Abstract
Background
Frailty and sarcopenia are age‐associated syndromes that have been associated with the risk of several adverse events, mainly functional decline and death, that usually coexist. However, the potential role of one of them (sarcopenia) in modulating some of those adverse events associated to the other one (frailty) has not been explored. The aim of this work is to assess the role of sarcopenia within the frailty transitions and mortality in older people.
Methods
Data from the Toledo Study of Healthy Aging (TSHA) were used. TSHA is a cohort of community‐dwelling older adults ≥65. Frailty was assessed according with the Frailty Phenotype (FP) and the Frailty Trait Scale‐5 (FTS5) at baseline and at follow‐up. Basal sarcopenia status was measured with the standardized Foundation for the National Institutes of Health criteria. Fisher's exact test and logistic regression model were used to determine if sarcopenia modified the transition of frailty states (median follow‐up of 2.99 years) and Cox proportional hazard model was used for assessing mortality.
Results
There were 1538 participants (74.73 ± 5.73; 45.51% men) included. Transitions from robustness to prefrailty and frailty according to FP were more frequent in sarcopenic than in non‐sarcopenic participants (32.37% vs. 15.18%, P ≤ 0.001; 5.76% vs. 1.12%; P ≤ 0.001, respectively) and from prefrailty‐to‐frailty (12.68% vs. 4.27%; P = 0.0026). Improvement from prefrail‐to‐robust and remaining robust was more frequent in non‐sarcopenic participants (52.56% vs. 33.80%, P ≤ 0.001; 80.18% vs 61.15%, P ≤ 0.001, respectively). When classified by FTS5, this was also the case for the transition from non‐frail‐to‐frail (25.91% vs. 4.47%, P ≤ 0.001) and for remaining stable as non‐frail (91.25% vs. 70.98%, P ≤ 0.001). Sarcopenia was associated with an increased risk of progression from robustness‐to‐prefrailty [odds ratio (OR) 2.34 (95% confidence interval, CI) (1.51, 3.63); P ≤ 0.001], from prefrailty‐to‐frailty [OR(95% CI) 2.50 (1.08, 5.79); P = 0.033] (FP), and from non‐frail‐to‐frail [OR(95% CI) 4.73 (2.94, 7.62); P‐value ≤ 0.001]. Sarcopenia does not seem to modify the risk of death associated with a poor frailty status (hazard ratios (HR, 95%) P > 0.05).
Conclusions
Transitions within frailty status, but not the risk of death associated to frailty, are modulated by the presence of sarcopenia.
Keywords: Frailty, Sarcopenia, Transitions, Death, Aging
Introduction
In old age, pathways leading to disability could be accelerated by certain conditions as frailty and sarcopenia. 1 , 2 , 3 Frailty is an age‐associated, biological syndrome characterized by decreased biological reserves and is associated with adverse outcomes (i.e. disability, institutionalization, death, and hospitalization). 4 In 2006, Gill and colleagues proved that frailty is a dynamic state that changes over time, mainly impairing, but also improving. 5 These results, confirmed in other longitudinal studies 6 , 7 , 8 and in a recent meta‐analysis, 9 opened an ample opportunity for the prevention of frailty and its consequences. 5 Moreover, some studies have suggested that different factors influence the frailty transitions (such as older age, previous diseases, 8 , 10 physical activity and mobility levels, 6 socio‐economic and clinical factors, 6 , 11 vitamin D levels, 11 or hospitalizations 8 ). A better knowledge of these factors and how they could modulate the frailty transitions would help to both refine the prognosis of frailty but also to develop effective strategies for the prevention and restoration of frailty, improving the quality of life of older people. 4
Sarcopenia, the loss of lean muscle mass and muscle strength and/or function, 2 has been proposed as the biological substrate of frailty. 12 Although frailty and sarcopenia could coexist and have been related as states of increased vulnerability due to degradation on multiple systems 2 , 13 , 14 , 15 and physical function impairment, 16 both entities have been clearly distinguished. 17
We previously described that only a minority of people with sarcopenia has frailty and, by opposite, that between a third and a quarter of frail people do not have sarcopenia, suggesting an association between sarcopenia and frailty beyond the simple coexistence of the two entities. 17 More recently, another group, using data from the Hertfordshire cohort study have reported similar findings in a cross‐sectional study. 18 This fact opens the possibility of different risks for the outcomes depending upon the coexistence of sarcopenia in frail patients, raising the chance of the existence of different clinical phenotypes, 17 but to date, no study has addressed this hypothesis.
Methods
Participant's data were taken from the Toledo Study of Healthy Aging (TSHA). This study was conformed according to the ethical standards defined in the 1964 Declaration of Helsinki and approved by the Clinical Research Ethics Committee of the Toledo Hospital, Spain. Participants signed an informed consent form previous to recruitment.
