Abstract
One aspect of skeletal muscle memory is the ability of a previously trained muscle to hypertrophy more rapidly following a period of detraining. Although the molecular basis of muscle memory remains to be fully elucidated, one potential mechanism thought to mediate muscle memory is the permanent retention of myonuclei acquired during the initial phase of hypertrophic growth. However, myonuclear permanence is debated and would benefit from a meta‐analysis to clarify the current state of the field for this important aspect of skeletal muscle plasticity. The objective of this study was to perform a meta‐analysis to assess the permanence of myonuclei associated with changes in physical activity and ageing. When available, the abundance of satellite cells (SCs) was also considered given their potential influence on changes in myonuclear abundance. One hundred forty‐seven peer‐reviewed articles were identified for inclusion across five separate meta‐analyses; (1–2) human and rodent studies assessed muscle response to hypertrophy; (3–4) human and rodent studies assessed muscle response to atrophy; and (5) human studies assessed muscle response with ageing. Skeletal muscle hypertrophy was associated with higher myonuclear content that was retained in rodents, but not humans, with atrophy (SMD = −0.60, 95% CI −1.71 to 0.51, P = 0.29, and MD = 83.46, 95% CI −649.41 to 816.32, P = 0.82; respectively). Myonuclear and SC content were both lower following atrophy in humans (MD = −11, 95% CI −0.19 to −0.03, P = 0.005, and SMD = −0.49, 95% CI −0.77 to −0.22, P = 0.0005; respectively), although the response in rodents was affected by the type of muscle under consideration and the mode of atrophy. Whereas rodent myonuclei were found to be more permanent regardless of the mode of atrophy, atrophy of ≥30% was associated with a reduction in myonuclear content (SMD = −1.02, 95% CI −1.53 to −0.51, P = 0.0001). In humans, sarcopenia was accompanied by a lower myonuclear and SC content (MD = 0.47, 95% CI 0.09 to 0.85, P = 0.02, and SMD = 0.78, 95% CI 0.37–1.19, P = 0.0002; respectively). The major finding from the present meta‐analysis is that myonuclei are not permanent but are lost during periods of atrophy and with ageing. These findings do not support the concept of skeletal muscle memory based on the permanence of myonuclei and suggest other mechanisms, such as epigenetics, may have a more important role in mediating this aspect of skeletal muscle plasticity.
Keywords: Muscle memory, Myonuclei, Satellite cell, Hypertrophy, Ageing, Meta‐analysis
Introduction
Skeletal muscle fibres are some of the largest cells in the body and uniquely multinucleated with more than one hundred myonuclei per mm length of fibre. 1 In order to maximize the distance between neighbouring nuclei, all nuclei within the syncytium are evenly positioned, adjacent to the plasma membrane. 2 More interestingly, skeletal muscle is an extraordinary tissue with the ability to respond to intrinsic and extrinsic stimuli by changing its size. 3 Myonuclei have an important role in skeletal muscle size adaptation through the production of transcripts that support the synthesis of proteins for use in the immediate vicinity surrounding each nucleus. 4
In response to exercise, new myonuclei can be acquired by myofibres as the result of fusion by muscle stem cells (known as satellite cells), which are normally in a quiescent state and become activated upon exposure to external stimuli, such as exercise or injury. Once activated, satellite cells (SCs) proliferate, differentiate into myogenic progenitor cells, and subsequently fuse to existing myofibres, providing additional nuclei to the growing myofibres. 5 , 6 , 7 Studies have provided evidence showing that each nucleus within a myofibre oversees a given amount of cytoplasm, which is referred to as the myonuclear domain. 3 , 4 The notion of a myonuclear domain is based on the concept that each nucleus has a limited capacity to control transcriptional characteristics over a finite volume of cytoplasm. 4 , 8 Further, other studies have suggested the size of the myonuclear domain may not be as fixed as is often indicated. 9 , 10
Skeletal muscle possesses the remarkable ability to ‘recall’ a previous hypertrophic state upon resumption of training following a period of detraining, a phenomenon that has been called ‘muscle memory’. 11 , 12 , 13 Scientists first attributed the phenomenon of muscle memory to motor learning via the central nervous system. 14 The findings from more recent studies have proposed that muscle memory is related to the abundance of myonuclei, with the new myonuclei added during the initial hypertrophy being permanent, thereby providing enhanced transcriptional output in response to training following a bout of detraining. 15 , 16 It has been hypothesized that the retention of the hyper‐nucleated condition might be responsible for the accelerated regeneration and return of myofibre size and function even after a prolonged period of inactivity in previously trained skeletal muscle. 16 Current available evidence regarding muscle memory is quite conflicting with some reports confirming myonuclear permanence, 15 , 16 , 17 , 18 although other studies showing myonuclei could be lost during detraining. 19 , 20 , 21 , 22 Some studies have reported that myonuclear content in skeletal muscle is not permanent and undergoes apoptosis with atrophy in response to hindlimb suspension, 23 , 24 denervation, 25 , 26 exposure to microgravity, 27 and immobilization. 28 Moreover, recent studies in both rodents 21 and humans 22 , 29 , 30 have shown that myonuclei acquired during hypertrophy are not permanent following long‐term inactivity with myonuclear abundance returning to previously untrained state.
To the best of our knowledge, no systematic review and meta‐analysis has yet assessed whether hypertrophy‐induced myonuclear accretion is maintained after exercise cessation or inactivity in both humans and rodents. The aim of this systematic review and meta‐analysis was to assess myonuclear and SC content in skeletal muscle that underwent hypertrophy or atrophy in both humans and rodents. Finally, the long‐term myonuclear permanence in human was assessed by the inclusion of ageing studies in the meta‐analyses.
Methods
The present preclinical and clinical review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number: CRD42020152068 and was performed in accordance with PRISMA guidelines. 31
Research question
In the present systematic review and meta‐analysis, we sought to answer the following questions: (i) Is hypertrophy‐induced myonuclear accretion maintained after exercise cessation in either humans and/or rodents? (ii) Does myonuclear content and/or SC abundance change during atrophy in either humans or rodents? (iii) Is there any difference in myonuclear content and/or SC abundance between elderly and young adults?
Data sources and searches
A systematic literature search for relevant studies was carried out using the following databases: CINAHL, MEDLINE, CENTRAL, PEDro, ProQuest, and Scopus, from the earliest record of each database up to February 2022. Search terms included a combination of the following keywords related to muscle memory: ‘muscle memory’ and ‘memory’; related to muscle CSA: ‘muscle hypertrophy’, ‘muscle atrophy’, ‘myonuclei’, ‘myonuclear domain’, ‘satellite cell’, and ‘muscle stem cell’; related to training: ‘resistance exercise’, ‘resistance training’, ‘strength training’, ‘power training’, ‘endurance exercise’, and ‘endurance training’; related to atrophy stimuli: ‘loading’, ‘unloading’, ‘hindlimb suspension’, ‘suspension’, ‘leg immobilization’, ‘immobilization’, ‘step reduction’, ‘denervation’, ‘spinal cord injury’ and ‘spinal cord transaction’; and related to human ageing: ‘sarcopenia’, ‘human Aging’, ‘aging’, and ‘elderly’.
Study selection
We included all studies involving human and animal models independent of sex, age, and intervention (except steroid administration) that evaluated satellite cell or myonuclear abundance. In terms of study design, both controlled and uncontrolled clinical trials were included in the systematic review and meta‐analysis (Figure 1 ).
Figure 1.
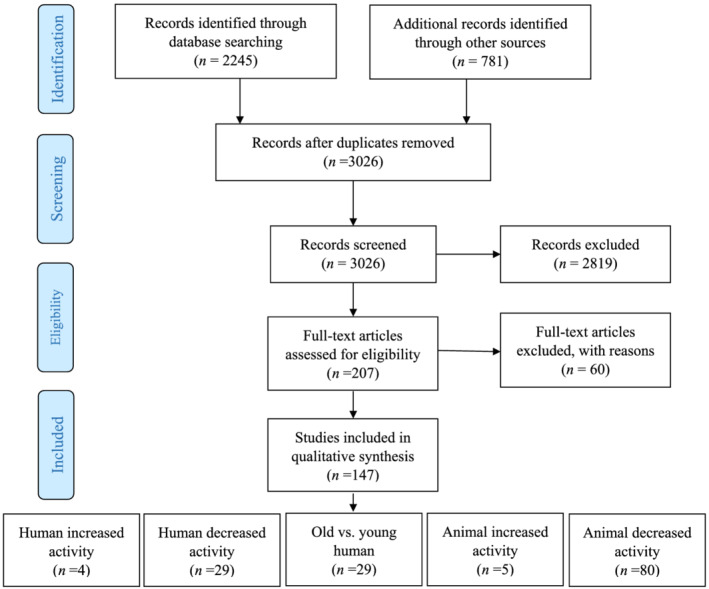
PRISMA flow diagram of study selection.
Quality assessment
We assessed potential study bias using Physiotherapy Evidence Database (PEDro) scale for human studies by two independent researchers. 32 All included human studies presented a score of ≤5.0. We also used the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool for assessing the risk of bias in animal studies. 33 The results of quality assessments in both human and animal studies are outlined in Figure S1A – S1E .
Data extraction
Two reviewers independently (MR and FM) extracted all related information with disagreements between reviewers resolved by discussion. The included information was collected and organized into Tables 1 , 2 , 3 . Information was extracted on study design characteristics (rodent species, sex, age, hypertrophy or atrophy model, etc.), type of intervention (training or atrophy duration), and outcome data (myonuclear content and satellite cell abundance). Included studies were grouped according to the following experiments: human subjects experienced hypertrophy, human subjects experienced atrophy, comparison of old vs. young people, animal models experienced hypertrophy, and animal models experienced atrophy.
Table 1.
The effect of hypertrophy or atrophy on myonuclear content and domain size and satellite cell content in humans
| Author | Participants (number, sex) | Age | Muscle | Hypertrophy/Atrophy model | Training/Atrophy duration | Detraining duration | Muscle fibre size | Myonuclear content | Myonuclear domain | SC content |
|---|---|---|---|---|---|---|---|---|---|---|
| Kadi et al. (2004) 36 | Young (15, M) | 24 ± 1 | VL | Resistance training | 12 wk | 12 wk |
Training: Mixed: ↑ Detraining: Mixed: ↓ |
Training: Mixed: ↔ Detraining: Mixed: ↔ |
NM |
