Abstract
Background
Activation of sphingomyelinase (SMase) as a result of a general inflammatory response has been implicated as a mechanism underlying disease‐related loss of skeletal muscle mass and function in several clinical conditions including heart failure. Here, for the first time, we characterize the effects of SMase activity on human muscle fibre contractile function and assess skeletal muscle SMase activity in heart failure patients.
Methods
The effects of SMase on force production and intracellular Ca2+ handling were investigated in single intact human muscle fibres. Additional mechanistic studies were performed in single mouse toe muscle fibres. RNA sequencing was performed in human muscle bundles exposed to SMase. Intramuscular SMase activity was measured from heart failure patients (n = 61, age 69 ± 0.8 years, NYHA III‐IV, ejection fraction 25 ± 1.0%, peak VO2 14.4 ± 0.6 mL × kg × min) and healthy age‐matched control subjects (n = 10, age 71 ± 2.2 years, ejection fraction 60 ± 1.2%, peak VO2 25.8 ± 1.1 mL × kg × min). SMase activity was related to circulatory factors known to be associated with progression and disease severity in heart failure.
Results
Sphingomyelinase reduced muscle fibre force production (−30%, P < 0.05) by impairing sarcoplasmic reticulum (SR) Ca2+ release (P < 0.05) and reducing myofibrillar Ca2+ sensitivity. In human muscle bundles exposed to SMase, RNA sequencing analysis revealed 180 and 291 genes as up‐regulated and down‐regulated, respectively, at a FDR of 1%. Gene‐set enrichment analysis identified ‘proteasome degradation’ as an up‐regulated pathway (average fold‐change 1.1, P = 0.008), while the pathway ‘cytoplasmic ribosomal proteins’ (average fold‐change 0.8, P < 0.0001) and factors involving proliferation of muscle cells (average fold‐change 0.8, P = 0.0002) where identified as down‐regulated. Intramuscular SMase activity was ~20% higher (P < 0.05) in human heart failure patients than in age‐matched healthy controls and was positively correlated with markers of disease severity and progression, and with several circulating inflammatory proteins, including TNF‐receptor 1 and 2. In a longitudinal cohort of heart failure patients (n = 6, mean follow‐up time 2.5 ± 0.2 years), SMase activity was demonstrated to increase by 30% (P < 0.05) with duration of disease.
Conclusions
The present findings implicate activation of skeletal muscle SMase as a mechanism underlying human heart failure‐related loss of muscle mass and function. Moreover, our findings strengthen the idea that SMase activation may underpin disease‐related loss of muscle mass and function in other clinical conditions, acting as a common patophysiological mechanism for the myopathy often reported in diseases associated with a systemic inflammatory response.
Keywords: Sphingomyelinas, Skeletal muscle, Ca2+ sensitivity RNAseq, Heart failure
Introduction
Activation of sphingomyelinase (SMase) has been suggested to underlie human disease‐related loss of skeletal muscle mass and function. In humans, disease‐related elevations in circulating SMase activity have been correlated with muscle atrophy and contractile dysfunction. 1 In rodents, SMase has been demonstrated to perturb skeletal muscle force production and fatigue resistance 2 , 3 , 4 possibly by an impairment of intracellular Ca2+‐regulated processes. 3 Moreover, studies in myogenic cellular linages and animal models demonstrate that SMase and its downstream products partake in the regulation of muscle cell differentiation and protein metabolism. 5 , 6 , 7 However, the effects of SMase on intracellular Ca2+ handling was not addressed in these earlier studies, and the effects of SMase on human muscle fibre force production and muscle atrophy processes have yet to be investigated. In addition, intramuscular SMase activity has not been studied in human disease. Hence, whether local SMase activation is involved in disease‐related loss of muscle function and mass is unknown.
In mammals, several alternative forms of SMase proteins exists including neutral SMase (nSMase), and a lysosomal acid SMase (aSMase) that can be exocytosed, 8 a process promoted by inflammatory stimuli. 9 The enzymatic activity of SMase is regulated by cytokines and other factors implicated in inflammatory responses. 10 , 11 Circulating SMase activity is elevated in patients with conditions characteristically associated with skeletal muscle dysfunction, including heart failure, 1 rheumatoid arthritis, 12 and critical illness myopathy, 13 but the underlying mechanisms remain uncertain. A number of circulating proinflammatory cytokines are known to be elevated in these clinical conditions. 14 , 15 , 16 In heart failure patients, circulating SMase activity is positively correlated to both circulating TNFα and sTNF‐R1 while negatively correlated to muscle strength measures and lean tissue mass. 1 Hence, SMase activation may be related to a general inflammatory response, which is one plausible mechanism contributing to disease‐related skeletal muscle dysfunction.
The aims of the present study were (i) to study the effects of SMase activity on the intracellular Ca2+ handling and force production in muscle fibres, and (ii) to investigate the effects of SMase activity on the gene expression of factors related to the regulation of muscle mass and atrophy processes in human muscle bundles, and (iii) to characterize the intramuscular SMase activity in heart failure patients and its association to circulating inflammatory markers. This was conducted by investigating the effects of SMase on single intact human muscle fibres and muscle bundles, combined with in depth mechanistic experiments in single intact fibres from mouse toe muscles. The activity of tissue SMase activity was measured in skeletal muscle of heart failure patients and compared with healthy age‐matched controls and related to previously measured circulatory factors known to be associated with progression and severity of disease.
