Summary
Background
Vaccines are an important means to overcome the SARS-CoV-2 pandemic. They induce specific antibody and T-cell responses but it remains open how well vaccine-induced immunity is preserved over time following homologous and heterologous immunization regimens. Here, we compared the dynamics of humoral and cellular immune responses up to 180 days after homologous or heterologous vaccination with either ChAdOx1-nCoV-19 (ChAd) or BNT162b2 (BNT) or both.
Methods
Various tests were used to determine the humoral and cellular immune response. To quantify the antibody levels, we used the surrogate neutralization (sVNT) assay from YHLO, which we augmented with pseudo- and real virus neutralization tests (pVNT and rVNT). Antibody avidity was measured by a modified ELISA. To determine cellular reactivity, we used an IFN-γ Elispot, IFN-γ/IL Flurospot, and intracellular cytokine staining.
Findings
Antibody responses significantly waned after vaccination, irrespective of the regimen. The capacity to neutralize SARS-CoV-2 – including variants of concern such as Delta or Omicron – was superior after heterologous compared to homologous BNT vaccination, both of which resulted in longer-lasting humoral immunity than homologous ChAd immunization. All vaccination regimens induced stable, polyfunctional T-cell responses.
Interpretation
These findings demonstrate that heterologous vaccination with ChAd and BNT is a potent alternative to induce humoral and cellular immune protection in comparison to the homologous vaccination regimens.
Funding
The study was funded by the German Centre for Infection Research (DZIF), the European Union's “Horizon 2020 Research and Innovation Programme" under grant agreement No. 101037867 (VACCELERATE), the “Bayerisches Staatsministerium für Wissenschaft und Kunst” for the CoVaKo-2021 and the For-COVID projects and the Helmholtz Association via the collaborative research program “CoViPa”. Further support was obtained from the Federal Ministry of Education and Science (BMBF) through the “Netzwerk Universitätsmedizin”, project “B-Fast” and “Cov-Immune”. KS is supported by the German Federal Ministry of Education and Research (BMBF, 01KI2013) and the Else Kröner-Stiftung (2020_EKEA.127).
Keywords: Heterologous vaccination, COVID-19, vaccine, BNT162b2, ChAdOx1-nCoV-19, SARS-CoV-2, long-term, maintenance, T cell immunity, antibody avidity
Research in context.
Evidence before this study
Due to some rare severe side effects after the administration of the adenoviral vaccine, ChAdOx1 nCoV-19, many countries recommended a heterologous vaccination scheme including mRNA vaccines like BNT162b2 for the second dose. We performed a PubMed search (with no restrictions on time span) using the search terms “SARS-CoV-2” and “heterologous vaccination” and obtained 247 results. Only a fraction of manuscripts included direct comparisons of patient cohorts that received either a heterologous or a homologous vaccination regimen. Of those, the vast majority investigated only short-term immunogenicity after vaccination. Thus, little is known about the preservation of immunity by heterologous compared to homologous vaccination.
Added value of this study
We add a very comprehensive and comparative study investigating heterologous and homologous vaccination regimens early and late after vaccination. Key features include the number of patients (n = 472), the number of vaccination cohorts (n = 3), the fact that samples were derived from three independent study centers and comparative analyses were performed at two independent study centers, as well as in-depth investigation of humoral and T cellular immunity.
Implications of all the available evidence
The recent data creates a line of evidence that heterologous vaccination, compared to homologous vaccination regimens, results in at least non-inferior maintenance of humoral and cellular immunity. The enhanced understanding of immunity induced by individual vaccination regimens is crucial for further recommendations regarding the necessity, timing and choice of additional vaccinations and public health policies.
Alt-text: Unlabelled box
Introduction
The widespread use of safe and effective vaccines is essential for overcoming the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‑CoV‑2) pandemic. As of today, billions of doses of Coronavirus Disease 19 (COVID-19) vaccines, based on adenoviral vectors or mRNA, have been administered worldwide. In very rare cases, the administration of the adenoviral vector-based ChAdOx1-nCov-19 (ChAd) vaccine has been associated with the induction of a vaccine-induced thrombocytopenic thrombosis syndrome, particularly in young women.1 Consequently, the vaccination authorities of several countries recommended that persons under the age of 60 years who had received a primary dose of ChAd should receive an mRNA-based Covid-19 vaccine for the second immunization.2
We and others have previously shown that the heterologous combination of ChAd and mRNA vaccination results in a non-inferior or even superior humoral and cellular immune response compared to homologous mRNA or ChAd vaccination regimens.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 While homologous ChAd vaccination elicited a strong T-cell response shortly after the second immunization, antibody responses were inferior to homologous or heterologous regimens with mRNA vaccines. Furthermore, in the case of homologous vaccination regimens, various studies have shown a decline in antibody and T-cell levels a few months after the second dose.13 For heterologous vaccination regimens, however, follow-up data on how long B- and T-cell immunity persists are limited.14,15 This particularly applies to the immune response against newly emerged SARS-CoV-2 variants of concern (VoC) such as the Delta or Omicron mutant.8 Currently, it therefore remains unclear how the heterologous combination of ChAd and mRNA vaccination compares to homologous mRNA or ChAd vaccination in terms of persistence of humoral and cellular immunity.
Here, we examined humoral and cellular immunity in up to 472 participants from three different study centers at different time points before, and up to 180 days after heterologous and homologous vaccination with mRNA with BNT162b2 (BNT) and ChAd. While T-cell responses showed only modest contraction, significant waning of humoral immunity was observed over time in all three vaccination regimens. Compared to homologous vaccination with ChAd or BNT, the heterologous regimen generally resulted in more constant antibody responses both in terms of quantity and quality.
Methods
Study design and participants
The study is a follow-up analysis of 472 homologously or heterologously vaccinated participants that were previously only assessed for the production of antibodies using sVNT.5 A priori, no power analysis was performed on the number of participants. This was due to the fact that at that time there were only a very small number of people who could have been included according to the criteria, as well as the voluntary basis of participation.
Study participants (all of European Caucasian ethnicity) were divided into three different cohorts according to their vaccination regimen. Subjects of the two homologous groups received two doses of BNT or ChAd, respectively. In contrast, subjects of the third group received the heterologous vaccination regimen consisting of ChAd vaccine for the first and BNT for the second dose. Participants’ sera and peripheral blood mononuclear cells (PBMCs) were analyzed at two different study centers in Germany. Blood sampling schedules varied by study center and cohort. Not all tests were performed at every point in time. In general, four different points in time can be distinguished. Time point “before” is the initial time point at the day of the first vaccination. “Early after #1” refers to the moment of the second vaccination, “early after #2” corresponds in median 13 – 15 days after this vaccination and the “late after #2” time point analysis was carried out between 98 – 158 days in the median after the second vaccination, depending on the study center and vaccination regimen. The cohorts of homologous BNT and ChAd vaccinated people mainly include healthcare workers, whereas the heterologous vaccinated cohort did not comprise a specific professional group. Subjects were not pre-selected by the study team, but could voluntarily enroll for study participation. This was on condition that they had been vaccinated in accordance with the defined vaccination regimens. Accordingly, there was no selection bias by the study team. Subjects who reported SARS-CoV-2 infection at follow-up were excluded from the analysis. Likewise, those who were positive for N-specific antibodies after natural infection would have been excluded.
For longitudinal characterizations of the T-cell responses at the Munich study center (heterologous ChAd-BNT cohort), a separate cohort of vaccinees was included for the time points “early after #1”, “early after #2”, and “late after #2”. For longitudinal characterizations of the T-cell responses at the Erlangen study center (homologous BNT cohort), a separate cohort of vaccinees was included for the time points “before”, “early after #1”, and “early after #2” for contextualization. A detailed description of the cohorts can be found in Table 1.
Table 1.