As detailed elsewhere previously, 19 TSHA is a longitudinal cohort aimed at studying different aging phenotypes through socio‐demographic, clinical and genetical variables and its relationship with physical and neuropsychological assessments and lifestyle components, such as physical activity, diet, tobacco, and alcohol consumption. TSHA was designed to study frailty prevalence and its underlying causes in rural and urban community‐dwelling older adults aged 65 years or older. Subjects from the cohort were selected by a two‐stage random sampling of the municipal census of the province of Toledo. Sampling was conducted within census sections in six strata according to sex, age and town‐size groups, recruiting 24% of the population aged 65 and older in the Toledo province.
For the purposes of the current study, data from basal (2011–2013) and follow‐up (2014–2017; median time of 2.99 years, range 2.0–5.4) face‐to‐face visits were analysed.
Study variables
Frailty status was assessed according to two established criteria: the Frailty Phenotype 1 and the Frailty Trait Scale 5 (FTS5) 20 at baseline and at follow‐up.
Frailty status
Fried scale
Frailty Phenotype (FP) was assessed according to its five criteria fitted to Spanish population 21 :
Weight loss: defined as self‐reported unintentional weight loss of ≥4.5 kg in the last year.
Exhaustion: was measured by self‐report using two questions: (‘How many days during the last week have you felt that anything you did was a big effort?’ and ‘How many times during the last week have you felt that you could not keep on doing things?’). The criterion was met when participant answered at a score of 2 or higher (0–4).
Weakness: handgrip strength was measured using JAMAR Hydraulic Hand Dynamometer (Sammons Preston Rolyan, Bolingbrook, IL). Best peak strength of three performances was selected and gathered using international standard procedures. 22 Between performances, at least 1 min of resting was permitted. Results was adjusted for sex and body mass index. Low grip strength ≤20th percentile.
Slowness: was defined using the 3 m walking test at their usual pace, according to the standard protocol. Best time of two performances was chosen. Cut‐offs were adjusted by sex and height.
Low physical activity: defined as being in the lowest quintile using the Physical Activity Scale for the Elderly (PASE) scale, 23 stratified by gender.
The stages of frailty were defined as robust or not frail in those whose score was 0. A score of 1 or 2 indicated that someone is pre‐frail. People were considered frail when they met three or more domains. Differences in the cut‐off points of the frailty criteria in the TSHA according to Fried's originals are shown in Supporting Information S1.
Frailty Trait Scale 5 (FTS5)
FTS5 20 is a recent tool developed and validated in the TSHA. It seems to improve the accuracy of the Frailty Phenotype to predict adverse events (death, hospitalization, incident frailty, and disability) 20 in older adults even better than classical frailty tools, as the two most used tool 24 : the Frailty Phenotype 1 and the Frailty Index. 25 In addition, it allows continuous assessment of frailty levels, being sensitive to small changes that have been shown to be related to the risk of different adverse events such as disability, hospitalization and mortality, potentially overcoming several of the pitfalls of previous frailty assessment. 26 It is composed by five domains [gait speed, grip strength, physical activity, body mass index (BMI), and balance].
Gait speed, handgrip strength, and physical activity were performed, as has been detailed previously, and scored according to the rules of this scale (Supporting Information S2).
BMI was estimated as body weight in kg (adjusted to the nearest 0.1) divided by height in meter squared. Height was measured using a stadiometer at head level to the nearest centimetre.
Balance was evaluated using the progressive Romberg test. 27 This battery test consists of testing the balance ability of the participant in three position (side‐by‐side, semi‐tandem and full‐tandem position), each one more challenging than previous and with the goal of maintaining balance for at least 10 s.
FTS5 score ranges from 0 to 50, being 0 the lowest frailty score and 50 the highest. Each of the five domains scores from 0 to 10. Scores to 25.25 or higher were considered as frail and lower as no frail.
Sarcopenia
Sarcopenia was measured at baseline and defined according to the Foundation for the National Institutes of Health (FNIH), fitted to the cut‐off points of our population (standardized FNIH [sFNIH]). 17 An individual was qualified as sarcopenic if he or she had a low muscle mass, in addition to a low gait speed and a grip strength below the cut‐off points.
Muscle mass was determined using dual‐energy X‐ray absorptiometry (DEXA) (Hologic, Serie Discovery QDR, Bedford, MA, USA). DEXA scans were analysed using the software Physician's Viewer (apex System Software, version 3.1.2: Bedford, USA).
BMI‐adjusted by appendicular lean mass (BMI/ALM), derived as the sum of the muscle mass of the arms and legs was finally determined. According to sFNIH diagnosis algorithm, low muscle mass was present in men and women when ALM/BMI is below 0.65 and 0.54, respectively.
Gait speed and handgrip strength measurement methodology has been explained previously. Gait speed cut‐off point was <0.8 m/s and handgrip strength cut‐off points were <25.51 kg for men and <19.19 kg for women.
Mortality
Vital status was ascertained by the Spanish National Death Index (Ministry of Health, Consumer Affairs and Social Welfare), hospital records, and phone contact during the study follow‐up. Mortality follow‐up time was right censored at 4 years. Median follow‐up time was 2.64 years (range from 0.60 to 4.00).