Methods
Ethical approval
All experiments involving human subjects were performed in accordance with the Declaration of Helsinki and the study was approved by the Regional Ethical Review Board in Stockholm (Dnr 2012/2181‐31/2 and 2016/2326‐31/4). Planned experiments and procedures were explained before subjects gave their informed written consent to participate. The parts of the study involving mice were approved by the Stockholm North Ethical Committee on Animal Experiments (DNR 1047/2017). All experiments involving mice were performed at the Karolinska Institutet and were carried out according to the guidelines laid down by the animal welfare committee at Karolinska Institutet. All experiments on mice complied with the Swedish Animal Welfare Act, the Swedish Welfare Ordinance, and applicable regulations and recommendations from Swedish authorities.
Study participants
Intercostal muscle biopsies were obtained during lobectomy of two men and three women (age 65 ± 5 years) suffering from, or suspected to suffer from, lung cancer. Surgery was performed at the Karolinska University Hospital in Stockholm, Sweden. None of the subjects recruited for experiments on intercostal muscles had any overt skeletal muscle problems and they were at least recreationally active as indicated by the self‐reported weekly physical activity. A second cohort of 71 subjects (49 men and 22 women, age 69 ± 6 years, see Table 1 for clinical characteristics) were recruited for SMase analysis in heart failure patients (61 subjects) and controls (10 subjects).
Table 1.
Characteristics of patients and controls included in the study.
| Parameter | Heart failure (n = 61) | Control (n = 10) | P value |
|---|---|---|---|
| Age (years) | 69 ± 0.8 | 71 ± 2.2 | 0.55 |
| Male/female | 48/13 | 1/9 | |
| Coronary artery disease | 34/61 | 0/10 | |
| Dilated cardiomyopathy | 10/61 | 0/10 | |
| Atrial fibrillation | 35/61 | 0/10 | |
| Hypertension | 33/61 | 4/10 | |
| Diabetes | 28/61 | 1/10 | |
| LVEF (%) | 25 ± 1.0 | 60 ± 1.2 | <0.05 |
| LVEDD (mm) | 64.5 ± 1.7 | 43.9 ± 2.0 | <0.05 |
| NT‐proBNP (ng/L) | 3907 ± 589 | 120 ± 28 | <0.05 |
| Peak VO2 (mL/kg/min) | 14.4 ± 0.6 | 25.8 ± 1.1 | <0.05 |
| RQ value | 1.07 ± 0.01 | 1.03 ± 0.02 | 0.13 |
| ACE inhibitor/ARB | 58/61 | 4/10 | |
| Aldosterone antagonist | 37/61 | 0/10 | |
| Loop diuretic | 58/61 | 0/10 | |
| CRT | 25/61 | 0/10 |
Animals
In total seven adult (10–15 weeks) female C57BL/6J mice (Scanbur Research, Stockholm, Sweden) were used for these studies. Animals were housed in a temperature‐controlled environment with a 12 h light–dark cycle and were provided with standard rodent food and water ad libitum.
Muscle biopsies and dissection
External and internal intercostal muscle biopsies were obtained during thoracotomies. Biopsies were sampled at the midaxillary line in the 4th intercostal space during thoracoscopy or in the 5th intercostal space during open surgical procedures. Biopsies had intact periostium and thus preserved tendons at both ends. Biopsies were placed in Dulbecco's modified eagle medium (DMEM, Gibco® Invitrogen, Life Technologies, Carlsbad, CA, USA) containing 0.2% fetal bovine serum ((FBS) Gibco® Invitrogen, Life Technologies) bubbled with a mixture of 95% O2 and 5% CO2 prior to collection and immediately transported to the laboratory. Mice were killed by cervical dislocation and whole flexor digitorum brevis (FDB) muscles from the hindleg were removed. Intact single rodent and human muscle fibres were dissected as previously described for mouse toe muscle fibres 17 , 18 and human intercostal muscle fibres. 19
Force and [Ca2+]i measurements
Individual muscle fibres were mounted in a stimulation chamber between an Akers 801 force transducer (Kronex Technologies, Oakland, CA, USA) and an adjustable holder, 18 and the length was adjusted to that giving maximal tetanic force. The diameter of the fibre at this length was measured at the widest and narrowest parts and used to calculate the cross‐sectional area. The fibre was electrically stimulated by supramaximal current pulses (duration 0.5 ms) delivered from a Pulsemaster A300 (WPI, Hertfordshire, England) and a lab‐built power amplifier to a pair of platinum plate electrodes lying parallel to the long axis of the muscle fibre. Contractions were evoked using stimulus trains with a duration of 500 ms (human fibres) or 350 ms (mouse fibres). Maximal tetanic [Ca2+]i and force during contractions were measured as the mean over 100 ms where they were maximal. Forces are expressed relative to the cross‐sectional area. Force‐[Ca2+]i curves were obtained by stimulating the fibre at 10–120 Hz at 1 min intervals.