Training: Mixed: ↑ Detraining: Mixed: ↓ |
| Psilander et al. (2019) a , 29 | Young (10, W & 9, M) | 25 ± 1 | VL | Resistance training | 10 wk | 20 wk |
Training: Mixed, I, II: ↔ Detraining: Mixed, I, II: ↔ |
Training: Mixed, I, II: ↔ Detraining: Mixed, I, II: ↔ |
Training: Mixed, I: ↔; II: ↔ Detraining: Mixed, I, II: ↔ |
NM |
| Snijders et al. (2019) 30 | Old (53, M/W) | 70 ± 6 | VL | Resistance training | 24 wk | 48 wk |
Training: Mixed, II: ↑; I: ↔ Detraining: Mixed, II: ↓; I: ↔ |
Training: Mixed, I: ↔; II: ↑ Detraining: Mixed, I: ↔; II: ↓ |
Training: Mixed, I, II: ↔ Detraining: Mixed, I, II: ↔ |
Training: Mixed, I: ↔; II: ↑ Detraining: Mixed, I: ↔; II: ↓ |
| Blocquiaux et al. (2020) 35 | Old (30, M) | 66 ± 5 | VL | Resistance training | 12 wk | 12 wk |
Training: I, II: ↔ Detraining: I, II: ↔ |
Training: I, II: ↔ Detraining: I: ↓; II: ↔ |
Training: I, II: ↔ Detraining: I, II: ↔ |
Training: I, II: ↔ Detraining: I, II: ↔ |
| Carlson et al. (2009) 38 | Young (11, M); Old (9,M) | 22 ± 2; 71 ± 3 | VL | Leg immobilization | 2 wk | NA | Young and Old : Mixed: ↓ | NM | NM | Young and Old : Mixed: ↔ |
| Dirks et al. (2014a) 39 | Old (12, M) | 69 ± 1 | VL | Leg immobilization | 5 d | NA | Mixed: ↓; I, II: ↔ | I, II: ↔ | I, II: ↓ | I, II: ↔ |
| Snijders et al. (2014) 40 | Young (12, M) | 24 ± 1 | VL | Leg immobilization | 2 wk | NA | I, II: ↓ | I, II: ↔ | I, II: ↔ | I, II: ↔ |
| Dirks et al. (2014b) 41 | Young (12, M) | 23 ± 1 | VL | Leg immobilization | 5 d | NA | Mixed: ↓; I, II: ↔ | I, II: ↔ | I, II: ↔ | I, II: ↔ |
| Suetta et al. (2013) 42 | Young (11, M); Old (9,M) | 25 ± 4; 67 ± 6 | VL | Leg immobilization | 2 wk | NA | Young: I, II: ↓; Old: I: ↔, II: ↓ | NM | NM | Young: I, II: ↑; Old: I, II: ↔ |
| Ohira et al. (1999) 43 | Young (13, M) | 33 ± 3 | Soleus | Bed rest | 2 and 4 mos | NA | Mixed: 2mon: ↔, 4mon: ↓ | Mixed: 2 and 4 mos: ↔ | Mixed: 2 mos: ↔, 4 mos: ↓ | NM |
| Brooks et al. (2010) 44 | Young (7, M) | 40 ± 15 | VL | Bed rest | 28 d | NA | Mixed: ↓ | Mixed: ↔ | NM | Mixed: ↔ |
| Arentson‐Lantz et al. (2016) 19 | Young (7, M/W) | 51 ± 1 | VL | Bed rest | 2 wk | NA | Mixed, I, II: ↓ | Mixed, I, II: ↓ | Mixed: ↓ | Mixed, I, II: ↓ |
| Reidy et al. (2017) 45 | Old (9, M/W) | 69 ± 2 | VL | Bed rest | 5 d | NA | Mixed, I: ↓; II: ↔ | Mixed, I, II: ↔ | Mixed, I, II: ↔ | Mixed, I: ↓; II: ↔ |
| Reidy et al. (2018) 46 | Young (14, M/W); Old (9, M/W) | 23 ± 1; 66 ± 1 | VL | Bed rest | 5 d | NA | Young and Old: Mixed, I, II: ↔ | NM | NM | Young and Old: Mixed, I, II: ↔ |
| Moore et al. (2018) 47 | Old (14, M) | 71 ± 5 | VL | Step reduction | 14 d | NA | I: ↔, II: ↓ | I, II: ↔ | I, II: ↓ | I: ↔, II: ↓ |
| Reidy et al. (2019) 48 | Old (12, M) | 70 ± 2 | VL | Step reduction | 7 and 14 d | NA | 7 and 14 d in Mixed: ↔ | 7 and 14 d in Mixed, I, II: ↔ | NM | 7 and 14 d in I: ↑; II: ↔ |
| Smith et al. (2013) 49 | Young (8, M/W); CP (8, M/W) | 16 ± 2; 11 ± 4 | VL | CP | NA | NA | NM | NM | NM | Mixed: ↓ |
| Dayanidhi et al. (2015) 50 | Children (6, M) | 13 ± 3 | Gracilis | CP | NA | NA | Mixed: ↓ | Mixed: ↔ | Mixed: ↔ | Mixed: ↓ |
| Von Walden et al. (2018) 51 | Children and adolescents (22, M/W) | 15 ± 7 | VL | CP and brain injury | NR | NA | Mixed: ↑ | NM | NM | Mixed: ↓ |
| Eliason et al. (2009) 52 | Old (12, M/W); Moderate COPD (12, M/W); Severe COPD (11, M/W) | 62 ± 6.6 | Tibial anterior | COPD | NR | NA |
Moderate COPD: I, II: ↔ Severe COPD: I: ↔; II: ↓ |
NM | NM |
Moderate COPD: Mixed: ↔ Severe COPD: Mixed: ↔ |
| Menon et al. (2012) 53 | Old (7, M/W); COPD (12, M/W) | 67 ± 2 | VL | COPD | NR | NA | I, II: ↔ | NM | NM | Mixed: ↔ |
| Thériault et al. (2012) 54 | Old (12, M/W); Moderate COPD (12, M/W); Severe COPD (11, M/W) |
67 ± 3; 64 ± 2 70 ± 2 |
VL | COPD | NR | NA |
Moderate COPD: I: ↔, II: ↓ Severe COPD: I, II: ↓ |
NM | NM |
Moderate COPD: Mixed: ↔ Severe COPD: Mixed: ↔ |
| Sancho‐Muñoz et al. (2021) 55 | Old (13, M/W); Non SAR (19, M/W); SAR (26, M/W) |
66 ± 5; 65 ± 7 62 ± 8 |
VL | COPD | NR | NA |
Non SAR: I: ↓, II: ↔ SAR: I, II: ↓ |
NM | NM |
Non SAR in Mixed: ↔ SAR in Mixed: ↔ |
| Noehren et al. (2016) 56 | Young (10, M/W) | 23 ± 5 | VL | ACL injury | 12 wk | NA | I: ↔; II: ↓ | NM | NM | Mixed: ↓ |
| Fry et al. (2017) 57 | Young (10, M/W) | 23 ± 5 | VL | ACL injury | 8 wk | NA | NM | NM | NM | Mixed, I, II: ↓ |
| Parstorfer et al. (2021) 58 | Young (1, W; 15, M) | 26 ± 4 | VL | ACL injury | 12 wk | NA | I, II: ↔ | NM | NM | Mixed, I, II: ↓ |
| Day et al. (1995) b , 20 | Young (5, M/W) | 40 ± 7 | VL | Space flight | 11 d | NA | I: ↔; II: ↓ | I: ↔; II: ↓ | I: ↔; II: ↓ | NM |
| Dirks et al. (2015) 59 | Old (6, M/W) | 63 ± 6 | VL | ICU patients | NA | NA | I, II: ↓ | I, II: ↔ | I, II: ↔ | I, II: ↔ |
| Kramer et al. (2017) 60 | Old (30, F) | 80 ± 2 | VL | Hip fracture | NR | NA | I, II: ↓ | I, II: ↔ | I, II: ↓ | I, II: ↔ |
| Farup et al. (2016) 61 | Young (32, NR) | 46 ± 1 | VL | Multiple sclerosis | NR | NA | Mixed, I, II: ↓ | I, II: ↔ | NM | I, II: ↔ |
| Shao et al. (2020) 62 | Young (12, M/W) | 14 ± 4 | Bilateral thoracic multifidus | Idiopathic scoliosis | NR | NA |
Mixed, I: ↓ II: ↔ |
Mixed, I, II: ↓ | NM | Mixed, I, II: ↓ |
| Verdijk et al. (2012) 63 | Young (8, M) | 31 ± 3 | VL | Spinal cord injury | 9 years | NA | I, II: ↓ | I: ↔;II: ↓ | I, II: ↔ | I: ↔; II: ↓ |
| D'Souza et al. (2016) 64 | Young (11, M) | 20 ± 2 | VL | Type 1 diabetes | NR | NA | NM | NM | NM | Mixed: ↓ |
↑, significantly higher compared with control values; ↓, significantly lower compared with control values; ↔, no difference between experiment and control values; ACL, anterior cruciate ligament; COPD, chronic obstructive pulmonary patients; CP, cerebral palsy; I, Type I muscle fibres; II, Type II muscle fibres; M, men; M/W, men and women combined; Mixed, mixed muscle fibre type; NA, not applicable; NM, not measured; NR, not reported; SAR, sarcopenic patients; VL, vastus lateralis; W, women.
This study is performed in both muscle cross section and single muscle fibre.
This study is performed in single muscle fibre.
Table 2.
The effect of ageing on myonuclear content and domain size and satellite cell content in humans
| Author | Age, years (number) | Gender | Muscle | Muscle fibre size | Myonuclear content | Myonuclear domain | SC number |
|---|---|---|---|---|---|---|---|
| Vassilopoulos et al. (1977) 68 | 12–30 (6) vs. 60–71 (6) | M/W | VL | Mixed: ↔ | Mixed: ↔ | NM | NM |
| Manta et al. (1987) 69 | 17–30 (4) vs. >60 (7) | M/W | VL | Mixed: ↓ | Mixed: ↔ | Mixed: ↑ | NM |
| Hikida et al. (1998) 70 | 17–26 (7) vs. 59–71 (8) | M | VL | Mixed, I, II,: ↓ | Mixed: ↔ | NM | Mixed: ↔ |
| Roth et al. (2000) 71 |
22–28 (7) vs. 66–72 (8) 25–27 (7) vs. 64–71 (7) |
M W |
VL | NM | NM | NM |
Mixed: ↔ Mixed: ↔ |
| Renault et al. (2002) 72 | 22–24 (6) vs. 70–78 (6) | M/W |
Biceps Masseter |
NM |
Mixed: ↔ Mixed: ↔ |
NM |
Mixed: ↓ Mixed: ↓ |
| Sajko et al. (2002) 73 | 24–38 (4) vs. 67–73 (6) | M | VL | NM | Mixed: ↓ | NM | Mixed: ↓ |
| Kadi et al. (2004) 74 |
23–29 (15) vs. 70–78 (13) 20–26 (16) vs. 73–79 (14) |
M W |
VL | NM |
Mixed: ↑ Mixed: ↑ |
NM |
Mixed: ↓ Mixed: ↓ |
| Sajko et al. (2004) 75 | 26–30 (6) vs. 69–71 (6) | M | VL | NM | NM | NM | Mixed: ↓ |
| Dreyer et al. (2006) 76 | 21–35 (10) vs. >60 (9) | M | VL | I: ↔, II: ↓ | Mixed: ↔ | NM | Mixed: ↔ |
| Petrella et al. (2006) 77 |
20–35 (15) vs. 60–75 (13) 20–35 (16) vs. 60–75 (14) |
M W |
VL |
Mixed: ↔ Mixed: ↔ |
Mixed: ↔ Mixed: ↔ |
Mixed: ↔ Mixed: ↔ |
Mixed: ↔ Mixed: ↔ |
| Mohamed et al. (2007) 78 | 24–50 (7) vs 65–81 (9) | NR | Triceps | Mixed: ↓ | NM | NM | Mixed: ↓ |
| Verdijk et al. (2007) 79 | 19–21 (8) vs. 69–71 (8) | M | VL | I: ↔, II: ↓ | I, II: ↑ | I, II: ↓ | I: ↔, II: ↓ |
| Cristea et al. (2010) a , 80 |
21–32 (6) vs. 72–96 (9) 24–32 (6) vs. 65–96 (9) |
M W |
VL |
I: ↑, II: ↓ I: ↑, II: ↓ |
I: ↑, II: ↔ I: ↑, II: ↔ |
I: ↑, II: ↓ I, II: ↔ |
NM |
| McKay et al. (2012) 81 | 18–24 (9) vs. 66–74 (9) | M | VL | I: ↔, II: ↓ | NM | I: ↔, II: ↓ | Mixed, II: ↓, I: ↔ |
| Verdijk et al. (2012) 63 | 28–34 (8) vs. 73–77 (8) | M | VL | I: ↔, II: ↓ | I: ↔, II: ↓ | I: ↔, II: ↔ | I: ↔, II: ↓ |
| Walker et al. (2012) 82 |
25–29 (5) vs. 68–72 (6) 25–29 (5) vs. 68–72 (6) |
M W |
VL |
I: ↔, II: ↓ I: ↔, II: ↓ |
Mixed: ↔ Mixed: ↔ |
Mixed: ↓ Mixed: ↔ |
Mixed: ↔ Mixed: ↓ |
| Suetta et al. (2013) 42 | 21–30 (11) vs. 61–74 (9) | M | VL | I, II: ↔ | NM | NM | I, II: ↔ |
| McKay et al. (2014) 83 | 21–27 (12) vs. 62–70 (12) | M | VL | I, II: ↔ | I, II: ↔ | I, II: ↔ | I, II: ↔ |
| Snijders et al. (2014) 65 | 21–23 (10) vs. 72–74 (10) | M | VL | I: ↔, II: ↓ | NM | NM | I: ↔, II: ↓ |
| Verdijk et al. (2014) 84 | 18–49 (50) vs. ≥70 (49) | M | VL | I: ↔, II: ↓ | I: ↔, II: ↓ | I, II: ↔ | I: ↔, II: ↓ |
| Verdijk et al. (2016) 85 | 24–28 (14) vs. 71–73 (16) | M | VL | I: ↔, II: ↓ | I, II: ↓ | NM | I: ↔, II: ↓ |
| Nederveen et al. (2016) 86 | 21–24 (23) vs. 63–71 (22) | M | VL | I: ↔, II: ↓ | NM | NM | I: ↔, II: ↓ |
| Kramer et al. (2017) 60 | 18–25 (15) vs. ≥65 (15) | W | VL | I: ↔, II: ↓ | I: ↔, II: ↓ | I: ↑, II: ↔ | I, II: ↔ |
| Kelly et al. (2018) 87 | 22–30 (27) vs. 62–70 (91) | M | VL | I: ↔, II: ↓ | I, II: ↔ | I: ↔, II: ↓ | I, II: ↔ |
| Reidy et al. (2018) 46 | 18–35 (14) vs. 60–75 (9) | M/W | VL | Mixed: ↔ | NM | NM | Mixed, I, II: ↔ |
| Karlsen et al. (2019) 88 | 19–23 (9) vs. 70–84 (18) | M | VL | I: ↔, II: ↓ | I: ↔, II: ↓ | I: ↔, II: ↓ | I: ↔, II: ↓ |
| Naro et al. (2019) b , 89 | 22–28 (6) vs. 81–96 (6) | M/W | VL | I, II: ↔ | I, II: ↔ | I, II: ↔ | NM |
| Karlsen et al. (2020) 90 | 22–28 (7) vs. 63–71 (19) | M | VL | I: ↔, II: ↓ | NM | NM | Mixed, I: ↔, II: ↓ |
| Perez et al. (2021) 91 | 20–24 (6) vs. 65–78 (11) | M/W | VL | NM | NM | NM | Mixed: ↓ |
↑, significantly higher compared with control values; ↓, significantly lower compared with control values; ↔, no difference between experiment and control values; I, Type I muscle fibres; II, Type II muscle fibres; M, men; M/W, men and women combined; Mixed, mixed muscle fibre type; NM, not measured; VL, vastus lateralis; W, women.
This study is performed in single muscle fibre.
This study is performed in both muscle cross‐section and single muscle fibre.
Table 3.
