Abstract
Background
Whether adiposity and muscle function are associated with mortality risk in patients with non‐alcoholic fatty liver disease (NAFLD) remains unknown. We examine the independent and combined associations of body mass index (BMI) and muscle strength with overall mortality in individuals with NAFLD.
Methods
We analysed data from 7083 participants with NAFLD in the Thai National Health Examination Survey and their linked mortality. NAFLD was defined using a lipid accumulation product in participants without significant alcohol intake. Poor muscle strength was defined by handgrip strength of <28 kg for men and <18 kg for women, according to the Asian Working Group on Sarcopenia. The Cox proportional‐hazards model was constructed to estimate the adjusted hazard ratio (aHR) for overall mortality.
Results
The mean age was 49.3 ± 13.2 years, and 69.4% of subjects were women. According to the Asian‐specific criteria, 1276 individuals (18.0%) were classified as lean NAFLD (BMI 18.5–22.9 kg/m2), 1465 (20.7%) were overweight NAFLD (BMI 23–24.9 kg/m2), and 4342 (61.3%) were obese NAFLD (BMI ≥ 25 kg/m2). Over 60 432 person‐years, 843 participants died. In Cox models adjusted for physiologic, lifestyle, and comorbid factors, individuals with lean NAFLD [aHR 1.18, 95% confidence interval (CI): 0.95–1.48; P = 0.138] and subjects with overweight NAFLD (aHR 1.28, 95% CI: 0.89–1.84; P = 0.158) had mortality risk estimates similar to their obese counterparts, whereas participants with lower handgrip strength had significantly higher mortality risk than those with higher handgrip strength in men and women. Compared with obese individuals with the highest handgrip strength, elevated mortality risk was observed among men (aHR 3.21, 95% CI: 1.35–7.62, P = 0.011) and women (aHR 2.22, 95% CI, 1.25–3.93, P = 0.009) with poor muscle strength. Among men, poor muscle strength was associated with increased risk of mortality with obese NAFLD (aHR 3.94, 95% CI, 1.38–11.3, P = 0.013), overweight NAFLD (aHR 2.93, 95% CI, 1.19–7.19, P = 0.021), and lean NAFLD (aHR 2.78, 95% CI, 0.93–8.32, P = 0.065). Among women, poor muscle strength was associated with increased mortality risk with obese NAFLD (aHR 2.25, 95% CI, 1.06–4.76, P = 0.036), overweight NAFLD (aHR 1.69, 95% CI, 0.81–3.51, P = 0.153), and lean NAFLD (aHR 2.47, 95% CI, 1.06–5.73, P = 0.037).
Conclusions
In this nationwide cohort of individuals with NAFLD, muscle strength, but not BMI, was independently associated with long‐term overall mortality. Measuring handgrip strength can be a simple, non‐invasive risk stratification approach for overall mortality in patients with NAFLD.
Keywords: Non‐alcoholic fatty liver disease, Sarcopenia, Muscle strength, Body mass index, Mortality
Introduction
Paralleling the increasing global prevalence of obesity and metabolic syndrome, non‐alcoholic fatty liver disease (NAFLD) has been recognized as the most common liver disease worldwide. 1 NAFLD has become a significant public health concern because it is associated with increased mortality. 2 Obesity is a common clinical phenotype associated with NAFLD, which is linked to metabolic syndrome and related comorbidities. However, a significant proportion of patients with NAFLD have a normal body mass index (BMI), denoted as lean NAFLD. This sub‐phenotype of NAFLD patients appears to be recognized more frequently in Asians, even when strict ethnicity‐specific criteria define obesity. Several studies demonstrated that lean subjects with NAFLD have milder features of metabolic syndrome when compared with obese individuals. 3 , 4 , 5 , 6 , 7 The available data on liver disease progression and overall mortality between lean and obese NAFLD are limited and contradictory. In a multi‐ethnic NAFLD cohort from the United States, the development of advanced fibrosis was lower in non‐obese individuals compared with their obese counterparts. 8 A Swedish cohort of patients with lean NAFLD showed no increased mortality compared with patients with a higher BMI. 9 In contrast, an international cohort study reported increased mortality for patients with lean NAFLD compared with those with higher BMI. 10 The differences in clinical outcomes observed in these studies could be due to residual confounding by only including patients with biopsy‐proven NAFLD. Consequently, the clinical phenotype of NAFLD determined by BMI as the measure of adiposity is still debatable.
The mechanisms underlying the development and progression of NAFLD are largely unknown. Emerging evidence suggests that low skeletal muscle mass or pre‐sarcopenia contributes to the risk of developing NAFLD and advanced fibrosis. 11 , 12 , 13 , 14 Indeed, skeletal muscle is the primary tissue responsible for insulin‐mediated glucose disposal and plays an important role in glucose homeostasis and insulin resistance, which are vital in the pathogenesis of NAFLD. 15 Muscle strength as a marker of muscle quality is closely related to muscle mass, which is reduced with ageing. It has also been demonstrated that muscle function is more important than muscle mass when estimating mortality risk. 16 Many epidemiological studies highlight that lower muscle strength is associated with higher mortality. 17 , 18 , 19 Resistance training has been widely studied in NAFLD as an exercise intervention that improves hepatic fat, insulin sensitivity, and muscle strength, illustrating the significance of skeletal muscle in health. 20 Nonetheless, we still do not know whether muscle strength affects mortality varied by BMI status in individuals with NAFLD. Therefore, this longitudinal study aimed to investigate the independent and combined associations of BMI and muscle strength on all‐cause mortality in persons with NAFLD using nationwide health examination cohort data.
Methods
Study population
The Fourth Thai National Health Examination Survey is a nationally representative survey that employs a complex multistage, stratified strategy to monitor the general health and nutrition status of the Thai civilian, non‐institutionalized population. 21 Of 21 960 persons from the Fourth Thai National Health Examination Survey during 2008–2009, we initially selected 18 323 persons aged ≥ 18 years (Figure 1 ). Subjects with BMI < 18.5 kg/m2 (n = 1689) and missing data for handgrip strength, BMI, and alcohol use (n = 473) were excluded. In addition, 3430 subjects who had weekly alcohol consumption of >210 g/week for men and >140 g/week for women or other possible causes of chronic liver disease were excluded. Of 12 731 individuals without significant alcohol intake, 7083 participants were diagnosed with NAFLD, and 5648 subjects were classified as non‐NAFLD according to the lipid accumulation product (LAP) (Figure 1 ).
Figure 1.
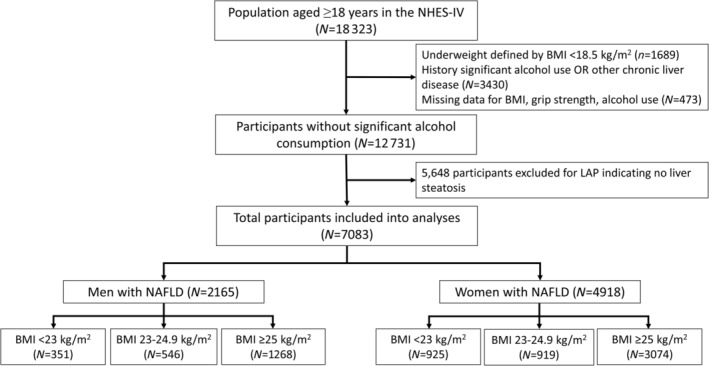
Flow diagram of the study population. LAP, lipid accumulation product; NAFLD, non‐alcoholic fatty liver disease; NHES‐IV, National Health Examination Survey IV.