[Ca2+]i was measured with the fluorescent Ca2+ indicator indo‐1 (Thermo Fisher Scientific, Stockholm, Sweden), which was microinjected into the fibre. After injection of indo‐1, the fibre was allowed to rest for at least 20 min. Tetanic force was tested before injection, 20 min after injection, and at regular intervals to verify stable force production; fibres were discarded if tetanic force was decreased by more than 10%. Indo‐1 was excited with light at 360 nm, and the light emitted at 405 ± 5 and 495 ± 5 nm was measured with two photomultiplier tubes. The fluorescence ratio of the light emitted at 405 nm to that emitted at 495 nm (R) is monotonically related to [Ca2+]i according to the following equation (Equation 1) 20 :
| (1) |
where KD is the apparent dissociation constant of indo‐1, β is the ratio of the 495 nm signals at very low and saturating [Ca2+]i, Rmin, and Rmax are the ratios at very low and saturating [Ca2+]i, respectively. Resting and tetanic ratios of Indo‐1 were converted to [Ca2+]i as described previously. 18
Force‐[Ca2+]i curves were constructed by fitting the data points obtained to the following 3‐parameter Hill equation (Equation 2):
| (2) |
where P is the measured force, P max is the fitted peak tetanic force at saturating [Ca2+]i, Ca50 is the [Ca2+]i producing 50% of P max, and N is a constant related to the steepness of the relation.
Experimental solutions
Muscle fibres were superfused with Tyrode solution (mM): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4; NaHCO3, 24.0; EDTA, 0.1; glucose, 5.5. FBS (0.2%) was added to the solution. The solution was bubbled with a mixture of 5% CO2 and 95% O2, which gives an extracellular pH of 7.4. For single fibre SMase experiments, fibres were exposed to 0.05 U/mL bacterial SMase ( Staphylococcus aureus ; Sigma‐Aldrich, St. Louis, MO). The temperature of the solution flowing through the muscle bath was kept constant at 37°C by passing it through the inner glass coil of a heated Graham condenser. The temperature of the bath solution was routinely measured in front of the intact fibre at a point furthest from the solution inflow. Caffeine (5 mM) was prepared fresh in Tyrode each day. For the RNA‐sequencing experiments, dissected fibre bundles were exposed to 0.25 U/mL bacterial SMase in Tyrode, or control solution without SMase, for 1 h at 37°C.
RNA sequencing
Total RNA from muscle samples was extracted using the RNeasy Mini Kit (Qiagen Venlo, Netherlands) and TRIzol™ reagent according to the manufacturer's instructions (Invitrogen). Muscle bundles of approximately 0.5 mg muscle tissue were used as input material for each assay. RNA quality control was performed using the RNA 6000 Pico chip on the 2100 Bioanalyzer automated electrophoresis system (Agilent Technologies Inc.). The mean RNA Integrity Number (RIN) was 7.1 (range 6.0–7.8). The preparation of the cDNA library and the RNA sequencing was performed by the core facility for Bioinformatics and Expression Analysis at Karolinska Institutet (Stockholm, Sweden). RNA was sequenced as single‐end, 50 base pairs, on the high‐throughput sequencing platform Illumina HiSeq 2000, and generated an average sequencing depth across samples of ~49 million single‐end reads. The expression levels of each sample were normalized as transcripts per kilobase per million (TPKM) by dividing the read count of each transcript model with its length and scaling the total per sample to one million using Kalisto V0.4.4. Quality control of raw and mapped reads was performed using FastQC version 0.11.8. Differential gene expression analysis was performed on the mapped read counts using the edgeR package in R, correcting for common, trended and tagwise disparity with the formula log logCPM = 0 + Treatment with each patient as random factor to account for the repeated measures design. The analysis rendered P‐values, two‐base logarithmic fold change (log2FC) differences and false discovery rate (FDR) values where FDR of <1% were considered significant. Pathway analysis was performed using gene‐set enrichment analysis (GSEA) where pathway annotation of genes was retrieved from WikiPathways and Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8, current as of May 2020. Pathway‐level comparison of the different conditions was calculated using two‐sided Wilcoxon signed‐rank tests on empirical cumulative distributions of log2FC where a nominal P value of <0.05 was considered a significant difference.
Sphingomyelinase activity assay
Tissues were homogenized using a protein homogenizer in lysis buffer (mM): Tris–HCl pH 7.6, 25; NaCl, 150; EDTA, 10; Na4P2O7, 10; β‐glycerophosphate, 25; NaF, 100; 10% glycerol; Na3VO4, 2, phosphatase and proteinase inhibitor cocktail (Phosphatase Inhibitor Cocktails 2 and 3, Sigma‐Aldrich, St. Louis, MO; Complete tablet, Roche Applied Science, Indianapolis, IN). In agreement with the findings from Moylan et al., 21 we found Triton X‐100 to inhibit nSMase activity. Hence, detergent was omitted from all isolation and assay buffers.