Detailed representation of different study cohorts, separated by study center.
| Study Center Munich |
Study Center Erlangen |
||||
|---|---|---|---|---|---|
| BNT162b2 mRNA prime, BNT162b2 mRNA boost | ChAdOx1 nCoV-19 prime, BNT162b2 mRNA boost | BNT162b2 mRNA prime, BNT162b2 mRNA boost | ChAdOx1 nCoV-19 prime, ChAdOx1 nCoV-19 boost | ChAdOx1 nCoV-19 prime, BNT162b2 mRNA boost | |
| MUC n = 50 | CGN n = 50 | ERL n = 119 | ERL n = 52 | ERL n = 201 | |
| Volunteer source | Healthcare worker | General population at vaccination center | Healthcare worker | General population at vaccination center | General population at vaccination center |
| Age in years, median (IQR) [range] | 40.5 (32-52.75) [22-75] | 47 (33.25-55) [23-61] | 44 (30-54) [17-85] | 59 (46-64) [31-64] | 42 (33-52) [19-60] |
| Sex, n (%) Female | 31 (62%) | 37 (74%) | 81 (68.1%) | 35 (67.3%) | 127 (63.2%) |
| Sex, n (%) Male | 19 (38%) | 13 (26%) | 38 (31.9%) | 17 (32.7%) | 74 (36.8%) |
| Time from prime to second dose in days, median (IQR) [range] | 21 (20-22) [19-24] | 63 (63-64) [60-84] | 23 (21-25) [13-29] | 63 (63-63) [63-63] | 63 (63-63) [60-63] |
| Time from second dose to blood collection in days, median (IQR) [range] | Early: 13 (13-14) [11-16] Late: 98 (96-102) [91-158] |
Early: 15 (14-15) [13-15] Late: 110 (105,5-111) [104-113] |
Early: 14 (14-16) [10-36] Late: 158 (153-167) [140-180] |
Early: 13 (13-15) [13-16] Late: 142 (141-144) [140-144] |
Early: 14 (14-15) [12-17] Late: 142 (141-144) [140-146] |
| lost to follow-up, n | 4 | 7 | 0 | 0 | 0 |
MUC, Munich; CGN, Cologne; ERL, Erlangen; IQR: interquartile range.
Antibody response using surrogate virus neutralization assay
We used the iFlash-1800 CLIA Analyzer (YHLO Shenzhen, China) for the quantification of the antibody response. For the detection of neutralizing antibodies, we applied the iFlash-2019-nCoV NAb assay according to the manufacturer's instructions. The test principle is a competitive immunoassay. The iFlash-2019-nCoV NAb assay is only validated up to a level of 800 AU/ml according to the WHO standard. Therefore, all results exceeding this limit have been set to 800 AU/ml.
Antibody avidity
Binding strength of the SARS-Cov-2 IgG antibodies was determined by adaptation of the commercial IgG agile SARS-CoV-2 ELISA (Virion/Serion, Germany) using ammonium thiocyanate (NH4SCN) (Roth, Germany) as previously described.16, 17, 18 Serum samples were measured using the IgG agile SARS-CoV-2 ELISA and diluted to 100 U/mL according to the standard curve provided by the manufacturer to exclude an influence of variable antibody concentrations. Thereafter, serum samples were incubated in the plates pre-coated with Wuhan SARS-CoV-2-spike-ectodomain S1, S2 and RBD recombinant antigens for 1h at 37°C in a humid chamber. After washing, antigen-antibody complexes were incubated in the presence of 1.0 M ammonium thiocyanate or PBS as control for 10 min at room temperature. After washing to remove antibodies bound with low avidity, the ELISA was completed according to the manufacturer's instructions. The relative avidity index was calculated as follows: IgG concentrations (NH4SCN) / IgG concentrations (PBS) x 100, and is given in percent.
Real virus neutralization assay
Based on a previously established infection inhibition assay,16 VeroE6 cells (ATCC, US, RRID: CVCL_YQ49) were seeded in 10% fetal calf serum Dulbecco's Modified Eagles medium (Thermo Fisher Scientific, Germany) at 15,000 cells per well one day before incubation. Infection was started using SARS-CoV-2 at a multiplicity of infection (MOI) of 0.03 plaque-forming units (PFU) / cell. To detect virus-neutralization activity, serum samples were serially diluted 1:2 with DMEM starting from 1:20 up to a 1:2560 or 1:5120 dilution, respectively. SARS-CoV-2 virus (GISAID EPI ISL: 2772700 [Delta/ B1.617.2], 7808190 [Omicron/ B1.1.529 BA.1]) (480 PFU/15,000 cells/well) was added in a total volume of 50 µL at 37°C. After one hour of preincubation, the inoculum was transferred to the pre-seeded VeroE6 cells for another one-hour incubation at 37°C before the inoculum was replaced by supplemented DMEM. SARS-CoV-2 infection was terminated after 23 hours by adding 4% paraformaldehyde to fix the cells, and infection rate was analyzed by an in-cell ELISA.
After fixation, cells were washed with PBS and permeabilized with 0.5% saponin (Sigma-Aldrich, Germany). Blocking buffer, consisting of 0.1% saponin-10% goat serum (Sigma-Aldrich) in PBS, was added and incubated for one hour on fixed cells to avoid unspecific binding of antibodies. As a primary antibody, the SinoBiological anti-SARS-CoV-2-N T62 antibody (40143-T62, RRID:AB2892769) was used. The antibody was diluted with 1% FCS-PBS to 1:1500 ratio and 50µl were added in each well and incubated at room temperature for 2 hours. After washing, the second antibody was added. Goat anti-rabbit IgG2a-HRP antibody (EMD Millipore / order number 12-348, RRID:AB_390191) with 1% FCS-PBS was diluted to 1:4000 ratio. 50µl were added and incubated at room temperature for 1-2 hours. After the final washing step 100µl tetramethylbenzidine (TMB) were incubated for 20 min at room temperature. As final step 2M H2SO4 were added to stop the reaction. The result was quantified using optical detection with a Tecan Infinite 200 reader (TECAN, Switzerland) at 450 nm wavelength. The inhibition curve of each sample was analyzed by statistical analysis software Graph Pad Prism (GraphPad Software, USA), and 50% inhibitory concentration (IC50) was determined using non-linear regression.
FACS-based analysis of anti-S binding antibodies
A modified version of our previously published serological assay was used, in which HEK 293T (RRID:CVCL_4U22) cells either stably expressing the spike protein from the original Wuhan strain or transiently expressing the spike protein of B.1.167.2 or B1.1.529, respectively, were used as target cells.19 To quantify antigen-specific antibodies, 5 × 105 HEK 293T cells were incubated with serum samples diluted in 100 µl FACS-PBS (PBS with 0.5% BSA and 1 mM sodium azide) for 20 minutes at 4°C to bind to spike protein on the surface. After washing with 200 µl buffer, bound S-specific antibodies were detected with anti-human IgG-AF647 (4°C, 30 min incubation; clone HP6017, Biolegend, Cat #409320, RRID:AB_2563330). After further washing, samples were measured on an AttuneNxt (ThermoFisher) and analyzed using FlowJo software (Tree Star Inc.). A standard plasma sample with a defined concentration of 1,01mg/ml anti-SARS-CoV-2S IgG was used as reference control. The median fluorescence intensity (MFI) correlates with the level of bound antibodies.19
Pseudotype neutralization assay
Neutralization of the early D614G (WT) and the B1.617.2 variants was assessed with the help of spike-pseudotyped simian immunodeficiency virus particles as described before.20 To produce pseudotyped reporter particles, HEK293T cells were transfected with the SIV-based self-inactivating vector encoding luciferase (pGAE-LucW, RRID:Addgene_21375), the SIV-based packaging plasmid (pADSIV3,), and the respective spike variant-encoding plasmid as described previously.21
For the assessment of pseudotype neutralization, HEK293T-ACE2 cells were seeded at 2 × 104 cells/well in a 96well flat bottom plate. 24 h later, 60 µl of serial dilutions of the serum samples were incubated with 60 µl lentiviral particles for 1 h at 37°C. HEK293T cells were washed with PBS and the particle-sample mix was added to the cells. 48 h later, medium was discarded, and the cells washed twice with 200 µl PBS. Following 50µl PBS and 25µl ONE-Glo™ (Promega Corp, Madison, USA) was added and after 3 minutes the luciferase signal was assessed on a microplate luminometer (VICTOR X5, PerkinElmer) and analyzed using PerkinElmer 2030 Manager software. The reciprocal serum ID50 was determined with Prism GraphPad 9 (San Diego, California, USA) by application of the Sigmoidal 4PL function. For sera that did not reach neutralization by at least 50% at the highest serum dilution, the ID50 was set to the highest reciprocal serum dilution, namely 20.