Co‐morbidity
Charlson index 28 was used to assess co‐morbidity.
Nutritional status
Mini‐Nutritional Assessment 29 was used to assess nutritional status. Participants were categorized according to their score as well‐nourished (≥24), at risk of malnutrition (17–23.5), or undernourished (<17). Due to the small number of undernourished subjects we merged this category with at‐risk of malnutrition in the same category.
Cognitive status
The Mini‐Mental State Examination 30 was used to evaluate the cognitive status. Participants were classified into two categories (normal cognitive status and cognitive impairment) according to their cut‐off point based on their educational level adjusted to the Spanish population. 31
Statistical analysis
Characteristics of the subjects at baseline were stratified according to frailty status and presence, or absence, of sarcopenia.
Descriptive statistics were shown as mean (standard deviation, SD) and number (N, %). Differences between sarcopenic and non‐sarcopenic were tested using Mann–Whitney and χ 2 test.
We used Fisher exact test to assess if transitions between frailty status were modified by the presence of sarcopenia.
The association between sarcopenia and basal frailty status with the outcomes were assessed using Cox proportional hazard model for death and logistic regression model for improvement, maintenance and worsening in frailty category: robust, prefrail and frail in FP; and no frail and frail in FTS5. We used two models. Model 1 was the univariate model. Then, we adjusted by age, gender and Charlson index (Model 2). Additionally, in a sensitivity analysis, we included to Model 2 cognitive or nutritional status as confounders.
Analyses were performed using the Statistical Package R version 3.6.1 for Windows (Vienna, Austria). Statistical significance was set at P‐value <0.05.
Results
Study population
There were 1538 participants (700 men) included in the analysis, with a mean age of 74.73 (5.73 SD) years old. Three hundred forty‐eight met criteria of sarcopenia according to the sFNIH. Participant's characteristics are shown in Table 1. Sarcopenia rates were statistically higher in individuals with frailty. While 77.64% (FTS5) and 56.82% (FP) of subjects with frailty were sarcopenic, only 16.19% (FTS5) and 15.02% (FP) of non‐frail participants met sarcopenic criteria. Moreover, frailty rates within the sarcopenic individuals were statistically higher than in non‐sarcopenic individuals (Supporting Information S3). The status of the participants was successfully assessed along the follow‐up in 1349 subjects (87.71%). Participants lost for the follow‐up did not show any difference in their baseline characteristics regarding age (P‐value 0.904), gender (P‐value 0.348), Charlson index (P‐value 0.792), frailty (FP: P‐value 0.718; FTS5: P‐value 0.775), and sarcopenia (P‐value 0.331) stata.
Table 1.
Characteristics of the participants
| Frailty Phenotype | FTS5 | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Robust Fried | Prefrail Fried | Frail Fried | P‐value | No frail | Frail | P‐value | |
| N (%) | 1,538 | 1,058 (68.79) | 436 (28.35) | 44 (2.86) | 1,377 (89.53) | 161 (10.47) | ||
| Age (SD) | 74.73 (5.73) | 73.55 (5.19) | 76.99 (6.01) | 80.70 (4.95) | <0.001 | 74.18 (5.48) | 79.42 (5.74) | <0.001 |
| Gender, male (%) | 700 (45.51) | 477 (45.09) | 200 (45.87) | 23 (52.27) | 0.78 | 661 (48.00) | 39 (24.22) | <0.001 |
| Charlson index (SD) | 1.18 (1.59) | 1.01 (1.42) | 1.52 (1.83) | 1.89 (2.27) | <0.001 | 1.13 (1.55) | 1.65 (1.86) | <0.001 |
| Sarcopenia (%) | 348 (22.62) | 159 (15.02) | 164 (37.61) | 25 (56.82) | <0.001 | 223 (16.19) | 125 (77.64) | <0.001 |
| Cognitive impairment or dementia (%) | 241 (15.98) | 132 (12.67) | 89 (20.99) | 20 (47.62) | <0.001 | 184 (13.58) | 57 (37.25) | <0.001 |
| At‐risk of malnutrition and malnourished (%) | 368 (24.93) | 154 (15.13) | 182 (43.75) | 32 (76.19) | <0.001 | 282 (21.36) | 86 (55.13) | <0.001 |
Data are expressed as mean (SD) and frequency (%). In bold: P‐value < 0.05. N, number of the sample; FTS5, Frailty Trait Scale 5; SD, standard deviation.
Frailty transitions
Transitions in frailty status and mortality according to the presence or absence of sarcopenia at baseline are included in Table 2. Subjects who met sarcopenia criteria showed a higher probability to impair their frailty status, disregarding the tool used to assess their condition. When FP was used, people with sarcopenia showed a higher percentage of transitions from robust to prefrail (32.37% vs. 15.18%; P ≤ 0.001) and frail (5.76% vs. 1.13%; P ≤ 0.001) and from prefrail to frail (12.68% vs. 4.27%; P = 0.003). When FTS5 was used, the percentage of those progressing from non‐frail to frail achieved the figure of 25.91% in those with sarcopenia, while it was 4.47% in those non‐sarcopenic (P ≤ 0.001). When we looked to those remaining in the same frailty status, we again observed the same trend. Those without sarcopenia had the highest probability of remaining robust than their sarcopenic counterparts (FP: 80.18% vs. 61.15%, P ≤ 0.001; FTS5: 91.25% vs. 70.98%, P ≤ 0.001).
