Abstract
The compound β‐hydroxy‐β‐methyl butyrate (HMB) is proposed to increase or mitigate the loss of skeletal muscle and improve muscle function. We undertook a review of systematic reviews of HMB supplementation to promote gains or mitigate muscle loss in ageing and clinical populations. Following PRISMA guidelines, we searched for systematic reviews reporting the effect of HMB in our target populations. Dual‐energy X‐ray absorptiometry (DXA) measured lean soft‐tissue mass (LSTM) was accepted as a proxy for muscle. We identified 15 systematic reviews that met our inclusion criteria, which were independently evaluated. The methodological quality of the reviews was assessed using A Measurement Tool to Assess Systematic Reviews (AMSTAR), and standardized effectiveness statements were generated. Five of 15 studies found some evidence that HMB augmented LSTM; the remaining 10 studies reported some evidence favouring no difference (6/10 studies) or insufficient evidence to determine an effect (4/10 studies). Of the 12 studies that evaluated strength, 4/12 found some evidence, 5/12 found some evidence of no effect with one article finding some evidence in favour of patients in peri‐hospitalized and no evidence for those that are community‐dwelling, 4/12 had insufficient evidence to determine an effect, and 1/12 had insufficient evidence. No]study reported a positive effect of HMB on physical function; however, 2/10 studies found some evidence favouring no effect, and 7/10 studies reported insufficient evidence to determine an effect. The effectiveness of HMB supplementation in augmenting LSTM was heterogeneous, with most reviews finding no effect or inconclusive evidence to determine an effect. Most reviews concluded that HMB supplementation did not affect strength outcome measures or studies were inconclusive. The current evidence is insufficient to assess the impact of HMB supplementation on functional outcome measures. Our analysis shows minor, inconsistent support for HMB as part of an oral nutritional supplement or as a stand‐alone supplement (or combined with other amino acids) to increase or promote retention of LSTM, improve strength, and no evidence that it improves physical function in older persons or clinical populations.
Keywords: Sarcopenia, Muscle mass, Strength, Function, Supplement
Introduction
The compound β‐hydroxy‐β‐methyl butyrate (HMB) is a metabolite of the amino acid leucine formed in vivo through a series of enzyme‐catalysed reactions. In humans, the biosynthesis of HMB is rate limited, such that only an estimated 5% of leucine is converted to HMB. The branched‐chain amino acids (BCAA) act in a pro‐anabolic and anti‐catabolic manner, especially in skeletal muscle (for review, see Choudry et al. 1 ). These effects of BCAA are, however, predominantly (or solely) due to leucine, which is a potent stimulator of skeletal muscle protein synthesis (MPS) 2 , 3 and suppressor of muscle protein breakdown (MPB). 4
There are abundant data from cells, pre‐clinical models, and humans to indicate that HMB is a potent stimulator of MPS and inhibits MPB. Several early studies showed a positive impact of HMB supplementation in mitigating age‐related losses of lean mass in older persons, 5 , 6 older hospitalized patients, 7 , 8 and potentially in older patients receiving critical care. 9 There are numerous systematic reviews of HMB and its effectiveness in older persons in mitigating sarcopenia and in clinical practice to attenuate muscle loss or promote muscle gain. The main aim of this review was to conduct an umbrella review of these systematic reviews in which HMB was examined for its effects on older persons and clinical populations. We examined HMB as a compound alone or combined with macronutrients (usually as part of an oral nutritional supplement—ONS) and other amino acids to stimulate gains or mitigate losses in muscle mass. In most reports, it is not muscle mass that is measured but fat‐free and bone‐free lean soft‐tissue mass (LSTM), which is most often measured by dual‐energy X‐ray absorptiometry (DXA) or fat‐free mass (FFM) using bioelectrical impedance analysis (BIA). Hence, in this review, we accepted DXA‐measured LSTM and FFM as proxies for muscle mass. We also sought to determine the role of HMB in improving muscle strength or function, manifesting either as an improvement in mobility or physical function. Improvements in these outcomes would be beneficial for mitigating sarcopenia and improving outcomes in clinical populations. The quality of each systematic review was scored according to the 11‐item AMSTAR tool. 10 We also generated standardized effectiveness statements (i.e. sufficient evidence, some evidence, insufficient evidence, insufficient evidence to determine) about the treatment effect of the intervention(s) in the individual systematic reviews, based on methods previously outlined. 11 The quality of evidence (QoE) was subsequently evaluated using a method based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for primary evidence.
Methods
This review, along with searches and planned analyses, was registered on the International Platform of Registered Systematic Review and Meta‐analysis Protocols (INPLASY; https://inplasy.com/) as INPLASY2021100072 (https://inplasy.com/inplasy‐2021‐10‐0072/). We searched Embase, PubMed, and the Web of Science core collection (see supporting information for search strategies). The search was restricted to English‐language systematic reviews of HMB supplementation and was confined to humans. We included studies per the PICOS statement outlined in Table 1 and followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.
Table 1.
PICOS criteria for the inclusion of studies
| Criteria | Description | |
|---|---|---|
| Study Design | Is the study a systematic review? |
A. Only systematic reviews B. No narrative reviews are considered |
| Participants | Does the study involve older people or people with clinical conditions? |
Adults aged ≥50 years are considered Groups that may be covered: A. Healthy older adults B. Older adults within clinical populations C. Clinical populations |
| Intervention |
3. Does the study evaluate HMB interventions? 4. Does the study evaluate the mechanisms of HMB? 5. Are these interventions aimed at prevention or treatment of sarcopenia? 6. Are the interventions aimed at treating people losing muscle mass due to disease? 7. Are the interventions aimed at treating people losing muscle mass while in the ICU? |
HMB supplementation includes: A. Studies in which the effect of HMB supplementation is compared with no supplementation B. Studies in which HMB supplementation is added to an exercise program and compared with a control group of exercise without supplementation |
| Outcomes |
8. Does the study report effects on sarcopenia‐related outcomes? 9. Does the study report effects on ICU‐related outcomes? |
Relevant outcomes include: A. Muscle mass* B. Muscle strength C. Muscle endurance D. Flexibility E. Mobility F. Physical function G. Disability H. Function and participation |
Muscle mass or its proxies as LSTM, FFM (however derived).