Isolation and cultivation of peripheral blood mononuclear cell (PBMC)
PBMCs were isolated from citrate peripheral blood of vaccinated individuals by density gradient centrifugation using Biocoll® separating solution, density 1.077 g/ml (Bio&Sell) and frozen in heat-inactivated FCS + 10% DMSO (Sigma-Aldrich) for liquid nitrogen storage. Thawed PBMCs were cultured in complete RPMI medium (RPMI 1640 medium (Thermo Fisher) supplemented with 10% heat-inactivated FCS, 50 µM ß-Mercaptoethanol, 0.05 mg/ml gentamicin, 1.192 g/l HEPES, 0.2 g/l L-glutamine, and 100 U/ml penicillin-streptomycin) at 37°C and 5% CO2.
IFN-γ Enzyme-linked immunospot (ELISPOT)
Cryopreserved PBMCs were thawed and rested overnight at 1 × 106 cells/ml in complete RPMI medium. ELISPOT plates (Millipore) were coated with anti-human IFN-γ monoclonal antibody (clone 1-DIK, Mabtech) at 0.5 µg/well overnight at 4°C. Plates were washed with sterile PBS and subsequently blocked with complete RPMI medium for 1-2 h at 37°C. 400,000 PBMCs/well were seeded and stimulated with 11aa overlapping 15-mer PepMixTM SARS-CoV-2 spike glycoprotein peptide pool (1 µg/ml), provided in two peptide sub-pools S1 and S2 (JPT), for 20 h at 37°C. For the unstimulated condition, PBMCs were cultured in complete RPMI medium and respective dilution of solvent DMSO. As a positive control, PBMCs were stimulated with 25 ng/ml phorbol myristate acetate (PMA) (Sigma-Aldrich) and 1 µg/ml ionomycin (Sigma-Aldrich). Following this incubation, all steps were performed at room temperature. Plates were washed with PBS containing 0.05% Tween-20 (Sigma-Aldrich) and incubated with biotinylated anti-human IFN-γ monoclonal antibody (clone 7-B6-1, Mabtech, RRID:AB_907272) at 0.2 µg/well for 2 h. Following a second wash step with PBS containing 0.05% Tween-20, plates were incubated with an avidin-biotinylated peroxidase complex (VECTASTAIN® Elite ABC-HRP Kit, Vector Laboratories) for 1-2 h. After final washing steps with first PBS containing 0.05% Tween-20 and then PBS, plates were developed by the addition of AEC substrate solution (Sigma-Aldrich) for 15 minutes. Subsequently, plates were washed with water, dried for 24 h in the dark, and analyzed on an ImmunoSpot® Analyzer (Cellular Technologies Limited). A positive peptide-specific response was quantified by subtraction of mean spots of the unstimulated control and depicted as spot forming units (SFU)/106 PBMCs.
IFN-γ/IL-2 Fluorospot assay
Cryopreserved PBMCs were thawed and rested overnight at 2 × 106 cells/ml in complete RPMI medium. Human IFN-γ/IL-2 Fluorospot assays (CTL Europe, Germany) were performed according to the manufacturer's instructions. One day before the Fluorospot assays were performed, the plates were activated by adding 70% ethanol for less than one minute. Followed by a washing step and addition of IFN-γ/IL-2 capture antibodies overnight. After decanting the plate, 200,000 PBMCs/well were seeded and stimulated with 11aa overlapping 15-mer PepMixTMSARS-CoV-2 spike glycoprotein peptide pool (1 µg/ml), provided in two peptide sub-pools S1 and S2 (JPT), for 20 h at 37°C. As antigen-specific positive control, we used a CEF pool of in total 32 15mer peptides derived from Cytomegalovirus (5 peptides), Epstein-Barr virus (15 peptides), and Influenza virus (Flu) (12 peptides) proteins (National Institute for Biological Standards and Control (NIBSC), UK). For the unstimulated condition, PBMCs were cultured in complete RPMI medium. After the stimulation period, the plates were washed and 80 µL of anti-human IFN-γ (FITC, RRID:AB_2733588)/anti-human IL-2 (Hapten2,) detection antibody solution was added for 2h at room temperature. For the visualization of secreted cytokines, plates were washed and a tertiary solution including anti-FITC Alexa Fluor® 488 (visualizes IFN-γ) and anti-Hapten2 CTL-Red™ (visualizes IL-2) was added for one hour. The staining procedure was stopped by washing the plate. After drying the plates for 24h on paper towels on bench top, Fluorospot plates were scanned and analyzed using an automated reader system (ImmunoSpot Ultimate UV Image analyzer/ImmunoSpot 7.0.17.0 Professional DC Software, CTL Europe GmbH, Germany). Positive reactivity to experimental stimulatory agents was given when the spot count in antigen-stimulated cells was greater than twice the spot count in unstimulated (background) wells.
Intracellular cytokine staining (ICCS)
Cryopreserved PBMCs were thawed and rested overnight at 1 × 106 cells/ml in complete RPMI medium. 106 PBMCs were stimulated with spike glycoprotein peptide pool as described above for 20 h at 37°C in the presence of 1 µl/ml GolgiPlug™ (BD Biosciences). For the unstimulated condition, PBMCs were cultured in complete RPMI medium and respective dilution of solvent DMSO. As a positive control, PBMCs were stimulated with 25 ng/ml PMA and 1 µg/ml ionomycin. Following this incubation, all steps were performed at 4°C. PBMCs were washed twice with FACS buffer (PBS containing 0.5% BSA) and stained with ethidium-monoazide-bromide (EMA) (Thermo Fisher) for 15 minutes for live/dead discrimination. After two washing steps with FACS buffer, PBMCs were stained for surface markers CD8-eFluor450 (clone OKT8, Thermo Fisher, dilution 1:200, RRID:AB_2535439) and CD4-PE (clone RPA-T4, Thermo Fisher, 1:400, RRID:AB_1257144) for 20 minutes. Excess antibody was removed by two washing steps with FACS buffer followed by fixation/permeabilization using Cytofix/Cytoperm (BD Biosciences). PBMCs were washed twice with 1x Perm Wash buffer (BD Biosciences) and subsequently stained intracellularly for IL-2-APC (clone 5344.11, BD Biosciences, 1:20, RRID:AB_400574) and IFN-γ-FITC (clone 25723.11, BD Biosciences, 1:10, RRID:AB_400425) for 30 minutes. Following washing steps with first 1x Perm Wash buffer and then FACS-buffer, PBMCs were filtered through a nylon mesh and acquired on a LSRFortessaTM flow cytometer (BD Biosciences). A positive peptide-specific response was quantified by subtracting the mean frequency of IL-2 and IFN-γ double-positive T cells of the unstimulated control.
Ethics
Ethics approval was granted by the local ethics committees in Erlangen (Az. 340_21B) and Munich (Az. 26/21 and Az. 330/21 S). All subjects were informed about the study and confirmed their participation by means of an informed consent form.
Statistics
The following statistical tests were used:
Mann-Whitney test: Applied to all comparisons comparing a time point consisting of only two groups whose data sets were not paired and for which we could not assume a normal distribution due to sample size.
Kruskal-Wallis test followed by Dunn's multiple comparison test: Applied to all comparisons comparing a time point consisting of more than two groups whose data sets were not paired and for which we could not assume a normal distribution due to the sample size.
Wilcoxon test: Applied to all comparisons that compared two time points of a cohort and that had different sample sizes at time points one and two. The reason for this is the loss to follow-up.
Friedman test with multiple comparisons: Applied to all comparisons that compared two time points in a cohort that had identical sample sizes at time one and two.
Due to the a priori determination of the vaccination regimens, randomization and blinding were not possible. Sample size determination was limited by the number of available subjects, as well as the available testing capacity.
Data are presented with frequencies and percentages for categorial variables and with median, interquartile range (IQR) and range for continuous variables. Categorical data were compared using chi-squared test or Fisher's exact test as appropriate. Median comparison was performed with Mann-Whitney U test (2 categories) or Kruskal-Wallis H (3 categories) as appropriate. A linear regression model analysis was built in order to determine which variables had impact in the late IC50. In case any of these variables had a p value <0.1 in the univariable model it was included in the multivariable one. The multivariable model was performed with the backwards Wald methods. A p value ≤0.05 was considered statistically significant.