Table 2.
Number and rates of transition in frailty status and mortality according to the presence or absence of sarcopenia
| Fried phenotype | Follow‐up group | |||||||
|---|---|---|---|---|---|---|---|---|
| Basal frailty state | Basal sarcopenia state | Robust | Prefrail | Frail | P‐value | Death | P‐value | Total |
| From robust | No sarcopenic to (%) | 639 (80.17) | 121 (15.18) | 9 (01.12) | <0.001 | 28 (03.51) | 0.13 | 797 |
| Sarcopenic to (%) | 85 (61.15) | 45 (32.37) | 8 (05.76) | 1 (00.72) | 139 | |||
| From prefrail | No sarcopenic to (%) | 123 (52.56) | 85 (36.32) | 10 (04.27) | <0.001 | 16 (06.84) | 0.13 | 234 |
| Sarcopenic to (%) | 48 (33.80) | 59 (41.55) | 18 (12.68) | 17 (11.97) | 142 | |||
| From frail | No sarcopenic to (%) | 2 (11.11) | 9 (50.00) | 2 (11.11) | 0.32 | 5 (27.78) | 0.89 | 18 |
| Sarcopenic to (%) | 3 (15.79) | 7 (36.84) | 4 (21.05) | 5 (26.32) | 19 | |||
| FTS5 | Follow‐up group | ||||||
|---|---|---|---|---|---|---|---|
| Basal frailty state | Basal sarcopenia state | No frail | Frail | P‐value | Death | P‐value | Total |
| From no frail | No sarcopenic | 939 (91.25) | 46 (04.47) | <0.001 | 44 (04.28) | 0.53 | 1,029 |
| Sarcopenic | 137 (70.98) | 50 (25.91) | 6 (03.11) | 193 | |||
| From frail | No sarcopenic | 8 (27.59) | 14 (48.28) | 1.00 | 7 (24.14) | 0.63 | 29 |
| Sarcopenic | 32 (30.19) | 56 (52.83) | 18 (16.98) | 106 | |||
Data are shown as number and percentage. In bold: p‐value <0.05. Percentage adds up to 100 horizontally. FTS5: Frailty Trait Scale 5.
Although most of these spontaneous transitions were toward a worse state of frailty (Figure 1), some individuals improved, especially those who did not meet the criteria for sarcopenia. In this line, a lower number of prefrail sarcopenic persons (according to the FP) improved in their frailty status (29.27% vs. 45.22%; P ≤ 0.001).
Figure 1.
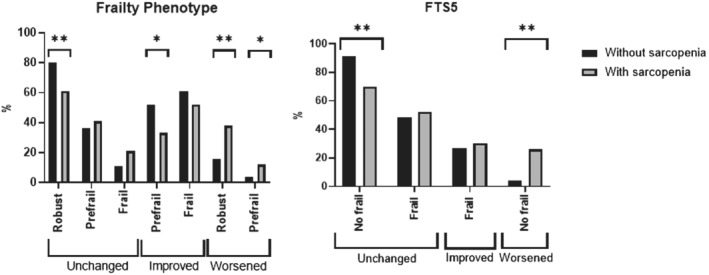
Rates of transitions in frailty status, assessed by FP and FTS5, according to the presence of sarcopenia. FP, frailty phenotype; FTS5, Frailty Trait Scale 5. *P < 0.005; **P < 0.001. Columns without symbol means non‐significant difference P ≥ 0.05.
Risk of worsening, maintenance or improving within the same frailty status at baseline according to the presence or absence of sarcopenia is shown in Table 3. Sarcopenic subjects were significantly more likely to worsen in their frailty status (FP: robust OR (95% CI) 2.34 (1.51; 3.63). P‐value < 0.001, prefrail OR (95% CI) 2.50 (1.08; 5.79), P‐value = 0.033; FTS5 non‐frail OR (95% CI) 4.73 (2.94; 7.62), P‐value < 0.001). Robust non‐sarcopenic subjects were significantly more likely to maintain their robustness regardless of the tool used. According to the FP, prefrail individuals with sarcopenia were more likely to maintain their prefrailty status or worsening while those without sarcopenia improved in their frailty status twice as much.
Table 3.