Definition of non‐alcoholic fatty liver disease
Lipid Accumulation Product was used as a parameter for the diagnosis of NAFLD. 22 LAP was calculated using different formulae for women (waist circumference (WC)[cm] − 58) × (triglyceride[mmol/L]) and men (WC[cm] − 65) × (triglyceride[mmol/L]), which include the minimum sex‐specific WC values. 22 This model is a validated method for diagnosing NAFLD in the general population. 22 Subjects were presumed to have NAFLD if their LAP score was ≥30.5 in men and ≥23.0 in women.
Handgrip strength measurement
Handgrip strength, as a proxy for muscle strength, was measured by using a digital dynamometer (TKK 5401, GRIP‐A; Takei, Niigata, Japan). Isometric grip force was assessed from a single 3 s maximal grip effort of the right‐side and left‐side arms with participants seated upright with their elbow by their side and flexed at 90°. The average of the right‐side and left‐side values, expressed in absolute units (kilograms), was used for the analysis. To account for biological differences in handgrip strength within sex groups, poor muscle strength was determined using the values of handgrip strength < 28 kg for men and <18 kg for women, according to the Asian Working Group on Sarcopenia 2019 consensus. 23
Data collection measurements
Face‐to‐face interviews with research nurses utilizing standardized questions were used to obtain all data. Never smokers, former smokers, and current smokers were all categorized. Alcohol use was calculated based on self‐reported questionnaire items about the frequency and amount of alcohol consumed per day during the previous 12 months. Physical activity was assessed by using the Global Physical Activity Questionnaire, and a high‐leisure‐time physical activity (LTPA) level was defined as engaging in moderate or vigorous‐intensity activity for ≥20 min at a time and at least three times weekly.
Blood pressure, weight, height, and WC were measured using standard procedures. BMI was calculated as weight (kg) divided by the square of the standing height (m2), and subjects were categorized into lean (BMI 18.5–22.9 kg/m2), overweight (BMI 23–24.9 kg/m2), and obese (BMI ≥ 25 kg/m2) following the Asian‐specific criteria. 24 Metabolic syndrome was diagnosed in individuals meeting three of the five following criteria: (1) hyperglycaemia (fasting glucose ≥ 100 mg/dL) or previously diagnosed type 2 diabetes mellitus (fasting glucose ≥ 126 mg/dL or treatment with antidiabetic medications), (2) hypertriglyceridemia (fasting triglycerides ≥ 150 mg/dL), (3) hypertension (systolic blood pressure ≥ 130 and/or diastolic blood pressure ≥ 85 mmHg), (4) low high‐density lipoprotein cholesterol (HDL‐C) (<40 mg/dL in men & <50 mg/dL in women), and (5) central obesity (WC ≥ 80 cm in women and ≥90 cm in men for Asian). 25 Charlson comorbidity index (CCI) score was computed for each patient to measure comorbidity. 26
Blood samples were obtained from an antecubital vein after an overnight fast. The samples were transferred for the determination of fasting plasma glucose using the hexokinase method. Total cholesterol, low‐density lipoprotein cholesterol, HDL‐C, and triglyceride were measured by homogeneous enzymatic colorimetric methods.
Mortality status
The mortality status was determined by linking the NHES data to the Ministry of Interior's National Civil Registration and Vital Statistics System, which systematically gathers data on the death of all Thai residents. Time to death was counted from the date of the NHES survey participation to the date of death or 31 December 2019, whichever came first. All participants provided written informed consent during the initial assessment. The study was carried out following the Declaration of Helsinki and was approved by the Institutional Review Board.
Statistical analysis
STATA Version 14.0 was used for all analyses (StataCorp LP, College Station, Texas, USA). The statistical analyses were weighted to account for the complex survey design. Baseline characteristics of participants with NAFLD across the BMI category and handgrip strength quartile were compared by one‐way analysis of variance or Kruskal–Wallis tests or by χ 2 tests, when appropriate. Post hoc multiple comparison analysis was performed with Bonferroni correction. The survival analysis of subjects with NAFLD stratified by BMI category and muscle strength quartile was performed using the Kaplan–Meier method, and the log‐rank test was used to compare survival distribution between groups.
The rates of overall mortality were calculated and expressed as deaths per 1000 person‐years at risk. Cox proportional‐hazards regression models were used to estimate multivariable‐adjusted hazard ratios (aHRs) for overall mortality. Models were adjusted for age and then further adjusted for sex, smoking status, alcohol use, physical activity, and handgrip strength (for models related to BMI) or BMI (for models related to handgrip strength). Because it has been suggested that insulin resistance and comorbid diseases are in the causal pathway between low muscle strength and death, the second model was then adjusted for hyperglycaemia/diabetes and CCI. Furthermore, the relationship between handgrip strength and all‐cause mortality was examined using the penalized spline smoothing method with multivariate adjustment. To investigate the relationship between BMI and handgrip strength on overall mortality, the P value for interaction was calculated. Finally, we analysed the combined effects of handgrip strength and BMI on overall mortality for both men and women.
In sensitivity analyses, individuals who died within 12 months of enrolment were excluded from the multivariate analysis to reduce the possibility of reverse causation. Subjects with serious illness at the time of the survey may have reduced grip strength due to the disease being more likely to die. To address the possibility that NAFLD per se confounded the effect of BMI and handgrip strength on mortality, multivariate Cox proportional hazard models were constructed for a cohort of 12 731 participants who did not consume significant amounts of alcohol in which full models were finally adjusted for NAFLD status.
Results
Population characteristics
A total of 7083 participants met the definition of NAFLD. Mean age at baseline was 49.3 ± 13.2 years, and mean BMI was 26.8 ± 4.04 kg/m2. A total of 1276 individuals (18.0%) were classified as lean NAFLD, 1465 (20.7%) were overweight NAFLD, and 4342 (61.3%) were obese NAFLD. The baseline characteristics of the study population according to the BMI category are in Table 1 .
Table 1.
Baseline characteristics of patients with NAFLD stratified by BMI category
| Characteristics | BMI category | P value | Comparison | |||
|---|---|---|---|---|---|---|
| Overall | Lean(a) (<23 kg/m2) | Overweight(b) (23–24.9 kg/m2) | Obese(c) (≥25 kg/m2) | |||
| Number (%) | 7083 | 1276 (18.0) | 1465 (20.7) | 4342 (61.3) | ||
| Male gender, n (%) | 2165 (30.6) | 351 (26.6) | 546 (36.1) | 1268 (28.8) | 0.003 | a ≠ b, b ≠ c |
| Age, years | 49.5 ± 13.2 | 53.2 ± 15.2 | 50.4 ± 13.4 | 48.3 ± 12.4 | <0.001 | a ≠ b ≠ c |
| Waist circumference, cm | 87.3 ± 9.5 | 78.2 ± 5.4 | 82.4 ± 5.4 | 91.2 ± 9.0 | <0.001 | a ≠ b ≠ c |
| Alcohol intake, n (%) | 1334 (18.8) | 233 (24.7) | 314 (25.9) | 787 (22.3) | 0.118 | |
| Current smoking, n (%) | 804 (11.4) | 178 (14.1) | 201 (16.2) | 425 (11.5) | <0.001 | a ≠ c, b ≠ c |
| High LTPA, n (%) | 5187 (74.2) | 869 (77.8) | 1072 (78.6) | 3246 (82.2) | 0.055 | |
| Charlson comorbidity index | 1.33 ± 0.66 | 1.39 ± 0.75 | 1.33 ± 0.67 | 1.31 ± 0.63 | 0.003 | a ≠ c |
| Metabolic syndrome, n (%) | 3557 (50.2) | 501 (26.9) | 604 (31.1) | 2452 (51.8) | <0.001 | a ≠ c, b ≠ c |
| Hyperglycaemia/diabetes | 2398 (33.9) | 368 (21.8) | 483 (24.4) | 1547 (28.1) | 0.039 | a ≠ c |
| Hypertension | 4279 (60.4) | 682 (37.1) | 847 (44.2) | 2750 (52.7) | <0.001 | a ≠ b ≠ c |
| Hypertriglyceridemia | 4279 (60.4) | 920 (72.7) | 932 (64.8) | 2,427 (57.4) | <0.001 | a ≠ b ≠ c |
| Low HDL‐C | 4613 (65.1) | 873 (68.2) | 959 (64.4) | 2781 (67.2) | 0.282 | |
| Central obesity | 5638 (70.8) | 551(38.9) | 1035 (67.4) | 4052 (92.8) | <0.001 | a ≠ b ≠ c |
| Glucose, mg/dL | 91 (83–104) | 89 (82–100) | 91 (82–103) | 92 (84–105) | 0.001 | a ≠ b ≠ c |
| Total cholesterol, mg/dL | 221 (192–251) | 220 (187–253) | 221 (192–253) | 221 (193–250) | 0.643 | |
| LDL‐C, mg/dL | 140 (112–168) | 135 (107–166) | 140 (109–167) | 141 (115–168) | 0.001 | a ≠ c |
| HDL‐C, mg/dL | 43 (37–50) | 42 (36–49) | 42 (36–49) | 43 (37–50) | 0.001 | a ≠ c, b ≠ c |
| Triglycerides, mg/dL | 168 (127–226) | 183 (145–243) | 174 (135–241) | 160 (120–214) | <0.001 | a ≠ b ≠ c |
| Handgrip strength, kg | 25.1 (20.2–31.1) | 22.6 (18.2–27.4) | 25.1 (20.1–31.8) | 25.9 (21–32.1) | <0.001 | a ≠ b ≠ c |
BMI, body mass index; BP, blood pressure; LDL‐C, low‐density lipoprotein cholesterol; LTPA; leisure‐time physical activity; HDL‐C, high‐density lipoprotein cholesterol.