Statistics as well as figures were created with PRISM GraphPad 9.3.1 as well as IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY, United States.
Role of Funders
The funders did not interfere with the study design, data collection, data analysis, interpretation, or writing of report.
Results
Heterologous COVID-19 vaccination induced strong antibody responses which are superior or comparable to homologous mRNA vaccination regimens
We compared humoral and cellular immune responses in 472 healthy individuals in median 13 – 15 days (“early after #2”) and 98 – 158 days (“late after #2”) after heterologous or homologous vaccination with ChAd and BNT (Table 1). Previously, we had reported limited data on antibody responses early after second vaccination.5 Analyses were performed at the study centers in Munich (Munich and Cologne samples) and Erlangen (Erlangen samples) (Table 1). There are differences in sample collection time points and other study participant characteristics and only for the Erlangen study center exist data from all three vaccination regimens (Suppl. Table 1). Furthermore, a proportion analysis comparing the cohorts receiving different vaccination regimens revealed higher age and body mass index (BMI) for the ChAd-ChAd group (Suppl. Table 2). This might be due to the specific recommendation in Germany at that time to not use the ChAd vaccine in people under the age of 60. In light of the above-mentioned differences, the results were stratified for the two study sites.
We first assessed the quantities of antibody levels by sVNT (Figure 1). This assay correlates well with a real virus neutralization assay not only for the EU-strain SARS-CoV-2 D614G virus, as previously described,5 but also for the Delta VoC (Suppl. Figure 1). Regardless of the vaccination schedule, we observed significant (p < 0,0001 for all comparisons [Wilcoxon test]) waning of antibody levels in almost all individuals at the late time point.
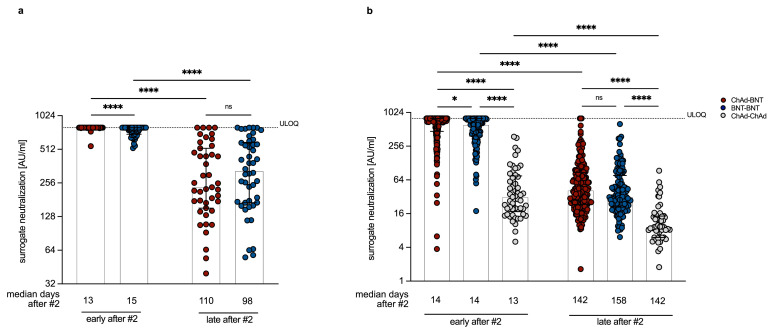
Figure 1.
Neutralizing antibody levels after heterologous ChAd-BNT vaccination are non-inferior compared to homologous vaccination regimens. Surrogate virus neutralization levels measured at the Munich (a) and Erlangen (b) study site after heterologous ChAd-BNT or homologous BNT-BNT or ChAd-ChAd vaccination. The sVNT is only validated up to a maximum of 800 AU/ml, therefore all values measured as greater than 800 AU/ml were set to 800 AU/ml. (a) „Early after #2“ refers to a median of 15 days (for BNT-BNT) or 13 days (for ChAd-BNT) after the second vaccination. „Late after #2“ refers to sampling at median 98 days (for BNT-BNT) or 110 days (for ChAd-BNT) after the second vaccination. n = 50 and 43 (ChAd-BNT; “early after #2” and „late “, respectively) and n = 50 and 46 (BNT-BNT; “early after #2” and „late after #2“, respectively). (b) „Early after #2“ refers to sampling at median 14 days (for BNT-BNT and ChAd-BNT) and 13 days (for ChAd-ChAd) after the second vaccination. „Late after #2“ refers to a median of 158 days (for BNT-BNT) or 142 days (for ChAd-BNT and ChAd-ChAd) after the second vaccination. n = 201 (ChAd-BNT), 119 (BNT-BNT) and 52 (ChAd-ChAd). ULOQ = upper limit of quantification (800 AU/ml). For inter-group statistics concerning one time point Mann-Whitney (a) or Kruskal-Wallis followed by Dunn's multiple comparisons test was used (b). Bars represent group medians, whiskers interquartile range. Over-time comparison within one group was done by Wilcoxon test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and n.s. indicates not significant. A detailed description of the data can be found in Supplemental Table for Figure 1a and b.
At the study center in Munich (Figure 1a), antibody neutralization capacity at the follow-up time point was significantly (p < 0,0001 [Wilcoxon test]) reduced compared to the time point early after second vaccination, but the remaining antibody levels were similar after ChAd-BNT (median = 234.65 AU/ml; n = 43) and BNT-BNT (median = 328.17 AU/ml; n = 46) vaccination. At an independent study center in Erlangen, these results were confirmed (Figure 1b). Furthermore, additional analyses of samples from a homologous ChAd-ChAd vaccination scheme cohort showed that neutralizing antibody levels late after homologous ChAd-ChAd vaccination (median of 9.32 AU/ml; n = 52) were still significantly (p < 0,0001 [Kruskal-Wallis test]) lower compared to homologous BNT-BNT (median = 31.74 AU/ml; n = 119) or heterologous ChAd-BNT (median = 41.72 AU/ml; n = 201) vaccination (Figure 1b). Thus, the heterologous ChAd-BNT vaccination regimen results in neutralizing antibody levels against SARS-CoV-2 WT virus which are as high as after homologous BNT-BNT and higher than after homologous ChAd-ChAd immunization. Using a linear regression analysis, we confirmed that neither body mass index, comorbidities, time intervals between the vaccinations, nor blood sampling significantly affected the neutralization levels at the late time points, whereas age (p = 0.022) and the vaccination regimen (p < 0.001) were significantly associated using a univariable model (Table 2). However, in the multivariable model age was not statistically significantly associated anymore (p = 0.206), leaving vaccination regimen as the only parameter in the model that is statistically significantly associated with the late IC50 values (p < 0.001). With this outcome, the model is not multivariable anymore, but it underlines the interpretation that the vaccination regimens strongly influence the IC50 values at late time points after 2nd vaccination.
Table 2.
Linear regression analysis for study center Erlangen.
| UNIVARIABLE MODEL |
MULTIVARIABLE MODEL |
|||||||
|---|---|---|---|---|---|---|---|---|
| p value | B | 95% CI for B |
p value | B | 95% CI for B |
|||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Vaccination regimen | <.001 | −30,630 | −45,973 | −15,287 | <.001 | −30,630 | −45,973 | −15,287 |
| Age | 0,022 | −1,045 | −1,936 | −0,154 | 0,206 | −0.590 | −1.507 | 0.327 |
| Sex | 0,210 | 15,112 | −8,556 | 38,780 | ||||
| BMI | 0,745 | 0,445 | −2,247 | 3,137 | ||||
| Comorbidities | 0,340 | −14,632 | −44,742 | 15,479 | ||||
| Time from prime to second dose | 0,586 | 0,169 | −0,441 | 0,778 | ||||
| Time to blood collection, early after 2nd | 0,366 | −1,884 | −5,972 | 2,205 | ||||
| Time to blood collection, late after 2nd | 0,664 | −0,261 | −1,443 | 0,921 | ||||
Serum neutralization capacity of variants of concern is superior after heterologous vaccination
To investigate potential differences in serological responses between the different cohorts in more detail, we next applied rVNT for the most relevant SARS-CoV-2 VoC. At the Munich study center, neutralization of VoC B.1.617.2 (Delta) and B.1.1.529 (Omicron) was investigated using sera collected 13 – 15 days and 98 – 110 days in median after heterologous ChAd-BNT or homologous BNT-BNT vaccination (Figure 2a). Early after the second vaccine dose, heterologous ChAd-BNT vaccination resulted in significantly (p = 0,0003 [Mann-Whitney test]) better serum neutralization capacity of Delta, and to a lesser extent (p = 0,0122 [Mann-Whitney test]) also Omicron, than homologous BNT-BNT vaccination (ChAd-BNT median IC50 = 929.15; n = 50; BNT-BNT median IC50 = 432.85; n = 50). Serum neutralization capacity for Omicron compared to Delta was reduced 25.8-fold and 21.6-fold for ChAd-BNT (median IC50 = 36) and BNT-BNT (median IC50 = 20), respectively, in an analysis of sub-cohorts consisting of 15 participants each. 98 – 110 days in median after the second vaccination, serum neutralization capacity for Delta still significantly (p < 0,0001 [Mann-Whitney test]) differed between ChAd-BNT (median IC50= 398.20, n = 43) and BNT-BNT vaccination (median IC50 = 72.93, n = 46). However, there was barely any neutralization capacity left against Omicron in either cohort (Figure 2a).