The original search yielded 230 articles in August 2021, which, when screened by title and abstract, yielded 34 reports. These papers were retrieved and reviewed in greater detail yielding 14 systematic reviews (Figure 1) pertinent to the research question 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 25 , 26 and according to the PICOS statement (Table 1). One additional study was added, 24 totalling 15 studies. Two authors screened all reviews (K. J. L. and A. C. D.), and a third author checked their results (S. M. P.). Each report was scrutinized, data were extracted, reviewed by two authors (K. J. L. and A. C. D.), and re‐reviewed by a third (S. M. P.). Any disagreements over inclusion, scores, or criteria were settled by consensus amongst the three authors. Each review was given an AMSTAR score, which ranges from 1 to 11 and is based on common characteristics detailed previously. 10 The evidence was synthesized systematically to yield standardized effectiveness statements 11 (sufficient evidence, some evidence, insufficient evidence, insufficient evidence to determine; see supporting information) about the treatment effect of the intervention(s) in the individual systematic reviews. The QoE supporting each conclusion (see Table 2) was rated by using a method based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for primary evidence (1 = very low; 2 = low; 3 = moderate; or 4 = high). This method considers design issues, a meta‐analysis performed (yes or no), and the AMSTAR rating of the included systematic reviews 11 (see supporting information).
Figure 1.
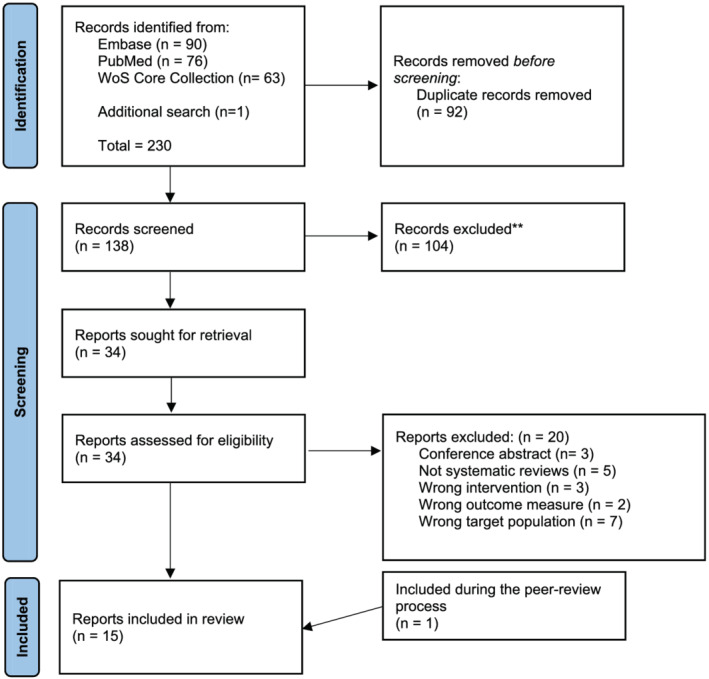
PRISMA flowchart of papers identified, screened, removed, and included in the review. WoS, Web of Science.
Table 2.
Summary of studies included
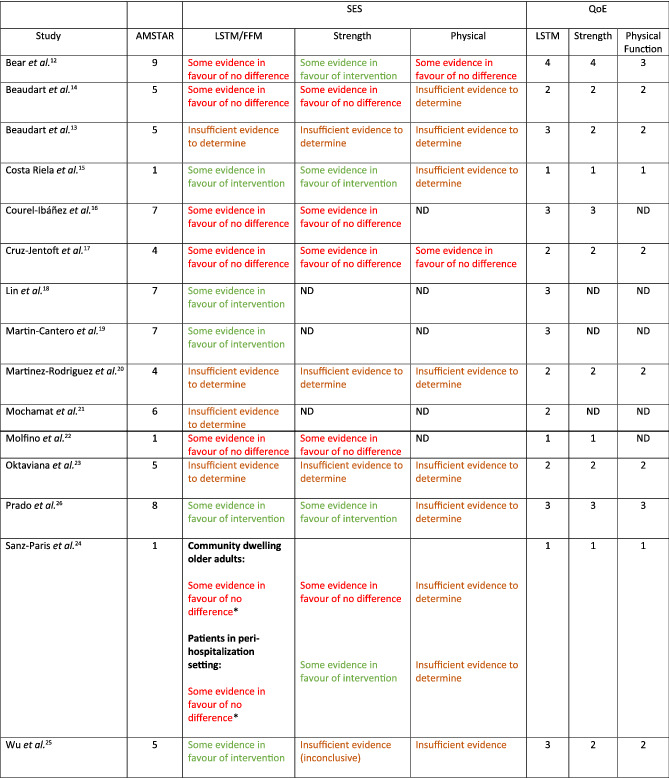
Applicable to favourable changes in body composition, not necessarily changes in LSTM.
A Measurement Tool to Assess Systematic Reviews (AMSTAR) rating, standardized effectiveness statements (SES), and quality of evidence (QoE) (1 = very low; 2 = low; 3 = moderate; or 4 = high); see supporting information for definitions. Green text indicates some evidence in favour of interventions. Brown text indicates some evidence in favour of no difference. Red text indicates insufficient evidence to determine (see supporting information to explain how the SES are derived).
Results
A total of 231 studies were screened for eligibility, 92 were removed as duplicates, 104 articles were excluded based on title and abstract screening, and 20 were excluded upon full‐text assessment (see supporting information). The 15 systematic reviews 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 that met our PICOS criteria (Table 1) were included in our analysis (for details of studies, see supporting information). AMSTAR scores for the included systematic reviews range from 1 to 9 (Figure 2, Table 2). The 15 systematic reviews examined the effects of HMB supplementation, either as part of an ONS or a stand‐alone supplement, on body composition (assessed by DXA or bioelectrical impedance analysis; BIA), strength and functional outcomes in older persons and in various clinical populations.
Figure 2.