The effect of hypertrophy or atrophy on myonuclear content and domain size and satellite cell content in rodents
| Author | Specie (sex) | Muscle | Hypertrophy/atrophy model | Training/atrophy duration | Detraining duration | Myonuclear content | Satellite cell number |
|---|---|---|---|---|---|---|---|
| Bruusgaard et al. (2010) a , 16 | NMRI mice (F) | EDL | Synergist ablation | 14 d | 2/8 wk denervation |
In vivo Training: ↑, Detraining: ↔ Ex vivo Training: ↑, Detraining: ↔ |
NM |
| Lee et al. (2018) a , 18 | Sprague–Dawley rats (F) | FHL | Weight loaded‐ladder climbing | 8 wk | 20 wk | Training: ↑, Detraining: ↔ | NM |
| Dungan et al. (2019) b , 21 | C57BL/6J mice (F) | Plan | Weighted wheel running | 8 wk | 12 wk |
Single muscle fibre Training: ↑, Detraining: ↓ Muscle cross section Training: ↑, Detraining: ↓ |
Muscle cross section Training: ↑ Detraining: ↔ |
| Murach et al. (2020) b , 22 | C57BL/6J mice (F) | Sol, Gas, Plan | Weighted wheel running | 8 wk | 24 wk |
Single muscle fibre Training: Sol, Gas: ↑ Detraining: Sol: ↔, Gas: ↓ Muscle cross section Training: Sol: ↑, Gas: ↔ Detraining: Sol, Gas: ↔ |
NM |
| Eftestøl et al. (2021) 92 | Sprague–Dawley rats (M) | Sol | Climbing | 5 wk | 10 wk |
Training: ↑ Detraining: ↑ |
NM |
| Hyatt et al. (2003)S1 | Sprague–Dawley rats (F) | MG, TA |
Denervation Spinal cord transection |
3, 14, 28 d | NA | NM |
Denervation 3 d in MG, TA: ↔ 14, 28 d in MG, TA: ↑ Spinal cord transection 3, 14, 28 d in MG: ↔ 3, 28 d in TA: ↔ 14 d in TA: ↑ |
| Kasper et al. (1996a) a S69 | Sprague–Dawley rats (F) | Gas, TA |
Suspension Space flight |
5.4 d | NA |
Suspension: Gas, TA: ↔ Space flight: Gas, TA: ↑ |
NM |
| Bruusgaard et al. (2008) b , 17 | NMRI mice (F) | EDL, Sol |
Suspension Denervation TTX blockade |
3, 7, 14, and 21 d | NA |
Single muscle fibre Suspension: 14 d in EDL: ↔ Denervation: 7, 14, and 21 d in EDL: ↔, 14 and 21 d in Sol: ↔ TTX blockade: 14 and 21 d in EDL: ↔ Muscle cross‐section Denervation: 3, 7, 14, and 21 d in EDL& Sol: ↔ |
NM |
| Ontell (1974) a S2 | Wistar rats (M) | EDL | Denervation | 2 and 3 wk | NA | 2 and 3 wk: ↔ | 2 and 3 wk: ↑ |
| Cardasis & Cooper (1975) a S3 | Princeton‐Rockefeller mice (M) | Gas | Denervation | 1, 2, 3, 7, 14, 18, and 28 d | NA | 1, 2, 3, 7, 14, 18, and 28 d: ↔ | NM |
| Snow (1983)S4 | C57BL/6 mice (M/F) | EDL, Sol | Denervation | 3, 7, 14, 23, 30, 45, and 65 d | NA | NM | 3, 7, 14, 23, and 65 d in EDL & Sol: ↔, 30d in EDL: ↑, 30 and 45 d in Sol: ↑ |
| Maltin et al. (1992)S5 | Hooded Lister rats (M) | Sol | Denervation | 4 d | NA | ↓ | ↓ |
| Irintchev et al. (1994)S6 | CBA/J and Balb/c mice | Sol | Denervation | 5 and 7 d | NA | NM | 5 and 7 d: ↑ |
| Allen et al. (1995) a S7 | Cats (F) | Sol | Denervation | 6 mos | NA | Sol: ↔ | NM |
| Viguie et al. (1997) a S8 | WI/HicksCar rats (M) | EDL | Denervation | 2, 4, and 7 mos | NA | 2, 4, and 7 mos: ↓ |
2 mos: ↑ 4 mos: ↔ 7 mos: ↓ |
| Dupont‐Versteegden et al. (1999) 25 | Sprague–Dawley rats (F) | Sol | Denervation | 10 d | NA | ↓ | NM |
| Milanic et al. (1999) a S9 | Wistar rats (F) | Sternomastoideus | Denervation | 4 and 7 d | NA | 4 and 7 d: ↔ | NM |
| Dupont‐Versteegden et al. (2000)S10 | Sprague–Dawley rats (F) | Plan, Sol | Denervation | 8 wk | NA | Plan: ↔, Sol: ↓ | NM |
| Schmalbruch et al. (2000)S11 | Wistar rats (M) | EDL, Sol | Denervation | 10 wk | NA | EDL: ↓, Sol: ↓ | NM |
| Dedkov et al. (2001)S12 | WI/HicksCar rats (M) | EDL | Denervation | 25 mos | NA | ↓ | ↓ |
| Nnodim (2001) a S13 | WI/HicksCar rats (M) | Levator ani | Denervation | 8 wk | NA | ↔ | ↑ |
| Wada et al. (2002) a S14 | ICR mice (M) | Plan | Denervation | 5, 10, and 120 d | NA |
3 weeks old (5, 10 d): ↓ 4 months old (10, 120 d): ↔ |
NM |
| Dedkov et al. (2003)S15 | Young and old WI/HicksCar rats (M) | TA | Denervation | 2 mos | NA | NM | Young: ↑, Old: ↑ |
| Roy et al. (2005) a S16 | Sprague–Dawley rats (F) | MG, TA | Denervation | 4 and 60 d | NA |
4 d in MG: ↔ 60 d in MG: ↓ 4 and 60 d in TA: ↔ |
NM |
| Zhong et al. (2005) a S17 | Sprague–Dawley rats (F) | Sol | Denervation | 4 and 60 d | NA |
4 d: ↓ 60 d: ↔ |
NM |
| Aravamudan et al. (2006) a S18 | Sprague–Dawley rats (M) | Diaphragm | Denervation | 14 d | NA | ↔ | NM |
| Van Der Merr et al. (2011)S19 | Wistar rats (M) | Gas | Denervation | 1, 2, and 4 wk | NA |
5‐month‐age rats 1, 2, 4 wk: ↔ 25‐month‐age rats 1, 2, and 4 wk: ↔ |
5‐month‐age rats 1 and 2 wk: ↔, 4 wk: ↑ 25‐month‐age rats 1 wk: ↑, 2 and 4 wk: ↔ |
| Liu et al. (2015)S20 | C57BL/6 mice (M) | TA | Denervation | 6 wk | NA | NM | ↑ |
| Aguera et al. (2019)S21 | Wistar rats (M) | Sol | Denervation | 10 d | NA | NM | ↑ |
| Choi et al. (2020) a S22 | TA | Denervation | Denervation | 7 d | NA | NM | ↓ |
| Hansson et al. (2020) a , 1 | NMRI mice (F) | EDL | Denervation | 14 d | NA | ↔ | NM |
| Xing et al. (2020)S23 | Sprague–Dawley rats (M) | Gas | Denervation | 2, 4, and 6 wk | NA | 2, 4, and 6 wk: ↓ | 2, 4, and 6 wk: ↔ |
| Wong et al. (2021)S24 | C57BL/6 mice (M) | TA | Denervation | 3,6, and 12 mos | NA | NM | 3 and 6 mos: ↑, 12 mos: ↔ |
| Darr et al. (1989) b S26 | Sprague Dawley rats (M) | EDL, Sol | Suspension | 3, 10, 20, and 30 d | NA |
Single muscle fibre 3 d in Sol: ↔ 10, 20, 30 d in Sol: ↓ 3, 10, and 30 d in EDL: ↔ 20 d in EDL: ↓ Muscle cross‐section 3 d in Sol: ↔ 10, 20, 30 d in Sol: ↓ 3, 10, 20, and 30 d in EDL: ↔ |
Single muscle fibre 3,10, 20, and 30 d in Sol: ↓ 3 d in EDL: ↓ 10, 20, and 30 d in EDL: ↔ Muscle cross‐section 3 d in Sol, EDL: ↓ 10, 20, and 30 d in Sol: ↔ 10, 20, and 30 d in EDL:↔ |
| Kasper et al. (1996b) a S27 | Wistar rats (F) | Sol, Plan | Suspension | 28 d | NA | Sol: ↑, Plan: ↔ | NM |
| Allen et al. (1997) a S28 | Sprague–Dawley rats (F) | Sol | Suspension | 14 d | NA | ↓ | NM |
| Mozdziak et al. (2000) a S29 | Sprague–Dawley rats (M) | Sol | Suspension | 28 d | NA | ↓ | NM |
| Mitchell et al. (2001) a S30 | BALB/c mice (F) | Sol | Suspension | 14 d | NA | ↓ | NM |
| Yamazaki (2003)S31 | Wistar rats (M) | Sol | Suspension | 14 d | NA | ↓ | NM |
| Mitchell and Pavlath (2004)S32 | C57BL/6 mice (F) | Sol | Suspension | 14 d | NA | ↓ | NM |
| Leeuwenburgh et al. (2005) 24 | Fischer 344 Norway rats(M) | Sol | Suspension | 14 d | NA | 6 mos: ↓, 32 mos: ↔ | NM |
| Ferreira et al. (2006)S33 | Charles River mice (M) | Gas | Suspension | 6,12,24, 48, and 72 h and 1 wk | NA | NM |
6, 12, 24, and 48 h: ↑ 72 h, 1 wk: ↓ |
| Wang et al. (2006)S34 | Wistar rats (M) | Sol | Suspension | 16 d | NA | ↓ | ↓ |
| Kawano et al. (2007) a S35 | Wistar rats (M) | Sol | Suspension | 14 d | NA | ↓ | NM |
| Kawano et al. (2008) a S36 | Wistar rats (M/F) | Sol | Suspension | 3 mos | NA | ↓ | ↓ |
| Oishi et al. (2008) b S37 | Wistar rats (M) | Sol | Suspension | 14 d | NA | ↓ | NM |
| Tarakina et al. (2008)S38 | Wistar rats (M) | Sol | Suspension | 14 d | NA | ↓ | ↓ |
| Matsuba et al. (2009)S39 | C57BL/6 mice (M) | Sol | Suspension | 14, 28, and 42 d | NA | 14, 28, and 42 d: ↔ | 14, 28, and 42 d: ↓ |
| Kartashkina et al. (2010)S40 | Wistar rats (M) | Sol | Suspension | 14 d | NA | ↓ | ↓ |
| Zhang et al. (2010)S41 | Wistar rats (M) | EDL, Sol | Suspension | 28 d | NA | EDL: ↔, Sol: ↓ | EDL, Sol: ↓ |
| Kachaeva et al. (2011)S42 | Wistar rats (M) | Sol | Suspension | 14 d | NA | ↓ | ↓ |
| Ohira et al. (2011)S43 | Wistar rats (M) | Adductor longus | Suspension | 16 and 32 d | NA | 16 and 32 d: ↓ | 16 and 32 d: ↔ |
| Teixeira et al. (2011)S44 | Charles River mice (M) | Sol | Suspension | 1, 2, 3, and 8 d | NA | 1 d: ↔, 2, 3, and 8 d: ↓ | NM |
| Bruusgaard et al. (2012) 15 | Wistar rats (F) | Sol | Suspension | 2, 4, and 14 d | NA | ↔ | NM |
| Jackson et al. (2012) a S25 | Pax7‐DTA mice (F) | Sol | Suspension | 14 d | NA | ↔ | NM |
| Guo et al. (2012) 23 | BALB/c mice (M) | Sol | Suspension | 14 d | NA | NM | ↓ |
| Lomonosova et al. (2012)S45 | Wistar rats (M) | Sol | Suspension | 14 d | NA | NM | ↓ |
| Zushi et al. (2012)S46 | Wistar rats (M) | Sol | Suspension | 14 d | NA | ↓ | NM |
| Itoh et al. (2014)S47 | ICR mice (M) | Sol | Suspension | 14 d | NA | ↓ | NM |
| Park et al. (2014)S48 | C57BL/6 mice (F) | Sol | Suspension | 7 d | NA | NM | ↔ |
| Babcock et al. (2015)S49 | Wistar rats (M) | TA | Suspension | 10 d | NA | ↓ | ↓ |
| Ohira et al. (2015) a S50 | Osteopetrotic mice (M) | Sol | Suspension | 10 d | NA | In +/+, +/op and op/op: ↓ | In +/+, +/op and op/op: ↓ |
| Nakanish et al. (2016)S51 | Wistar rats (F) | Sol | Suspension | 7 d | NA | ↓ | ↓ |
| Itoh et al. (2017)S52 | ICR mice (M) | Sol | Suspension | 14 d | NA | ↔ | NM |
| Anderson et al. (2018)S53 | C57BL/6 mice (M/F) | Gas | Suspension | 18 d | NA | NM | ↓ |
| Brooks et al. (2018) 66 | C57BL/6 mice (M/F) | Gas | Suspension | 14 d | NA | ↓ | ↔ |
| Miller et al. (2018)S54 | Norway–F344 rats (M) | Gas | Suspension | 14 d | NA | ↔ | ↓ |
| Kneppers et al. (2019)S55 | C57BL/6 mice (M) | Gas | Suspension | 14 d | NA | ↓ | ↔ |
| Nakanishi et al. (2021)S56 | Wistar rats (F) | Sol | Suspension | 14 d | NA | NM | ↓ |
| Petrocelli et al. (2021)S57 | C57BL/6 mice (M) | Gas, Sol | Suspension | 14 d | NA | NM | Gas, Sol: ↔ |
| Smith et al. (2000)S58 | Californian rabbits (F) | Sol | Immobilization | 2 and 6 d | NA | 2 d: ↔, 6 d: ↓ | NM |
| Wanek and Snow (2000)S59 | Sprague–Dawley rats (M/F) | Sol | Immobilization | 2, 4, and 8–10 wk | NA | NM | 2 and 4 wk:↔, 8–10 wk: ↓ |
| Ye et al. (2013)S60 | C57BL/6 mice (F) | Sol | Immobilization | 14 d | NA | NM | ↓ |
| Matsumoto et al. (2014)S61 | Wistar rats (M) | Gas | Immobilization | 4 wk | NA | ↓ | NM |
| Li et al. (2016) 28 | Wistar rats (M) | Sol | Immobilization | 14 d | NA | NM | ↓ |
| Guitart et al. (2018)S62 | C57BL/6 mice (F) | Gas, Sol | Immobilization | 7 d | NA | NM | Gas: ↓, Sol: ↓ |
| Usuki et al. (2019)S63 | Wistar rats (M) | Sol | Immobilization | 7 d | NA | NM | ↓ |
| Suzuki et al. (2020)S64 | Sprague–Dawley rats (M) | Plan, Sol | Immobilization | 7 d | NA | Plan, Sol: ↔ | NM |
| Zazula et al. (2020)S65 | Wistar rats (M) | TA | Immobilization | 7 d | NA | ↓ | NM |
| Honda et al. (2021)S66 | Wistar rats (M) | Sol | Immobilization | 14 d | NA | ↓ | NM |
| Allen et al. (1996) a S67 | Sprague–Dawley rats (M) | Sol | Space flight | 14 d | NA | ↓ | NM |
| Hikida et al. (1997)S68 | Fisher 344 rats (M) | Sol | Space flight | 10 d | NA | ↓ | NM |
| Sandonà et al. (2012) 67 | C57BL/10J mice (M) | EDL, Sol | Space flight | 91 d | NA | EDL: ↔, Sol: ↓ | NM |
| Radugina et al. (2018) 27 | C57BL/6 mice (M) | Quadriceps | Space flight | 30 d | NA | ↓ | NM |
| McClung et al. (2006)S70 | Sprague–Dawley rats (F) | Diaphragm | Mechanical ventilation | 12 h | NA | ↓ | NM |
↑, significantly higher compared with control values; ↓, significantly lower compared with control values; ↔, no difference between experiment and control values; EDL, extensor digitorum longus; F, female; FHL, flexor hallucis longus; Gas, gastrocnemius muscle; ICR, Institute of Cancer Research (ICR) mice (Japan SLC, Shizuoka, Japan); M, male; M/F, male and female combined; MG, medial gastrocnemius; NA, not applicable; NM, not measured; Plan, plantaris muscle; Sol, soleus muscle; TA, tibialis anterior muscle.