Protein concentrations were assessed with a Lowry total protein determination kit (DC Protein Assay; BioRad, Hercules, CA, USA), and 25 μg were used in each assay. nSMase activity was measured in reaction buffer (mM): Tris–HCl pH 7.4, 50; MgCl2, 7.5; NaF, 15; Na3VO4, 1; C6‐NBD‐SM (N‐[6‐[(7‐nitro‐2‐1,3‐benzoxadiazol‐4‐yl)amino]hexanoyl]‐sphingosine‐1‐phosphocholine), 25; phosphatase and proteinase inhibitor cocktail (Phosphatase Inhibitor Cocktails 2 and 3, Sigma‐Aldrich, St. Louis, MO; Complete tablet, Roche Applied Science, Indianapolis, IN) in a final volume of 50 μL for 90 min at 37°C. Reaction was stopped by adding 500 μL diluent solution containing chloroform and MeOH (3:1) plus 100 μL dH2O. Samples were subsequently vortexed and centrifuged at 1000 g for 5 min at 4°C. Organic phase was then transferred to GC‐vials and dried in a rotary evaporator (Speedvac) for 45 min. Samples were resuspended in 5 μL diluent solution and separated on a TLC‐plate. The generation of fluorescent product, NBD‐ceramide, was then assessed using a CCD‐camera by calculating the enzyme activity by the use of a standard curve. 22
Heart failure plasma proteome analysis
The heart failure patients in whom the skeletal muscle SMase activity was estimated have been previously characterized with regard to circulating factors using targeted protemics, 23 and thus, the plasma levels of 92 circulating proteins from 61 patients were available and could be related to skeletal muscle SMase activity. Correlations between SMase activity and plasma proteins were assessed through robust linear regression where a nominal P‐value of <0.05, corresponding to a correlation coefficient of 0.30, was considered significant.
Statistical analysis
For analysis of RNA sequencing data, the statistical software R was employed (for details of statistical analysis see details above). The statistical software SigmaPlot version 13.0 (Systat Software, Inc., San Jose, CA, USA) was used for all other statistical analysis. All variables were examined for normal distribution by Shapiro–Wilk's normality test and when needed, values were log‐transformed before analyses to better approximate a normal distribution. Statistically significant differences when comparing two groups were evaluated by the use of an unpaired or paired Student's t‐test for independent and dependent variables, respectively. Statistically significant differences when assessing more than two measurements in the same fibre were evaluated by one‐way repeated measures ANOVA for dependent variables. The effect of SMase on force and tetanic [Ca2+]i in single muscle fibres was analysed using two‐way repeated‐measures ANOVA with the factors condition (control and SMase) × time (0, 5, 10, 15, and 20 min). Tukey's post hoc test was then used to locate differences and interactions between groups. Differences were considered significant at P < 0.05. Data are presented as means ± SEM unless stated otherwise.
Results
Sphingomyelinase depresses force in single intact human muscle fibres by reducing both sarcoplasmic reticulum Ca2+ release and myofibrillar Ca2+ sensitivity
Exposure to SMase has been previously demonstrated to reduce the force production and fatigue tolerance of rodent diaphragm muscle bundles and skinned fibres. 2 , 3 , 4 To test whether SMase exposure depresses force in human muscle fibres, and the mechanisms whereby SMase‐induced force depression occurs, we performed experiments in single intact muscle fibres from human intercostal and mouse toe muscles. In intact single human and mouse muscle fibres, the effects of SMase on force production and [Ca2+]i were investigated. Results revealed a time‐dependent depression of force production and tetanic [Ca2+]i in both human and mouse single muscle fibres (Figure 1A,B). In intact single human and mouse muscle fibres, SMase caused a time‐dependent depression of tetanic [Ca2+]i and force production (Figure 1A,B). This demonstrates that SMase impaired SR Ca2+ release.
Figure 1.

SMase depresses force and tetanic [Ca2+]i i in single intact fibres. (A) Representative [Ca2+]i and force records during 20 Hz contractions in a single intact human intercostal muscle fibre exposed to SMase for up to 20 min. (B) Mean data of [Ca2+]i and force as a percentage of control in single mouse toe muscle fibres exposed to SMase. Data are means ± SEM. Significant effects (P < 0.05): a = interaction; b = time; c = treatment, 40 or 100 Hz control versus 40 or 100 Hz SMase, respectively, with two‐way RM ANOVA.
Analysis of mean force‐[Ca2+]i values before and after exposure to SMase revealed that SMase shifted the mean force‐[Ca2+]i values to the right of the control values (Figure 2A–D). Thus, in addition to impairing SR Ca2+ release, SMase also induced a decrease in myofibrillar Ca2+ sensitivity in both human and mouse muscle fibres. Following application of 5 mM caffeine in the mouse fibres, tetanic force and [Ca2+]i were fully restored to control values (Figure 2D), demonstrating that the maximal force producing capacity was not affected by SMase exposure.
Figure 2.