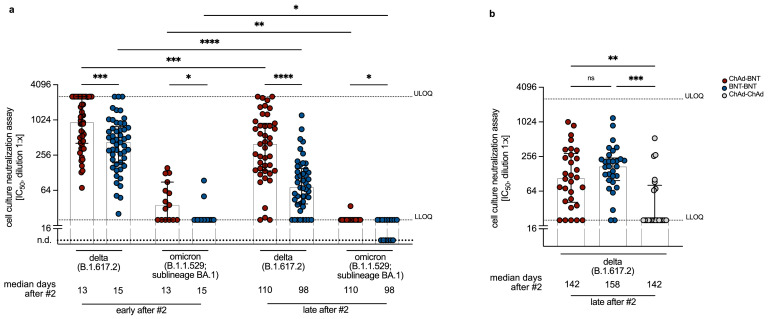
Figure 2.
Individuals of the heterologous cohort neutralize variants of concerns more efficiently than individuals from homologous cohorts. Real virus neutralization levels measured against Delta [B.1.617.2] and Omicron [B.1.1.529 BA.1] at the Munich (a) or Erlangen (b) study site after heterologous ChAd-BNT or homologous BNT-BNT or ChAd-ChAd vaccination. (a) „Early after #2“ refers to on average (median) 13-15 days after second BNT vaccination. „Late after #2“ refers to on average (median) 98-110 days after second vaccination. n = 50 and 43 (ChAd-BNT; “early after #2” and „late after #2“, respectively) and n = 50 and 46 (BNT-BNT; “early after #2” and „late after #2“, respectively). Each group was measured against Delta and Omicron at each time point. (b) „late after #2“ refers to on average (median) 158 days after second BNT (for BNT-BNT) and 142 days after second BNT or ChAd (for ChAd-BNT and ChAd-ChAd) vaccination. n = 30 (ChAd-BNT), n = 30 (BNT-BNT) and n = 21 (ChAd-ChAd). Here, only neutralization of Delta was tested. Bars represent group medians, whiskers interquartile range. ULOQ = upper limit of quantification (2560). LLOQ = lower limit of quantification (20). n.d. = not detected. (a) For inter-group statistics Kruskal-Wallis followed by Dunn's multiple comparisons test was used (b). Over-time comparison within one group was done by Wilcoxon test ((a) (b)). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and n.s. indicates not significant. A detailed description of the data can be found in Supplemental Table for Figure 2a and b.
To confirm these results, serum samples from the Erlangen study center collected at 142 – 158 days in median after second vaccination („late after #2“) were analyzed in the rVNT assay for the ability to neutralize the Delta variant. These analyses again additionally included a cohort of homologous ChAd-ChAd vaccinated participants. Late after ChAd-ChAd immunization, barely any neutralization capacity against Delta was detectable (median IC50 = 20; n = 21), which was significantly different from the ChAd-BNT and BNT-BNT cohorts (p = 0,0072 and p = 0.0002 [Kruskal-Wallis test]). In contrast to the results obtained at the Munich study center, there was no significant (p = 0.8119 [Kruskal-Wallis test]) difference in neutralization capacity against Delta between the ChAd-BNT (median IC50 = 107.8; n = 30) and BNT-BNT (median IC50 = 172; n = 30) group (Figure 2b). These findings were further confirmed using pVNT, although overall the neutralization titers were slightly lower than in the rVNT (Suppl. Figure 2). Whether the discrepancy between the two centers are due to the later sampling time point of the Erlangen cohort, the lower numbers of participants, or variations in the conditions for the neutralization assay, cannot be clarified. However, the differences between vaccination regimens at one site are not affected by this, since analyses for a single given time point were conducted in the same experiment. The reduced capacity to recognize the spike (S) proteins of VoC in comparison to the S protein of the original Wuhan strain observed in the pVNT assay (Suppl. Figure 2) was confirmed by a flow cytometric analysis using HEK293 cells expressing the corresponding S proteins in their natural conformation on the cell surface (Suppl. Figure 3). Overall, these results demonstrate that humoral immunity against VoC was reduced irrespective of the vaccination regimen, but that differences in antibody neutralization capacity between the immunization cohorts remained unchanged.
Heterologous vaccination results in increased antibody avidity
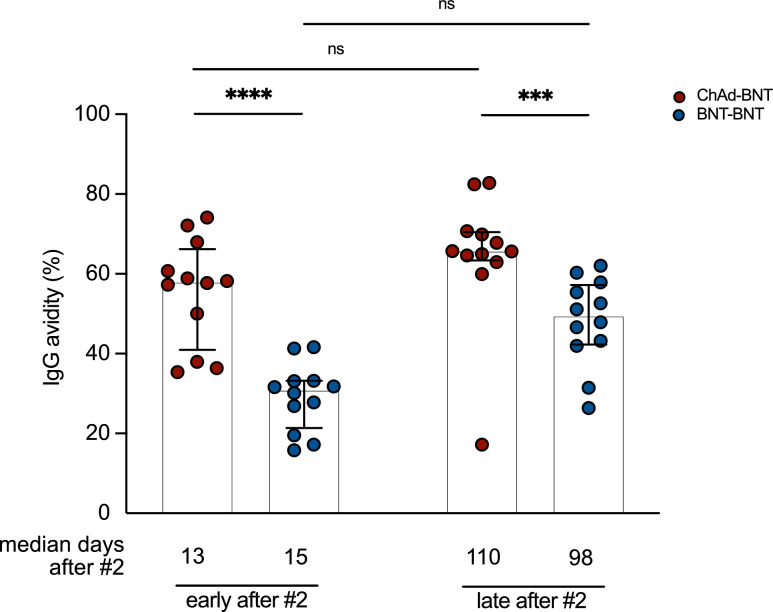
The neutralization capacity of antibodies depends not only on their quantity, but also on their quality.16 We therefore next applied a modified quantitative anti-S ELISA to determine antibody avidity against the S1 domain of the SARS-CoV-2 WT spike antigen. To this end, we used samples of the sub-cohorts from the Omicron rVNT analysis (Figure 3). 13-15 days after second vaccination we observed a higher avidity of antibodies in the heterologous ChAd-BNT (median = 57.96 %) compared to the homologous BNT-BNT (median = 30.86 %) cohort. This difference remained constant at follow-up (Figure 3). Over time, there was a tentative increase in antibody avidity for both groups (BNT-BNT median = 49.49 %; ChAd-BNT median = 65.69 %) which was, however, not statistically significant (p = 0.0537 and p = 0.4118 [Friedman test]). (Figure 3). These results indicate that higher antibody avidity after heterologous ChAd-BNT compared to homologous BNT-BNT vaccination contributes to non-inferior neutralization capacity (Figure 1).
Figure 3.
Higher antibody avidity upon heterologous ChAd-BNT compared to homologous BNT-BNT vaccination. Antibody avidity of a subcohort (n = 12) from study center Munich after heterologous ChAd-BNT and homologous BNT-BNT vaccination. „Early after #2“ refers to on average (median) 13-15 days after secondary BNT vaccination. „Late after #2“ refers to on average (median) 98 and 110 days after second vaccination. Bars represent group medians, whiskers interquartile range. For inter-group statistics concerning one time point Mann-Whitney test was used. Over-time comparison within one group was done by Friedman test. A detailed description of the data can be found in Supplemental Table for Figure 3.
Homologous and heterologous vaccination induce stable polyfunctional SARS-CoV-2 spike-reactive T-cell responses
Given the critical role of T lymphocytes in protection against SARS-CoV-2 infection, we next also characterized the T-cell response elicited by heterologous or homologous vaccination regimens. We acquired peripheral blood mononuclear cells (PBMCs) from vaccinated individuals at the two study centers and characterized CD4 and CD8 T-cell responses by IFN-γ enzyme-linked immunospot (ELISPOT), IFN-γ/IL-2 Fluorospot as well as intracellular cytokine staining followed by flow cytometry analysis (ICCS). To this end, PBMCs were stimulated overnight with two 15mer peptide pools covering the S1 and S2 domains of the full-length SARS-CoV-2 spike glycoprotein, respectively.