This study is performed in single muscle fibre.
This study is performed in both muscle cross‐section and single muscle fibre.
Data analysis
All data analyses were conducted using Review Manager Software (RevMan 5.3, Cochrane Collaboration, Copenhagen, Denmark) as previously described in detail by us. 34 For instance, when data was only available in a graphic format, we used WebPlotDigitizer software to extract quantitative data from the figure. Results were expressed as standardized mean difference (SMD) and 95% confidence intervals (CI) when the outcome is measured in different ways; otherwise, the mean difference (MD) and 95% CI were calculated. 31 , 34 When there was a sufficient number of studies, subgroup analysis was performed on muscle type, atrophy model, atrophy duration, and hypertrophy percentage in the animal model and on age (young and old) and atrophy model in human subjects. To evaluate and ensure the robustness of the results, sensitivity analysis was carried out by removing studies from the meta‐analysis. Sensitivity analysis showed that no results were affected by any study (data not shown). Finally, funnel plots with Egger weighted regression test were used for assessing publication bias using STATA version 16.
Results
Evidence from human studies
Skeletal muscle responses to hypertrophy
Four reports involving 117 participants assessed the response of skeletal muscle (vastus lateralis) to resistance training followed by a period of detraining. 29 , 30 , 35 , 36 Resistance training duration ranged from 10 to 24 weeks in these studies. However, detraining duration ranged from 12 to 48 weeks. Currently, the general consensus is that myonuclear content tends to be lower in older adults (≥60 year) compared with young adults (18–55 year). 37 Thus, we performed a subgroup analysis to clarify the effects of an episode of overload hypertrophy and subsequent disuse atrophy on the present review outcomes in terms of the different age categories. The details of the included studies are shown in Table 1 .
Myofibre size following training and detraining
Resistance training significantly increased cross‐sectional area (CSA) compared with baseline values (mean: MD = 650.32, 95% CI 355.30–945.34, P = 0.0001; Type I: MD = 470.83, 95% CI 168.29–773.37, P = 0.002; Type II: MD = 723.93, 95% CI 358.02–1089.84, P = 0.0001; Figure S2 A– S2 C). Further, CSA after a detraining period following resistance training returned to the pre‐training values (mean: MD = 83.46, 95% CI −649.41 to 816.32, P = 0.82; Type I: MD = 104.39, 95% CI −604.64 to 813.23, P = 0.77; Type II: MD = 190.74, 95% CI −882.92 to 1264.40, P = 0.73; Figure S2 D– S2 F). Subgroup analysis in mixed and Type II fibres showed no statistically significant difference between young and old adults after training and detraining periods (mixed: P = 0.50 and P = 0.20; Type II: P = 0.97 and P = 0.31, respectively). Further, subgroup analysis showed that the reduction of Type I fibre CSA of young adults was significantly higher following a detraining period than old subjects (P = 0.03) (Figure S2 A– S2 F).
Myonuclear content following training and detraining
Resistance training significantly increased myonuclear content in mixed and Type II fibres compared with baseline values (mean: MD = 0.12, 95% CI 0.00–0.23, P = 0.04; Type I: MD = 0.04, 95% CI −0.08 to 0.15, P = 0.55; Type II: MD = 0.23, 95% CI 0.07–0.40, P = 0.006; Figure S2 G– S2 I). Compared with pre‐training, there was a significant difference in myonuclear content after a detraining period (mean: MD = −0.14, 95% CI −0.26 to −0.02, P = 0.02; Type I: MD = −0.14, 95% CI −0.28 to −0.0, P = 0.05; Type II: MD = −0.23, 95% CI −0.37 to −0.10, P = 0.0009; Figure S2 J– S2 L), indicating that myonuclear content after a detraining period was less than the baseline. Subgroup analysis showed no statistically significant difference between young and old adults after training and detraining periods (mixed: P = 0.56 and P = 0.73; Type I: P = 0.42 and P = 0.86; Type II: P = 0.37 and P = 0.73; respectively; Figure S2 G– S2 L).
Myonuclear content in single muscle fibre following training and detraining
A single report with 19 participants assessed myonuclear content in single muscle fibre using 44–57 fibres from each biopsy sample. 29 This study reported no change in myonuclear content in response to resistance training (i.e. +5%) and after detraining (i.e. +3%).
Myonuclear domain following training and detraining
Resistance training significantly increased myonuclear domain (MND) only in mixed fibres compared with baseline values (mean: MD = 110.91, 95% CI 24.93–196.89, P = 0.01; Type I: MD = 5.67, 95% CI −133.51 to 144.85, P = 0.94; Type II: MD = 73.87, 95% CI −62.35 to 210.09, P = 0.29; Figure S2 M– S2 O). Moreover, MND after a detraining period returned to pre‐training levels (mean: MD = 43.16, 95% CI −42.14 to 128.47, P = 0.32; Type I: MD = −9.26, 95% CI −166.29 to 147.77, P = 0.91; Type II: MD = 55.98, 95% CI −138.18 to 250.14, P = 0.57; Figure S2 P– S2 R). Subgroup analysis in mixed fibres showed no statistically significant difference between young and old adults after training and detraining periods (P = 0.06 and P = 0.33, respectively). The number of studies was too small to permit subgroup analyses of Type I or Type II fibres (Figure S2 M– S2 R).
Satellite cell number following training and detraining
Three studies involving 94 participants assessed SC abundance. 30 , 35 , 36 Resistance training significantly increased SC abundance in mixed and Type II fibres compared to baseline values (mean: SMD = 0.75, 95% CI 0.33–1.18, P = 0.0005; Type I: SMD = 0.36, 95% CI −0.14 to 0.85, P = 0.16; Type II: SMD = 0.81, 95% CI 0.30–1.32, P = 0.002; Figure S2 S– S2 U). Additionally, SC abundance after a detraining period returned to pre‐training levels (mean: SMD = 0.16, 95% CI −0.32 to 0.64, P = 0.52; Type I: SMD = −0.01, 95% CI −0.66 to 0.65, P = 0.99; Type II: SMD = 0.09, 95% CI −0.57 to 0.74, P = 0.79; Figure S2 W– S2 Y). Subgroup analysis in mixed fibres showed no statistically significant difference between young and old adults after training and detraining periods (P = 0.29 and P = 0.58, respectively). The number of studies was too small to permit subgroup analyses of Type I or Type II fibres (Figure S2 S– S2 Y).
Skeletal muscle responses to atrophy
Twenty‐nine studies assessed skeletal muscle growth in whole muscle cross section in response to leg immobilization, 38 , 39 , 40 , 41 , 42 bed rest, 19 , 43 , 44 , 45 , 46 step reduction, 47 , 48 space flight, 20 and patients suffering from cerebral palsy, 49 , 50 , 51 chronic obstructive pulmonary disease (COPD), 52 , 53 , 54 , 55 anterior ligament reconstruction, 56 , 57 , 58 , fully sedating ICU patients 59 hip fracture, 60 multiple sclerosis, 61 adolescent idiopathic scoliosis, 62 spinal cord injury, 63 and Type1 diabetes. 64 The details of the included studies are shown in Table 1 . We performed subgroup analyses to determine the potential impact that differences in the age of the participants (old vs. young), the duration of the intervention (≤5 days, 7–14 days, 20–30 days, and ≥60 days), and the model of atrophy used had on the atrophic response.
Myofibre size following atrophy
Analysis of 19 studies involving 460 participants 19 , 39 , 41 , 42 , 43 , 45 , 46 , 47 , 50 , 52 , 53 , 54 , 55 , 56 , 59 , 60 , 62 , 63 , 65 found there was lower skeletal muscle CSA following the aforementioned (‘Skeletal muscle responses to atrophy’ section) interventions (mixed: MD = −497.24, 95% CI −734.13 to −260.35, P = 0.0001; Type I: MD = −743.63, 95% CI −1059.28 to −427.98, P = 0.00001; Type II: MD = −908.11, 95% CI −1268.67 to −547.54, P = 0.00001; Figure S3 A– S3 C). Subgroup analysis showed no statistically significant difference between young and old adults for CSA of mixed, Type I, and Type II fibres in response to atrophy (P = 0.52, P = 0.93, and P = 0.60, respectively). Stratifying studies based on the duration of the intervention period found that myofibre CSA was decreased after 7 days in different atrophy models (mixed: MD = −914.33, 95% CI −1528.91 to −299.75, P = 0.004; Type I: MD = −710.72, 95% CI −1217.05 to −204.38, P = 0.006; Type II: MD = −1126.26, 95% CI −1618.85 to −633.68, P = 0.00001; Figure S3 D– S3 F). Subgroup analysis that stratified studies based on the model of atrophy showed that bed rest, COPD, idiopathic scoliosis, and hip fracture induced a significant decrease in fibre CSA (Figure S3 G– S3 I).
Myonuclear content following atrophy
Analysis of 13 studies involving 260 participants 19 , 39 , 40 , 41 , 43 , 44 , 45 , 47 , 50 , 59 , 60 , 61 , 62 found lower myonuclear content in response to skeletal muscle atrophy (mean: MD = −11, 95% CI −0.19 to −0.03, P = 0.005; Type I: MD = −0.09, 95% CI −0.17 to −0.00, P = 0.04; Type II: MD = −0.13, 95% CI −0.22 to −0.05, P = 0.003; Figure S3 J– S3 L). Interestingly, subgroup analysis showed myonuclear content in mixed, Type I, and Type II fibre only decreased in young adults and not in old adults who experienced atrophy (old adults: P = 0.61, P = 0.58, and P = 0.77, respectively). Subgroup analysis showed no difference between different period of interventions in mixed, Type I, and Type II fibres (P = 0.69, P = 0.81, and P = 0.64, respectively; Figure S3 M– S3 O). Stratifying studies based on the model of atrophy showed that bed rest, idiopathic scoliosis, and cerebral palsy induced a significant decrease in myonuclear content (Figure S3 P– S3 R).
Myonuclear content in single muscle fibre following atrophy
A single report with five astronauts assessed myonuclear content in single muscle fibre using 42–81 fibres from each biopsy sample before and after 11 days of space flight. 20 This study reported no change in the myonuclear content of Type I fibres, whereas lower myonuclear content was found in Type II fibres.
Myonuclear domain following atrophy
Analysis of 10 studies involving 202 participants 19 , 39 , 40 , 41 , 43 , 47 , 50 , 59 , 60 , 63 found a significant decrease in MND in response to skeletal muscle atrophy (mean: MD = −1.92, 95% CI −2.72 to −1.12, P = 0.00001; Type I: MD = −0.65, 95% CI −0.97 to −0.32, P = 0.0001; Type II: MD = −0.72, 95% CI −1.03 to −0.40, P = 0.0001; Figure S3 S– S3 W). The results from a single study with five astronauts showed lower MND in single muscle fibres after 11 days of space flight. 20 Subgroup analysis showed no difference between the reduction of MND in mixed, Type I, and Type II fibres in old and young adults and different periods of intervention (Figure S3 X– S3 Z). Stratifying studies based on the model of atrophy showed that leg immobilization, step reduction, cerebral palsy, and hip fracture induced a significant decrease in myonuclear content ( Figure S3A1 – S3C1 ).
Satellite cell number following atrophy
Analysis from 24 studies involving 611 participants 19 , 38 , 39 , 41 , 45 , 46 , 47 , 50 , 51 , 52 , 53 , 54 , 56 , 57 , 58 , 59 , 60 , 61 , 64 , 66 , 67 found there was lower SC abundance in response to skeletal muscle atrophy (mean: SMD = −0.49, 95% CI −0.77 to −0.22, P = 0.0005; Type I: SMD = −0.20, 95% CI −0.59 to 0.20, P = 0.33; Type II: SMD = −0.37, 95% CI −0.71 to −0.02, P = 0.04; Figure S3D1 – S3F1 ). In agreement with changes in myonuclear content, subgroup analysis showed SC content in mixed, Type I, and Type II fibre only decreased in young adults and not in old adults who experienced atrophy (old adults: P = 0.07, P = 0.76, and P = 0.35, respectively). Stratifying studies based on duration of the intervention period found that SC content was decreased after 60 days (mixed: MD = −0.85, 95% CI −1.61 to −0.09, P = 0.03; Type I: MD = −0.87, 95% CI −0.48 to −0.43, P = 0.01; Type II: MD = −1.02, 95% CI −1.61 to −0.44, P = 0.0006; Figure S3G1 – S3I1 ). Stratifying studies based on the model of atrophy showed that bed rest, cerebral palsy, idiopathic scoliosis, and ACL injury induced a significant decrease in SC content ( Figure S3J1 – S3L1 ).
Skeletal muscle responses in ageing compared with young adults
Next, we assessed the impact of ageing on myonuclear content, MND, and SC abundance. Twenty‐nine studies measured the aforementioned skeletal muscle characteristics in young and old adults. 42 , 60 , 63 , 65 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 The details of the included studies are shown in Table 2 .