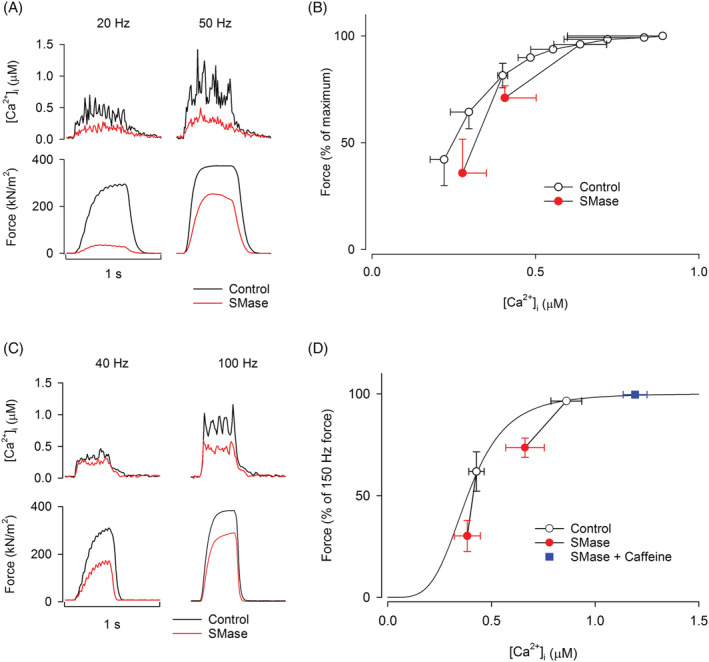
SMase‐induced force depression is due to the combined effect of a reduction in SR Ca2+ release and myofibrillar Ca2+ sensitivity. (A,B) Representative [Ca2+]i and force records in a single intact human intercostal and mouse toe muscle fibre, respectively, before and after exposure to SMase. (C,D) In human intercostal (n = 3) and mouse toe muscle (n = 8) fibres, SMase caused a shift of the mean force‐[Ca2+]i values to the right of the control force‐[Ca2+]i curve (i.e. an apparent decrease in myofibrillar Ca2+ sensitivity), and a shift downward to the left compared with frequency‐matched control force‐[Ca2+]i values (i.e. an apparent decrease in tetanic force and [Ca2+]i). (D) Increased SR Ca2+ release following application of caffeine fully offsets the SMase‐induced force depression in mouse toe muscle fibres. The hill plot in (D) was generated from the P max, N and Ca50 mean values of all mouse toe muscle fibres analysed (n = 8).
Sphingomyelinase activates the transcription of genes involved in proteasome degradation while reducing the transcription of ribosomal proteins
Sphingomyelinase activity is elevated in cachectic compared with non‐cachectic human heart failure patients, 1 and elevated activity is associated with a loss of muscle mass and contractile function. 1 , 24 To investigate the putative role of SMase activation in disease‐related muscle atrophy, we studied the expression of factors associated with the regulation of muscle mass and regeneration in human muscle bundles exposed to SMase. RNA sequencing analysis revealed that SMase exposure resulted in increased expression of 180 genes at a FDR of 1%. Gene‐set enrichment analysis identified four significantly up‐regulated pathways, including ‘proteasome degradation’ (average fold‐change 1.1, P = 0.008, Figure 3A). Meanwhile, 291 genes enriched for 62 pathways and 203 biological processes were down‐regulated. These included the pathway ‘cytoplasmic ribosomal proteins’ (average fold‐change 0.8, P < 0.0001, Figure 3B). In addition, factors involving proliferation of muscle cells (average fold‐change 0.8, P = 0.0002) and locomotion (average fold‐change 0.9, P < 0.0001) were located among those down‐regulated (see Supporting Information, Table S1 for a complete list of all differentially expressed genes, pathways and processes).
Figure 3.
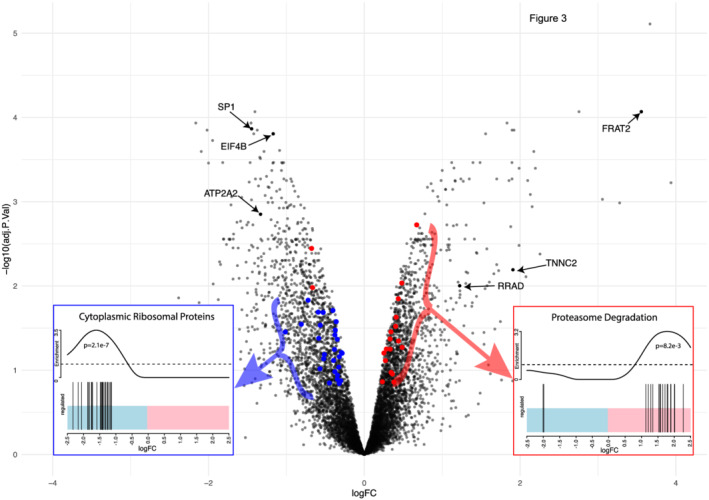
SMase activity induces the transcription of factors promoting protein degradation while transcription of ribosomal proteins is supressed. Volcano‐plot showing SMase‐induced changes in gene expression in isolated human muscle fibres with 471 differentially expressed genes (180 up‐regulated and 291 down‐regulated FDR <1%). Several genes of importance for muscle fibre structural integrity such as the transcription factor SP1 and eukaryotic translation initiation factor 4B, but also transcripts involved in calcium handling such as ATP2A2 were down‐regulated. Up‐regulated genes included transcripts associated with denervation (FRAT2, RRAD) and fibre‐type switching (TNNC2). (C) On the pathway level, the most notable up‐regulated pathway was proteasome degradation (P = 0.008) whereas cytoplasmic ribosomal proteins was down‐regulated (P < 0.0001). Transcripts belonging to these pathways are highlighted in red and blue on the volcano. The barcode plots illustrate the changes in expression of the members of these two pathways where most of all transcripts involved in ‘proteasome degradation’ and ‘cytoplasmic ribosomal proteins’ were up‐and down‐regulated respectively following SMase treatment.
Skeletal muscle neutral sphingomyelinase activity is elevated in human heart failure patients
The enzyme activities of nSMase and aSMase were analysed in skeletal muscle vastus lateralis biopsies from human heart failure patients (n = 61) and healthy age‐matched controls (n = 10). Results revealed a higher activity of nSMase in human heart failure patients, while the activity of aSMase was similar between groups (Figure 4A,B). SMase activity was further investigated in a subset (n = 16) of the study participants with a mean follow‐up time at the second biopsy of 2.5 (±0.2) years. Results show that the enzyme activities of both nSMase and aSMase were increased with duration of disease (Figure 5C,D).