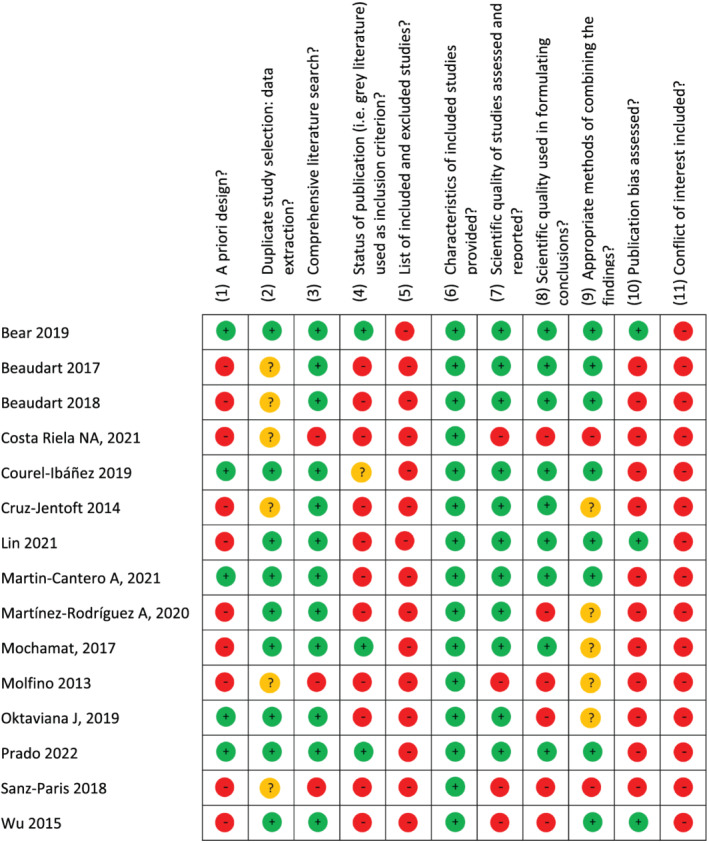
A Measurement Tool to Assess Systematic Reviews (AMSTAR) scores. ‘‐’ indicates no; ‘?’ indicates cannot answer/not applicable; and ‘+’ indicates yes for included reviews.
Body composition
All 15 reviews, ranging from very‐low quality of evidence (QoE: level 1) to high (level 4), looked at the influence of HMB supplementation on body composition measures. Six of the 15 reviews, one of high quality, 12 one of moderate quality, 16 two of low quality, 13 , 17 and two of very low quality, 22 , 25 provided evidence suggesting that HMB supplementation does not affect muscle mass (Table 2). A meta‐analysis carried out by Bear et al. 12 containing nine heterogeneous studies found a non‐significant effect of HMB supplementation, alone as part of an ONS, on what they defined as ‘skeletal muscle mass (either FFM or lean mass)’ (SMD = 0.25; 95% CI: −0.00, 0.50; z = 1.93; P = 0.05; QoE: level 4). In a meta‐analysis by Courel‐Ibanez et al., 16 they similarly found evidence supporting the argument that HMB supplementation has no significant effect on skeletal muscle mass, defined by the authors as FFM, appendicular skeletal muscle mass (ASMM), ASMM index and the muscle area, measured via CT, DXA, and BIA, (ES = 0.07; 95% CI: −0.69, 0.82; P = 0.833; QoE: level 3). Cruz‐Jentoft et al. 17 found that most RCTs included in their review (3/4) observed that HMB supplementation did not affect the prevention of muscle loss in frail/sarcopenic older adults (QoE: level 2). Beaudart et al. 13 highlighted that while muscle mass improved with exercise in 3/3 included RCT, an interactive effect of HMB was only found in 1/3 RCT (QoE: level 2). Sanz‐Paris et al. 25 found HMB supplementation to be associated with improvements in body composition in only 1/3 studies using community‐dwelling older adults and 2/5 studies using patients in peri‐hospitalized settings (QoE: level 1). Finally, Molfino et al. 22 emphasized that HMB supplementation did not affect body mass, FFM, or fat mass in 9/11, 6/10, and 7/8 of the included RCT, respectively (QoE: level 1). These authors 22 concluded that the heterogeneity of evidence and small numbers of subjects in the RCT did not warrant a meta‐analysis.
Despite the appearance from some systematic reviews that HMB supplementation does not affect LSTM/FFM/muscle mass, four moderate quality, 18 , 19 , 24 , 26 and one very‐low‐quality review, 15 supported the thesis that HMB supplementation does result in favourable changes in these variables. Martin‐Cantero et al. 19 carried out a meta‐analysis with three studies and found HMB (or CaHMB) plus essential amino acids (EAA) supplementation significantly increased LM/FFM (SMD = 0.522; 95% CI: 0.175, 0.868; P = 0.003) (QoE: level 3); however, it is difficult to know the role of HMB per se from that of added EAA. Prado et al. 24 reported that HMB supplementation was beneficial to prevent muscle mass loss in 3/4 studies (QoE: level 3). Nevertheless, in one of the three RCT, HMB was effective only in a sub‐analysis containing participants losing 2–5% bodyweight prior to starting the trial. 24 Wu et al. 26 showed in their meta‐analysis using six articles, and seven studies that CaHMB supplementation had a significant positive effect on what they labelled as muscle mass (SMD = 0.352 kg; 95% CI: 0.11, 0.594; z = 2.85; P = 0.004) (QoE: level 3). In a meta‐analysis by Lin et al., 18 the pooled results of eight articles (nine studies), found HMB supplementation to have a favourable effect on FFM (ES = 0.37; 95% Cl: 0.16, 0.58; z = 3.47; P = 0.001); however, subgroup analysis revealed that this significant effect was only present with HMB supplementation alone (ES = 0.59; 95% CI: 0.32, 0.87; z = 4.24; P < 0.001) and not when HMB was combined with an exercise intervention (ES = 0.06; 95% CI: −0.26, 0.38; z = 0.38; P = 0.705; QoE: level 3). Consistent with the results from other reviews, 18 , 19 , 24 , 26 Costa Riela et al. 15 too found that HMB supplementation significantly increased lean mass in 3/4 of their included studies (QoE: level 1).