Myofibre size following ageing
Analysis of 25 studies involving 724 participants 42 , 46 , 60 , 63 , 65 , 68 , 69 , 70 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 found that except Type I fibres, CSA in mixed and Type II fibres decreased with ageing (mean: SMD = 0.91, 95% CI 0.25–1.56, P = 0.007; Type I: MD = −131.7, 95% CI −353.91 to 90.51, P = 0.25; Type II: MD = 1313.31, 95% CI 995.45–1631.16, P = 0.00001; Figure S4 A– S4 C).
Myonuclear content following ageing
Analysis of 17 studies involving 494 participants 60 , 63 , 68 , 69 , 70 , 72 , 74 , 76 , 77 , 79 , 82 , 83 , 84 , 85 , 86 , 87 , 88 found no change in myonuclear content of mixed and Type I fibres with ageing (MD = −0.03, 95% CI −0.24 to 0.19, P = 0.8; MD = −0.01, 95% CI −0.31 to 0.29, P = 0.95; respectively; Figure S4 D and S4 E). A pooled analysis from eight studies involving 274 participants found lower myonuclear content in Type II fibres with ageing (MD = 0.47, 95% CI 0.09–0.85, P = 0.02; Figure S4 F).
Myonuclear content in single muscle fibre following ageing
Two studies assessed muscle response to ageing at the single muscle fibre level. Cristea et al. 80 in a separate analysis of men and women reported a significant increase in myonuclear content of Type I fibres with no change in Type II fibres. In another study, Naro et al. 89 reported no change in myonuclear content and MND of Type I and II fibres.
Myonuclear domain following ageing
Analysis of 11 studies involving 346 participants 60 , 63 , 69 , 77 , 79 , 81 , 82 , 83 , 84 , 87 , 88 found no change in MND of mixed and Type I fibres with ageing (MD = 236.01, 95% CI −11.78 to 483.79, P = 0.06; MD = −26.75, 95% CI −207.05 to 153.56, P = 0.77; respectively; Figure S4 G and S4 H). In contrast, there was lower MND in Type II fibres with ageing (MD = 296.19, 95% CI 109.08–483.29, P = 0.002; Figure S4 I).
Satellite cell number following ageing
Analysis of 25 studies involving 717 participants 42 , 46 , 63 , 65 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 90 , 91 found lower SC abundance in mixed fibres with ageing (SMD = 0.78, 95% CI 0.37–1.19, P = 0.0002; Figure S4 J). There was no change in SC content associated with Type I fibres, whereas SC content associated with Type II fibres was lower with ageing (SMD = 0.09, 95% CI −0.11 to 0.28, P = 0.38; SMD = 1.23, 95% CI 0.86–1.60, P = 0.00001; respectively; Figure S4 K and S4 L).
Evidence from animal studies
Skeletal muscle responses to hypertrophy
Five studies assessed skeletal muscle growth in response to a hypertrophic stimulus induced by synergist ablation, 16 weight loaded‐ladder climbing, 18 climbing, 92 or weighted wheel running 21 , 22 in extensor digitorum longus (EDL), 16 flexor hallucis longus (FHL), 18 plantaris, 21 , 22 soleus, tibialis anterior (TA), 92 and gasterocnemius 22 , 92 muscles. Following exposure to an episode of overload‐induced hypertrophy, skeletal muscle was subsequently exposed to disuse atrophy as a model of detraining 18 , 21 , 22 , 92 or denervation. 16 Given that young mice (<4 months old) have been shown to display a different response to overload‐induced hypertrophy relative to mature mice (>4 months old), 93 we performed a subgroup analysis to determine the effects of age on an episode of overload‐induced hypertrophy followed by disuse atrophy on the aforementioned outcome variables. The details of the included studies are shown in Table 3 .
Myofibre size following training and detraining
Five studies assessed CSA response to increased activity. 16 , 18 , 21 , 22 , 92 An episode of overload‐induced hypertrophy significantly increased fibre CSA (SMD = 1.25, 95% CI 0.83–1.67, p = 0.00001; Figure S5 A). Compared with control, there was no significant difference in fibre CSA after a detraining period (SMD = −0.60, 95% CI −1.71 to 0.51, P = 0.29), demonstrating that fibre CSA after a detraining period returns to baseline levels (Figure S5 B). Subgroup analysis showed a significant difference between young and mature animals after training and detraining (P = 0.04 and P = 0.03, respectively), indicating that fibre CSA in young animals increases by a higher extent following training and decreases by a larger extent following detraining.
Myonuclear content following training and detraining
Three studies assessed myonuclear content in muscle cross section. 21 , 22 , 92 In response to a hypertrophic stimulus, there was a significant increase in myonuclear content (MD = 0.17, 95% CI 0.09–0.25, P = 0.0001; Figure S5 C). Myonuclear content remained significantly elevated after a period of detraining compare with control animals (MD = 0.11, 95% CI 0.02–0.20, P = 0.01; Figure S5 D). The number of studies was too small to permit subgroup analysis.
Myonuclear content in single muscle fibre following training and detraining
Four studies assessed myonuclear content in single muscle fibre. 16 , 18 , 21 , 22 An episode of overload‐induced hypertrophy significantly increased myonuclear content (SMD = 2.26, 95% CI 1.28–3.23, P = 0.00001; Figure S5 E). Myonuclear content following a period of detraining remained significantly elevated compared with control animals (SMD = 1.46, 95% CI 0.60–2.32, P = 0.0008; Figure S5 F). Subgroup analysis of maturational age showed no statistically significant difference after overload‐induced hypertrophy or detraining periods (P = 0.60 and P = 0.43, respectively).
Skeletal muscle responses to atrophy
Eighty studies assessed skeletal muscle atrophy in response to different duration of denervation, 1 , 17 , 25 ,S1–S24 hindlimb suspension, 15 , 17 , 23 , 24 , 66 ,S25–S57 immobilization, 28 ,S58–S66 space flight, 27 , 67 ,S67–S69 tetrodotoxin blockage, 17 and mechanical ventilation.S70 We performed subgroup analyses to determine the potential impact that differences in the muscle under investigation, the duration of the intervention, and the model of atrophy used had on the atrophic response.
Myofibre size following atrophy
Analysis of 53 studies found lower CSA in response to skeletal muscle atrophy with a mean reduction of ~−36.9% (SMD = −1.96, 95% CI −2.21 to −1.71, P = 0.00001; Figure S6 A). Subgroup analysis for different muscles showed fibre CSA was significantly decreased in plantaris, soleus, gastrocnemius, pectoralis major, EDL, and TA (Figure S6 B). Subgroup analysis that stratified studies based on duration of the intervention period (≤5 days, 7–14 days, 20–30 days, and ≥42 days) found that myofibre CSA was decreased for all periods (Figure S6 C). Subgroup analysis that stratified studies based on the model of atrophy showed that except for mechanical ventilation, all models induced a significant decrease in fibre CSA (Figure S6 D). Subgroup analysis that stratified studies based on different methods of atrophy showed that myonuclear content was decreased in studies that performed hindlimb suspension, denervation, and immobilization (Figure S6 E). Additionally, subgroup analysis based on %CSA reduction (<20, 20–29, 30–39, 40–49, and >50%) showed that when %CSA reduction reach ≥30%, myonuclear content decreased significantly (Figure S6 F). A final subgroup analysis that stratified studies based on different methods of atrophy showed that SC content was decreased only in studies that performed hindlimb suspension (~−24.4%) and immobilization (~−30.1%). More interestingly, SC abundance was increased (~+113.3%) in response to denervation (Figure S6 G).
Myonuclear content following atrophy
Analysis of 40 studies found lower myonuclear content in muscle cross section with a mean reduction of ~−20.6% (SMD = −1.03, 95% CI −1.30 to −0.76, P = 0.00001; Figure S6 H). Subgroup analysis that stratified studies based on different muscles showed that myonuclear content was decreased only in gastrocnemius, EDL, and soleus (Figure S6 I). Subgroup analysis that stratified studies based on different intervention periods (≤5, 7–14, 20–30, and ≥42 days) showed that myonuclear content was decreased in all periods (Figure S6 J).
Myonuclear content in single muscle fibre following atrophy
Analysis of 22 studies found lower myonuclear content in single muscle fibres with a mean reduction of approximately −10.1% (SMD = −0.52, 95% CI −0.81 to −0.23, P = 0.0005; Figure S6 K). Subgroup analyses that stratified studies based on differences in muscle under investigation, duration of the intervention, and model of atrophy used found myonuclear content was only decreased in the soleus (Figure S6 L). Subgroup analysis that stratified studies based on different intervention periods (≤5 days, 7–14 days, 20–30 days, and ≥42 days) showed that myonuclear content was decreased in studies that lasted between 7–14 and more than 42 days (Figure S6 M). Subgroup analysis that stratified studies based on different models of atrophy showed that myonuclear content was decreased in studies that performed hindlimb suspension and denervation (Figure S6 N). Considering the different muscle type responses to atrophy, the discrepancy between the results for myonuclear content in whole muscle cross section and single muscle fibres may be due to the lower and selected fibre measurements in the studies that used single muscle fibre, as no more than 100 fibres were evaluated in any study.
Satellite cell number following atrophy
Analysis of 41 studies found no change in SC content in cross‐section (SMD = −0.13, 95% CI −0.50 to −0.24, P = 0.48) (Figure S6 O). Subgroup analysis that stratified studies based on different muscles showed that SC content was decreased in soleus, whereas in TA it increased, and in EDL tend to increase (Figure S6 P). Subgroup analysis that stratified studies based on different intervention periods (≤5, 7–14, 18–30, and ≥42 days) showed a trend for lower SC content only in studies that lasted between 7 and 14 days (Figure S6 Q).
Sensitivity analysis and publication bias
In regard to sensitivity analysis, the overall pooled estimates of the respective outcomes obtained in each analysis closely resembled the preliminary associations. Further, funnel plots were checked for the included studies in the meta‐analysis, which suggested that in almost all analyses in human studies, there is no noticeable bias (Figure S7A – S7D ). Additionally, Begg's correlation rank and Egger's regression did not show significant publication bias in almost all analyses in human studies (Table 4 ). In contrast, we found noticeable publication bias in most analyses of animal studies with significant Begg's correlation rank and Egger's regression results ( Figure S7E and S7F ; Table 4 ).
Table 4.