Risk of worsening, maintenance, and improvement in frailty status according to the presence or absence of sarcopenia
| Frailty tool | Basal frailty state | Basal sarcopenia state | Worsening | Staying the same | Improving | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |||
| Frailty Phenotype | Robust | No Sarcopenic | 1 | <0.001 | 1 | <0.001 | 3.06 (2.07; 4.53) | <0.001 | 2.48 (1.63; 3.75) | <0.001 | NA | NA | ||
| Sarcopenic | 3.06 (2.07; 4.53) | 2.34 (1.51; 3.63) | 1 | 1 | NA | NA | ||||||||
| Prefrail | No Sarcopenic | 1 | 0.002 | 1 | 0.033 | 1 | 0.14 | 1 | 0.018 | 2.09 (1.24; 3.52) | 0.001 | 2.09 (1.24; 3.53) | 0.006 | |
| Sarcopenic | 3.50 (1.56; 7.85) | 2.50 (1.08; 5.79) | 1.40 (0.90; 2.18) | 1.77 (1.10; 2.83) | 1 | 1 | ||||||||
| Frail | No Sarcopenic | NA | NA | 1 | 0.42 | 1 | 0.35 | 2.20 (0.33; 14.73) | 0.42 | 2.96 (0.41; 21.20) | 0.28 | |||
| Sarcopenic | NA | NA | 2.20 (0.33; 14.73) | 2.51 (0.37; 17.13) | 1 | 1 | ||||||||
| Frailty Trait Scale 5 | No frail | No Sarcopenic | 1 | <0.001 | 1 | <0.001 | 7.45 (4.80; 11.55) | <0.001 | 5.29 (3.30; 8.46) | <0.001 | NA | NA | ||
| Sarcopenic | 7.45 (4.80; 11.55) | 4.73 (2.94; 7.62) | 1 | 1 | NA | NA | ||||||||
| Frail | No Sarcopenic | NA | NA | 1 | 1.00 | 1 | 0.61 | 1 | 1.00 | 1 | 0.49 | |||
| Sarcopenic | NA | NA | 1.00 (0.38; 2.64) | 1.31 (0.47; 3.63) | 1.00 (0.38; 2.64) | 1.50 (0.48; 4.73) | ||||||||
In bold: P‐value <0.05. NA, not applicable. Model 1: univariate regression model. Model 2: adjusted by age and gender and Charlson index. OR, odds ratio; LL, lower limit; UL, upper limit. LL and UL were calculated according to 95% confidence interval.
In frail participants, people without sarcopenia had a higher possibility of improving frailty [FP: OR (95% CI) 2.96 (0.41; 21.20); FTS5: OR 1.50 (0.48; 4.73)] and those with sarcopenia of maintaining their frail condition [FP: OR 2.51 (0.37; 17.13); FTS5: OR 1.31 (0.47; 3.63)]. In no case, these increases in risk achieved statistical significance. Sensitivity analysis models adding cognitive and nutritional status are shown in the Supporting Information S4.
Mortality
Even though frailty was associated to a higher mortality (FP: robust 2.74%; prefrail 7.57%; frail 22.73%, P < 0.001; FTS5: no frail 3.63%; frail 15.53%, P < 0.001), there were no significant differences in mortality between sarcopenics and non‐sarcopenics within the same frailty status (Table 2). According to this finding, there were no statistically significant differences in the risk of mortality between sarcopenic and non‐sarcopenic older adults within the same frailty category (Table 4), as expected. These findings were not modified when nutritional and cognitive status were added to the model (Supporting Information S5).
Table 4.
Risk of death within the same frailty status according to the presence or absence of sarcopenia
| Frailty tool | Basal frailty state | Basal sarcopenia state | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |||
| Frailty phenotype | Robust | No Sarcopenic to (%) | 3.39 (0.82; 14.07) | 0.09 | 3.00 (0.71; 12.69) | 0.14 |
| Sarcopenic to (%) | 1 | 1 | ||||
| Prefrail | No Sarcopenic to (%) | 0.67 (0.37; 1.20) | 0.18 | 0.71 (0.38; 1.30) | 0.26 | |
| Sarcopenic to (%) | 1 | 1 | ||||
| Frail | No Sarcopenic to (%) | 2.29 (0.75; 7.00) | 0.15 | 1.50 (0.48; 4.63) | 0.48 | |
| Sarcopenic to (%) | 1 | 1 | ||||
| Frailty Trait Scale 5 | No frail | No Sarcopenic to (%) | 1.72 (0.79; 3.76) | 0.17 | 1.73 (0.78; 3.87) | 0.18 |
| Sarcopenic to (%) | 1 | 1 | ||||
| Frail | No Sarcopenic to (%) | 1.42 (0.63; 3.20) | 0.40 | 0.99 (0.43; 2.27) | 0.98 | |
| Sarcopenic to (%) | 1 | 1 | ||||
In bold: P‐value <0.05. NA, not applicable. Model 1: univariate regression model. Model 2: adjusted by age and gender. OR, odds ratio; LL, lower limit; UL, upper limit. LL and UL were calculated according to 95% confidence interval.