Figure 4.

Skeletal muscle SMase activity is elevated in human heart failure patients and the activity of SMase increases with disease duration. (A,B) Skeletal muscle nSMase and aSMase activity, respectively, in human heart failure patients (n = 61) and healthy age‐matched controls (n = 10). (C,D) Skeletal muscle nSMase and aSMase activity, respectively, in human heart failure patients at the time of the 1st biopsy (time of inclusion in the study) and at the 2nd biopsy (follow‐up). Mean follow‐up time 2.5 (±0.2) years, n = 16. Bars indicate mean value and circles represent individual data points. *P < 0.05, age‐matched control subjects versus heart failure patients with unpaired t‐test; 1st versus 2nd biopsy with paired t‐test.
Figure 5.

Skeletal muscle SMase activity correlates to circulatory markers of inflammation and muscle atrophy. Correlation of skeletal muscle nSMase activity and serum protein quantities of factors analysed with targeted proteomics in human heart failure patients (n = 61). The figure depicts correlation coefficients of plasma proteins significantly (P < 0.05) correlated with skeletal muscle SMase activity. Several factors associated with systemic inflammation including TNF receptor 1 and 2 (TNF‐R1 and TNF‐R2) were significantly associated with skeletal muscle SMase activity in patients with heart failure.
Next, the putative correlation of skeletal muscle SMase activity with inflammatory and other atrophic factors in serum of heart failure patients, previously reported by us for the current patient cohort, 25 was investigated. Results revealed a positive correlation of skeletal muscle SMase activity with markers of inflammatory activity, including TNF receptor 1 and 2 (TNF‐R1 and TNF‐R2) but also factors associated with extracellular matrix remodelling such as MMP‐3 and Integrin beta‐2 (Figure 5).
Discussion
This is the first study to address the effects of SMase on the force production, intracellular Ca2+ handling and downstream effects of SMase in human muscle fibres. We report that (i) in intact human muscle fibres, SMase caused a prompt force depression by affecting intracellular Ca2+‐handling and induced marked changes in the gene expression promoting muscle atrophy processes, and (ii) in human heart failure patients, the intramuscular activity of SMase was elevated and positively correlated to circulatory markers of inflammation, atrophy and severity of disease.
Our data show that SMase markedly depressed muscle fibre force via two distinct effects; first, it reduced SR Ca2+ release and second, it attenuated myofibrillar Ca2+ sensitivity. Interestingly, impaired SR Ca2+ release and reduced myofibrillar Ca2+ sensitivity have previously been shown to contribute to disease‐related muscle weakness and fatigue intolerance in heart failure, 21 , 23 , 26 rheumatoid arthritis, 25 and critical illness. 27 Notably, these clinical conditions all display an increased activation of SMase.
In addition to the negative effects of SMase on the qualitative aspects of skeletal muscle function discussed above, we were interested in the plausible role of SMase in the disease‐related loss of muscle mass. Circulating SMase is elevated in several clinical conditions associated with muscle atrophy 1 , 12 , 13 and found to be negatively correlated to lean tissue mass. 1 To address the question of a causative role of SMase activation in this process, we investigated the effects of SMase on the gene expression of genes involved in the regulation of muscle mass. Results revealed that the transcripts of genes involved in muscle atrophy processes, including proteasome degradation pathways, were higher in human muscle fibre bundles exposed to SMase compared with controls. Meanwhile, the opposite relation was observed for transcripts of genes encoding ribosomal proteins that contribute to protein synthesis. In fibres exposed to SMase, a lower number of transcripts was also observed for factors associated with muscle cell proliferation and locomotion, which could be of relevance for the regenerative capacity of skeletal muscle. These results are coherent with existing reports from non‐human myogenic cell lines and animal models in which sphingolipids modified signalling pathways linked to the regulation of muscle mass, and affected the gene expression of members of the ubiquitin‐proteasome system. 2 , 3 , 4
The ubiquitin‐proteasome is one of the two major proteolytic systems reported to be involved in muscle atrophy. 28 Gene expression analyses of diverse clinical conditions associated with muscle atrophy have revealed common transcriptional changes of genes regulating the ubiquitin‐proteasome system. 29 , 30 These include an up‐regulation of transcripts encoding ubiquitin, ubiquitin‐conjugating enzymes, ubiquitin‐protein ligases and several proteasome subunits. 31 Notably, several of these common transcripts, including essential subunits of the 20s core and 19s regulatory proteasome components, as well as polyubiquitin C, were induced in human muscle fibres exposed to sphingomyelinase. Among the ribosomal proteins transcriptionally repressed following sphingomyelinase exposure were RPL6, a component of the 60s ribosomal subunit also known to be an intracellular partner of FGF2. 32 This is in line with previous observations from rodent models where heart failure is associated with decreased skeletal muscle expression of ribosomal RNA and increased sphingomyelinase activity. 33 Furthermore, such RPL6‐FGF2 interaction is thought to promote cell survival and regulation of RPL6 has been reported in relation to muscle atrophy via atrogin‐1, 34 which could imply a mechanistic link between SMase‐activity and changes in muscle mass. All in all, the present findings expand the role of SMase to involve transcriptional regulation of genes involved in the regulation of skeletal muscle mass. A direct link between SMase‐activity and muscle ceramide content has been previously shown in cell‐models, 2 but the canonical steps involved between SMase‐activity and transcription and whether increased ceramide production is a pivotal step remain to be elucidated.