Meta‐analysis of all studies
| Subgroup analysis | Classification | Heterogeneity | Model | Meta‐analysis | ||||
|---|---|---|---|---|---|---|---|---|
| P | I 2 (%) | SMD (95% CI) | P | Beggs' P value | Eggers' P value | |||
| Human studies: skeletal muscle responses to hypertrophy | ||||||||
| Outcome: CSA in whole cross section | ||||||||
| Mixed fibre | After training | 0.6 | 0% | Fixed | 650.32 (355.30, 945.34) | 0.0001 | 1.0000 | 0.324 |
| After detraining | 0.01 | 72% | Random | 83.46 (−649.41, 816.32) | 0.82 | 0.7341 | 0.144 | |
| Type I fibres | After training | 0.72 | 0% | Fixed | 470.83 (168.29, 773.37) | 0.002 | 1.0000 | 0.618 |
| After detraining | 0.07 | 62% | Random | 104.39 (−604.46, 813.23) | 0.77 | 0.2963 | 0.309 | |
| Type II fibres | After training | 0.32 | 13% | Fixed | 723.93 (358.02, 1089.84) | 0.0001 | 1.0000 | 0.363 |
| After detraining | 0.04 | 70% | Random | 190.74 (−882.92, 1264.40) | 0.73 | 0.2963 | 0.200 | |
| Outcome: Myonuclear content | ||||||||
| Mixed fibres | After training | 0.58 | 0% | Fixed | 0.12 (0.00, 0.23) | 0.04 | 0.7341 | 0.491 |
| After detraining | 0.52 | 0% | Fixed | −0.14 (−0.26, −0.02) | 0.02 | 1.0000 | 0.993 | |
| Type I fibres | After training | 0.70 | 0% | Fixed | 0.04 (−0.08, 0.15) | 0.55 | 1.0000 | 0.764 |
| After detraining | 0.96 | 0% | Fixed | ‐0.14 (−0.28, −0.00) | 0.05 | 1.0000 | −0.71 | |
| Type II fibres | After training | 0.4 | 0% | Fixed | 0.23 (0.07, 0.40) | 0.006 | 1.0000 | 0.349 |
| After detraining | 0.61 | 0% | Fixed | −0.23 (−0.37, −0.10) | 0.0009 | 1.0000 | 0.733 | |
| Outcome: Myonuclear domain | ||||||||
| Mixed fibres | After training | 0.2 | 36% | Fixed | 110.91 (24.93, 196.89) | 0.01 | 0.7341 | 0.437 |
| After detraining | 0.55 | 0% | Fixed | 43.16 (−42.14, 128.47) | 0.32 | 0.3082 | 0.349 | |
| Type I fibres | After training | 0.34 | 0% | Fixed | 5.67 (−133.51, 144.85) | 0.94 | IO | IO |
| After detraining | 0.42 | 0% | Fixed | −9.26 (−166.29, 147.77) | 0.91 | IO | IO | |
| Type II fibres | After training | 0.8 | 0% | Fixed | 73.87 (−62.35, 210.09) | 0.29 | IO | IO |
| After detraining | 0.48 | 0% | Fixed | 55.98 (−138.18, 250.14) | 0.57 | IO | IO | |
| Outcome: Satellite cells | ||||||||
| Mixed fibres | After training | 0.52 | 0% | Fixed | 0.75 (0.33, 1.18) | 0.0005 | 1.0000 | 0.814 |
| After detraining | 0.84 | 0% | Fixed | 0.16 (−0.32, 0.64) | 0.52 | 1.0000 | 0.808 | |
| Type I fibres | After training | 0.77 | 0% | Fixed | 0.36 (−0.14, 0.85) | 0.16 | IO | IO |
| After detraining | 0.58 | 0% | Fixed | −0.01 (−0.66, 0.65) | 0.99 | IO | IO | |
| Type II fibres | After training | 0.98 | 0% | Fixed | 0.81 (0.30, 1.32) | 0.002 | IO | IO |
| After detraining | 0.74 | 0% | Fixed | 0.09 (−0.57, 0.74) | 0.79 | IO | IO | |
| Human studies: skeletal muscle responses to atrophy | ||||||||
| Outcome: CSA | ||||||||
| Mixed fibre | NA | 0.002 | 61% | Random | −497.24 (−734.13, −260.35) | 0.0001 | 0.0022 | 0.005 |
| Type I fibres | NA | 0.0001 | 62% | Random | −735.16 (−1062.57, −407.75) | 0.0001 | 0.0369 | 0.089 |
| Type II fibres | NA | 0.00001 | 71% | Random | −919.18 (−1292.14, −546.22) | 0.00001 | 0.0241 | 0.102 |
| Outcome: Myonuclear content | ||||||||
| Mixed fibre | NA | 0.12 | 32% | Fixed | −0.11 (−0.19, −0.03) | 0.005 | 0.0160 | 0.052 |
| Type I fibres | NA | 0.71 | 0% | Fixed | −0.09 (−0.17, −0.00) | 0.04 | 0.2129 | 0.141 |
| Type II fibres | NA | 0.06 | 44% | Fixed | −0.13 (−0.22, −0.05) | 0.003 | 0.1367 | 0.164 |
| Outcome: Myonuclear domain | ||||||||
| Mixed fibre | NA | 0.0001 | 72% | Fixed | −1.92 (−2.72, −1.12) | 0.00001 | 0.7555 | 0.520 |
| Type I fibres | NA | 0.63 | 0% | Fixed | −0.65 (−0.97, −0.32) | 0.0001 | 0.5362 | 0.798 |
| Type II fibres | NA | 0.68 | 0% | Fixed | −0.72 (−1.03, −0.40) | 0.0001 | 0.3865 | 0.712 |
| Human studies: skeletal muscle responses to atrophy | ||||||||
| Outcome: Satellite cells | ||||||||
| Mixed fibre | NA | 0.0001 | 61% | Random | −0.49 (−0.77, −0.22) | 0.0005 | 0.0232 | 0.000 |
| Type I fibres | NA | 0.00001 | 71% | Random | −0.20 (−0.59, 0.20) | 0.33 | 0.5289 | 0.081 |
| Type II fibres | NA | 0.0001 | 63% | Random | −0.37 (−0.71, −0.02) | 0.04 | 0.4415 | 0.022 |
| Human studies: Skeletal muscle responses in ageing compared with young adults | ||||||||
| Outcome: CSA | ||||||||
| Mixed fibres | NA | 0.01 | 63% | Random | 0.91 (0.25, 1.56) | 0.007 | 0.0163 | 0.107 |
| Type I fibres | NA | 0.02 | 42% | Random | −131.70 (−353.91, 90.51) | 0.25 | 0.8215 | 0.283 |
| Type II fibres | NA | 0.0001 | 61% | Random | 1313.31 (995.45, 1631.16) | 0.00001 | 0.5728 | 0.189 |
| Outcome: Myonuclear domain | ||||||||
| Mixed fibres | NA | 0.0001 | 83% | Random | 236.01 (−11.78, 483.79) | 0.06 | 0.8065 | 0.955 |
| Type I fibres | NA | 0.0001 | 79% | Random | −26.75 (−207.05, 153.56) | 0.77 | 0.7105 | 0.646 |
| Type II fibres | NA | 0.0002 | 75% | Random | 296.19 (109.08, 483.29) | 0.002 | 0.2655 | 0.502 |
| Outcome: Satellite cells | ||||||||
| Mixed fibres | NA | 0.00001 | 67% | Random | 0.78 (0.37, 1.19) | 0.0002 | 0.0179 | 0.006 |
| Type I fibres | NA | 0.13 | 30% | Fixed | 0.09 (−0.11, 0.28) | 0.38 | 0.9212 | 0.933 |
| Type II fibres | NA | 0.0004 | 64% | Random | 1.23 (0.86, 1.60) | 0.00001 | 0.0478 | 0.560 |
| Outcome: Myonuclear content | ||||||||
| Mixed fibres | NA | 0.00001 | 82% | Random | −0.03 (−0.24, 0.19) | 0.8 | 0.2464 | 0.092 |
| Type I fibres | NA | 0.003 | 67% | Random | −0.07 (−0.53, 0.39) | 0.76 | 0.7105 | 0.708 |
| Type II fibres | NA | 0.01 | 61% | Random | 0.58 (0.15, 1.02) | 0.008 | 0.7105 | 0.353 |
| Animal studies: skeletal muscle responses to hypertrophy | ||||||||
| Outcome: CSA in whole cross‐section | ||||||||
| Mean CSA | Control vs training | 0.15 | 37% | Fixed | 1.25 (0.83, 1.67) | 0.00001 | 0.0163 | 0.091 |
| Control vs detraining | 0.00001 | 85% | Random | −0.60 (−1.71, 0.51) | 0.29 | 0.229 | 0.015 | |
| Outcome: Myonuclear content in whole cross section | ||||||||
| Myonuclear content | Control vs training | 0.06 | 60% | Random | 0.17 (0.09, 0.25) | 0.0001 | 0.0894 | 0.149 |
| Control vs detraining | 0.06 | 59% | Random | 0.11 (0.02, 0.20) | 0.01 | 0.0894 | 0.251 | |
| Outcome: Myonuclear content in single muscle fibre | ||||||||
| Myonuclear content | Control vs training | 0.01 | 66% | Random | 2.26 (1.28, 3.23) | 0.00001 | 0.0085 | 0.062 |
| Control vs detraining | 0.007 | 68% | Random | 1.46 (0.60, 2.32) | 0.0008 | 0.0085 | 0.033 | |
| Animal studies: skeletal muscle responses to atrophy | ||||||||
| Outcome: CSA in whole cross section | ||||||||
| Mean CSA | NA | 0.00001 | 0.63% | Random | −1.96 (−2.21, −1.71) | 0.00001 | 0.0000 | 0.000 |
| Outcome: Myonuclear content in whole cross section | ||||||||
| Myonuclear content | NA | 0.00001 | 65% | Random | −1.03 (−1.30, −0.76) | 0.00001 | 0.0000 | 0.000 |
| Outcome: Satellite cells in whole cross section | ||||||||
| Satellite cells | NA | 0.00001 | 81% | Random | −0.13 (−0.50, 0.24) | 0.48 | 0.5724 | 0.266 |
| Outcome: Myonuclear content in single muscle fibre | ||||||||
| Myonuclear content | NA | 0.00001 | 62% | Random | −0.52 [−0.81, −0.23] | 0.0005 | 0.0000 | 0.000 |
CSA, cross‐sectional area; IO, insufficient observation; NA, not applicable; SMD, standard mean difference.
Discussion
The objective of the current systematic review and meta‐analysis was to assess the myonuclear and SC content of either human or rodent skeletal muscle that had undergone hypertrophy, atrophy, or detraining. We found that both myonuclear and SC content in human skeletal muscle are lower with atrophy, ageing, and following a period of detraining; however, the change in myonuclear and SC content with detraining represents a return to pre‐training levels. Subgroup analyses that stratified studies based on the age of the subjects showed that following detraining, Type I CSA in young adults decreases to a higher extent than in old adults. Additionally, following atrophy in human studies, we found that both myonuclear and SC content in mixed, Type I, and Type II fibres only decreased in young adults. In rodent studies, myonuclear content after an episode of overload‐induced hypertrophy remains elevated during the subsequent detraining period. With atrophy in rodents, myonuclear content is sensitive to the muscle type and the model of atrophy. More interestingly, we found that in animals, an atrophy of myofibre CSA of ≥30% was associated with a significant decrease in myonuclei.
Skeletal muscle fibres have a memory of prior chronic contractile activity, termed ‘muscle memory’. Evidence suggests myonuclei acquired during an initial period of hypertrophy are associated with enhanced muscle growth upon resumption of training following a period of detraining. 1 , 15 , 16 , 17 An obvious, but debated, critical aspect of this proposed mechanism of muscle memory is the ‘new’ myonuclei must be retained throughout the period of detraining. 16 , 17 The present meta‐analysis found that exercise‐induced myonuclei were not retained during detraining in humans but were in rodents. The rodent finding should be viewed with some caution as only five studies were included in the analysis with one study using denervation as a model of detraining following synergist ablation‐induced hypertrophy. 16 The concern with denervation as a model of detraining stems from our meta‐analysis showing that denervation in rodent skeletal muscle causes a significant increase in SC content. Thus, it is not clear if the elevated myonuclear content reported by Bruusgaard et al. 16 after denervation‐induced atrophy was driven by enhanced SC fusion, which would mask any loss of myonuclei. Other concerns that need to be taken into consideration are the magnitude of the hypertrophic response and the age of animals. The 25–60% increase in skeletal muscle CSA in response to synergist ablationS71–S75 is much higher than 6–10% increase in quadriceps CSA in response to resistance training in humans.S75–S79 Furthermore, three of the five rodent studies used animals under 4 months old. 16 , 18 , 92 Considering the different SC requirements for hypertrophic growth in fully mature mice compared with juvenile mice, 93 the elevated myonuclear content during detraining might reflect a low level of SC fusion known to occur in juvenile mice.S80 Additional animal studies are needed to more definitively answer the question of whether or not myonuclei acquired during hypertrophy are permanent during periods of detraining. Moreover, evaluating the same muscle in human studies (vastus lateralis) and different muscles in rodent studies (including EDL, FHL, gastrocnemius, soleus, and plantaris) resulted in very high heterogeneity in myonuclear content analysis in rodents (I 2 = 60–80%) but absolute homogeneity in humans (I 2 = 0%).
The meta‐analysis for atrophy in humans found that young adults respond differently to atrophy stimuli than old adults; myonuclear and SC content in mixed, Type I, and Type II fibres only decreased in young adults in response to atrophy. The influence of age on skeletal muscle plasticity is also observed in rodent studies, which found that juvenile mice (8 weeks of age) display a different response to overload‐induced hypertrophy relative to mature (16 weeks of age) mice; SC depletion in juvenile mice prevents hypertrophic growth, whereas skeletal muscle fibres in mature mice grow following SC depletion despite the lack of myonuclear accretion. 93 The results of our meta‐analysis indicate that in young adults, skeletal muscle atrophy is accompanied by a decrease in myonuclear and SC content, although changes in the myonuclear domain control skeletal muscle size in old adults. The rodent meta‐analysis for atrophy found that myonuclear content is sensitive to muscle type as the abundance of myonuclei may not change in some muscles. In this regard, following a detraining period, some muscles (like gastrocnemius and plantaris) lose their myonuclei, whereas other muscles (such as the soleus) with different activation patterns are resistant to the loss of myonuclei. 22 Hence, more pre‐clinical research using the various interventions in the same muscle is warranted. Interestingly, the magnitude of myonuclear elevation in rodent studies was about 2.6 times higher than in human studies (23.2% in animals vs. 9% in humans) and is reduced by 6.6% in animals after a detraining period. This finding indicates that even in rodents, elevated myonuclear content is not retained indefinitely but may decrease to a lower extent compared with humans. Additionally, the greater magnitude of hypertrophy in rodents was associated with a higher myonuclear content of approximately 18%, whereas in humans, it was ~11%. This finding provides further support for the notion that changes in myonuclear content influence the magnitude of muscle hypertrophy. Meta‐analysis of atrophy in humans found that myonuclear content was lower with only 9% atrophy. The rodent studies that directly assessed muscle memory showed no change in myonuclear number with atrophy of 10%; however, when atrophy was ≥30% in rodents, myonuclear content was lower. These findings reveal that, in rodents, myonuclear content is stable, except under the most extreme atrophic conditions. 22
Needing more evidence in both humans and rodents, we decided to assess myonuclear content and SC numbers after exposure to atrophy. The results of our meta‐analysis showed that myonuclear content and SCs of atrophied human Type II fibres decrease following atrophy. This analysis also found that myonuclear content in rodents decreases in response to hindlimb suspension, denervation, and immobilization with SC content lower in response to hindlimb suspension and immobilization. These findings implicate that myonuclear and SC content in both humans and rodents are not maintained indefinitely and may be reduced with skeletal muscle atrophy. Interestingly, we found lower myonuclear content was associated with higher SC content in response to denervation. These results can be explained by a higher rate of atrophy and a lower rate of myonuclear reduction in response to denervation (44 vs. 16%, respectively) compare with hindlimb suspension (35 vs. 25%, respectively), and immobilization (28 vs. 19%, respectively). Finally, needing more evidence regarding the possibility of long‐term myonuclear permanence in humans, we assessed myonuclear content, MND, and SC numbers in studies that compared young and elderly adults. Interestingly, we found that human ageing is accompanied by reduction in myonuclear content, MND, and SC abundance in atrophied Type II myofibres. These results clearly demonstrate that myonuclei are not retained indefinitely throughout the human lifespan.
To better understand how skeletal muscle possesses a memory of prior chronic contractile activity, recent studies have focused on the potential role of epigenetics. Skeletal muscle may possess a long‐term DNA hypomethylation ‘memory’ of prior exercise training that could have consequences for future myofibres adaptability during retraining. 94 , 95 , 96 Future studies should evaluate the role of epigenetic ‘memory’ association with a first training period to extend our understanding of the molecular bases of ‘muscle memory’.