Discussion
This study directly addresses the relationship between sarcopenia and frailty regarding the prognosis of frailty. We show that sarcopenia seems to be a modulator of transitions in the frailty status: sarcopenic individuals had more than two‐fold the risk of non‐sarcopenics of worsen across the frailty continuum. In this continuum, sarcopenia was an independent predictor of frailty but not in mortality. These findings reinforce the hypothesis of the existence of different frailty phenotypes in which sarcopenia could be one of the major risk factors of developing functional decline along the frailty spectrum, with a less relevant role, if any, in the mortality associated to frailty.
One of the strengths of this study is the large community‐based population used. Moreover, frailty tools and sarcopenia diagnosis criteria used in this study have been standardized and adjusted to the study population prior to this analysis, showing a better predictive ability. 17 , 21 Furthermore, muscle mass has been determined by DEXA, which is considered the gold standard in assessing body composition. Finally, and to avoid biases linked to the tool used, frailty has been evaluated by using two different tools without detecting relevant differences in the findings depending upon the tool, thus reinforcing the strengths of the results. On the other hand, our study presents a reliable ascertainment of mortality. Using the Spanish National Death Index ensures that all the deaths occurring in the cohort are registered.
Some weaknesses can be found in our study, but they do not seem to significantly bias the results and/or the conclusions. Although grip strength and gait speed are used to assess both frailty and sarcopenia, a fact that could explain some of the overlapping between the two entities, the cut‐off points used for qualifying the participants as sarcopenic or frail are different, being higher for sarcopenia.
Another limitation regards the low prevalence of frailty when FP is used. This prevalence is lower to the one found in the whole TSHA cohort and is probably explained by the lower number of frail subjects who attended to the DEXA examination. The consequence of this low power is a decrease in the ability to detect some differences, making non‐significative some of our findings. In our study, this potential source of bias could account for the outcomes in frail subjects, a category only met by 44 individuals at baseline when using the frailty phenotype. The lack of significant differences impacts both changes in frailty status and the risk of death. Regarding death, although there is an increase in the percentage of deaths as the frailty status worsen from robust to prefrailty and frailty, there is no differences inside each category of frailty. Having measured frailty by two different tools makes this unlikely, taking into account that the amount of people qualified as frail using FTS5 is not so low (n = 161) and the number of events is high enough to make our results stable. The findings in our study are quite consistent and do not change depending upon the method of assessing frailty.
It must be highlighted that the design of our study suffers from a ceiling effect for those who are frail. As we are only assessing the frailty status, but not disability, this makes impossible for those who are frail to impair their functional condition toward disability.
Although sarcopenia is usually mentioned as a key factor in frailty and has been proposed as a biomarker to confirm frailty, 17 its role in the transitions of the frailty status has been broadly neglected. In fact, we have not found studies addressing the issue of how the presence of sarcopenia can influence the changes in the frailty status along the time in terms of worsening, improving or maintenance of that status. However, in a study assessing the role of muscle mass, by the determination of the Phase Angle, in the transition of frailty, they found a direct relationship between muscle mass and the improvement in the frailty status, 32 a finding supporting our results.
Although frailty generally increases with age, there is high variability between subjects, and not necessarily should increase over time. 9 , 26 According to the FP, a 57% of total participants remain in their basal frailty status. These results are similar to those found in other studies 5 , 7 or meta‐analysis. 9 Moreover, among those who started being prefrail, 23% improved in their frailty status and 18.2% worsened, with twice the risk of improvement in those prefrail subjects without sarcopenia, and twice the risk of worsening in sarcopenic ones. This higher prevalence of improvement in those who started being prefrail was found by Lee and colleagues after 2 years of follow‐up. 8 They showed that 23.4 and 26.6% of the prefrail men and women, respectively, improved in their frailty status; and 11.1 and 6.6% worsened.
Also, as we assessed subject at 3 years, there may be transitions in frailty status that we are not capturing because of the time between visits. Several studies in the literature have assessed the transitions in the frailty status, with separate times of observation and controversial results, not allowing to establish what is the best time to reassess it. 6 , 33 , 34
Unanswered questions
According to our results, it seems that the presence of sarcopenia influences the spontaneous transition of frailty over time, suggesting the existence of different clinical phenotypes (sarcopenic vs. non‐sarcopenic) with different prognosis within the framework of frailty. 17 The existence of some other phenotypes of frailty with different origin and course has been proposed by other authors recently. 35 , 36 , 37 These different phenotypes raise the need of studying the different pathogenic pathways leading to each of them, but also the need of doing further research for identifying risk 8 , 11 and protective 11 factors, and looking for different diagnostic and therapeutic approaches according to these different phenotypes, with the final aim of providing a most personalized and accurate clinical framework for prevention, detection, and intervention of frailty, 38 thus contributing to healthy aging.
Conclusions
These results show sarcopenia as a modulator of frailty suggesting the existence of two different clinical phenotypes of frailty (with and without sarcopenia) associated to different prognosis. This raises the need of assessing sarcopenia as a second step after diagnosing frailty in the daily clinical management of this condition.
Conflict of interest
Authors have no conflict of interest to declare.
