The SARS‐CoV‐2 B.1.1.529 variant (Omicron) has spread aggressively around the world with rapid transmission in Canada identified within days of its classification as a variant of concern by the World Health Organization. 1 , 2 The BA.5 subvariant in particular has quickly spread to over 50 countries with recent rapid propagation in Central Canada, increasing from 45.8% to 78.2% of all SARS‐CoV‐2 infections in Ontario in the first half of July. 3 Alongside, per cent positivity of testing increased from 8.4% to 14.8%, sufficient for the provincial government to declare a seventh wave driven by BA.5 on the 6th of July (Figure 1). 4 , 5 During the same time period, the Quebec government also reported a seventh wave, with BA.5 representing 75.5% of all SARS‐CoV‐2 infections in Quebec. 6 The predominance of BA.5 in the most densely populated area of Canada indicates yet another pandemic epidemiological shift.
FIGURE 1.
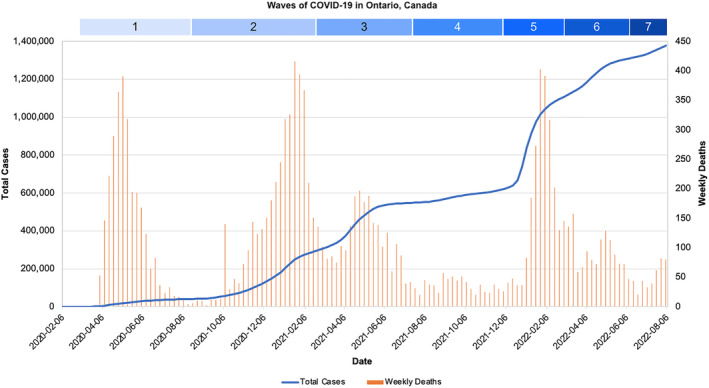
Total case count and weekly death count in Ontario, Canada, from 8 February 2020 to 30 July 2022 across seven COVID‐19 waves. COVID‐19 death count excludes deaths not caused by COVID‐19. Wave 7 is ongoing and only includes total cases and deaths up to and including 30 July 2022. Data obtained from the Ontario Government Data Catalogue
Omicron and its subvariants have shown progressing neutralization escape properties towards both immunization as well as previous COVID‐19 infection. 7 , 8 Initial lineages of Omicron already demonstrated lower vaccine effectiveness as compared with the Delta variant. 8 However, neutralizing activity of mRNA vaccines have been significantly lower against newer sublineages of Omicron as compared with the initial lineages, with BA.5 in particular having substantially lower serum neutralizing titres when compared with BA.1 and BA.2. 9 The spike‐F486V mutation associated with neutralizing antibody resistance in BA.4 has also been identified in BA.5. 7 These findings suggest subsequent sublineages of Omicron have continually expanded their neutralizing escape properties and have increased re‐infection risk for patients with endogenous anti‐SARS‐CoV‐2 antibodies. The successful development of bivalent vaccines, targeting both the original mRNA sequence as well as the newer sequence of Omicron and its subvariants, offers an alternative that may hinder escape properties. 10
In addition, rapid antigen tests (RATs), also referred to as lateral flow tests, have been noted to have reduced diagnostic performance towards the Omicron variant when compared with the Delta variant. 11 With decreased sensitivity towards Omicron and its descendent lineages, a single negative RAT result may not be a reliable indicator for ruling out infection. With RATs having superior accessibility, resource and cost efficiency, and turnaround time compared with the gold standard polymerase chain reaction test for the general public, this higher false negative rate may foster a false sense of security. This is particularly concerning given that cases have been documented as being infectious several days prior to testing positive on a RAT. 11 As such, there may be value in the development of RATs with increased sensitivity to the most prevalent COVID‐19 lineages based on regional epidemiological data.
With Omicron and its subvariants having ever‐increasing immune escape properties, considerable global resources have been invested in the development and production of neutralizing monoclonal antibodies (mAbs) that target specific components of the SARS‐CoV‐2 spike protein receptor as a therapeutic option in treating COVID‐19 infection. 12 , 13 While certain mAbs initially received rapid authorization for use, the propensity of COVID‐19 subvariants to develop mutations and mount resistance against therapeutic management has resulted in reduced efficacy and the removal of certain therapies from clinical practice guidelines. 13 , 14 BA.5 in particular has been identified as having considerably lower neutralization sensitivity to various mAbs compared with the initial Omicron lineages. 15 With the increasing development of resistance to existing therapy, there is considerable value in the research and development of therapeutic and prophylactic options that are targeted towards dominating global strains of COVID‐19.
Given the reduced protection from immunization and endogenous anti‐SARS‐CoV‐2 antibodies towards BA.5 in addition to decreased sensitivity of RATs and efficacy of mAbs, there is considerable room for the development of superior prophylactic, therapeutic, and testing options. There continues to be great importance in remaining vigilant by following infection prevention and control best practices including maintaining robust respiratory and hand hygiene, self‐isolating based on high‐risk exposure or symptoms, and limiting nonessential travel. Receiving booster doses of mRNA vaccination, if available and accessible, may offer an additional layer of protection especially for vulnerable populations. Bivalent vaccines including new mRNA sequences may also increase efficacy and length of protection. Careful disease surveillance of metrics such as wastewater signal monitoring and hospitalization rates will facilitate rapid identification and interventions for regional epidemiological changes.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest. The authors alone are responsible for the content and writing of the article.
