underwent liver resection or LT for HCC or ICC, that there was no significant association between total psoas volume and overall survival. 27
Comparison of previous studies is hindered by changing techniques and definitions of sarcopenia. Besides, not all previous studies stratified for gender and BMI, which could have led to bias similar to that of the Simpsons paradox, in which the measured effect is reduced or even reversed (Supporting Information, Figure S8). Lastly, previous studies relied on predominantly small single‐centre cohorts which also often included patients with a variety of indications for LT. Our study stands out in that regard, as we were able to analyse a large number of patients, from both eastern and western regions, making our results simultaneously more precise and generalizable. Inclusions from such a wide array of hospitals, however, require scrutiny of the assumption that the impact of muscle mass is equal for all centres. We investigated potential heterogeneity by means of a per‐centre analysis combined with meta‐analysis. This analysis did not change our conclusions; although we realize that for centres including only a few patients, the per‐centre estimates are subject to large variation. Another strength of our study is that results indicating a univariable association between SMI and long‐term survival rest on two objective variables only, the SMI and time until death or last follow‐up. These variables in particular were, despite the retrospective multicentre setup, subject to minimal measurement error as the intra‐ and inter‐rater reliability of the SMI measurements were qualified as excellent, and the survival data was based on national census data. With respect to the quality of the scan, the impact of scan phases was earlier studied and estimated to be negligible for skeletal muscle mass measurements. 43
Another strength is that SMI was measured in a period of 3 months prior to LT. This ensures that the measured muscle mass reflects the fitness of the patient at transplantation. In addition, the fact that the information regarding SMI is available prior to the‐transplantation is important to allow for clinical decision making regarding the surgery. The moment of measuring the biomarker should, however, be seen separate from how the study population is defined. In that regard, studying the association between SMI and survival in all patients beyond Milan, either at radiology or pathology, did not degrade its preoperative potential. Similarly, nor did the exclusion criteria that HCC needed to be confirmed upon pathology. How the study population was defined only affected to what group of patients the association could be generalized. In this research we focused on patients beyond the MC, as these are the patients affected by an extension of the transplant listing criteria. Concentrating on this subset allowed us to collect more data of relevant patients and therefore achieve more precise estimates for the population we are most interested in. Furthermore, in case the muscle mass of patients within the MC has a different impact on survival, compared with the one of patients beyond the MC, then including both groups would yield a less interpretable average association. Whereas, including only patients beyond the MC avoids this risk.
Our analysis could, however, be affected by selection bias, as patients that dropped out during listing were not included. Perhaps characteristics that were not accounted for (e.g. strong motivation and strong social support) affected the probability of sarcopenic patients to get transplanted. Therefore, sarcopenic patients without favourable characteristics might be underrepresented in our data, potentially leading to an under estimation of the effect of muscle mass.
Despite the large, yet likely conservative, estimate of the survival difference, predictive performance only marginally improved when the current prognostic models were supplemented with information on muscle mass. However, it is important to keep in mind that modelling efforts in this research were only explorative in nature. For instance, alternative cut‐off values for Sarcopenia or gender‐based normalization of SMI were outside the scope of this research. Furthermore, we recognize that the Metroticket 2.0 and AFP‐model are not the only models as a wide variety of alternative criteria are currently in use (e.g. Extended Toronto Criteria, Hangzhou criteria, up‐to‐seven criteria, University of California San Francisco criteria). Therefore, dedicated modelling studies should investigate in which format and model configuration the information captured by the muscle mass could best be exploited to aid prediction and patient selection.
Overall, these data demonstrate a significant association between high muscle mass and long‐term survival which should be taken into consideration in daily clinical practice. A low muscle mass should, however, be considered only as a relative contraindication for LT as the discriminatory performance was limited.
Conflict of interest
None declared.