The remaining four studies provided insufficient evidence to form a conclusion 14 , 20 , 21 , 23 (Table 2). For instance, Beaudart et al. 14 performed a meta‐analysis and found HMB supplementation to have no significant effect on muscle mass. However, due to the limited number (2) of studies included in this meta‐analysis, these results provide insufficient evidence to determine an effect.
All studies that looked at the effect of HMB supplementation on fat mass found no effect; QoE: level 1 22 QoE: level 3, 16 , 18 , 26 and QoE: level 4. 12
Strength
Twelve of the included systematic reviews looked at strength as an outcome measure. Five systematic reviews showed very‐low, 22 , 25 low, 13 , 17 and moderate 16 quality evidence supporting the notion that HMB does not affect muscular strength (Table 2). A meta‐analysis by Courel‐Ibanez et al. 16 found no significant difference in handgrip strength (ES = 0.19; 95% CI: −0.03, 0.40; P = 0.067, four studies) or leg strength (ES = −0.78; 95% CI: −3.16, 1.59; P = 0.291, three studies) between HMB supplemented and non‐supplemented groups (QoE: level 3). Beaudart et al. 13 (QoE: level 2), Cruz‐Jentoft et al. 17 (QoE: level 2), and Molfino 22 (QoE: level 1) showed similar results. The majority of RCT included in these reviews found that HBM supplementation did not affect muscular strength; 3/3, 13 3/4, 17 and 3/5, 22 respectively. Additionally, Sanz‐Paris et al. 25 found HMB supplementation to have no impact on hand‐grip strength in 2/3 studies conducted in community‐dwelling older adults (QoE: level 1).
Four systematic reviews 12 , 15 , 24 , 25 ranging from very low to high QoE found some evidence supporting the use of HMB supplementation to increase strength. A meta‐analysis carried out by Bear et al. 12 including six studies, found support for HMB supplementation to increase strength in clinical populations (SMD = 0.31; 95% CI: 0.12, 0.50; z = 1.95; P = 0.001; QoE: level 4). Only one of the studies included in this analysis looked at HMB supplementation alone, while the remaining RCT looked at HMB supplementation in combination with glutamine and arginine or incorporated into an oral nutrition supplement. Sanz‐Paris et al. 25 also found some evidence (2/3 studies) to support the use of HMB to improve strength in peri‐hospitalized patients (QoE: level 1). Prado et al. 24 found that two studies (2 non‐randomized trials) showed a statistically significant positive effect of HMB on hand‐grip strength in their systematic review (QoE: level 3). Costa Reila et al. 15 reported that the oral administration of CaHMB improved strength outcomes in 2/3 studies, whereas the one RCT found that HMB supplementation had no additional effect when paired with resistance exercise training (QoE: level 1). Three low‐quality reviews (QoE: level 2) 14 , 20 , 23 provided insufficient evidence to determine an effect, and one low‐quality review, Wu et al. 26 (QoE: level 2), reported inconclusive results with no effect present in 3/6 RCT included.
Functional outcomes
Ten of the included reviews investigated the effect of HMB supplementation on functional outcome measures. 12 , 13 , 14 , 15 , 17 , 20 , 23 , 24 , 25 , 26 Bear et al. (QoE: level 3), 12 and Cruz‐Jentoft et al. (QoE: level 2) 17 supported the null hypothesis that HMB supplementation has no impact on functional outcomes in older adult and clinical populations. Specifically, Bear et al. 12 found that 4/4 included RCT showed HMB supplementation to not affect functional outcome measures in chronic disease populations. 12 Cruz‐Jentoft et al. 17 highlighted that 3/4 studies included in their review found no effect of HMB supplementation on functional outcomes in frail or sarcopenic older adults (QoE: level 2). The eight remaining reviews provided insufficient evidence to determine an effect as they either included too few RCT to derive a conclusion (i.e. <3 RCT) 13 , 14 , 15 , 20 , 23 , 24 , 26 or, in the case of Wu et al., 26 they reported inconclusive results, with no effect present in 2/4 RCT included (QoE: level 2).
Discussion
We found inconsistent evidence that HMB supplementation, in various forms, augmented the gain or mitigated losses of LSTM. The quality of evidence provided for LSTM effects was variable, and effect sizes were small (i.e. <0.2). Hence, based on the best available evidence, there appears to be no clear consensus as to whether HMB supplementation can increase or prevent the loss of muscle mass (assessed by various proxies) in older persons or clinical populations. There was a clear consensus that HMB supplementation could not augment resistance training‐induced gains in muscle mass or strength. Importantly, only a few systematic reviews concluded that supplementation with HMB effectively promoted gains in strength. We found no evidence that HMB supplementation augmented physical function in older persons, and only one 24 review suggested an increment in muscle function (muscle strength and function combined) in cancer patients.
β‐Hydroxy‐β‐methyl butyrate supplementation to old or sarcopenic participants
We observed inconsistent and relatively low effect sizes reported for augmentation of gains or mitigation of loss of LSTM and FFM across the systematic reviews (Table 2). All reviews included RCT that used LSTM and FFM as an ostensible proxy outcome for skeletal muscle. While sarcopenia has been a concept for several decades, 27 its definition is still debated. 28 The main issue with defining sarcopenia is whether the inclusion of muscle mass (most usually lean LSTM), described as a core part of sarcopenia, 27 is still relevant. 28 Studies have compared the associations of grip strength and gait speed versus expert group definitions 28 , 29 , 30 , 31 for sarcopenia with falls and all‐cause mortality. 32 , 33 , 34 The findings showed that the association of the expert group definitions for sarcopenia with the outcomes were similar using grip strength and gait speed alone; however, including lean mass as well as grip strength or gait speed had neither a positive nor negative impact on the identification of individuals at risk for falls or all‐cause mortality. 32 , 33 , 34 Such findings raise a general question.
Interestingly, a recent Position Statement of the Sarcopenia Definition and Outcomes Consortium issued 13 statements on diagnosing sarcopenia but could not agree on whether LSTM should be included. 28 The main reason for the lack of consensus may be that LSTM, and more importantly, changes in this tissue compartment, are only a proxy for actual muscle mass. Muscle mass, when measured accurately, is associated with disability, poor physical function, hospitalization, and mortality. 35 , 36 The lack of consensus 28 as to whether LSTM is part of the sarcopenia paradigm is relevant in light of our findings relating to HMB, as it was most often the primary outcome of many of the reviews we analysed.