AUTHOR CONTRIBUTIONS
Toni Li: Conceptualization; data curation; supervision; visualization. Adrian Yung: Data curation. Carolyn Tran: Data curation. Maximilien Boulet: Supervision.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/irv.13046.
Funding Information
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. The authors received no financial support for the research, authorship, and/or publication of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Ontario Government Data Catalogue at https://data.ontario.ca/en/ and the Quebec Government Data Dashboard at https://www.quebec.ca/en/health/health-issues/a-z/2019-coronavirus/situation-coronavirus-in-quebec/.
REFERENCES
- 1. Pacchiarini N, Sawyer C, Williams C, et al. Epidemiological analysis of the first 1000 cases of SARS‐CoV‐2 lineage BA.1 (B.1.1.529, Omicron) compared with co‐circulating Delta in Wales, UK. Influenza Other Respir Viruses. 2022;irv.13021. doi: 10.1111/irv.13021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li A, Maier A, Carter M, Guan TH. Omicron and S‐gene target failure cases in the highest COVID‐19 case rate region in Canada—December 2021. J Med Virol. 2022;94(5):1784‐1786. doi: 10.1002/jmv.27562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ontario Agency for Health Protection and Promotion (Public Health Ontario) . Epidemiologic summary: SARS‐CoV‐2 Genomic Surveillance in Ontario, July 29, 2022. Queen’s Print Ont. Published online 2022.
- 4. Ontario Agency for Health Protection and Promotion (Public Health Ontario) . Weekly epidemiologic summary: COVID‐19 in Ontario – July 24, 2022 to July 30, 2022. Queen's Print Ont. Published online 2022.
- 5. Dyer O. Covid‐19: Ontario hospitals close wards as nursing shortage bites. BMJ. 2022;o1917. doi: 10.1136/bmj.o1917 [DOI] [PubMed] [Google Scholar]
- 6. Gouvernement du Québec . Data on COVID‐19 in Québec. Published 2022. Accessed August 6, 2022. https://www.quebec.ca/en/health/health-issues/a-z/2019-coronavirus/situation-coronavirus-in-quebec/
- 7. Tegally H, Moir M, Everatt J, et al. Emergence of SARS‐CoV‐2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022. doi: 10.1038/s41591-022-01911-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim SS, Chung JR, Talbot HK, et al. Effectiveness of two and three mRNA COVID‐19 vaccine doses against Omicron‐ and Delta‐related outpatient illness among adults, October 2021–February 2022. Influenza Other Respir Viruses. 2022;irv.13029. doi: 10.1111/irv.13029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hachmann NP, Miller J, Collier ARY, et al. Neutralization escape by SARS‐CoV‐2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387(1):86‐88. doi: 10.1056/NEJMc2206576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee IJ, Sun CP, Wu PY, et al. A booster dose of Delta × Omicron hybrid mRNA vaccine produced broadly neutralizing antibody against Omicron and other SARS‐CoV‐2 variants. J Biomed Sci. 2022;29(1):49. doi: 10.1186/s12929-022-00830-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jüni P, Baert S, Corbeil A, et al. Use of rapid antigen tests during the omicron wave. Ontario COVID‐19 Science Advisory Table. 2022;3:56. doi: 10.47326/ocsat.2022.03.56.1.0 [DOI] [Google Scholar]
- 12. Yang M, Li A, Wang Y, Tran C, Zhao S, Ao G. Monoclonal antibody therapy improves severity and mortality of COVID‐19 in organ transplant recipients: a meta‐analysis. J Infect. 2022;S016344532200384X. doi: 10.1016/j.jinf.2022.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ao G, Li A, Wang Y, Tran C, Qi X. Lack of efficacy for sotrovimab use in patients with COVID‐19: a meta‐analysis. J Infect. 2022;85(1):e10‐e12. doi: 10.1016/j.jinf.2022.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ontario COVID‐19 Drugs and Biologics Clinical Practice Guidelines Working Group . Clinical practice guideline summary: recommended drugs and biologics in adult patients with COVID‐19. Ontario COVID‐19 Science Advisory Table. 2022. doi: 10.47326/ocsat.cpg.2022.11.0 [DOI] [Google Scholar]
- 15. Yamasoba D, Kosugi Y, Kimura I, et al. Neutralisation sensitivity of SARS‐CoV‐2 omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect Dis. 2022;22(7):942‐943. doi: 10.1016/S1473-3099(22)00365-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the Ontario Government Data Catalogue at https://data.ontario.ca/en/ and the Quebec Government Data Dashboard at https://www.quebec.ca/en/health/health-issues/a-z/2019-coronavirus/situation-coronavirus-in-quebec/.