Supporting information
Table S1 – STROBE Statement
Table S2 – Number of final inclusions per center
Table S3 – Baseline characteristics included vs. excluded
Table S4 – Aetiology
Table S5 – Univariable correlation with SMI
Table S6 – Multivariable correlation with SMI
Table S7 – Distribution of the SMI stratified per Gender and BMI
Table S8 – Univariable Cox regression
Table S9 – Non‐linearity
Table S10 – Effect modification
Table S11 – Predictive performance
Figure S1 – Causal graph
Figure S2 – Flowchart of inclusion
Figure S3 – Per center clinicopathological characteristics
Figure S4 – Distribution of the SMI stratified per Gender and BMI
Figure S5 – Per center univariate analysis
Figure S6 – Sarcopenia multivariate
Figure S7 – Effect modification survival curves
Figure S8 – Illustration of the Simpson's paradox
Acknowledgements
The help of Eleonora Fresina in the logistics of the study and proofreading the manuscript are gratefully acknowledged. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 44
Beumer B. R., Van Vugt J. L. A., Sapisochin G., Yoon P., Bongini M., Lu D., Xu X., De Simone P., Pintore L., Golse N., Nowosad M., Bennet W., Tsochatzis E., Koutli E., Abbassi F., Claasen M. P. A. W., Merli M., O'Rourke J., Gambato M., Benito A., Majumdar A., Tan E. K., Ebadi M., Montano‐Loza A. J., Berenguer M., Metselaar H. J., Polak W. G., Mazzaferro V., IJzermans J. N. M., and Collaborators (2022) Impact of muscle mass on survival of patients with hepatocellular carcinoma after liver transplantation beyond the Milan criteria, Journal of Cachexia, Sarcopenia and Muscle, 13, 2373–2382, 10.1002/jcsm.13053
References
- 1. Liver EAFTSOT . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 2. Burra P, Burroughs A, Graziadei I, Pirenne J, Valdecasas JC, Muiesan P, et al. EASL clinical practice guidelines: liver transplantation. J Hepatol 2016;64:433–485. [DOI] [PubMed] [Google Scholar]
- 3. Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence‐based analysis of 15 years of experience. Liver Transpl 2011;17:S44–S57. [DOI] [PubMed] [Google Scholar]
- 4. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–700. [DOI] [PubMed] [Google Scholar]
- 5. Elshamy M, Aucejo F, Menon KVN, Eghtesad B. Hepatocellular carcinoma beyond Milan criteria: Management and transplant selection criteria. World J Hepatol 2016;8:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu D‐W, Wan P, Xia Q. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria: A review. World J Gastroenterol 2016;22:3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, et al. Influence of tumour characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl 2001;7:631–636. [DOI] [PubMed] [Google Scholar]
- 8. Silva M, Moya A, Berenguer M, Sanjuan F, López‐Andujar R, Pareja E, et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl 2008;14:1449–1,460. [DOI] [PubMed] [Google Scholar]
- 9. Onaca N, Davis GL, Goldstein RM, Jennings LW, Klintmalm GB. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumours in Liver Transplantation. Liver Transpl 2007;13:391–399. [DOI] [PubMed] [Google Scholar]
- 10. Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, et al. Long‐term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimetres. Ann Surg 2002;235:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kneteman NM, Oberholzer J, Al Saghier M, Meeberg GA, Blitz M, Ma MM, et al. Sirolimus‐based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl 2004;10:1301–1311. [DOI] [PubMed] [Google Scholar]
- 12. Zheng S‐S, Xu X, Wu J, Chen J, Wang WL, Zhang M, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726–1732. [DOI] [PubMed] [Google Scholar]
- 13. Duvoux C, Roudot‐Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including α‐fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986–994. e3. [DOI] [PubMed] [Google Scholar]
- 14. Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl 2007;13:1637–1,644. [DOI] [PubMed] [Google Scholar]
- 15. Shen W, Punyanitya M, Wang Z, Gallagher D, St.‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol 2004;97:2333–2,338. [DOI] [PubMed] [Google Scholar]
- 16. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: aetiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010;21:543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 2009;55:539–549. [DOI] [PubMed] [Google Scholar]
- 18. Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci 2003;58:M911–M916. [DOI] [PubMed] [Google Scholar]
- 19. van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Murad SD, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol 2018;68:707–714. [DOI] [PubMed] [Google Scholar]
- 20. Salinas‐Miranda E, Deniffel D, Dong X, Healy GM, Khalvati F, O'Kane GM, et al. Prognostic value of early changes in CT‐measured body composition in patients receiving chemotherapy for unresectable pancreatic cancer. Eur Radiol 2021;1–9. [DOI] [PubMed] [Google Scholar]