The activity of circulating SMase has previously been shown to be elevated in human diseases associated with increased inflammatory response 1 , 12 , 13 and to be associated with muscle weakness in patients with rheumatoid arthritis. 25 However, we are not aware of any previous study addressing the intramuscular SMase activity in these conditions. When we investigated this local activity of SMase in the skeletal muscle of human heart failure patients, we found that the intramuscular nSMase activity was elevated by approximately 20% as compared with controls. No significant increase was observed in aSMase. These findings are coherent with rodent data 35 and suggest a conserved mechanism of intramuscular nSMase activation in human and rodent disease. Given the specific abundance of the nSMase3 protein in skeletal muscle tissues, 26 intramuscular nSMase may prove a potential future pharmaceutical target in order to combat disease‐related loss of skeletal muscle mass and function.
Several agents of inflammatory stimulation, including inflammatory cytokines and LPS, have been demonstrated to increase aSMase and nSMase activity both in vivo 10 and ex vivo 11 and to increase the exocytosis of aSMase from endothelial cells. 11 Inflammatory cytokines exert endocrine effects on skeletal muscle acting via sarcolemmal TNF subtype 1 receptors to stimulate intramuscular nSMase activity. 36 , 37 To elaborate on plausible mechanisms, we focused on previously measured circulatory factors in heart failure patients. Intramuscular nSMase activity was positively correlated to circulatory markers of inflammation and atrophy, including sTNF‐R1 and GDF 15, in heart failure patients. Both of these factors were earlier demonstrated to link echocardiographic assessment of myocardial function, physical capacity, and daily physical activity as well as carried independent prognostic information in the Cox regression analysis of mortality in the same cohort. 38 Thus, the present findings support the importance of inflammatory mediators in the increase of SMase activity also in skeletal muscle tissue.
Collectively, when integrating our results from human and mouse single fibres, and muscle biopsies from human heart failure patients, we conclude that elevated SMase activity is a potential mechanism underlying heart failure‐induced loss of skeletal muscle contractility and possibly also muscle mass. Furthermore, our findings strengthen the overall role of SMase as a key player in disease‐related loss of muscle mass and function, plausibly acting as a common patophysiological mechanism for the myopathy often reported in diseases associated with a systemic inflammatory response.
Conflict of interest
The authors have no competing interests.
Funding
This study was supported by funds from: the Swedish Research Council for Sports Science to A.J.C. (D2016‐0036, FO2017‐0018, and FO2018‐0019) and to H.W. (P2019‐0060); the Swedish Research Council to H.W. (2018‐02576), N.T. (2019‐01629), and T.G. (2015‐02338); and Swedish Heart Lung Foundation to T.G.
Supporting information
Data S1. Supporting Information
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 38
Olsson K., Cheng A. J., Al‐Ameri M., Tardif N., Melin M., Rooyackers O., Lanner J. T., Westerblad H., Gustafsson T., Bruton J. D., and Rullman E. (2022) Sphingomyelinase activity promotes atrophy and attenuates force in human muscle fibres and is elevated in heart failure patients, Journal of Cachexia, Sarcopenia and Muscle, 13, 2551–2561, 10.1002/jcsm.13029
References
- 1. Doehner W, Bunck AC, Rauchhaus M, von Haehling S, Brunkhorst FM, Cicoira M. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J 2007;28:821–828. [DOI] [PubMed] [Google Scholar]
- 2. Ferreira LF, Moylan JS, Gilliam LA, Smith JD, Nikolova‐Karakashian M, Reid MB. Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue. Am J Physiol Cell Physiol 2010;299:C552–C560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferreira LF, Moylan JS, Stasko S, Smith JD, Campbell KS, Reid MB. Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers. J Appl Physiol 2012;112:1538–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loehr JA, Abo‐Zahrah R, Pal R, Rodney GG. Sphingomyelinase promotes oxidant production and skeletal muscle contractile dysfunction through activation of NADPH oxidase. Front Physiol 2014;5:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mebarek S, Komati H, Naro F, Zeiller C, Alvisi M, Lagarde M. Inhibition of de novo ceramide synthesis upregulates phospholipase D and enhances myogenic differentiation. J Cell Sci 2007;120:407–416. [DOI] [PubMed] [Google Scholar]
- 6. Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down‐regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 2005;19:461–463. [DOI] [PubMed] [Google Scholar]
- 7. De Larichaudy J, Zufferli A, Serra F, Isidori AM, Naro F, Dessalle K. TNF‐alpha‐ and tumor‐induced skeletal muscle atrophy involves sphingolipid metabolism. Skeletal Muscle 2012;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrews NW. Solving the secretory acid sphingomyelinase puzzle: Insights from lysosome‐mediated parasite invasion and plasma membrane repair. Cell Microbiol 2019;21:e13065. 10.1111/cmi.13065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cowart LA. A novel role for sphingolipid metabolism in oxidant‐mediated skeletal muscle fatigue. Focus on “Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue”. Am J Physiol Cell Physiol 2010;299:C549–C551. [DOI] [PubMed] [Google Scholar]