Funding
This work was supported by grants from the Spanish Ministry of Economy, Industry and Competitiveness, cofinanced by the European Regional Development Funds and the Centro de Investigación Biomédica en Red en Fragilidad y Envejecimiento Saludable (CB16/10/00464), and the Papel de la disfunción MITOcondrial en la relación entre multimorbilidad crónica y deterioro FUNcional en ancianos. El Proyecto MITOFUN, Fundación Francisco Soria Melguizo (Section 2/2020). The research leading to these results has also received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115621, resources of which are composed of financial contribution from the European Union Seventh Framework Programme (FP7/2007‐2013) and EFPIA companies' in‐kind contribution. This funding sources had no role in the design of the study, its execution, analyses and data interpretation or decision to submit results.
Supporting information
Supporting Information S1. Differences in the cut‐off points of the criteria for defining frailty in the TSHA according to Fried's originals.
Supporting Information S2. Frailty Trait Scale 5 (FTS5) score.
Supporting Information S3. Characteristics of participants according to the presence or absence of sarcopenia.
Supporting Information S4. Risk of worsening, maintenance, and improvement in frailty status according to the presence or absence of sarcopenia, nutritional and cognitive status.
Supporting Information S5. Risk of death within the same frailty status according to the presence or absence of sarcopenia and cognitive and nutritional status.
Acknowledgements
We would like to thank the participants, cohort members and team researcher members. We certify that we comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 39
Álvarez‐Bustos A., Carnicero‐Carreño J. A., Davies B., Garcia‐Garcia F. J., Rodríguez‐Artalejo F., Rodríguez‐Mañas L., and Alonso‐Bouzón C. (2022) Role of sarcopenia in the frailty transitions in older adults: a population‐based cohort study, Journal of Cachexia, Sarcopenia and Muscle, 13, 2352–2360, 10.1002/jcsm.13055
References
- 1. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the E.G. for E.: Sarcopenia: revised European consensus on definition and diagnosis. Age and Aging 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez‐Mañas L, Fried LP. Frailty in the clinical scenario. Lancet 2015;385:e7–e9. [DOI] [PubMed] [Google Scholar]
- 4. Rodríguez‐Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko‐Zajko W, et al. Searching for an Operational Definition of Frailty: A Delphi Method Based Consensus Statement. The Frailty Operative Definition‐Consensus Conference Project. The Journals of Gerontology: Series A 2013;68:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community‐living older persons. Arch Intern Med 2006;166:418–423. [DOI] [PubMed] [Google Scholar]
- 6. Ahmad NS, Hairi NN, Said MA, Kamaruzzaman SB, Choo WY, Hairi F, et al. Prevalence, transitions and factors predicting transition between frailty states among rural community‐dwelling older adults in Malaysia. PLOS ONE 2018;13:e0206445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bentur N, Sternberg SA, Shuldiner J. Frailty transitions in community‐dwelling older people. Israel Medical Association Journal 2016;18:449–453. [PubMed] [Google Scholar]
- 8. Lee JSW, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community‐living older adults and their associated factors. J Am Med Dir Assoc 2014;15:281–286. [DOI] [PubMed] [Google Scholar]
- 9. Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community‐dwelling older people: A systematic review and meta‐analysis. Ageing Res Rev 2019;50:81–88. [DOI] [PubMed] [Google Scholar]
- 10. Pollack LR, Litwack‐Harrison S, Cawthon PM, Ensrud K, Lane NE, Barrett‐Connor E, et al. Patterns and Predictors of Frailty Transitions in Older Men: The Osteoporotic Fractures in Men Study. J Am Geriatr Soc 2017;65:2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trevisan C, Veronese N, Maggi S, Baggio G, Toffanello ED, Zambon S, et al. Factors Influencing Transitions Between Frailty States in Elderly Adults: The Progetto Veneto Anziani Longitudinal Study. J Am Geriatr Soc 2017;65:179–184. [DOI] [PubMed] [Google Scholar]
- 12. Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Bernabei R, et al. Sarcopenia as the Biological Substrate of Physical Frailty. Clin Geriatr Med 2015;31:367–374. [DOI] [PubMed] [Google Scholar]
- 13. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. In The Lancet, Vol. 381. Lancet Publishing Group; 2013. p 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. J Gerontol A Biol Sci Med Sci 2004;59:M255–M263. [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez‐Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko‐Zajko W, et al. Appendix: Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition‐consensus conference project. J Gerontol A Biol Sci Med Sci 2013;68:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: Two sides of the same coin. Front Aging Neurosci 2014;6. 10.3389/fnagi.2014.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies B, García F, Ara I, Artalejo FR, Rodriguez‐Mañas L, Walter S. Relationship Between Sarcopenia and Frailty in the Toledo Study of Healthy Aging: A Population Based Cross‐Sectional Study. J Am Med Dir Assoc 2018;19:282–286. [DOI] [PubMed] [Google Scholar]