Data are presented as the mean ± standard deviation or number (percentage). Characteristics of participants with NAFLD across the BMI category were compared by one‐way analysis of variance or Kruskal–Wallis tests or by χ 2 tests, when appropriate. Post hoc multiple comparison analysis was performed with Bonferroni correction. a ≠ b ≠ c: There is statistical difference among BMI < 23 kg/m2 (a), BMI 23–24.9 kg/m2 (b), and BMI ≥ 25 kg/m2 (c). a ≠ b, a ≠ c: There is statistical difference between BMI < 23 kg/m2 (a) and BMI 23–24.9 kg/m2 (b) and between BMI < 23 kg/m2 (a) and BMI ≥ 25 kg/m2 (c), while there is no statistical difference between BMI 23–24.9 kg/m2 (b) and BMI ≥ 25 kg/m2 (c). b ≠ c: There is statistical difference between BMI 23–24.9 kg/m2 (b) and BMI ≥ 25 kg/m2 (c). a ≠ b, b ≠ c: There is statistical difference between BMI < 23 kg/m2 (a) and BMI 23–24.9 kg/m2 (b) and between BMI 23–24.9 kg/m2 (b) and BMI ≥ 25 kg/m2 (c), while there is no statistical difference between BMI < 23 kg/m2 (a) and BMI ≥ 25 kg/m2 (c). a ≠ b, b ≠ c: There is statistical difference between BMI < 23 kg/m2 (a) and BMI 23–24.9 kg/m2 (b) and between BMI 23–24.9 kg/m2 (b) and BMI ≥ 25 kg/m2 (c), while there is no statistical difference between BMI < 23 kg/m2 (a) and BMI ≥ 25 kg/m2 (c).
Participants with lean NAFLD were more likely to be older, current smokers, and had higher CCI scores but had lower low‐density lipoprotein cholesterol and handgrip strength values than those with obese NAFLD. Overweight subjects with NAFLD were also likely to be older, male gender, current smokers, and had lower handgrip strength than obese counterparts. The features of metabolic syndrome except hypertriglyceridemia were present less commonly among lean and overweight subjects compared with obese subjects. Conversely, lean and overweight subjects had higher triglyceride concentrations but lower HDL‐C values than obese individuals. The proportion of subjects with central obesity was significantly lower in the lean group than the overweight and obese groups.
All NAFLD phenotypes were of similar modest alcohol intake. LTPA was not substantially different in the lean and overweight groups compared with the obese group (77.8% vs. 78.6% vs. 82.2%, respectively), but there was a trend towards higher LTPA in the obese group (P = 0.055).
All‐cause mortality among non‐alcoholic fatty liver disease individuals stratified by body mass index categories
The mean follow‐up period for the study cohort was 8.52 ± 1.43 years (range: 0.76–8.96). During the 60 432 person‐years of follow‐up, 843 participants with NAFLD died, and the cumulative all‐cause mortality was 13.9 per 1000 person‐years. Overall mortality was higher among subjects with lean NAFLD than those with obese NAFLD [20.7 vs. 11.7 per 1000 person‐years, HR 1.32, 95% confidence interval (CI), 1.17–1.48; P < 0.001] (Figure 2 ). Compared with subjects with obese NAFLD, subjects with overweight NAFLD had significantly higher overall mortality (HR 1.53, 95% CI, 1.07–2.19; P = 0.022). However, after adjusting for age, sex, current smoking, alcohol use, high LTPA, CCI, hyperglycaemia/diabetes, and handgrip strength, participants with lean NAFLD (aHR 1.18, 95% CI, 0.95–1.48, P = 0.138) and subjects with overweight NAFLD (aHR 1.28, 95% CI: 0.89–1.84, P = 0.158) have non‐significantly higher overall mortality than persons with obese NAFLD.
Figure 2.
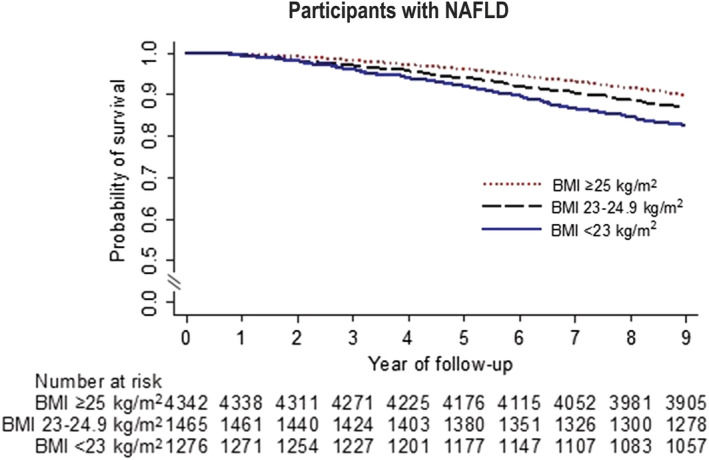
Kaplan–Meier survival curves for participants with non‐alcoholic fatty liver disease according to body mass index category.
In the multivariable‐adjusted model for mortality risk, we found significant effects of age (aHR 1.04 per 1 year increase, 95% CI, 1.01–1.07, P = 0.005), male gender (aHR 2.24, 95% CI, 1.61–3.40, P < 0.001), current smoking (aHR 1.37, 95% CI, 1.05–1.80, P = 0.024), CCI (aHR 1.42 per 1 score increase, 95% CI, 1.28–1.58, P < 0.001), hyperglycaemia/diabetes (aHR 1.30, 95% CI, 1.08–1.56, P = 0.009), and handgrip strength (aHR 0.96 per 1 kg increase, 95% CI, 0.93–0.98, P = 0.002) on all‐cause death among participants with NAFLD (Supporting Information, Figure S1 ).