Limitations
There are several limitations of the systematic review and meta‐analysis. First, despite the intense interest in the concept of ‘muscle memory’, the evidence to support the concept remains anecdotal as illustrated by the paucity of human and animal studies (i.e. only five studies in animals and four studies in humans). Second, different muscles were analysed across the animal studies, which confounded the results. Third, the different rates of muscle hypertrophy and myonuclear accretion between humans and animals make it quite challenging to translate animal results to in vivo human setting. Fourth, the small number of human studies made it challenging to determine the relationship between myonuclear content and the degree of atrophy as observed in rodents. Fifth, the analysis of SC content during atrophy in human studies associated with different diseases or models of atrophy was unable to identify a loss of SC content is related to a particular disease state or model of atrophy. Sixth, the current meta‐analysis is based on the assumption that all studies accurately measured myonuclear content. To accurately quantify myonuclear abundance by muscle cross section (which represents the vast majority of the studies analysed), it is critical to clearly identify the myofibre cell border; yet this approach can be hampered by the fact that a three‐dimensional structure, that is, the myofibre is being assessed in two dimensions. This can lead to the mis‐identification of a satellite cell nucleus being inside the myofibre or, alternatively, a bona fide myonucleus not being counted as it appears outside the dystrophin border. While this scenario is possible, it is assumed to have a minor impact, if at all, on the quantification of myonuclear content. We generated a new transgenic mouse model that allows for the definitive identification of myonuclei via nuclear GFP‐labelling, which should help to further minimize this inherent limitation of quantifying myonuclear content by muscle cross section. 97 Finally, the meta‐analysis of animal studies should be interpreted with caution as publication bias may be present.
Conclusion
The findings of this study extend and add new information to the field's knowledge regarding the concept of ‘muscle memory’ based on the idea that, once myonuclei are acquired, they are permanent. In humans, myonuclear content is not stable as it was found to change in response to a bout of detraining or atrophy. This finding suggests that other mechanisms are operative in mediating muscle memory. In rodents, the stability of myonuclei is less clear because of the limited number of studies and differences in experimental design across studies.
Conflict of interest
The authors declare that they have no conflicts of interest relevant to the content of this review.
Funding
This work has been supported by the Lorestan University.
Supporting information
Figure S1A: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that evaluated skeletal muscle responses to hypertrophy in humans. The items were scored on the Physiotherapy Evidence Database (PEDro) scale.
Figure S1B: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that evaluated skeletal muscle responses to atrophy in humans. The items were scored on the Physiotherapy Evidence Database (PEDro) scale.
Figure S1C: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that compared skeletal muscles of old versus young peoples. The items were scored on the Physiotherapy Evidence Database (PEDro) scale.
Figure S1D: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that evaluated skeletal muscle responses to hypertrophy in animals. The items in the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias assessment were scored with “yes” indicating low risk of bias, “no” indicating high risk of bias, or “unclear” indicating that the item was not reported, resulting in an unknown risk of bias.
Figure S1E: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that evaluated skeletal muscle responses to atrophy in anaimals. The items in the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias assessment were scored with “yes” indicating low risk of bias, “no” indicating high risk of bias, or “unclear” indicating that the item was not reported, resulting in an unknown risk of bias.
Figure S2. Meta‐analysis results for skeletal muscle responses to hypertrophy in human studies.
Figure S3. Meta‐analysis results for skeletal muscle responses to atrophy in human studies.
Figure S4. Meta‐analysis results for skeletal muscle responses in aging compared with young adults in human studies.
Figure S5. Meta‐analysis results for skeletal muscle responses to hypertrophy in animal studies.
Figure S6. Meta‐analysis results for skeletal muscle responses to atrophy in animal studies.
Figure S7A. Funnel plots for publication bias on skeletal muscle responses to hypertrophy in human studies after training.
Figure S7B. Funnel plots for publication bias on skeletal muscle responses to hypertrophy in human studies after detraining.
Figure S7C. Funnel plots for publication bias on skeletal muscle responses to atrophy in human studies.
Figure S7D. Funnel plots for publication bias on skeletal muscle responses in aging compared with young adults.
Figure S7E. Funnel plots for publication bias on skeletal muscle responses to hypertrophy in animal studies.
Figure S7F. Funnel plots for publication bias on skeletal muscle responses to atrophy in animal studies.
Data S1. Supporting Information
Rahmati M., McCarthy J. J., and Malakoutinia F. (2022) Myonuclear permanence in skeletal muscle memory: a systematic review and meta‐analysis of human and animal studies, Journal of Cachexia, Sarcopenia and Muscle, 13, 2276–2297, 10.1002/jcsm.13043
References
- 1. Hansson K‐A, Eftestøl E, Bruusgaard JC, Juvkam I, Cramer AW, Malthe‐Sørenssen A, Millay DP, Gundersen K. Myonuclear content regulates cell size with similar scaling properties in mice and humans. Nat Commun 2020;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruusgaard JC, Liestøl K, Ekmark M, Kollstad K, Gundersen K. Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J Physiol 2003;551:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murach KA, Fry CS, Kirby TJ, Jackson JR, Lee JD, White SH, Dupont‐Versteegden EE, McCarthy JJ, Peterson CA. Starring or supporting role? Satellite cells and skeletal muscle fiber size regulation. Phys Ther 2018;33:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pavlath GK, Rich K, Webster SG, Blau HM. Localization of muscle gene products in nuclear domains. Nature 1989;337:570–573. [DOI] [PubMed] [Google Scholar]
- 5. Enesco M, Puddy D. Increase in the number of nuclei and weight in skeletal muscle of rats of various ages. Am J Anat 1964;114:235–244. [DOI] [PubMed] [Google Scholar]
- 6. MacConnachie H, Enesco M, Leblond C. The mode of increase in the number of skeletal muscle nuclei in the postnatal rat. Am J Anat 1964;114:245–253. [DOI] [PubMed] [Google Scholar]
- 7. Reger JF, Craig AS. Studies on the fine structure of muscle fibers and associated satellite cells in hypertrophic human deltoid muscle. Anat Rec 1968;162:483–499. [DOI] [PubMed] [Google Scholar]
- 8. Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: Nuclei lost or paradigm lost? J Physiol 2008;586:2675–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz LM. Skeletal muscles do not undergo apoptosis during either atrophy or programmed cell death‐revisiting the myonuclear domain hypothesis. Front Physiol 2019;9:1887. 10.3389/fphys.2018.01887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Meer SF, Jaspers RT, Jones DA, Degens H. The time course of myonuclear accretion during hypertrophy in young adult and older rat plantaris muscle. Annal aAnatomy‐anatomischer Anzeiger 2011;193:56–63. [DOI] [PubMed] [Google Scholar]
- 11. Staron RS, Leonardi MJ, Karapondo DL, Malicky ES, Falkel JE, Hagerman FC, Hikida RS. Strength and skeletal muscle adaptations in heavy‐resistance‐trained women after detraining and retraining. J Appl Physiol 1991;70:631–640. [DOI] [PubMed] [Google Scholar]
- 12. Taaffe D, Marcus R. Dynamic muscle strength alterations to detraining and retraining in elderly men. Clin Physiol 1997;17:311–324. [DOI] [PubMed] [Google Scholar]
- 13. Gundersen K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J Exp Biol 2016;219:235–242. [DOI] [PubMed] [Google Scholar]
- 14. Rutherford O, Jones D. The role of learning and coordination in strength training. Eur J Appl Physiol Occup Physiol 1986;55:100–105. [DOI] [PubMed] [Google Scholar]
- 15. Bruusgaard JC, Egner IM, Larsen TK, Dupre‐Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol 2012;113:290–296. [DOI] [PubMed] [Google Scholar]
- 16. Bruusgaard JC, Johansen I, Egner I, Rana Z, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci 2010;107:15111–15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruusgaard JC, Gundersen K. In vivo time‐lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest 2008;118:1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee H, Kim K, Kim B, Shin J, Rajan S, Wu J, Chen X, Brown MD, Lee S, Park JY. A cellular mechanism of muscle memory facilitates mitochondrial remodelling following resistance training. J Physiol 2018;596:4413–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arentson‐Lantz EJ, English KL, Paddon‐Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle‐aged adults. J Appl Physiol 2016;120:965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Day MK, Allen DL, Mohajerani L, Greenisen MC, Roy RR, Edgerton VR. Adaptations of human skeletal muscle fibers to spaceflight. J Gravitat Physiol 1995;2:P47–P50. [PubMed] [Google Scholar]
- 21. Dungan CM, Murach KA, Frick KK, Jones SR, Crow SE, Englund DA, Vechetti IJ Jr, Figueiredo VC, Levitan BM, Satin J, McCarthy JJ, Peterson CA. Elevated myonuclear density during skeletal muscle hypertrophy in response to training is reversed during detraining. Am J Physiol Cell Physiol 2019;316:C649–C654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murach KA, Mobley CB, Zdunek CJ, Frick KK, Jones SR, McCarthy JJ, Peterson CA, Dungan CM. Muscle memory: myonuclear accretion, maintenance, morphology, and miRNA levels with training and detraining in adult mice. J Cachexia Sarcopenia Muscle 2020;11:1705–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo B‐S, Cheung K‐K, Yeung SS, Zhang B‐T, Yeung EW. Electrical stimulation influences satellite cell proliferation and apoptosis in unloading‐induced muscle atrophy in mice. PLoS ONE 2012;7:e30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leeuwenburgh C, Gurley CM, Strotman BA, Dupont‐Versteegden EE. Age‐related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 2005;288:R1288–R1296. [DOI] [PubMed] [Google Scholar]
- 25. Dupont‐Versteegden EE, Murphy RJ, Houlé JD, Gurley CM, Peterson CA. Activated satellite cells fail to restore myonuclear number in spinal cord transected and exercised rats. Am J Physiol Cell Physiol 1999;277:C589–C597. [DOI] [PubMed] [Google Scholar]
- 26. Siu PM, Alway SE. Mitochondria‐associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 2005;565:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radugina E, Almeida E, Blaber E, Poplinskaya V, Markitantova Y, Grigoryan E. Exposure to microgravity for 30 days onboard Bion M1 caused muscle atrophy and impaired regeneration in murine femoral Quadriceps. Life Sci Space Res 2018;16:18–25. [DOI] [PubMed] [Google Scholar]
- 28. Li T‐S, Shi H, Wang L, Yan C‐Z. Effect of bone marrow mesenchymal stem cells on satellite cell proliferation and apoptosis in immobilization‐induced muscle atrophy in rats. Med Sci Monit 2016;22:4651–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Psilander N, Eftestøl E, Cumming KT, Juvkam I, Ekblom MM, Sunding K, Wernbom M, Holmberg HC, Ekblom B, Bruusgaard JC, Raastad T, Gundersen K. Effects of training, detraining, and retraining on strength, hypertrophy, and myonuclear number in human skeletal muscle. J Appl Physiol 2019;126:1636–1645. [DOI] [PubMed] [Google Scholar]
- 30. Snijders T, Leenders M, de Groot L, van Loon LJ, Verdijk LB. Muscle mass and strength gains following 6 months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp Gerontol 2019;121:71–78. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Shamseer L, Clarke M, PRISMA‐P Group , Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003;83:713–721. [PubMed] [Google Scholar]
- 33. Hooijmans CR, Rovers MM, De Vries RB, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahmati M, Malakoutinia F. Aerobic, resistance and combined exercise training for patients with amyotrophic lateral sclerosis: A systematic review and meta‐analysis. Physiotherapy 2021;113:12–28. [DOI] [PubMed] [Google Scholar]
- 35. Blocquiaux S, Gorski T, Van Roie E, Ramaekers M, van Thienen R, Nielens H, Delecluse C, de Bock K, Thomis M. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp Gerontol 2020;133:110860. 10.1016/j.exger.2020.110860 [DOI] [PubMed] [Google Scholar]
- 36. Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 2004;558:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Snijders T, Aussieker T, Holwerda A, Parise G, van Loon LJ, Verdijk LB. The concept of skeletal muscle memory: Evidence from animal and human studies. Acta Physiol 2020;229:e13465. 10.1111/apha.13465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, Conboy I. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med 2009;1:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dirks ML, Wall BT, Nilwik R, Weerts DH, Verdijk LB, van Loon LJ. Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J Nutr 2014;144:1196–1203. [DOI] [PubMed] [Google Scholar]
- 40. Snijders T, Wall BT, Dirks ML, Senden JMG, Hartgens F, Dolmans J, Losen M, Verdijk LB, van Loon LJC. Muscle disuse atrophy is not accompanied by changes in skeletal muscle satellite cell content. Clin Sci 2014;126:557–566. [DOI] [PubMed] [Google Scholar]
- 41. Dirks ML, Wall BT, Snijders T, Ottenbros CL, Verdijk LB, Van Loon LJ. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol 2014;210:628–641. [DOI] [PubMed] [Google Scholar]
- 42. Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schrøder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re‐growth and myogenic precursor cell expansion early after immobility‐induced atrophy in human skeletal muscle. J Physiol 2013;591:3789–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ohira Y, Yoshinaga T, Ohara M, Nonaka I, Yoshioka T, Yamashita‐Goto K, Shenkman BS, Kozlovskaya IB, Roy RR, Edgerton VR. Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J Appl Physiol 1999;87:1776–1785. [DOI] [PubMed] [Google Scholar]