To evaluate the dynamics of spike-specific T cells, the frequency of antigen-reactive, IFN-γ-producing T cells was first longitudinally characterized within the ChAd-BNT in Munich (Figure 4a) and the BNT-BNT cohort in Erlangen (Figure 4b) early after first and second vaccination, as well as at the late follow-up time point. Limited T-cell responses to spike peptide stimulation were observed in some individuals already before vaccination (Figure 4b), which might result from cross-reactive clonotypes derived from exposure to common cold coronaviruses.22,23 Induction of spike-specific T cells was observed 55-137 days after one vaccination with ChAd (Munich study site, Figure 4a) or 9-12 days after one vaccination with BNT (p < 0.0001 [Mann-Whitney test]) (Erlangen study site, Figure 4b) in almost all individuals (“early after #1”). T-cell responses peaked 12-36 days (p < 0.0001 [Mann-Whitney test]) (Munich study site, Figure 4a) or 9-11 days (p < 0.0001 [Mann-Whitney test]) (Erlangen study site, Figure 4b) after second immunization with BNT (“early after #2”). 148–210 days (Erlangen study site) and 91–153 days (Munich study site) after second immunization with BNT (“late after #2”), comparable responses of spike-reactive T cells were observed for both homologous vaccination regimens as well as the heterologous vaccine cohort.
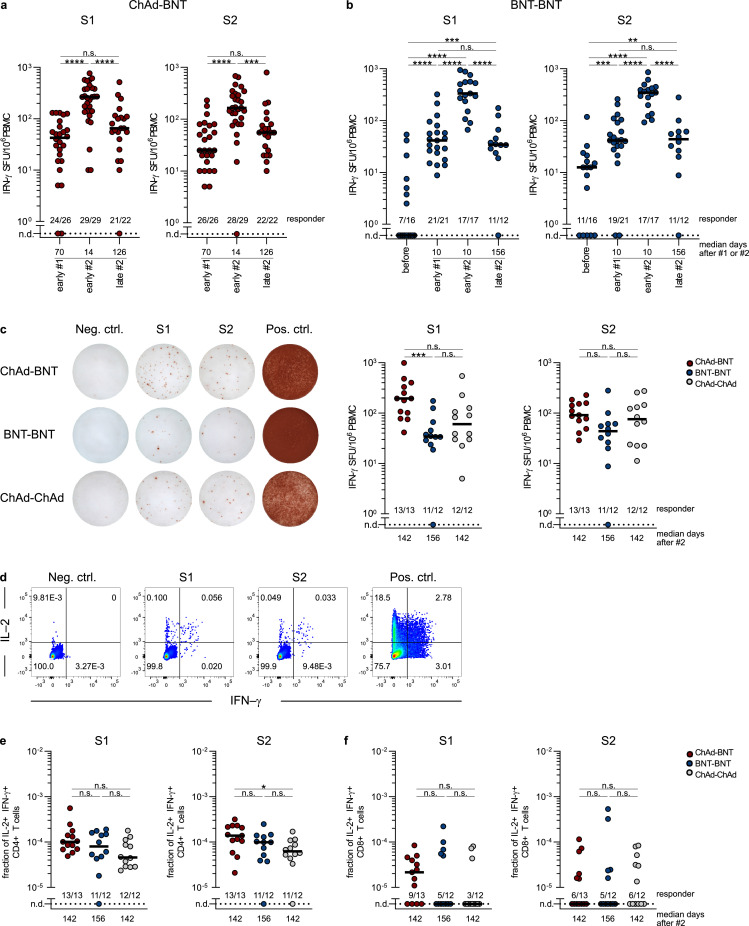
Figure 4.
Long-term maintenance of SARS-CoV-2 spike-reactive T cells after homologous and heterologous vaccination. (a)-(b) Longitudinal characterization of spike-specific T cells, quantified by IFN-γ spot forming units (SFU) after stimulation with SARS-CoV-2 spike peptide pools S1 and S2. (a) Vaccinees of Munich study center quantified by IFN-γ Fluorospot. “early after #1”: 55 to 137 days after initial ChAd vaccination, n = 26. “early after #2”: 12 to 36 days after second BNT vaccination, n = 29. “late after #2”: 91 to 153 days after second BNT vaccination, n = 22. (b) Vaccinees of Erlangen study center quantified by IFN-γ ELISPOT. “before”: pre-vaccination, n = 16. “early after #1”: 9-12 days after first BNT vaccination, n = 21. “early after #2”: 9-11 days after second BNT vaccination, n = 17. “late after #2”: 148–210 days after second BNT vaccination, n = 12. The time points “before”, “early after #1”, and “early after #2” refer to a separate cohort of vaccinees that was included for contextualization. (c) Cohort comparison of spike-specific T cells after stimulation with SARS-CoV-2 spike peptide pools S1 and S2, in dilution of solvent (Neg. ctrl.), or with PMA/ionomycin (Pos. ctrl.). Vaccinees of Erlangen study center 141–210 days after second vaccination (late after #2). Representative data (left) and quantification of IFN-γ SFU for all donors of indicated vaccination cohorts (right) are displayed. (d)-(f) Flow cytometric analyses of polyfunctional spike-specific T cells after stimulation with SARS-CoV-2 spike peptide pools S1 and S2, in dilution of solvent (Neg. ctrl.), or with PMA/ionomycin (Pos. ctrl.). Vaccinees of Erlangen study center 141–210 days after second vaccination (late after #2). (d) Representative flow cytometry data. Shown gates are pre-gated for CD4+ living lymphocytes. Quantification of IL-2 and IFN-γ double-positive CD4 (e) and CD8 (f) T cells for all donors of indicated vaccination cohorts. Dots represent individual vaccinees. Numbers indicate vaccinees with a positive response defined by a detectable T-cell response above background. Non-responsive vaccinees are represented as not detected (n.d.). For inter-group statistics concerning one time point Kruskal-Wallis test was performed followed by Dunn's multiple comparisons test ((c), (e), (f)). Over-time comparison within one group was done by Mann-Whitney test ((a), (b)). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and n.s. indicates not significant.
Having demonstrated that spike-reactive T cells were detectable at least 4 months after the second vaccination, we next examined the effect of different vaccination regimens on the quality of the T-cell response in more detail. We therefore quantified IFN-γ secreting, spike-specific T cells 141–210 days after vaccination with the different vaccine regimens in study participants at the Erlangen study center (Figure 4c). IFN-γ ELISPOT detected reactive T cells in almost all individuals of ChAd-BNT, BNT-BNT and ChAd-ChAd vaccination cohorts. After heterologous ChAd-BNT vaccination, S1-reactive T cells were detected at higher frequencies compared to the homologous BNT-BNT vaccination (p = 0.0010 [Kruskal-Wallis test]) cohort while there was no difference for S2-specific T cells and the other vaccination schemes (ChAd-BNT vs. BNT-BNT: p = 0.0529, ChAd-ChAd vs. BNT-BNT: p = 0.4797, ChAd-BNT vs. ChAd-ChAd: p>0.9999 [Kruskal-Wallis test]). Thus, heterologous vaccination was at least as efficient as homologous vaccination in inducing spike-reactive T-cell responses that are stable over time (Figure 4c).
T-cell polyfunctionality is a hallmark of high-quality immunity and predictive of protective immune responses.24 To examine whether heterologous and homologous vaccination regimens induce and maintain polyfunctional T lymphocytes equally well, T cells were characterized for simultaneous production of the effector cytokines IL-2 and IFN-γ (Figure 4d; Suppl. Figure 4). Quantification of these double-positive T cells revealed a dominant, polyfunctional CD4 T-cell response that persisted in the majority of individuals irrespective of the vaccination regimen used (S1: ChAd-BNT vs. BNT-BNT: p = 0.9115, ChAd-ChAd vs. BNT-BNT: p = 0.7499, ChAd-BNT vs. ChAd-ChAd: p = 0.0831; S2: ChAd-BNT vs. BNT-BNT: p = 0.3462, ChAd-ChAd vs. BNT-BNT: p>0.9999, ChAd-BNT vs. ChAd-ChAd: p = 0.0429 [Kruskal-Wallis test]) (Figure 4e). For CD8 T cells, we observed a greater inter-individual variability (Figure 4f) with 40-60% of cells not reacting to peptide stimulation at all. This effect was most probably due to variable recognition of CD8 epitopes within the 15mer peptides that were used for antigenic stimulation.