Handgrip strength and the risk of all‐cause mortality
We further explored characteristics of the study population by handgrip strength quartile among each gender (Tables S1 and S2 ). In both men and women with NAFLD, subjects in the lowest quartile for strength (Q1) were older and had higher CCI scores and more frequently hyperglycaemia/diabetes and hypertension than those in the highest handgrip strength group (Q4). Subjects in the highest quartile for strength were younger, more likely to be obese, and often had modest alcohol consumption and higher levels of LTPA in comparison with those in the lowest handgrip strength group. Men with high handgrip strength and women with low handgrip strength had significantly higher total cholesterol and triglyceride levels. Current smoking was observed in a more significant proportion of men among the higher quartile of strength and women among the lower quartile of strength.
When handgrip strength was treated as an ordinal variable using quartile, participants with lower handgrip strength had significantly lower survival than those with higher handgrip strength in men and women (Figure 3 ). Adjusting for age, BMI category, alcohol use, current smoking, high LTPA, CCI, and hyperglycaemia/diabetes did not markedly change these associations. Among men, adjusted HRs for all‐cause mortality were 3.21 (95% CI, 1.35–7.62, P = 0.011) for the lowest handgrip strength quartile (Q1), 2.78 (95% CI, 1.39–5.58, P = 0.006) for the Q2, and 2.76 (95% CI, 1.41–5.41, P = 0.005) for the Q3 in comparison with the highest handgrip strength quartile (Q4). For women, the Q1 (aHR 2.22, 95% CI, 1.25–3.93, P = 0.009) and the Q2 (aHR 1.95, 95% CI, 1.12–3.38, P = 0.021) was associated significantly with higher mortality risk in comparison with the Q4, whereas the Q3 did not exhibit a significant association with increased mortality (aHR 1.64, 95% CI, 0.77–3.51, P = 0.190).
Figure 3.
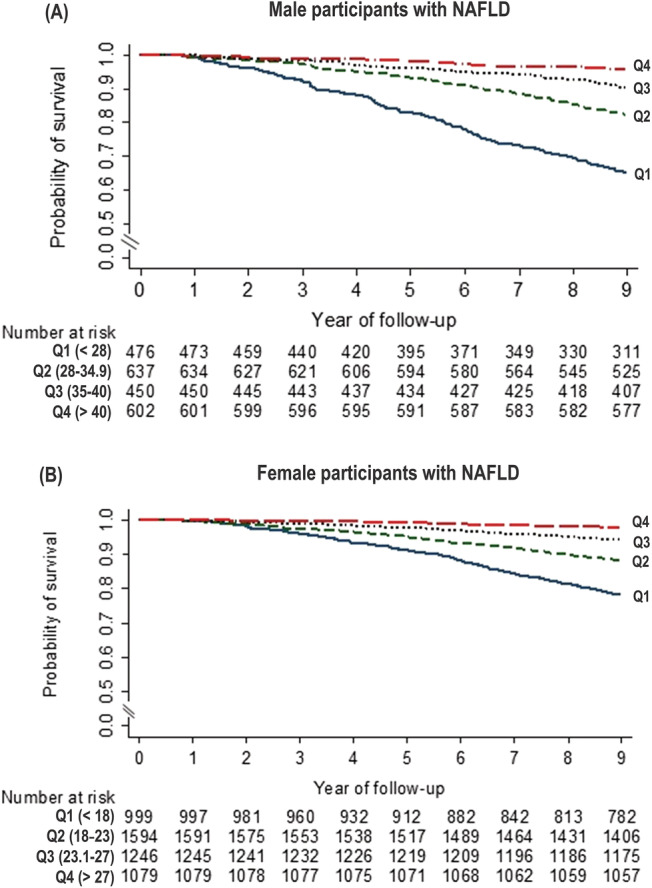
Kaplan–Meier survival curves for men (A) and women (B) with non‐alcoholic fatty liver disease according to quartile of handgrip strength.
Furthermore, the association between the continuous measure of handgrip strength at baseline and all‐cause death demonstrated an inverse association between handgrip strength and risk of death after adjusting for all potential confounders both in men and women (Figure 4 ). The curve showed a significantly increased risk of mortality in persons with poor muscle strength defined by handgrip strength of <28 kg for men and <18 kg for women compared with those with the strongest handgrip strength in the fully adjusted model.
Figure 4.
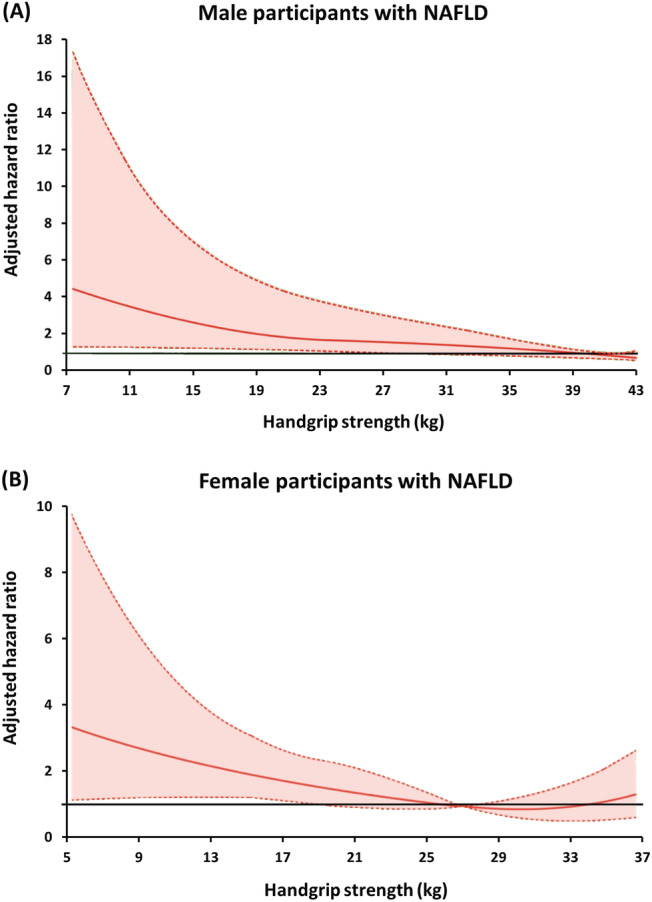
The relationship between handgrip strength at baseline and all‐cause mortality in (A) men and (B) women using the penalized spline smoothing method with multivariate adjustment. Models are adjusted for age, BMI category, current smoking, alcohol use, leisure‐time physical activity, Charlson comorbidity index, and hyperglycaemia/diabetes. The coloured area represents 95% confidence intervals to the relative risk (adjusted hazards ratio) estimate.
All‐cause mortality of non‐alcoholic fatty liver disease according to combinations of body mass index and handgrip strength
To determine whether BMI was a significant factor in the association between handgrip strength and overall mortality, tests for interactions between the BMI category and the handgrip strength quartile in full models did not show evidence of interactions between BMI and handgrip strength on mortality among men and women (P values for interaction = 0.693 and 0.615, respectively). We further investigated the combined associations of muscle strength and BMI with mortality in persons with NAFLD and performed the stratified analysis of mortality risk for each BMI category according to the handgrip strength quartile (Table 2 ). Obese subjects with the highest handgrip strength quartile were the reference group. Among male participants, weaker handgrip strength was associated with higher mortality risk across the BMI category after adjusting for age, smoking status, alcohol use, and high LTPA. Additional adjustment for pre‐existing comorbidities using CCI and hyperglycaemia/diabetes underscored the significant associations between low handgrip strength and high mortality risk for obese and overweight NAFLD. The highest mortality risk was among overweight participants with the handgrip strength between 35 and 40 kg (Q3) (aHR 4.89, 95% CI, 2.10–11.4, P = 0.001) relative to obese participants with the highest handgrip strength. Poor muscle strength defined by a handgrip strength of <28 kg (Q1) was associated significantly with overall mortality among men with obese NAFLD (aHR 3.94, 95% CI, 1.38–11.3, P = 0.013) and overweight NAFLD (aHR 2.93, 95% CI, 1.19–7.19, P = 0.021) after full adjustment (Table 2 ). Men with lean NAFLD and poor muscle strength had a significantly higher mortality risk (aHR 3.02, 95% CI, 1.05–8.73, P = 0.042) in comparison with obese NAFLD men with the highest grip strength in Model 1 but did not exhibit a significant association (aHR 2.78, 95% CI, 0.93–8.32, P = 0.065) in the fully adjusted model.