- 44. Brooks NE, Cadena SM, Vannier E, Cloutier G, Carambula S, Myburgh KH, Roubenoff R, Castaneda‐Sceppa C. Effects of resistance exercise combined with essential amino acid supplementation and energy deficit on markers of skeletal muscle atrophy and regeneration during bed rest and active recovery. Muscle Nerve 2010;42:927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reidy PT, McKenzie AI, Brunker P, Nelson DS, Barrows KM, Supiano M, LaStayo PC, Drummond MJ. Neuromuscular electrical stimulation combined with protein ingestion preserves thigh muscle mass but not muscle function in healthy older adults during 5 days of bed rest. Rejuvenation Res 2017;20:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reidy PT, Lindsay CC, McKenzie AI, Fry CS, Supiano MA, Marcus RL, LaStayo PC, Drummond MJ. Aging‐related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp Gerontol 2018;107:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moore DR, Kelly RP, Devries MC, Churchward‐Venne TA, Phillips SM, Parise G, Johnston AP. Low‐load resistance exercise during inactivity is associated with greater fibre area and satellite cell expression in older skeletal muscle. J Cachexia Sarcopenia Muscle 2018;9:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reidy PT, Yonemura NM, Madsen JH, McKenzie AI, Mahmassani ZS, Rondina MT, Lin YK, Kaput K, Drummond MJ. An accumulation of muscle macrophages is accompanied by altered insulin sensitivity after reduced activity and recovery. Acta Physiol 2019;226:e13251. 10.1111/apha.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith LR, Chambers HG, Lieber RL. Reduced satellite cell population may lead to contractures in children with cerebral palsy. Dev Med Child Neurol 2013;55:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dayanidhi S, Dykstra PB, Lyubasyuk V, McKay BR, Chambers HG, Lieber RL. Reduced satellite cell number in situ in muscular contractures from children with cerebral palsy. J Orthop Res 2015;33:1039–1045. [DOI] [PubMed] [Google Scholar]
- 51. Von Walden F, Gantelius S, Liu C, Borgström H, Björk L, Gremark O, Stål P, Nader GA, PontéN E. Muscle contractures in patients with cerebral palsy and acquired brain injury are associated with extracellular matrix expansion, pro‐inflammatory gene expression, and reduced rRNA synthesis. Muscle Nerve 2018;58:277–285. [DOI] [PubMed] [Google Scholar]
- 52. Eliason G, Abdel‐Halim S, Arvidsson B, Kadi F, Piehl‐Aulin K. Physical performance and muscular characteristics in different stages of COPD. Scand J Med Sci Sports 2009;19:865–870. [DOI] [PubMed] [Google Scholar]
- 53. Menon MK, Houchen L, Singh SJ, Morgan MD, Bradding P, Steiner MC. Inflammatory and satellite cells in the quadriceps of patients with COPD and response to resistance training. Chest 2012;142:1134–1142. [DOI] [PubMed] [Google Scholar]
- 54. Thériault M‐E, Paré M‐È, Maltais F, Debigaré R. Satellite cells senescence in limb muscle of severe patients with COPD. PLoS ONE 2012;7:e39124. 10.1371/journal.pone.0039124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sancho‐Muñoz A, Guitart M, Rodríguez DA, Gea J, Martínez‐Llorens J, Barreiro E. Deficient muscle regeneration potential in sarcopenic COPD patients: Role of satellite cells. J Cell Physiol 2021;236:3083–3098. [DOI] [PubMed] [Google Scholar]
- 56. Noehren B, Andersen A, Hardy P, Johnson DL, Ireland ML, Thompson KL, Damon B. Cellular and morphological alterations in the vastus lateralis muscle as the result of ACL injury and reconstruction. J Bone Jt Surg Am 2016;98:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fry CS, Johnson DL, Ireland ML, Noehren B. ACL injury reduces satellite cell abundance and promotes fibrogenic cell expansion within skeletal muscle. J Orthop Res 2017;35:1876–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parstorfer M, Profit F, Weiberg N, Wehrstein M, Barié A, Friedmann‐Bette B. Increased satellite cell apoptosis in vastus lateralis muscle after anterior cruciate ligament reconstruction. J Rehabil Med 2021;53. 10.2340/16501977-2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJ. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci 2015;128:357–365. [DOI] [PubMed] [Google Scholar]
- 60. Kramer IF, Snijders T, Smeets JS, Leenders M, van Kranenburg J, den Hoed M, Verdijk LB, Poeze M, van Loon LJC. Extensive type II muscle fiber atrophy in elderly female hip fracture patients. J Gerontol Ser A 2017;72:1369–1375. [DOI] [PubMed] [Google Scholar]
- 61. Farup J, Dalgas U, Keytsman C, Eijnde BO, Wens I. High intensity training may reverse the fiber type specific decline in myogenic stem cells in multiple sclerosis patients. Front Physiol 2016;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shao X, Chen J, Yang J, Sui W, Deng Y, Huang Z, Hu P, Yang J. Fiber type‐specific morphological and cellular changes of paraspinal muscles in patients with severe adolescent idiopathic scoliosis. Med Sci Monit 2020;26:e924415–e924411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Verdijk LB, Dirks ML, Snijders T, Verdijk LB, Dirks ML, Snijders T, Prompers JJ, Beelen M, Jonkers R, Thijssen DHJ, Hopman MTE, van Loon LJC. Reduced satellite cell numbers with spinal cord injury and aging in humans. Med Sci Sports Exerc 2012;44:2322–2330. [DOI] [PubMed] [Google Scholar]
- 64. D'Souza DM, Zhou S, Rebalka IA, MacDonald B, Moradi J, Krause MP, al‐Sajee D, Punthakee Z, Tarnopolsky MA, Hawke TJ. Decreased satellite cell number and function in humans and mice with type 1 diabetes is the result of altered notch signaling. Diabetes 2016;65:3053–3061. [DOI] [PubMed] [Google Scholar]
- 65. Snijders T, Verdijk LB, Smeets JS, McKay BR, Senden JMG, Hartgens F, Parise G, Greenhaff P, van Loon LJC. The skeletal muscle satellite cell response to a single bout of resistance‐type exercise is delayed with aging in men. Age 2014;36:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brooks MJ, Hajira A, Mohamed JS, Alway SE. Voluntary wheel running increases satellite cell abundance and improves recovery from disuse in gastrocnemius muscles from mice. J Appl Physiol 2018;124:1616–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sandonà D, Desaphy J‐F, Camerino GM, Bianchini E, Ciciliot S, Danieli‐Betto D, Dobrowolny G, Furlan S, Germinario E, Goto K, Gutsmann M, Kawano F, Nakai N, Ohira T, Ohno Y, Picard A, Salanova M, Schiffl G, Blottner D, Musarò A, Ohira Y, Betto R, Conte D, Schiaffino S. Adaptation of mouse skeletal muscle to long‐term microgravity in the MDS mission. PLoS ONE 2012;7:e33232. 10.1371/journal.pone.0033232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vassilopoulos D, Lumb E, Emery A. Karyometric changes in human muscle with age. Eur Neurol 1977;16:31–34. [DOI] [PubMed] [Google Scholar]
- 69. Manta P, Vassilopoulos D, Spengos M. Nucleo‐cytoplasmic ratio in ageing skeletal muscle. Eur Arch Psychiatry Neurol Sci 1987;236:235–236. [DOI] [PubMed] [Google Scholar]
- 70. Hikida RS, Walsh S, Barylski N, Campos G, Hagerman FC, Staron RS. Is hypertrophy limited in elderly muscle fibers? A comparison of elderly and young strength‐trained men. BAM‐PADOVA 1998;8:419–428. [Google Scholar]
- 71. Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell populations in healthy young and older men and women. Anatom Rec 2000;260:351–358. [DOI] [PubMed] [Google Scholar]
- 72. Renault V, Thorne LE, Eriksson PO, Butler‐Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell 2002;1:132–139. [DOI] [PubMed] [Google Scholar]
- 73. Sajko Š, Kubínová L, Kreft M, Dahmane R, Wernig A, Eržen I. Improving methodological strategies for satellite cells counting in human muscle during ageing. Image Anal Stereol 2002;21:7–12. [Google Scholar]
- 74. Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 2004;29:120–127. [DOI] [PubMed] [Google Scholar]
- 75. Sajko Š, Kubinova L, Cvetko E, Kreft M, Wernig A, Eržen I. Frequency of M‐cadherin‐stained satellite cells declines in human muscles during aging. J Histochem Cytochem 2004;52:179–185. [DOI] [PubMed] [Google Scholar]
- 76. Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 2006;33:242–253. [DOI] [PubMed] [Google Scholar]
- 77. Petrella JK, J‐s K, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance‐trained young and older men and women. Am J Physiol Endocrinol Metab 2006;291:E937–E946. [DOI] [PubMed] [Google Scholar]
- 78. Mohamed R, El Aasar H, Mohamed L, Abbas A. Morphological features of normal human skeletal muscle in different age groups: A histological and ultrastructural study. J Med Dent Sci 2007;7:69–169. [Google Scholar]
- 79. Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 2007;292:E151–E157. [DOI] [PubMed] [Google Scholar]
- 80. Cristea A, Qaisar R, Edlund PK, Lindblad J, Bengtsson E, Larsson L. Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell 2010;9:685–697. [DOI] [PubMed] [Google Scholar]
- 81. McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age‐related human muscle stem cell dysfunction. FASEB J 2012;26:2509–2521. [DOI] [PubMed] [Google Scholar]
- 82. Walker DK, Fry CS, Drummond MJ, Dickinson JM, Timmerman KL, Gundermann DM, Jennings K, Volpi E, Rasmussen BB. PAX7+ satellite cells in young and older adults following resistance exercise. Muscle Nerve 2012;46:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mackey A, Karlsen A, Couppe C, Couppé C, Mikkelsen UR, Nielsen RH, Magnusson SP, Kjaer M. Differential satellite cell density of type I and II fibres with lifelong endurance running in old men. Acta Physiol 2014;210:612–627. [DOI] [PubMed] [Google Scholar]
- 84. Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, Van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age 2014;36:545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Verdijk LB, Snijders T, Holloway TM, van Loon LJ. Resistance training increases skeletal muscle capillarization in healthy older men. Med Sci Sports Exerc 2016;48:2157–2164. [DOI] [PubMed] [Google Scholar]
- 86. Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, Parise G. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 2016;7:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve 2018;57:E52–E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Karlsen A, Bechshøft RL, Malmgaard‐Clausen NM, Andersen JL, Schjerling P, Kjaer M, Mackey AL. Lack of muscle fibre hypertrophy, myonuclear addition, and satellite cell pool expansion with resistance training in 83‐94‐year‐old men and women. Acta Physiol 2019;227:e13271. 10.1111/apha.13271 [DOI] [PubMed] [Google Scholar]
- 89. Naro F, Venturelli M, Monaco L, Toniolo L, Muti E, Milanese C, Zhao J, Richardson RS, Schena F, Reggiani C. Skeletal muscle fiber size and gene expression in the oldest‐old with differing degrees of mobility. Front Physiol 2019;10:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Karlsen A, Soendenbroe C, Malmgaard‐Clausen NM, Wagener F, Moeller CE, Senhaji Z, Damberg K, Andersen JL, Schjerling P, Kjaer M, Mackey AL. Preserved capacity for satellite cell proliferation, regeneration, and hypertrophy in the skeletal muscle of healthy elderly men. FASEB J 2020;34:6418–6436. [DOI] [PubMed] [Google Scholar]
- 91. Perez K, McGuirr J, Limbad C, Doi R, Nederveen JP, Nilsson MI, et al. Single nuclei profiling identifies cell specific markers of skeletal muscle aging, sarcopenia and senescence. medRxiv; 2021. [DOI] [PMC free article] [PubMed]
- 92. Eftestøl E, Ochi E, Juvkam IS, Gundersen K. A juvenile climbing exercise establishes a muscle memory boosting the effects of exercise in adult rats. bioRxiv; 2021. [DOI] [PubMed]
- 93. Murach KA, White SH, Wen Y, Ho A, Dupont‐Versteegden EE, McCarthy JJ, Peterson CA. Differential requirement for satellite cells during overload‐induced muscle hypertrophy in growing versus mature mice. Skeletal Muscle 2017;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Seaborne RA, Strauss J, Cocks M, Shepherd S, O'Brien TD, van Someren KA, Bell PG, Murgatroyd C, Morton JP, Stewart CE, Sharples AP. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci Rep 2018;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sharples AP. Skeletal muscle possesses an epigenetic memory of exercise: Role of nucleus type‐specific DNA methylation. Function 2021;2:zqab047. 10.1093/function/zqab047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Turner DC, Seaborne RA, Sharples AP. Comparative transcriptome and methylome analysis in human skeletal muscle anabolism, hypertrophy and epigenetic memory. Sci Rep 2019;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Iwata M, Englund DA, Wen Y, Dungan CM, Murach KA, Vechetti IJ, Mobley CB, Peterson CA, McCarthy JJ. A novel tetracycline‐responsive transgenic mouse strain for skeletal muscle‐specific gene expression. Skeletal Muscle 2018;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1A: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that evaluated skeletal muscle responses to hypertrophy in humans. The items were scored on the Physiotherapy Evidence Database (PEDro) scale.
Figure S1B: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that evaluated skeletal muscle responses to atrophy in humans. The items were scored on the Physiotherapy Evidence Database (PEDro) scale.
Figure S1C: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that compared skeletal muscles of old versus young peoples. The items were scored on the Physiotherapy Evidence Database (PEDro) scale.
Figure S1D: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that evaluated skeletal muscle responses to hypertrophy in animals. The items in the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias assessment were scored with “yes” indicating low risk of bias, “no” indicating high risk of bias, or “unclear” indicating that the item was not reported, resulting in an unknown risk of bias.
Figure S1E: Results of the risk of bias and methodological quality indicators at an individual level (A) and all included studies in the systematic review (B) that evaluated skeletal muscle responses to atrophy in anaimals. The items in the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias assessment were scored with “yes” indicating low risk of bias, “no” indicating high risk of bias, or “unclear” indicating that the item was not reported, resulting in an unknown risk of bias.
Figure S2. Meta‐analysis results for skeletal muscle responses to hypertrophy in human studies.
Figure S3. Meta‐analysis results for skeletal muscle responses to atrophy in human studies.
Figure S4. Meta‐analysis results for skeletal muscle responses in aging compared with young adults in human studies.
Figure S5. Meta‐analysis results for skeletal muscle responses to hypertrophy in animal studies.
Figure S6. Meta‐analysis results for skeletal muscle responses to atrophy in animal studies.
Figure S7A. Funnel plots for publication bias on skeletal muscle responses to hypertrophy in human studies after training.
Figure S7B. Funnel plots for publication bias on skeletal muscle responses to hypertrophy in human studies after detraining.
Figure S7C. Funnel plots for publication bias on skeletal muscle responses to atrophy in human studies.
Figure S7D. Funnel plots for publication bias on skeletal muscle responses in aging compared with young adults.
Figure S7E. Funnel plots for publication bias on skeletal muscle responses to hypertrophy in animal studies.
Figure S7F. Funnel plots for publication bias on skeletal muscle responses to atrophy in animal studies.
Data S1. Supporting Information