Overall, the frequency of polyfunctional T cells quantified by ICS correlated with the frequency of spike-reactive T cells determined by IFN-γ ELISPOT, further validating the findings (S1: r = 0.7, p < 0.0001; S2: r = 0.5, p = 0.0047 [Spearman correlation]) (Suppl. Figure 5). Fluorospot assays further confirmed the induction of IL-2 and IFN-γ secreting polyfunctional T cells after primary immunization with ChAd and secondary immunization with BNT, as well as the persistence of a polyfunctional CD4-dominated T-cell response at the level of primary vaccination throughout the entire observation period (Suppl. Figure 6). In summary, all vaccination regimens induce stable and polyfunctional T-cell responses.
Discussion
We here analyzed the humoral and cellular immune response of 472 participants from three different study sites, 13-15 days and 98-158 days in median after homologous and heterologous ChAd and BNT vaccination. Overall, heterologous vaccination with ChAd followed by BNT induced equal or even superior humoral and cellular immune responses compared to homologous BNT-BNT or ChAd-ChAd vaccination.
We and others had previously reported enhanced neutralization capacity early after heterologous ChAd-BNT vaccination compared to homologous BNT-BNT-vaccination, both of which in turn induced clearly higher neutralizing antibody titers than a homologous ChAd vaccination regimen.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Apart from significant waning of neutralization capacity towards WT virus at late time points for all regimens, we here observed that differences in humoral immunity towards WT virus between ChAd-BNT and BNT-BNT vaccination vanished, while homologous ChAd-ChAd still showed reduced neutralization titers compared to the other two vaccination schemes. One caveat in this regard is that we did not analyze time points longer than 5 months. In light of the unusually long germinal center reaction especially after mRNA vaccination,25, 26, 27, 28, 29 it is conceivable that at even later time points further differences between the vaccination regimens may emerge.
In line with previous reports,16,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 S-specific antibodies induced by the current vaccines encoding the S protein from the original Wuhan strain have significantly reduced neutralizing activity against the SARS-CoV-2 VoC Delta, and even less activity against Omicron. Of note, we here detect such loss of neutralization for all vaccination schedules. In terms of differences between the immunization regimens, ChAd-BNT and BNT-BNT groups showed higher neutralizing antibody response against VoCs than the ChAd-ChAd group, as observed for WT virus.
The relative binding capacity to the different spike variants might be also indicative for the degree of immune evasion by the different VoCs. Since neutralizing antibody levels directly correlate with the level of protection against infection,41 vaccine efficacy (VE) against infection with VoCs also decreased dramatically over time.42, 43, 44 Nevertheless, 98 – 158 days in median after the second vaccination, VE were reported to be comparable for BNT-BNT and ChAd-BNT, but lower for ChAd-ChAd schedules.45,46
Antibody quality might be even more important than the mere quantity for potent vaccine responses, as demonstrated by the high avidity of anti-spike antibodies after a third exposition.16 In our study, antibody avidity increased slightly from the early to the late time point for both the ChAd-BNT and the BNT-BNT cohort, which might indicate ongoing B-cell maturation. It has been reported that this process could last up to 6 months in recipients of homologous mRNA vaccines or convalescent patients, while comparable data on heterologous vaccinations are missing.25, 26, 27, 28, 29 A higher avidity of antibodies induced by heterologous ChAd-BNT vaccination offers an explanation why they show a superior neutralization capacity against VoC at both study centers. One potential reason for differential affinity maturation of memory B cells is the difference in the interval between the first and second vaccine dose, which was in the median 63 days for the ChAd-BNT vaccinees and 21-23 days for homologous BNT-BNT vaccinated individuals. Furthermore, the duration of antigen presentation in the germinal centers might be different after viral vector immunization or mRNA vaccination. The presence of vaccine-derived mRNA and spike protein has been shown in lymph node biopsies from mRNA vaccinated individuals up to 8 weeks.29 Of note, we cannot rule out that the larger time difference between the individual immunizations for the ChAd-BNT vaccinees could also influence the quantity of the antibody response, as reported recently for homologous vaccination regimens.47 Further investigations to elucidate the differences for the current vaccines are highly relevant for the implementation of future vaccine regimens using gene-based vaccines.
Although the VE against symptomatic infections wanes over time due to the reduced neutralizing capacity of vaccine-induced antibodies, and despite the fact that Omicron by now dominates SARS-CoV-2 case numbers worldwide, protection from severe disease progression currently still prevails. Apart from boosters of humoral immunity through a third vaccination, a central reason for this is a more long-lasting48, 49, 50, 51, 52 and conserved53, 54, 55, 56, 57, 58 T-cell response. In this context, we also addressed the question to which extent SARS-CoV-2 spike protein-specific T cells will persist in response to different vaccination regimens. Maintenance of spike-reactive T cells was observed for the vast majority of individuals after homologous and heterologous immunization at the late time point. Longitudinal characterization of the frequency in individual vaccinees indicated longer lasting quantities of these spike-specific T cells at a level obtained after the first immunization. This observation was made at both independent study centers. Depending on the readout, the heterologous vaccination regimen was consistently non-inferior and sometimes statistically significantly superior to the homologous BNT immunization. It has already been shown that a priming dose of ChAd induces a stronger T-cell response compared to a primary immunization with BNT, which was however no longer the case after a secondary BNT immunization.59 Nevertheless, this could still indicate that the overall superiority of humoral and cellular immunogenicity through the heterologous vaccination regimen results from a more potent primary immune response. For example, strong CD4 T-cell responses induced by primary ChAd vaccination may also explain why serological antibody responses after heterologous ChAd-BNT vaccination are more prominent than after homologous BNT-BNT vaccination.59
Polyfunctionality as a predictor of an effective T-cell immune response was demonstrated for persisting T cells after all vaccination regimens.24 Especially polyfunctional CD4 T cells were well maintained 141–210 days after the second vaccination. For CD8 T cells this was less clear, most probably owing to variable recognition of (shorter) CD8 epitopes within the 15mer peptides that were used for antigenic stimulation. Overall, our data show that heterologous vaccination is at least as capable as homologous vaccination regimens in inducing longer lasting maintenance of polyfunctional spike-specific T cells, which are likely to convey protective immunity.
In summary, these data document at least non-inferior humoral and cellular immunogenicity after heterologous ChAd-BNT vaccination compared to the respective homologous regimens. While waning of humoral immunity and reduced neutralization capacity against VoC was detected for all vaccination regimens, T-cell responses were more consistently conserved. An enhanced understanding of humoral and cellular immunity induced by individual vaccination regimens is crucial for further recommendations regarding the necessity, timing, and choice of additional vaccinations and public health policies.
Contributors
Conceptualization U.P., O.A.C., K.Ü., M.T., P.S.
Methodology P.I., P.S., S.B., J.H.; M.W.
Formal Analysis P.I., M.T., E.V., A.P., N.K., C.C.C., A.W., B.H.L., T.B., J.S.
Investigation P.I., M.T., E.V., A.P., N.K., C.C.C., A.W., B.H.L., S.H., T.B., R.B., S.J., C.M., V.S., C.C., S.S., K.T., S.Y., P.S.
Resources K.Ü., M.T., U.P., P.K.
Data Curation S.B., P.S., E.V., M.T., N.K., C.C.C., A.W., B.H.L., T.B., H.M., M.W.
Writing M.T., K.S., E.V., K.K., A.P., U.P., C.B.
Visualization A.P., E.V., K.K., M.T.
Supervision K.Ü., U.P., M.T., K.S., N.K., T.B., P.K., P.S.
Project Administration O.A.C., K.Ü., U.P., M.T., C.B.
Funding Acquisition O.A.C., K.Ü., U.P.
All authors read and approved the final version of the manuscript.
M.T., K.S. and U.P. have directly accessed and verified the underlying data.