Table 2.
The overall mortality of NAFLD participants stratified by BMI category and handgrip strength
| Person‐years | Deaths | Deaths per 1000person‐years | Age‐adjusted HR (95% CI) | P value | Multivariable‐adjusted HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | P value | Model 2 | P value | ||||||
| Men (n = 2165) | |||||||||
| BMI ≥ 25 kg/m2 (n = 1268) | |||||||||
| Quartile 4 (>40 kg) | 3714.4 | 17 | 4.58 | Reference | Reference | Reference | |||
| Quartile 3 (35–40 kg) | 2490.7 | 26 | 10.4 | 2.42 (1.33–4.38) | 0.006 | 2.50 (1.31–4.76) | 0.008 | 2.47 (1.27–4.80) | 0.010 |
| Quartile 2 (28–34.9 kg) | 2988.8 | 59 | 19.7 | 2.77 (0.97–7.95) | 0.057 | 2.87 (1.02–8.10) | 0.047 | 2.60 (0.91–7.42) | 0.071 |
| Quartile 1 (<28 kg) | 1563.5 | 75 | 48.0 | 4.14 (1.36–12.6) | 0.015 | 4.33 (1.50–12.5) | 0.009 | 3.94 (1.38–11.3) | 0.013 |
| BMI 23–24.9 kg/m2 (n = 546) | |||||||||
| Quartile 4 (>40 kg) | 1277.8 | 5 | 3.91 | 0.94 (0.17–5.14) | 0.940 | 1.02 (0.18–5.73) | 0.978 | 1.06 (0.18–6.14) | 0.948 |
| Quartile 3 (35–40 kg) | 921.2 | 12 | 13.0 | 4.52 (1.66–12.3) | 0.005 | 4.68 (2.11–10.4) | 0.001 | 4.89 (2.10–11.4) | 0.001 |
| Quartile 2 (28–34.9 kg) | 1329.7 | 29 | 21.8 | 3.56 (1.94–6.54) | <0.001 | 3.83 (2.15–6.81) | <0.001 | 3.58 (1.89–6.77) | <0.001 |
| Quartile 1 (<28 kg) | 1005.1 | 42 | 41.8 | 3.03 (1.26–7.31) | 0.016 | 3.18 (1.39–7.26) | 0.008 | 2.93 (1.19–7.19) | 0.021 |
| BMI < 23 kg/m2 (n = 351) | |||||||||
| Quartile 4 (>40 kg) | 307.9 | 3 | 9.74 | 0.98 (0.19–5.00) | 0.985 | 1.15 (0.21–6.34) | 0.862 | 1.29 (0.26–7.06) | 0.758 |
| Quartile 3 (35–40 kg) | 478.8 | 5 | 10.4 | 0.80 (0.47–1.35) | 0.384 | 0.95 (0.54–1.66) | 0.849 | 0.98 (0.60–1.59) | 0.927 |
| Quartile 2 (28–34.9 kg) | 1011.9 | 24 | 23.7 | 2.51 (0.80–7.82) | 0.107 | 2.71 (0.77–9.55) | 0.115 | 2.78 (0.79–9.79) | 0.105 |
| Quartile 1 (<28 kg) | 1040.9 | 48 | 46.1 | 3.03 (1.05–8.75) | 0.042 | 3.02 (1.05–8.73) | 0.042 | 2.78 (0.93–8.32) | 0.065 |
| Women (n = 4918) | |||||||||
| BMI ≥ 25 kg/m2 (n = 3074) | |||||||||
| Quartile 4 (>27 kg) | 7199.5 | 16 | 2.22 | Reference | Reference | Reference | |||
| Quartile 3 (23.1–27 kg) | 7085.8 | 42 | 5.93 | 0.84 (0.41–1.74) | 0.630 | 0.82 (0.38–1.76) | 0.592 | 0.82 (0.38–1.73) | 0.579 |
| Quartile 2 (18–23 kg) | 8290.4 | 103 | 12.4 | 1.88 (0.90–3.91) | 0.088 | 1.84 (0.95–3.59) | 0.070 | 1.80 (0.93–3.52) | 0.080 |
| Quartile 1 (<18 kg) | 4108.2 | 99 | 24.1 | 2.70 (1.24–5.85) | 0.015 | 2.60 (1.28–5.29) | 0.011 | 2.25 (1.06–4.76) | 0.036 |
| BMI 23–24.9 kg/m2 (n = 919) | |||||||||
| Quartile 4 (>27 kg) | 1413.4 | 5 | 3.54 | 0.80 (0.25–2.53) | 0.685 | 0.81 (0.26–2.56) | 0.708 | 0.83 (0.26–2.68) | 0.748 |
| Quartile 3 (23.1–27 kg) | 2030.7 | 15 | 7.39 | 3.30 (0.92–11.9) | 0.066 | 3.36 (1.01–11.1) | 0.048 | 3.46 (1.09–11.0) | 0.036 |
| Quartile 2 (18–23 kg) | 2607.7 | 32 | 12.3 | 1.15 (0.54–2.46) | 0.707 | 1.13 (0.53–2.41) | 0.734 | 1.13 (0.52–2.47) | 0.739 |
| Quartile 1 (<18 kg) | 1820.2 | 47 | 25.8 | 2.27 (1.02–5.03) | 0.044 | 2.20 (1.08–4.50) | 0.032 | 1.69 (0.81–3.51) | 0.153 |
| BMI < 23 kg/m2 (n = 925) | |||||||||
| Quartile 4 (>27 kg) | 982.9 | 1 | 1.02 | 0.37 (0.08–1.71) | 0.192 | 0.40 (0.08–2.02) | 0.253 | 0.39 (0.09–1.63) | 0.185 |
| Quartile 3 (23.1–27 kg) | 1802.1 | 14 | 7.77 | 2.10 (0.85–5.22) | 0.103 | 2.05 (0.88–4.80) | 0.092 | 2.08 (0.85–5.09) | 0.104 |
| Quartile 2 (18–23 kg) | 2706.5 | 53 | 19.6 | 2.56 (0.98–6.66) | 0.054 | 2.52 (1.07–5.94) | 0.037 | 2.68 (1.13–6.38) | 0.028 |
| Quartile 1 (<18 kg) | 2254.0 | 71 | 31.5 | 2.74 (1.07–7.04) | 0.038 | 2.50 (1.10–5.68) | 0.030 | 2.47 (1.06–5.73) | 0.037 |
BMI, body mass index; HR, hazards ratio.
Age‐adjusted HR (95% CI) was HR adjusted for age. Multivariable model 1 was adjusted for age, current smoking, alcohol intake, and regular exercise. Multivariable model 2 was Model 1 plus an adjustment for Charlson comorbidity index and hyperglycaemia/diabetes.
Among female participants, the risk of death increased with decreasing handgrip strength across all BMI categories. Overweight women with handgrip strength between 23.1 and 27 kg (Q3) had the most substantial effect on death (aHR 3.46, 95% CI, 1.09–11.0, P = 0.036) after total adjustment. The excess mortality risk of poor muscle strength defined by a handgrip strength of <18 kg (Q1) was significantly high among women with obese NAFLD (aHR 2.25, 95% CI, 1.06–4.76, P = 0.036) and lean NAFLD (aHR 2.47, 95% CI, 1.06–5.73, P = 0.037) in the fully adjusted model. Overweight women with poor muscle strength had a significantly higher mortality risk (aHR 2.20, 95% CI, 1.08–4.50, P = 0.032) in comparison with obese women with the highest grip strength in Model 1 but did not exhibit a significant association with all‐cause mortality (aHR 1.69, 95% CI, 0.81–3.51, P = 0.153) in the fully adjusted model.