- 18. Laskou F, Fuggle NR, Patel HP, Jameson K, Cooper C, Dennison E. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle 2022;13:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia‐Garcia FJ, Gutierrez Avila G, Alfaro‐Acha A, Amor Andres MS, de La Torre Lanza MDLA, Escribano Aparicio M v, et al. The prevalence of frailty syndrome in an older population from Spain. the Toledo study for healthy aging. Journal of Nutrition, Health and Aging 2011;15:852–856. [DOI] [PubMed] [Google Scholar]
- 20. García‐García FJ, Carnicero JA, Losa‐Reyna J, Alfaro‐Acha A, Castillo‐Gallego C, Rosado‐Artalejo C, et al. Frailty Trait Scale‐Short Form: A Frailty Instrument for Clinical Practice. J Am Med Dir Assoc 2020;21:1260–1266. [DOI] [PubMed] [Google Scholar]
- 21. Alonso Bouzón C, Rodríguez‐Mañas L, Carnicero JA, García‐García FJ, Turín JG, Rodríguez‐Mañas L, et al. The Standardization of Frailty Phenotype Criteria Improves Its Predictive Ability: The Toledo Study for Healthy Aging. J Am Med Dir Assoc 2017;18:402–408. [DOI] [PubMed] [Google Scholar]
- 22. Ottenbacher KJ, Branch LG, Ray L, Gonzales VA, Peek MK, Hinman MR. The reliability of upper‐ and lower‐extremity strength testing in a community survey of older adults. Arch Phys Med Rehabil 2002;83:1423–1427. [DOI] [PubMed] [Google Scholar]
- 23. Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol 1993;46:153–162. [DOI] [PubMed] [Google Scholar]
- 24. Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly‐cited instruments. Ageing Res Rev 2016;26:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 26. Álvarez‐Bustos A, Carnicero‐Carreño JA, Sanchez‐Sanchez JL, Garcia‐Garcia FJ, Alonso‐Bouzón C, Rodríguez‐Mañas L. Associations between frailty trajectories and frailty status and adverse outcomes in community‐dwelling older adults. J Cachexia Sarcopenia Muscle 2021. 10.1002/JCSM.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. New England Journal of Medicine 1995;332:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 29. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment short‐form (MNA‐SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009;13:782–788. [DOI] [PubMed] [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 31. Escribano‐Aparicio M, Pérez‐Dively M, García‐García F, Pérez‐Martín A, Romero L, Ferrer G, et al. Validación del MMSE de Folstein en una población española de bajo nivel educativo1. Semantic Scholar. Rev Esp Geriatr Gerontol 1999;34:319–326. [Google Scholar]
- 32. Zanforlini BM, Trevisan C, Bertocco A, Piovesan F, Dianin M, Mazzochin M, et al. Phase angle and metabolic equivalents as predictors of frailty transitions in advanced age. Exp Gerontol 2019;122:47–52. [DOI] [PubMed] [Google Scholar]
- 33. Xue Q, Bandeen‐Roche K, Tian J, Kasper JD, Fried LP. Progression of Physical Frailty and the Risk of All‐Cause Mortality: Is There a Point of No Return? J Am Geriatr Soc 2021;69:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nari F, Jang BN, Youn HM, Jeong W, Jang SI, Park EC. Frailty transitions and cognitive function among South Korean older adults. Sci Rep 2021;11. 10.1038/S41598-021-90125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu LK, Guo CY, Lee WJ, Chen LY, Hwang AC, Lin MH, et al. Subtypes of physical frailty: Latent class analysis and associations with clinical characteristics and outcomes. Sci Rep 2017;7. 10.1038/srep46417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang S, Tange C, Otsuka R, Nishita Y, Peng L, Hsiao F, et al. Subtypes of physical frailty and their long‐term outcomes: a longitudinal cohort study. J Cachexia Sarcopenia Muscle 2020;jcsm.12577. 10.1002/jcsm.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pamoukdjian F, Laurent M, Martinez‐Tapia C, Rolland Y, Paillaud E, Canoui‐Poitrine F. Frailty Parameters, Morbidity and Mortality in Older Adults with Cancer: A Structural Equation Modelling Approach Based on the Fried Phenotype. J Clin Med 2020;9:1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Landi F, Cesari M, Calvani R, Cherubini A, Di Bari M, Bejuit R, et al. The “Sarcopenia and Physical fRailty IN older people: multi‐componenT Treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res 2017;29:89–100. [DOI] [PubMed] [Google Scholar]
- 39. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1. Differences in the cut‐off points of the criteria for defining frailty in the TSHA according to Fried's originals.
Supporting Information S2. Frailty Trait Scale 5 (FTS5) score.
Supporting Information S3. Characteristics of participants according to the presence or absence of sarcopenia.
Supporting Information S4. Risk of worsening, maintenance, and improvement in frailty status according to the presence or absence of sarcopenia, nutritional and cognitive status.
Supporting Information S5. Risk of death within the same frailty status according to the presence or absence of sarcopenia and cognitive and nutritional status.