As the tissue substrate of sarcopenia, the relative preservation of LSTM in older adults would, seemingly, be advantageous. Numerous systematic reviews, including meta‐analyses, have concluded that LSTM (often labelled as muscle mass) is augmented to a small degree in older persons with ingestion of an HMB‐containing supplement (Table 2). Several reviews have concluded that supplementation with HMB has a small‐to‐moderate effect on gains in LSTM in older persons, 18 , 26 possibly restricted to women only, 20 and in frail older persons with sarcopenia, 23 but there were no changes in muscle function. Wu et al. 26 concluded that HMB supplementation resulted in an additional 352 g of muscle mass but used a small sample for their meta‐analysis (147 supplemented and 140 controls). Importantly, their result 26 was not muscle mass, but LSTM and the precision of effect that these authors report is implausible using DXA or any other method. Closer inspection of this analysis 26 showed that a single trial by Baier et al., 5 which was 12 months in duration, strongly influenced the outcome of greater LSTM in the HMB supplemented groups; however, despite greater LSTM retention, this was not associated with any significant improvement in muscle strength and functionality in the treatment group. 5
The effects of HMB do not add to those of any physical activity or exercise program in terms of gains in muscle strength and LSTM. 16 This finding 16 is not surprising as the effects of resistance exercise alone, as an anabolic stimulus, are difficult, if not impossible, to improve upon with non‐pharmaceutical supplements. Very few nutritional or nutraceutical interventions augment resistance exercise‐induced anabolic or anti‐catabolic effects, particularly in older persons, 37 except for creatine. 38 Resistance exercise training is a remarkably potent anabolic and anti‐catabolic stimulus for skeletal muscle 39 and improves functional strength and stability. 40
Our analysis of HMB supplementation, which could be summarized as finding inconsistent effects on LSTM and no clinically meaningful differences in strength and function, does not align with recommendations that HMB effectively mitigates any aspect of sarcopenia. However, in a review of studies from the European Working Group on Sarcopenia in Older People (EWGSOP) and the International Working Group on Sarcopenia (IWGS) 17 concerning exercise, the authors stated that ‘Some nutrition interventions such as EAA (with ∼2.5 g of leucine) and HMB may improve muscle parameters’. The summarized evidence in the present review does not support such a statement for HMB use.
β‐Hydroxy‐β‐methyl butyrate supplementation in clinical settings
Systematic reviews of HMB use in hospitalized patients have reported significant effects compared with placebo. For example, Bear et al. 12 conducted a systematic review (13 randomized controlled trials with 2137 patients; however, only nine studies (653 participants) had LSTM data, and reported that supplementation with HMB increased LSTM (labelled as ‘muscle mass’ by the authors) with a standardized mean difference (SMD) of 0.25 (95% confidence interval [CI] 0.00, 0.50; P = 0.05) versus placebo or usual care. Interestingly, the forms of HMB were HMB alone, HMB including the amino acids arginine and glutamine (HMB/Arg/Gln), and HMB in an ONS. In subgroup analyses, only the HMB/Arg/Gln subgroup of studies showed a statistically significant but likely clinically irrelevant effect (SMD = 0.49; 95% CI: −0.01, 0.99; P = 0.05). Notably, there were no significant effects when the study durations were <12 weeks. Also, it is important to realize that the patient groups included in this analysis 12 were highly heterogeneous (older care‐home residents receiving tube feeding, hospitalized older people with malnutrition/sarcopenia, hospitalized older people undergoing orthopaedic intervention, critically ill persons, cancer cachexia, HIV patients, maintenance haemodialysis, rheumatoid cachexia, gastric bypass, and bronchiectasis), which makes it problematic to ascribe outcomes to any one specific condition.
Focussing only on the studies that included hospitalized older patients with malnutrition/sarcopenia 7 , 41 and hospitalized older people undergoing orthopaedic intervention, 42 , 43 , 44 the effects of HMB were low to moderate for changes in LSTM. There were no significant effects of HMB on strength or physical function, 12 and these findings generally align with most of the reviews we analysed. Given the heterogeneity of the populations studied, the small effects, and the lack of translation of changes in LSTM to strength, functional outcomes or mobility (Table 2), there is little evidence to support a role for HMB in the treatment of older hospitalized patients.
One large multi‐centre randomized controlled trial of HMB as part of a high protein ONS was the NOURISH (Nutrition effect On Unplanned ReadmIssions and Survival in Hospitalized patients) study, a multicenter study prospective, parallel‐group study. 7 The patients enrolled were >65 years and treated for congestive heart failure, acute myocardial infarction, pneumonia, or chronic obstructive pulmonary disease. The primary endpoint of the trial was a composite of 90 day post‐discharge incidence of death and non‐elective readmission; however, the primary endpoint results were not different between HMB‐ONS (26.8%) and the standard of care group (i.e. ‘placebo’) (31.1%). Aside from the lack of difference in the primary endpoint, the results of this trial were impressive in that those patients that received the HMB‐ONS showed no between‐group differences for 90‐day readmission rate, but 90‐day mortality was significantly lower with HMB‐ONS relative to placebo (4.8% vs. 9.7%; relative risk 0.49, 95% CI, 0.27 to 0.90; P = 0.02). Hospital length of stay and all measured activities of daily living (ADL) were similar between treatments. The results of the NOURISH trial cannot, in our view, be ascribed to HMB. As highlighted, 45 the higher protein‐containing HMB‐ONS provided substantial protein and energy. Based on the real intake data, the HP‐HMB group ingested an additional ~30 g of protein per day (~0.5 g protein/kg/day) and ~525 kcal/day in hospital and during 30 days of follow‐up. 7 Oral nutritional supplements mitigate the risk of malnutrition in older hospitalized patients 45 , 46 , 47 and reduce surgical complications and postoperative infection 48 and mortality. 49 Hence, the substantial differences in energy and protein intake between the HMB‐ONS and placebo groups in this trial 7 may have been responsible for some or all of the observed effects.