- 21. Parikh ND, Zhang P, Singal AG, Derstine BA, Krishnamurthy V, Barman P, et al. Body composition predicts survival in patients with hepatocellular carcinoma treated with transarterial chemoembolization. Cancer Res Treatment 2018;50:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faron A, Sprinkart AM, Kuetting DL, Feisst A, Isaak A, Endler C, et al. Body composition analysis using CT and MRI: Intra‐individual intermodal comparison of muscle mass and myosteatosis. Sci Rep 2020;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010;211:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waits SA, Kim EK, Terjimanian MN, Tishberg LM, Harbaugh CM, Sheetz KH, et al. Morphometric age and mortality after liver transplant. JAMA Surg 2014;149:335–340. [DOI] [PubMed] [Google Scholar]
- 25. Lee CS, Cron DC, Terjimanian MN, Canvasser LD, Mazurek AA, Vonfoerster E, et al. Dorsal muscle group area and surgical outcomes in liver transplantation. Clin Transplant 2014;28:1092–1,098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamaguchi Y, Kaido T, Okumura S, Fujimoto Y, Ogawa K, Mori A, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl 2014;20:1413–1,419. [DOI] [PubMed] [Google Scholar]
- 27. Valero V, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumours. J Gastrointest Surg 2015;19:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montano‐Loza AJ, Meza‐Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014;20:640–648. [DOI] [PubMed] [Google Scholar]
- 29. Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Gianneli V, et al. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual‐energy X‐ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol 2015;27:328–334. [DOI] [PubMed] [Google Scholar]
- 30. Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 31. van Vugt JLA, Levolger S, Gharbharan A, Koek M, Niessen WJ, Burger JW, et al. A comparative study of software programmes for cross‐sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle 2017;8:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Vledder M, Levolger S, Ayez N, Verhoef C, Tran TCK, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550–557. [DOI] [PubMed] [Google Scholar]
- 33. McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996;1:30. [Google Scholar]
- 34. Hallgren KA. Computing inter‐rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol 2012;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994;6:284. [Google Scholar]
- 36. Basagana X, Pedersen M, Barrera‐Gómez J, Gehring U, Giorgis‐Allemand L, Hoek G, et al. Analysis of multicentre epidemiological studies: contrasting fixed or random effects modelling and meta‐analysis. Int J Epidemiol 2018;47:1343–1,354. [DOI] [PubMed] [Google Scholar]
- 37. Pugh R, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–649. [DOI] [PubMed] [Google Scholar]
- 38. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Thernau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 39. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence‐based approach—the ALBI grade. J Clin Oncol 2015;33:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018;154:128–139. [DOI] [PubMed] [Google Scholar]
- 41. Van Vugt J, Levolger S, de Bruin RWF, van Rosmalen J, Metselaar HJ, IJzermans JNM. Systematic review and meta‐analysis of the impact of computed tomography–assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant 2016;16:2277–2,292. [DOI] [PubMed] [Google Scholar]
- 42. Meza‐Junco J, Montano‐Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861–870. [DOI] [PubMed] [Google Scholar]
- 43. van Vugt JL, van den Braak RRJC, Schippers HJW, Veen KM, Levolger S, de Bruin RW, et al. Contrast‐enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin Nutr 2018;37:1707–1714. [DOI] [PubMed] [Google Scholar]
- 44. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle, 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 – STROBE Statement
Table S2 – Number of final inclusions per center
Table S3 – Baseline characteristics included vs. excluded
Table S4 – Aetiology
Table S5 – Univariable correlation with SMI
Table S6 – Multivariable correlation with SMI
Table S7 – Distribution of the SMI stratified per Gender and BMI
Table S8 – Univariable Cox regression
Table S9 – Non‐linearity
Table S10 – Effect modification
Table S11 – Predictive performance
Figure S1 – Causal graph
Figure S2 – Flowchart of inclusion
Figure S3 – Per center clinicopathological characteristics
Figure S4 – Distribution of the SMI stratified per Gender and BMI
Figure S5 – Per center univariate analysis
Figure S6 – Sarcopenia multivariate
Figure S7 – Effect modification survival curves
Figure S8 – Illustration of the Simpson's paradox