We performed sensitivity analyses by excluding participants who died during the first year of their follow‐up (Table S3 ). Excluding these subjects had no discernible impact on the associations between handgrip strength and mortality among both genders of all BMI levels.
Combinations of body mass index and handgrip strength on mortality among persons without significant alcohol consumption
We conducted analyses to determine the possibility that the combined effects of BMI and handgrip strength on mortality might be confounded by NAFLD status (Table 3 ). Among subjects without significant alcohol use, the risk of death increased with decreasing handgrip strength across all BMI categories in men after adjusting for age, smoking status, alcohol use, high LTPA, CCI, and hyperglycaemia/diabetes. Further adjustment with NAFLD status had little effect on the association between handgrip strength and mortality in men for all BMI categories. The detrimental effect of low handgrip strength on mortality was weaker and did not reach statistical significance among obese and overweight women. Poor muscle strength was associated significantly with all‐cause mortality among lean women (aHR 2.55, 95% CI, 1.17–5.55, P = 0.021) after adjusting for age, lifestyle, comorbidities, and NAFLD.
Table 3.
The overall mortality of all participants who did not use significant amounts of alcohol stratified by BMI category and handgrip strength
| Deaths per 1000 person‐years | Adjusted HR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Total population (n = 12 731) | NAFLD (n = 7083) | Non‐NAFLD (5648) | Multivariable model | P value | Full model | P value | |
| Men (n = 5070) | |||||||
| BMI ≥ 25 kg/m2 (n = 1617) | |||||||
| Quartile 4 (>40 kg) | 4.09 | 4.58 | 2.15 | Reference | Reference | ||
| Quartile 3 (35–40 kg) | 9.14 | 10.4 | 4.39 | 2.66 (1.47–4.84) | 0.003 | 2.65 (1.46–4.81) | 0.003 |
| Quartile 2 (28–34.9 kg) | 18.2 | 19.7 | 12.5 | 3.01 (1.10–8.28) | 0.035 | 2.97 (1.08–8.18) | 0.037 |
| Quartile 1 (<28 kg) | 45.4 | 48.0 | 38.3 | 5.68 (2.26–14.3) | 0.001 | 5.50 (2.23–13.6) | 0.001 |
| BMI 23–24.9 kg/m2 (n = 1104) | |||||||
| Quartile 4 (>40 kg) | 3.69 | 3.91 | 3.50 | 1.62 (0.39–6.62) | 0.486 | 1.53 (0.38–6.21) | 0.536 |
| Quartile 3 (35–40 kg) | 11.4 | 13.0 | 9.76 | 4.15 (1.72–10.0) | 0.003 | 3.96 (1.69–9.31) | 0.003 |
| Quartile 2 (28–34.9 kg) | 16.4 | 21.8 | 11.0 | 3.32 (1.89–5.85) | <0.001 | 3.12 (1.76–5.51) | <0.001 |
| Quartile 1 (<28 kg) | 39.2 | 41.8 | 36.8 | 4.32 (1.82–10.3) | 0.002 | 4.03 (1.73–9.38) | 0.003 |
| BMI < 23 kg/m2 (n = 2349) | |||||||
| Quartile 4 (>40 kg) | 2.68 | 9.74 | 2.04 | 0.98 (0.31–3.12) | 0.966 | 0.85 (0.27–2.75) | 0.785 |
| Quartile 3 (35–40 kg) | 8.94 | 10.4 | 8.73 | 3.33 (1.37–8.13) | 0.011 | 2.97 (1.23–7.16) | 0.018 |
| Quartile 2 (28–34.9 kg) | 19.7 | 23.7 | 19.0 | 3.84 (1.72–8.57) | 0.002 | 3.40 (1.56–7.40) | 0.004 |
| Quartile 1 (<28 kg) | 45.1 | 46.1 | 44.9 | 6.60 (2.50–17.4) | 0.001 | 5.80 (2.30–14.6) | 0.001 |
| Women (n = 7661) | |||||||
| BMI ≥ 25 kg/m2 (n = 3547) | |||||||
| Quartile 4 (>27 kg) | 2.38 | 2.22 | 3.31 | Reference | Reference | ||
| Quartile 3 (23.1–27 kg) | 5.46 | 5.93 | 3.00 | 0.80 (0.41–1.52) | 0.463 | 0.78 (0.40–1.53) | 0.449 |
| Quartile 2 (18–23 kg) | 11.7 | 12.4 | 6.73 | 1.54 (0.90–2.64) | 0.108 | 1.50 (0.85–2.65) | 0.151 |
| Quartile 1 (<18 kg) | 22.8 | 24.1 | 11.1 | 1.81 (0.93–3.53) | 0.079 | 1.72 (0.85–3.51) | 0.125 |
| BMI 23–24.9 kg/m2 (n = 1443) | |||||||
| Quartile 4 (>27 kg) | 2.45 | 3.54 | 1.38 | 1.08 (0.35–3.27) | 0.891 | 1.01 (0.33–3.10) | 0.981 |
| Quartile 3 (23.1–27 kg) | 5.63 | 7.39 | 2.98 | 2.41 (0.81–7.12) | 0.107 | 2.28 (0.71–7.33) | 0.157 |
| Quartile 2 (18–23 kg) | 10.0 | 12.3 | 5.76 | 0.88 (0.46–1.68) | 0.686 | 0.84 (0.42–1.67) | 0.598 |
| Quartile 1 (<18 kg) | 24.6 | 25.8 | 20.6 | 1.59 (0.99–2.54) | 0.051 | 1.46 (0.84–2.55) | 0.166 |
| BMI < 23 kg/m2 (n = 2671) | |||||||
| Quartile 4 (>27 kg) | 2.28 | 1.02 | 2.65 | 1.44 (0.52–3.96) | 0.465 | 1.28 (0.47–3.48) | 0.617 |
| Quartile 3 (23.1–27 kg) | 3.98 | 7.77 | 2.36 | 1.57 (0.83–2.98) | 0.159 | 1.42 (0.69–2.93) | 0.328 |
| Quartile 2 (18–23 kg) | 13.7 | 19.6 | 10.3 | 2.31 (1.21–4.40) | 0.014 | 2.04 (0.90–4.62) | 0.086 |
| Quartile 1 (<18 kg) | 32.5 | 31.5 | 33.3 | 2.87 (1.51–5.46) | 0.003 | 2.55 (1.17–5.55) | 0.021 |
BMI, body mass index; HR, hazards ratio.
The multivariable model was adjusted for age, current smoking, alcohol intake, high‐leisure‐time physical activity, Charlson comorbidity index, and hyperglycaemia/diabetes. The full multivariable model was Model 1 plus an adjustment for NAFLD.
Discussion
The major finding from this nationwide, population‐based cohort study was the observation that subjects with lean or overweight NAFLD had a comparable risk of overall mortality as those with obese NAFLD when controlling for relevant confounders. This study also discovered that reduced muscle strength is linked to an increased risk of all‐cause mortality in subjects with NAFLD, even after adjusting for physiologic, lifestyle, and comorbid determinants. Low muscle strength predicts mortality in both men and women with NAFLD across BMI categories.