As compounds with related metabolism, it is perhaps unsurprising that leucine and HMB act as agonists of many similar metabolic and signalling pathways. 52 , 53 Namely, leucine triggers a rise in MPS through Sestrin2, 54 , 55 as does HMB 52 ; however, there may be some potentially important differences between how the two compounds exert their mechanism of action, at least in neonatal pigs. 53 Wilkinson et al. showed that ingestion of equivalent quantities of leucine and the free acid form of HMB, which is more rapidly absorbed and has a greater concentration maximum than the calcium salt form of HMB, 56 , 57 resulted in almost identical rises in MPS. 50 Interestingly, the calcium form of HMB also has virtually identical effects on MPS as the free acid (Figure 3). As Figure 3 illustrates, the stimulatory effects of HMB and leucine on MPS are completely redundant on a g‐for‐g basis. The two forms of HMB, the free acid form 57 and calcium salt, 50 suppressed proteolysis by 46% and 31%, respectively. Leucine also has anti‐proteolytic effects that are likely mediated in part by the amino acid itself 4 and via the rise in insulin seen with leucine ingestion, 58 which is not seen with ingestion of HMB. 50 While the effects of insulin are permissive for MPS, the process of MPB is remarkably sensitive even to moderate hyperinsulinaemia 58 that occurs with either ingestion of leucine alone 50 or leucine enriched protein. 59
Figure 3.
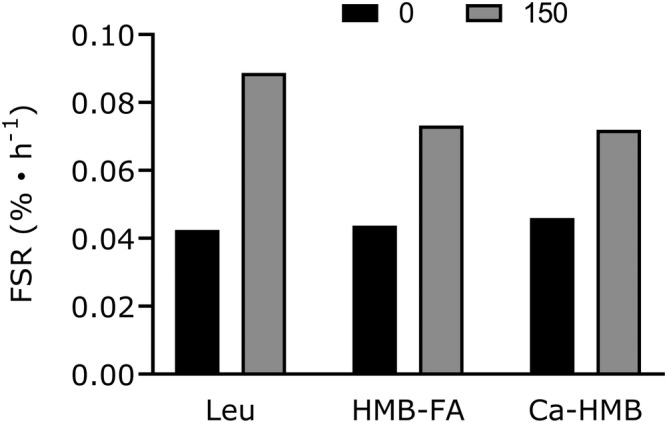
Comparative stimulation of muscle protein synthesis from resting (fasted) to 150 min post‐ingestion of leucine (3.42 g; Leu), HMB as a free acid (2.42 g; HMB‐FA), and HMB as a calcium salt (3.42 g; Ca‐HMB, equivalent to 2.72 g HMB‐FA). Data are from previous studies. 50 , 51 Values are means only.
In summary, our umbrella review of HMB supplementation in the treatment of sarcopenia and clinical practice revealed minor effects in mitigating the loss or promoting the retention of LSTM, with the evidence commonly scored as low (QoE = 2) or moderate quality (QoE = 3). Most reviews reported evidence of no effect or insufficient data to reach a definitive conclusion. The evidence regarding the effects of HMB supplementation on strength is conflicting with an equal number of reviews, most scored as very‐low (QoE = 1) or low quality (QoE = 2), pointing to no positive effect or insufficient data to conclude an effect. Supplementation with HMB shows no effects on physical function and an absence of data on the topic to provide further recommendations, with the evidence frequently scored as low‐quality (QoE = 2). Overall, more evidence is needed before HMB as a supplement, which appears mechanistically redundant with leucine in skeletal muscle in humans (Figure 3), can be recommended in managing sarcopenia or in patients in clinical care.
Conflict of interest
Dr. Phillips reports currently (or in the prior five years) held grants from the US National Dairy Council and a contract with Roquette during the conduct of the study; personal fees from US National Dairy Council, non‐financial support from Enhanced Recovery, outside the submitted work. In addition, Dr. Phillips has a patent Canadian 3052324 issued to Exerkine, and a patent US20200230197 pending to Exerkine but reports no financial gains. All other authors report no conflicts of interest.
Supporting information
Table S1 ‐ Standardized Effectiveness Statements, from [1]
Table S2 ‐ Method to rate the quality of the evidence (QoE) supporting each bottom‐line statement. From [1]
Table S3 ‐ Systematic reviews, including details, included in the analysis
Table S4 ‐ Papers screened, but not included and reason for exclusion
Table S5 ‐ Conflicts of interest and sources of funding reported by the systematic reviews included in the analysis
Acknowledgements
No specific sources of funding were used for this work. Everson A. Nunes is a Tier 2 research productivity fellow supported by the Brazilian National Council for Scientific and Technological Development (CNPq) grant number 308584/2019‐8. Stuart Phillips is Tier 1 Canada Research Chair and acknowledges the support from that award. Alysha D'Souza was supported by a Natural Science and Engineering Research Council of Canada GCS‐M award while completing this work.