- 10. Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A. Acute systemic inflammation up‐regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A 2000;97:8681–8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide‐mediated cell signaling. J Biol Chem 1998;273:4081–4088. [DOI] [PubMed] [Google Scholar]
- 12. Hanaoka BY, Ormseth MJ, Michael Stein C, Banerjee D, Nikolova‐Karakashian M, Crofford LJ. Secretory sphingomyelinase (S‐SMase) activity is elevated in patients with rheumatoid arthritis. Clin Rheumatol 2018;37:1395–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Claus RA, Bunck AC, Bockmeyer CL, Brunkhorst FM, Losche W, Kinscherf R. Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J 2005;19:1719–1721. [DOI] [PubMed] [Google Scholar]
- 14. Conraads VM, Bosmans JM, Schuerwegh AJ, De Clerck LS, Bridts CH, Wuyts FL, et al. Association of lipoproteins with cytokines and cytokine receptors in heart failure patients. Differences between ischaemic versus idiopathic cardiomyopathy. Eur Heart J 2003;24:2221–2226. [DOI] [PubMed] [Google Scholar]
- 15. Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 2000;102:3060–3067. [DOI] [PubMed] [Google Scholar]
- 16. Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM. The Sick and the Weak: Neuropathies/Myopathies in the Critically Ill. Physiol Rev 2015;95:1025–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lannergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol 1987;390:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng AJ, Westerblad H. Mechanical isolation, and measurement of force and myoplasmic free [Ca(2+)] in fully intact single skeletal muscle fibers. Nat Protoc 2017;12:1763–1776. [DOI] [PubMed] [Google Scholar]
- 19. Olsson K, Cheng AJ, Alam S, Al‐Ameri M, Rullman E, Westerblad H. Intracellular Ca(2+)‐handling differs markedly between intact human muscle fibers and myotubes. Skeletal Muscle 2015;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985;260:3440–3450. [PubMed] [Google Scholar]
- 21. Perreault CL, Gonzalez‐Serratos H, Litwin SE, Sun X, Franzini‐Armstrong C, Morgan JP. Alterations in contractility and intracellular Ca2+ transients in isolated bundles of skeletal muscle fibers from rats with chronic heart failure. Circ Res 1993;73:405–412. [DOI] [PubMed] [Google Scholar]
- 22. Loidl A, Claus R, Deigner HP, Hermetter A. High‐precision fluorescence assay for sphingomyelinase activity of isolated enzymes and cell lysates. J Lipid Res 2002;43:815–823. [PubMed] [Google Scholar]
- 23. Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol 2003;160:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Supinski GS, Alimov AP, Wang L, Song XH, Callahan LA. Neutral sphingomyelinase 2 is required for cytokine‐induced skeletal muscle calpain activation. Am J Physiol Lung Cell Mol Physiol 2015;309:L614–L624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamada T, Fedotovskaya O, Cheng AJ, Cornachione AS, Minozzo FC, Aulin C. Nitrosative modifications of the Ca2+ release complex and actin underlie arthritis‐induced muscle weakness. Ann Rheum Dis 2015;74:1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ward CW, Reiken S, Marks AR, Marty I, Vassort G, Lacampagne A. Defects in ryanodine receptor calcium release in skeletal muscle from post‐myocardial infarct rats. FASEB J 2003;17:1517–1519. [DOI] [PubMed] [Google Scholar]
- 27. Llano‐Diez M, Cheng AJ, Jonsson W, Ivarsson N, Westerblad H, Sun V. Impaired Ca(2+) release contributes to muscle weakness in a rat model of critical illness myopathy. Crit Care 2016;20:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 2013;6:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001;294:1704–1708. [DOI] [PubMed] [Google Scholar]
- 30. Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin‐1, a muscle‐specific F‐box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 2001;98:14440–14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 2004;18:39–51. [DOI] [PubMed] [Google Scholar]
- 32. Shen B, Arese M, Gualandris A, Rifkin DB. Intracellular association of FGF‐2 with the ribosomal protein L6/TAXREB107. Biochem Biophys Res Commun 1998;252:524–528. [DOI] [PubMed] [Google Scholar]
- 33. Coblentz PD, Ahn B, Hayward LF, Yoo JK, Christou DD, Ferreira LF. Small‐hairpin RNA and pharmacological targeting of neutral sphingomyelinase prevent diaphragm weakness in rats with heart failure and reduced ejection fraction. Am J Physiol Lung Cell Mol Physiol 2019;316:L679–L690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sluzalska KD, Slawski J, Sochacka M, Lampart A, Otlewski J, Zakrzewska M. Intracellular partners of fibroblast growth factors 1 and 2 ‐ implications for functions. Cytokine Growth Factor Rev 2020;57:93–111. [DOI] [PubMed] [Google Scholar]
- 35. Empinado HM, Deevska GM, Nikolova‐Karakashian M, Yoo JK, Christou DD, Ferreira LF. Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content. Eur J Heart Fail 2014;16:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Philipp S, Puchert M, Adam‐Klages S, Tchikov V, Winoto‐Morbach S, Mathieu S. The Polycomb group protein EED couples TNF receptor 1 to neutral sphingomyelinase. Proc Natl Acad Sci U S A 2010;107:1112–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rullman E, Melin M, Mandic M, Gonon A, Fernandez‐Gonzalo R, Gustafsson T. Circulatory factors associated with function and prognosis in patients with severe heart failure. Clin Res Cardiol 2020;109:655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