Our study shows that 18% of the NAFLD population were lean even using the conservative lower BMI for Asian populations. This finding is similar to a global prevalence of 19.2% (95% CI, 15.9–23.0) reported in a meta‐analysis. 27 About a quarter (26.9%) of lean individuals and one‐third (31.1%) of overweight subjects had metabolic syndrome as compared with a half (51.8%) of obese participants. This finding suggests that in a proportion of individuals with NAFLD, other pathophysiological factors may exist that link to NAFLD but are independent of the traditional insulin resistance‐related comorbidities. Sarcopenia or a loss of skeletal muscle mass and function seems to be associated with NAFLD among individuals with normal BMI. Growing evidence has shown that low skeletal muscle mass (pre‐sarcopenia) is associated with increased risks of NAFLD and advanced fibrosis, independent of obesity or metabolic control. 12 , 28 , 29 , 30 , 31 In a large Asian cohort, handgrip strength was used as a proxy for skeletal muscle function, and the results revealed an inverse relationship between handgrip strength and NAFLD. 32 In line with this finding, our study showed that muscle strength decrements were consistently associated with a higher prevalence of lean NAFLD. The association between weak muscle strength and lean NAFLD was shown to be more pronounced in men than in women. This might partly be explained by differences in lifestyle behaviours between men and women (e.g. modest alcohol intake, current smoking, and LTPA), which could affect the observed association. Several studies have uncovered associations between several genetic variants and the natural history of NAFLD, independent of insulin resistance 33 ; however, genetic analysis was not performed in our cohort. Further studies are needed to explore how environmental factors, including physical activity, diet composition, and drinking habits, interact with genetic variations among these individuals.
The prognosis of lean or non‐obese individuals with NAFLD continues to be debated. Liver disease progression, especially the development of advanced fibrosis, was less rapid in non‐obese patients with NAFLD from the Hong Kong cohort than their obese counterparts. 8 Furthermore, clinically relevant outcomes such as liver failure, hepatocellular carcinoma, and overall mortality were lower in non‐obese patients than those with obese NAFLD. 8 These findings are supported by data from a Swedish cohort demonstrating that patients with lean NAFLD had no increased risk of mortality throughout a 20 year follow‐up. 9 A Caucasian cohort from four counties reported no difference in survival between lean and non‐lean individuals. 34 In contrast, a multi‐ethnic and international cohort showed that lean patients with NAFLD had a considerably lower cumulative survival than their obese counterparts over an 11 year follow‐up period. 10 However, the results of these studies might be hampered by recruiting only individuals undergoing liver biopsies in academic centres, implying that there could be selection bias. In our nationwide, population‐based cohort of NAFLD, lean or overweight subjects with NAFLD had a higher incidence of all‐cause mortality despite having a more favourable metabolic profile than obese individuals with NAFLD. However, the prognostic significance of lean NAFLD and overweight NAFLD did not exist after adjusting for health, lifestyle, and comorbid factors. This suggests that pathophysiologic drivers of the disease may be variables other than adiposity as evaluated by BMI. Handgrip strength was revealed to be an independent risk factor for mortality in our Cox regression analysis, indicating that muscle quality is a significant prognostic marker for NAFLD.
Sarcopenia has been recognized as a comorbidity associated with poor prognosis in patients with liver cirrhosis. 35 The majority of studies assessing the relationship between sarcopenia and NAFLD rely only on objective measurements of skeletal muscle mass. 11 , 12 , 13 , 14 , 36 Low skeletal muscle index, a proxy for the loss of muscle mass, has been associated with an increased risk of overall mortality in individuals with NAFLD. 37 However, sarcopenia must be diagnosed by a decline in skeletal muscle strength or performance. Thus, the prognostic relevance of sarcopenia in patients with NAFLD is yet unknown. To our knowledge, this is the first study exploring the relationship between handgrip strength and mortality among patients with NAFLD. We found an inversed relationship between muscle strength and all‐cause mortality, with the substantial risk at the lowest handgrip strength quartile in the fully adjusted model incorporating age, BMI, lifestyle behaviours, and comorbidities. As defined by weak handgrip strength using the Asian Working Group on Sarcopenia definition, poor muscle strength was consistently associated with an increased risk of all‐cause death among NAFLD individuals across BMI categories. Surprisingly, the highest mortality risk was observed in overweight subjects at the third quartile of handgrip strength. This finding might be attributable to residual confounding from unmeasured factors, such as weight changes over time, which could cause estimates in our data to be non‐linearity. It was shown that weight loss or gain was associated with increased mortality risk in overweight people. 38
There are several potential mechanisms by which low muscle strength predispose to death. A progressive decline in muscle strength is often a consequence of subclinical illnesses and other unfavourable physiological processes in the body. Insulin resistance and chronic inflammation have also been demonstrated as pathophysiological pathways linked to sarcopenia. 39 In this regard, the adjustment for CCI and hyperglycaemia/diabetes attenuated the unfavourable effects of low muscle strength on mortality, suggesting an essential role of chronic conditions and insulin resistance in the pathway towards decreasing muscle strength. We performed sensitivity analyses by excluding subjects who died within the first year of follow‐up in order to account for other unmeasured confounders. The exclusion did not change the mortality risk associated with low muscle strength.
To ensure the prognostic significance of handgrip strength on overall mortality, we examined data from the cohort of participants who did not consume significant amounts of alcohol. The analyses revealed that decreasing handgrip strength significantly impacted overall mortality in men for all BMI categories after adjusting for age, lifestyle habits, and comorbidities. It has been shown that NAFLD is associated with low skeletal muscle mass. 11 , 12 , 13 , 14 Therefore, NAFLD can distort the studied association. In our full models, the adjustment of NAFLD had a minimal influence on the association of handgrip strength and BMI on mortality. These data support the notion that muscle strength is a physiologic activity marker related to mortality risk. However, only lean women with poor muscle strength had a considerably higher risk of death. No significant associations were found between handgrip strength and overall mortality among obese and overweight women. The obesity paradox, which refers to evidence showing that obesity in subjects with several chronic diseases may be protective and associated with decreased mortality, might be a possible explanation for this finding. 40
The strength of our study is the use of a large‐scale population with standardized measures for collecting covariates. In addition, the mortality risk with muscle strength was calculated after accounting for a number of known confounders to strengthen the validity of the results. Finally, sensitivity analyses were performed to ensure that the findings were reliable. However, this study has some limitations. First, we utilized LAP as a non‐invasive diagnostic tool for NAFLD. Using LAP score based on anthropometric and laboratory data allowed us to examine a large, population‐based sample and minimize ascertainment bias, which is common in studies using convenience samples. Second, we were unable to investigate the association between handgrip strength and disease‐specific mortality because the NHES database did not capture causes of death. Finally, the severity of liver fibrosis may influence the prognosis of muscle strength on mortality in individuals with NAFLD. However, this NHES did not collect variables for calculating non‐invasive fibrosis scores to identify persons with NAFLD at risk for advanced fibrosis. This could be mitigated by accounting for metabolic factors that impact liver fibrogenesis in multivariable‐adjusted analysis.
In conclusion, this nationally representative cohort provides crucial information on the mortality risk of adiposity as evaluated by BMI and muscle function determined by handgrip strength among individuals with NAFLD. We found that while overall survival of lean and overweight participants was lower than that of obese counterparts, the mortality risk was not significantly different between individuals with lean, overweight, and obese NAFLD when age, sex, lifestyle habits, comorbidities, hyperglycaemia/diabetes, and handgrip strength were taken into account. Our analysis also revealed a significant inverse relationship between handgrip strength and mortality risk among men and women with NAFLD. Substantial relationships exist, even when accounting for physiologic, lifestyle, and comorbid factors. Thus, it is important to classify this population according to muscle strength and reconsider a BMI‐based strategy for determining the prognosis of NAFLD. Measuring handgrip strength can be a simple, non‐invasive risk stratification approach for overall mortality in patients with NAFLD. Further research is required to determine its clinical value and explore whether improving muscle strength can reduce mortality in this population.
Conflict of interest
None declared.