Phillips S. M., Lau K. J., D'Souza A. C., and Nunes E. A. (2022) An umbrella review of systematic reviews of β‐hydroxy‐β‐methyl butyrate supplementation in ageing and clinical practice, Journal of Cachexia, Sarcopenia and Muscle, 13, 2265–2275, 10.1002/jcsm.13030
References
- 1. Choudry HA, Pan M, Karinch AM, Souba WW. Branched‐chain amino acid‐enriched nutritional support in surgical and cancer patients. J Nutr 2006;136:314–8s. [DOI] [PubMed] [Google Scholar]
- 2. Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin‐sensitive pathway. Journal of Nutrition 2000;130:2413–2419. [DOI] [PubMed] [Google Scholar]
- 3. Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV, Garlick PJ. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. Journal of Nutrition. 2009;139:1103–1109. [DOI] [PubMed] [Google Scholar]
- 4. Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, et al. A leucine‐supplemented diet restores the defective postprandial inhibition of proteasome‐dependent proteolysis in aged rat skeletal muscle. J Physiol 2005;569:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baier S, Johannsen D, Abumrad N, Rathmacher JA, Nissen S, Flakoll P. Year‐long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta‐hydroxy‐beta‐methylbutyrate (HMB), L‐arginine, and L‐lysine. JPEN J Parenter Enteral Nutr 2009;33:71–82. [DOI] [PubMed] [Google Scholar]
- 6. Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta‐hydroxy‐beta‐methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004;20:445–451. [DOI] [PubMed] [Google Scholar]
- 7. Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr 2016;35:18–26. [DOI] [PubMed] [Google Scholar]
- 8. Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, et al. Effect of beta‐hydroxy‐beta‐methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr 2013;32:704–712. [DOI] [PubMed] [Google Scholar]
- 9. Rahman A, Wilund K, Fitschen PJ, Jeejeebhoy K, Agarwala R, Drover JW, et al. Elderly persons with ICU‐acquired weakness: the potential role for β‐hydroxy‐β‐methylbutyrate (HMB) supplementation? JPEN J Parenter Enteral Nutr 2014;38:567–575. [DOI] [PubMed] [Google Scholar]
- 10. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gielen E, Beckwée D, Delaere A, De Breucker S, Vandewoude M, Bautmans I. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta‐analyses. Nutr Rev 2021;79:121–147. [DOI] [PubMed] [Google Scholar]
- 12. Bear DE, Langan A, Dimidi E, Wandrag L, Harridge SDR, Hart N, et al. β‐Hydroxy‐β‐methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta‐analysis. Am J Clin Nutr 2019;109:1119–1132. [DOI] [PubMed] [Google Scholar]
- 13. Beaudart C, Dawson A, Shaw S, Harvey NC, Kanis J, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017;28:1817–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beaudart C, Rabenda V, Simmons M, Geerinck A, Araujo De Carvalho I, Reginster JY, et al. Effects of Protein, Essential Amino Acids, B‐Hydroxy B‐Methylbutyrate, Creatine, Dehydroepiandrosterone and Fatty Acid Supplementation on Muscle Mass, Muscle Strength and Physical Performance in Older People Aged 60 Years and Over. A Systematic Review on the Literature. J Nutr Health Aging 2018;22:117–130. [DOI] [PubMed] [Google Scholar]
- 15. Costa Riela NA, Alvim Guimarães MM, Oliveira de Almeida D, Araujo EMQ. Effects of Beta‐Hydroxy‐Beta‐Methylbutyrate Supplementation on Elderly Body Composition and Muscle Strength: A Review of Clinical Trials. Ann Nutr Metab 2021;77:16–22. [DOI] [PubMed] [Google Scholar]
- 16. Courel‐Ibáñez J, Vetrovsky T, Dadova K, Pallarés JG, Steffl M. Health Benefits of β‐Hydroxy‐β‐Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta‐Analysis. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin Z, Zhao Y, Chen Q. Effects of oral administration of β‐hydroxy β‐methylbutyrate on lean body mass in older adults: a systematic review and meta‐analysis. Eur Geriatr Med 2021;12:239–251. [DOI] [PubMed] [Google Scholar]
- 19. Martin‐Cantero A, Reijnierse EM, Gill BMT, Maier AB. Factors influencing the efficacy of nutritional interventions on muscle mass in older adults: a systematic review and meta‐analysis. Nutr Rev 2021;79:315–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martínez‐Rodríguez A, Cuestas‐Calero BJ, Hernández‐García M, Martíez‐Olcina M, Vicente‐Martínez M, Rubio‐Arias J. Effect of Supplements on Endurance Exercise in the Older Population: Systematic Review. Int J Environ Res Public Health 2020;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mochamat CH, Marinova M, Kaasa S, Stieber C, Conrad R, et al. A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: a European Palliative Care Research Centre cachexia project. J Cachexia Sarcopenia Muscle 2017;8:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Molfino A, Gioia G, Rossi Fanelli F, Muscaritoli M. Beta‐hydroxy‐beta‐methylbutyrate supplementation in health and disease: a systematic review of randomized trials. Amino Acids 2013;45:1273–1292. [DOI] [PubMed] [Google Scholar]
- 23. Oktaviana J, Zanker J, Vogrin S, Duque G. The Effect of β‐hydroxy‐β‐methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J Nutr Health Aging 2019;23:145–150. [DOI] [PubMed] [Google Scholar]
- 24. Prado CM, Orsso CE, Pereira SL, Atherton PJ, Deutz NEP. Effects of β‐hydroxy β‐methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: a systematic review. J Cachexia Sarcopenia Muscle 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanz‐Paris A, Camprubi‐Robles M, Lopez‐Pedrosa JM, Pereira SL, Rueda R, Ballesteros‐Pomar MD, et al. Role of Oral Nutritional Supplements Enriched with β‐Hydroxy‐β‐Methylbutyrate in Maintaining Muscle Function and Improving Clinical Outcomes in Various Clinical Settings. J Nutr Health Aging 2018;22:664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, et al. Effect of beta‐hydroxy‐beta‐methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta‐analysis. Arch Gerontol Geriatr 2015;61:168–175. [DOI] [PubMed] [Google Scholar]
- 27. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990s–991s. [DOI] [PubMed] [Google Scholar]
- 28. Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc 2020;68:1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J AmMedDirAssoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85‐89 year old community‐dwelling men: a report from the ULSAM study. BMC Geriatr 2019;19:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayhew AJ, Phillips SM, Sohel N, Thabane L, McNicholas PD, de Souza RJ, et al. The impact of different diagnostic criteria on the association of sarcopenia with injurious falls in the CLSA. J Cachexia Sarcopenia Muscle 2020;11:1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han P, Chen X, Yu X, Zhang Y, Song P, Cai M, et al. The Predictive Value of Sarcopenia and Its Individual Criteria for Cardiovascular and All‐Cause Mortality in Suburb‐dwelling Older Chinese. J Nutr Health Aging 2020;24:765–771. [DOI] [PubMed] [Google Scholar]