Data sharing statement
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Declaration of interests
J.H. reports grants and speaker honoraria from Pfizer, outside the study. JSG received speaker honoraria from Gilead Nordica and Pfizer Ireland, outside the study. P.K. reports to serve as scientific adviser to Avexis and Freeline and a grant from Roche outside from this study. U.P. reports grants from ALiOS and VirBio, and personal fees from AbbVie, Arbutus, Gilead, GSK, Johnson & Johnson, Roche, Sobi, and Vaccitech, outside the study. U.P. is co-founder and shareholder of SCG Cell Therapy O.A.C. reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, Pfizer; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Jannsen, MedPace, Paratek, PSI, Shionogi; A patent at the German Patent and Trade Mark Office (DE 10 2021 113 007.7); Other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley, outside the submitted work.
All other authors declare no competing interests.
Acknowledgments
We thank Manuela Stefanie Glocker, Thomas Wochnig, Romina Bester, Philip Hagen, Kirsten Fraedrich, Norbert Donhauser, Manuela Laumer for excellent technical assistance.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104294.
Contributor Information
Ulrike Protzer, Email: protzer@tum.de.
Kilian Schober, Email: kilian.schober@uk-erlangen.de.
Matthias Tenbusch, Email: matthias.tenbusch@fau.de.
Appendix. Supplementary materials
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vygen-Bonnet S, Koch J, Bogdan C, et al. Robert Koch-Institut; 2021. Beschluss der STIKO zur 5. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung. [Google Scholar]
- 3.Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9(11):1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tenbusch M, Schumacher S, Vogel E, et al. Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 2021;21(9):1212–1213. doi: 10.1016/S1473-3099(21)00420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoo NKH, Lim JME, Gill US, et al. Differential immunogenicity of homologous versus heterologous boost in Ad26.COV2.S vaccine recipients. Med. 2022;3(2) doi: 10.1016/j.medj.2021.12.004. 104-118.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groß R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behrens GMN, Cossmann A, Stankov MV, et al. SARS-CoV-2 delta variant neutralisation after heterologous ChAdOx1-S/BNT162b2 vaccination. Lancet. 2021;398(10305):1041–1042. doi: 10.1016/S0140-6736(21)01891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaku CI, Champney ER, Normark J, et al. Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination. Science. 2022;375(6584):1041–1047. doi: 10.1126/science.abn2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27(9):1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Normark J, Vikström L, Gwon Y-D, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021;385(11):1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altmann DM, Boyton RJ. Waning immunity to SARS-CoV-2: implications for vaccine booster strategies. Lancet Respirat Med. 2021;9(12):1356–1358. doi: 10.1016/S2213-2600(21)00458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marking U, Havervall S, Greilert-Norin N, et al. Duration of SARS-CoV-2 immune responses up to six months following homologous or heterologous primary immunization with ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccines. Vaccines. 2022;10(3):359. doi: 10.3390/vaccines10030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho T-C, Chen Y-MA, Chan H-P, et al. The effects of heterologous immunization with prime-boost COVID-19 vaccination against SARS-CoV-2. Vaccines. 2021;9(10):1163. doi: 10.3390/vaccines9101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wratil PR, Stern M, Priller A, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. 2022;28:496–503. doi: 10.1038/s41591-022-01715-4. [DOI] [PubMed] [Google Scholar]
- 17.Almanzar G, Ottensmeier B, Liese J, Prelog M. Assessment of IgG avidity against pertussis toxin and filamentous hemagglutinin via an adapted enzyme-linked immunosorbent assay (ELISA) using ammonium thiocyanate. J Immunol Methods. 2013;387(1-2):36–42. doi: 10.1016/j.jim.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Kneitz R-H, Schubert J, Tollmann F, Zens W, Hedman K, Weissbrich B. A new method for determination of varicella-zoster virus immunoglobulin G avidity in serum and cerebrospinal fluid. BMC Infect Dis. 2004;4:33. doi: 10.1186/1471-2334-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapuente D, Maier C, Irrgang P, et al. Rapid response flow cytometric assay for the detection of antibody responses to SARS-CoV-2. Eur J Clin Microbiol Infect Dis. 2021;40(4):751–759. doi: 10.1007/s10096-020-04072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dispinseri S, Marzinotto I, Brigatti C, et al. Seasonal betacoronavirus antibodies’ expansion Post-BNT161b2 vaccination associates with reduced SARS-CoV-2 VoC neutralization. J Clin Immunol. 2022;42:448–458. doi: 10.1007/s10875-021-01190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapuente D, Fuchs J, Willar J, et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat Commun. 2021;12(1):6871. doi: 10.1038/s41467-021-27063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun J, Loyal L, Frentsch M, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 23.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 Coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7) doi: 10.1016/j.cell.2020.05.015. 1489-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 25.Sokal A, Chappert P, Barba-Spaeth G, et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell. 2021;184(5) doi: 10.1016/j.cell.2021.01.050. 1201-1213.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim W, Zhou JQ, Horvath SC, et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature. 2022:1–8. doi: 10.1038/s41586-022-04527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mudd PA, Minervina AA, Pogorelyy MV, et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell. 2022;185(4) doi: 10.1016/j.cell.2021.12.026. 603-613.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner JS, O'Halloran JA, Kalaidina E, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Röltgen K, Nielsen SC, Silva O, et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185(6) doi: 10.1016/j.cell.2022.01.018. 1025-1040.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muecksch F, Wang Z, Cho A, et al. Increased potency and breadth of SARS-CoV-2 neutralizing antibodies after a third mRNA vaccine dose. bioRxiv 2022.
- 31.Sheward DJ, Kim C, Ehling RA, et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: a cross-sectional study. Lancet Infect Dis. 2022;22(6):813–820. doi: 10.1016/S1473-3099(22)00129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185(3) doi: 10.1016/j.cell.2021.12.032. 447-456.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 34.Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399(10321):234–236. doi: 10.1016/S0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muik A, Lui BG, Wallisch A-K, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375(6581):678–680. doi: 10.1126/science.abn7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386(11):1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3) doi: 10.1016/j.cell.2021.12.046. 467-484.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruell H, Vanshylla K, Tober-Lau P, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022:1–4. doi: 10.1038/s41591-021-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng SMS, Mok CKP, Leung YWY, et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022:1–4. doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pérez-Then E, Lucas C, Monteiro VS, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:481–485. doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 42.Hansen CH, Schelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv 2021:2021.12.20.21267966.
- 43.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez-Baz I, Trobajo-Sanmartín C, Miqueleiz A, et al. Product-specific COVID-19 vaccine effectiveness against secondary infection in close contacts, Navarre, Spain, April to August 2021. Euro Surveill. 2021;26(39) doi: 10.2807/1560-7917.ES.2021.26.39.2100894. pii=2100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordström P, Ballin M, Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet. 2022;399(10327):814–823. doi: 10.1016/S0140-6736(22)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11 doi: 10.1016/j.lanepe.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei J, Pouwels KB, Stoesser N, et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022;28(5):1072–1082. doi: 10.1038/s41591-022-01721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woldemeskel BA, Garliss CC, Blankson JN. mRNA vaccine-elicited SARS-CoV-2-specific T cells persist at 6 months and recognize the delta variant. Clin Infect Dis. 2022;75(1):e898–e901. doi: 10.1093/cid/ciab915. [DOI] [PubMed] [Google Scholar]
- 49.Oberhardt V, Luxenburger H, Kemming J, et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597(7875):268–273. doi: 10.1038/s41586-021-03841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mateus J, Dan JM, Zhang Z, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science. 2021;374(6566):eabj9853. doi: 10.1126/science.abj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 52.Guerrera G, Picozza M, D'Orso S, et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci Immunol. 2021;6(66):eabl5344. doi: 10.1126/sciimmunol.abl5344. [DOI] [PubMed] [Google Scholar]
- 53.Gao Y, Cai C, Grifoni A, et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat Med. 2022:1–5. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603(7901):488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naranbhai V, Nathan A, Kaseke C, et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell. 2022 doi: 10.1016/j.cell.2022.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.GeurtsvanKessel CH, Geers D, Schmitz KS, et al. Divergent SARS CoV-2 Omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022:eabo2202. doi: 10.1126/sciimmunol.abo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5) doi: 10.1016/j.cell.2022.01.015. 847-859.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pozzetto B, Legros V, Djebali S, et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature. 2021;600(7890):701–706. doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.