Funding
This study was supported by the National Research Council of Thailand and a grant from the Siriraj Research Development Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supporting information
Table S1. Baseline characteristics of male participants stratified by handgrip strength
Table S2. Baseline characteristics of female participants stratified by handgrip strength
Table S3. The overall mortality of participants with NAFLD stratification by BMI category and handgrip strength (exclusion subjects who died within 1 year after survey).
Figure S1. Factors associated with the overall mortality of participants with NAFLD.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 41
Charatcharoenwitthaya P., Karaketklang K., and Aekplakorn W. (2022) Muscle strength, but not body mass index, is associated with mortality in patients with non‐alcoholic fatty liver disease, Journal of Cachexia, Sarcopenia and Muscle, 13, 2393–2404, 10.1002/jcsm.13001
Contributor Information
Phunchai Charatcharoenwitthaya, Email: phunchai@yahoo.com.
Wichai Aekplakorn, Email: wichai.aek@mahidol.ac.th.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2. Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, et al. Changing trends in etiology‐based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology 2018;155:1154, e3–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–327. [DOI] [PubMed] [Google Scholar]
- 4. Feng RN, Du SS, Wang C, Li YC, Liu LY, Guo FC, et al. Lean‐non‐alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J Gastroenterol 2014;20:17932–17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med 2004;164:2169–2175. [DOI] [PubMed] [Google Scholar]
- 6. Alam S, Gupta UD, Alam M, Kabir J, Chowdhury ZR, Alam AK. Clinical, anthropometric, biochemical, and histological characteristics of nonobese nonalcoholic fatty liver disease patients of Bangladesh. Indian J Gastroenterol 2014;33:452–457. [DOI] [PubMed] [Google Scholar]
- 7. Sookoian S, Pirola CJ. Systematic review with meta‐analysis: risk factors for non‐alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther 2017;46:85–95. [DOI] [PubMed] [Google Scholar]
- 8. Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology 2017;65:54–64. [DOI] [PubMed] [Google Scholar]
- 9. Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long‐term follow‐up study. Hepatol Commun 2018;2:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dela Cruz AC, Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P, et al. Characteristics and long‐term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology 2014;146:S‐ 909. [Google Scholar]
- 11. Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014;59:1772–1778. [DOI] [PubMed] [Google Scholar]
- 12. Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008‐2011). J Hepatol 2015;63:486–493. [DOI] [PubMed] [Google Scholar]
- 13. Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non‐alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123–131. [DOI] [PubMed] [Google Scholar]
- 14. Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008‐2011). Hepatology 2016;63:776–786. [DOI] [PubMed] [Google Scholar]
- 15. Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non‐diabetic patients with non‐alcoholic fatty liver disease: sites and mechanisms. Diabetologia 2005;48:634–642. [DOI] [PubMed] [Google Scholar]
- 16. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 17. Celis‐Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018;361:k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strand BH, Cooper R, Bergland A, Jorgensen L, Schirmer H, Skirbekk V, et al. The association of grip strength from midlife onwards with all‐cause and cause‐specific mortality over 17 years of follow‐up in the Tromso study. J Epidemiol Community Health 2016;70:1214–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leong DP, Teo KK, Rangarajan S, Lopez‐Jaramillo P, Avezum A Jr, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266–273. [DOI] [PubMed] [Google Scholar]
- 20. Keating SE, Adams LA. Exercise in NAFLD: Just do it. J Hepatol 2016;65:671–673. [DOI] [PubMed] [Google Scholar]
- 21. Aekplakorn W, Inthawong R, Kessomboon P, Sangthong R, Chariyalertsak S, Putwatana P, et al. Prevalence and trends of obesity and association with socioeconomic status in Thai adults: national health examination surveys, 1991‐2009. J Obes 2014;2014:410259. 10.1155/2014/410259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dai H, Wang W, Chen R, Chen Z, Lu Y, Yuan H. Lipid accumulation product is a powerful tool to predict non‐alcoholic fatty liver disease in Chinese adults. Nutr Metab (Lond) 2017;14:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuo YH, Wang TF, Liu LK, Lee WJ, Peng LN, Chen LK. Epidemiology of sarcopenia and factors associated with it among community‐dwelling older adults in Taiwan [Am J Med Sci. 2019; 357(2):124‐133]. Am J Med Sci 2019;358:169. [DOI] [PubMed] [Google Scholar]
- 24. Consultation WHOE . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 25. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 26. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 27. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non‐obese or lean non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol 2020;5:739–752. [DOI] [PubMed] [Google Scholar]
- 28. Kim HY, Kim CW, Park CH, Choi JY, Han K, Merchant AT, et al. Low skeletal muscle mass is associated with non‐alcoholic fatty liver disease in Korean adults: the Fifth Korea National Health and Nutrition Examination Survey. Hepatobiliary Pancreat Dis Int 2016;15:39–47. [DOI] [PubMed] [Google Scholar]
- 29. Moon JS, Yoon JS, Won KC, Lee HW. The role of skeletal muscle in development of nonalcoholic fatty liver disease. Diabetes Metab J 2013;37:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim G, Lee SE, Lee YB, Jun JE, Ahn J, Bae JC, et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a 7‐year longitudinal study. Hepatology 2018;68:1755–1768. [DOI] [PubMed] [Google Scholar]
- 31. Issa D, Alkhouri N, Tsien C, Shah S, Lopez R, McCullough A, et al. Presence of sarcopenia (muscle wasting) in patients with nonalcoholic steatohepatitis. Hepatology 2014;60:428–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meng G, Wu H, Fang L, Li C, Yu F, Zhang Q, et al. Relationship between grip strength and newly diagnosed nonalcoholic fatty liver disease in a large‐scale adult population. Sci Rep 2016;6:33255. 10.1038/srep33255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trepo E, Valenti L. Update on NAFLD genetics: from new variants to the clinic. J Hepatol 2020;72:1196–1209. [DOI] [PubMed] [Google Scholar]
- 34. Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Caucasian lean subjects with non‐alcoholic fatty liver disease share long‐term prognosis of non‐lean: time for reappraisal of BMI‐driven approach? Gut 2022;71(2):382–390. [DOI] [PubMed] [Google Scholar]
- 35. Bhanji RA, Carey EJ, Yang L, Watt KD. The long winding road to transplant: how sarcopenia and debility impact morbidity and mortality on the waitlist. Clin Gastroenterol Hepatol 2017;15:1492–1497. [DOI] [PubMed] [Google Scholar]
- 36. Petta S, Ciminnisi S, Di Marco V, Cabibi D, Camma C, Licata A, et al. Sarcopenia is associated with severe liver fibrosis in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;45:510–518. [DOI] [PubMed] [Google Scholar]
- 37. Kim D, Wijarnpreecha K, Sandhu KK, Cholankeril G, Ahmed A. Sarcopenia in nonalcoholic fatty liver disease and all‐cause and cause‐specific mortality in the United States. Liver Int 2021;41:1832–1840. [DOI] [PubMed] [Google Scholar]
- 38. Klenk J, Rapp K, Ulmer H, Concin H, Nagel G. Changes of body mass index in relation to mortality: results of a cohort of 42,099 adults. PLoS ONE 2014;9:e84817. 10.1371/journal.pone.0084817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055–2065. [DOI] [PubMed] [Google Scholar]
- 40. Donini LM, Pinto A, Giusti AM, Lenzi A, Poggiogalle E. Obesity or BMI paradox? Beneath the tip of the iceberg. Front Nutr 2020;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of male participants stratified by handgrip strength
Table S2. Baseline characteristics of female participants stratified by handgrip strength
Table S3. The overall mortality of participants with NAFLD stratification by BMI category and handgrip strength (exclusion subjects who died within 1 year after survey).
Figure S1. Factors associated with the overall mortality of participants with NAFLD.