- 35. Cawthon PM, Blackwell T, Cummings SR, Orwoll ES, Duchowny KA, Kado DM, et al. Muscle Mass Assessed by the D3‐Creatine Dilution Method and Incident Self‐reported Disability and Mortality in a Prospective Observational Study of Community‐Dwelling Older Men. J Gerontol A Biol Sci Med Sci 2021;76:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, et al. Strong Relation Between Muscle Mass Determined by D3‐creatine Dilution, Physical Performance, and Incidence of Falls and Mobility Limitations in a Prospective Cohort of Older Men. J Gerontol A Biol Sci Med Sci 2019;74:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta‐analysis and meta‐regression of the effect of protein supplementation on resistance training‐induced gains in muscle mass and strength in healthy adults. Br J Sports Med 2018;52:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults‐a meta‐analysis. Med Sci Sports Exerc 2014;46:1194–1203. [DOI] [PubMed] [Google Scholar]
- 39. McLeod JC, Stokes T, Phillips SM. Resistance Exercise Training as a Primary Countermeasure to Age‐Related Chronic Disease. Front Physiol 2019;10:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Talar K, Hernández‐Belmonte A, Vetrovsky T, Steffl M, Kałamacka E, Courel‐Ibáñez J. Benefits of Resistance Training in Early and Late Stages of Frailty and Sarcopenia: A Systematic Review and Meta‐Analysis of Randomized Controlled Studies. J Clin Med 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cramer JT, Cruz‐Jentoft AJ, Landi F, Hickson M, Zamboni M, Pereira SL, et al. Impacts of High‐Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double‐Blinded, Controlled Trial. J Am Med Dir Assoc 2016;17:1044–1055. [DOI] [PubMed] [Google Scholar]
- 42. Malafarina V, Uriz‐Otano F, Malafarina C, Martinez JA, Zulet MA. Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi‐centre randomized trial Maturitas 2017;101:42–50. [DOI] [PubMed] [Google Scholar]
- 43. Ekinci O, Yanık S, Terzioğlu Bebitoğlu B, Yılmaz Akyüz E, Dokuyucu A, Erdem Ş. Effect of Calcium β‐Hydroxy‐β‐Methylbutyrate (CaHMB), Vitamin D, and Protein Supplementation on Postoperative Immobilization in Malnourished Older Adult Patients With Hip Fracture: A Randomized Controlled Study. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition 2016;31:829–835. [DOI] [PubMed] [Google Scholar]
- 44. Nishizaki K, Ikegami H, Tanaka Y, Imai R, Matsumura H. Effects of supplementation with a combination of β‐hydroxy‐β‐methyl butyrate, L‐arginine, and L‐glutamine on postoperative recovery of quadriceps muscle strength after total knee arthroplasty. Asia Pac J Clin Nutr 2015;24:412–420. [DOI] [PubMed] [Google Scholar]
- 45. Reinders I, Volkert D, de Groot L, Beck AM, Feldblum I, Jobse I, et al. Effectiveness of nutritional interventions in older adults at risk of malnutrition across different health care settings: Pooled analyses of individual participant data from nine randomized controlled trials. Clin Nutr 2019;38:1797–1806. [DOI] [PubMed] [Google Scholar]
- 46. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev 2009;2009:Cd003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stratton RJ, Hébuterne X, Elia M. A systematic review and meta‐analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res Rev 2013;12:884–897. [DOI] [PubMed] [Google Scholar]
- 48. Liu M, Yang J, Yu X, Huang X, Vaidya S, Huang F, et al. The role of perioperative oral nutritional supplementation in elderly patients after hip surgery. Clin Interv Aging 2015;10:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hiesmayr M, Tarantino S, Moick S, Laviano A, Sulz I, Mouhieddine M, et al. Hospital Malnutrition, a Call for Political Action: A Public Health and NutritionDay Perspective. J Clin Med 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, et al. Effects of leucine and its metabolite beta‐hydroxy‐beta‐methylbutyrate on human skeletal muscle protein metabolism. J Physiol 2013;591:2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilkinson DJ, Hossain T, Limb MC, Phillips BE, Lund J, Williams JP, et al. Impact of the calcium form of beta‐hydroxy‐beta‐methylbutyrate upon human skeletal muscle protein metabolism. Clin Nutr 2018;37:2068–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suryawan A, Davis TA. Amino Acid‐ and Insulin‐Induced Activation of mTORC1 in Neonatal Piglet Skeletal Muscle Involves Sestin2‐GATOR2, Rag A/C‐mTOR, and RHEB‐mTOR Complex Formation. J Nutr 2018;148:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Suryawan A, Rudar M, Fiorotto ML, Davis TA. Differential regulation of mTORC1 activation by leucine and β‐hydroxy‐β‐methylbutyrate in skeletal muscle of neonatal pigs. J Appl Physiol 1985;2020:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar‐Peled L, et al. The Sestrins interact with GATOR2 to negatively regulate the amino‐acid‐sensing pathway upstream of mTORC1. Cell Rep 2014;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016;351:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fuller JC Jr, Sharp RL, Angus HF, Baier SM, Rathmacher JA. Free acid gel form of beta‐hydroxy‐beta‐methylbutyrate (HMB) improves HMB clearance from plasma in human subjects compared with the calcium HMB salt. Br J Nutr 2011;105:367–372. [DOI] [PubMed] [Google Scholar]
- 57. Fuller JC, Sharp RL, Angus HF, Khoo PY, Rathmacher JA. Comparison of availability and plasma clearance rates of β‐hydroxy‐β‐methylbutyrate delivery in the free acid and calcium salt forms. Br J Nutr 2015;114:1403–1409. [DOI] [PubMed] [Google Scholar]
- 58. Greenhaff PL, Karagounis L, Peirce N, Simpson EJ, Hazell M, Layfield R, et al. Disassociation between the effects of amino acids and insulin on signalling, ubiquitin‐ligases and protein turnover in human muscle. Am J Physiol Endocrinol Metab 2008;295:E595–E604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107:987–992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ‐ Standardized Effectiveness Statements, from [1]
Table S2 ‐ Method to rate the quality of the evidence (QoE) supporting each bottom‐line statement. From [1]
Table S3 ‐ Systematic reviews, including details, included in the analysis
Table S4 ‐ Papers screened, but not included and reason for exclusion
Table S5 ‐ Conflicts of interest and sources of funding reported by the systematic reviews included in the analysis


