Abstract
Proteins that have a structure similar to those of LuxR and FixJ comprise a large subfamily of transcriptional activator proteins. Most members of the LuxR-FixJ family contain a similar amino-terminal receiver domain linked by a small region to a carboxy-terminal domain that contains an amino acid sequence similar to the helix-turn-helix (HTH) motif found in other DNA-binding proteins. GerE from Bacillus subtilis is the smallest member of the LuxR-FixJ family. Its 74-amino-acid sequence is similar over its entire length to the DNA binding region of this protein family, including the HTH motif. Therefore, GerE provides a simple model for studies of the role of this HTH domain in DNA binding. Toward this aim, we sought to identify the amino acids within this motif that are important for the specificity of binding to DNA. We examined the effects of single base pair substitutions in the high-affinity GerE binding site on the sigK promoter and found that nucleotides at positions +2, +3, and +4 relative to the transcription start site on the sigK promoter are important for a high-affinity interaction with GerE. We next examined the effects of single alanine substitutions at two positions in the HTH region of GerE on binding to wild-type or mutant target sites. We found that the substitution of an alanine for the threonine at position 42 of GerE produced a protein that binds with equal affinity to two sites that differ by 1 bp, whereas wild-type GerE binds with different affinities to these two sites. These results provide evidence that the amino acyl residues in or near the putative HTH region of GerE and potentially other members of the LuxR-FixJ family determine the specificity of DNA binding.
Proteins that have a structure similar to those of LuxR and FixJ comprise a large subfamily of transcriptional activator proteins. Homologous proteins include UhpA, NarL, BvgA, MalT, and GerE. This family can further be subdivided into two groups. The first group includes response regulators of two-component systems, which are activated by phosphorylation. Examples of the response regulators are FixJ and UhpA. FixJ regulates nitrogen fixation in Sinorhizobium meliloti. It activates the transcription of nifA and fixK, stimulating nitrogen fixation (19). UhpA activates transcription from the uhpT promoter in order to allow Escherichia coli to accumulate sugar phosphates in an unaltered form (22). The second group includes proteins that are active constitutively or are regulated by low-molecular-weight cofactors. For example, MalT activates the maltose regulon in the presence of maltose and ATP in E. coli (6).
Most members of the LuxR-FixJ family contain a similar amino-terminal receiver domain linked by a small region to a carboxy-terminal domain that contains an amino acid sequence similar to the helix-turn-helix (HTH) motif (23, 24) found in other DNA-binding proteins. The HTH motif that is found in other families of DNA-binding proteins (i.e., catabolite gene activator protein and λcI) has been shown to interact directly with its target DNA sequences. RNA polymerase sigma factors also contain a region similar to the HTH motif, and genetic evidence supports the model that amino acids in the HTH motif of sigma factors interact specifically with the DNA at the −35 region of promoters (16, 20, 26). Though it is not known whether the putative HTH region in members of the LuxR-FixJ family plays a direct role in DNA binding, the carboxy-terminal region of these proteins, which contains this motif, has been shown to be important for DNA binding. Data from studies of several members of this family indicated that the amino terminus is not always necessary for the DNA binding activity of the protein. For example, deletion of the N terminus of FixJ results in a protein with much higher affinity for DNA than that of the full-length FixJ (19). A deletion in LuxR produced similar results (12). The amino-terminal domain of MalT is also nonessential for DNA binding (28), the carboxy-terminal 95 amino acids of MalT being sufficient for binding to MalT-dependent promoters (28). Moreover, point mutations in the putative HTH region of FixJ and UhpA probably affect the DNA binding activity of these proteins. However, in no case is there experimental evidence that the putative HTH regions are involved in determining the specificity of DNA binding by this family of proteins.
GerE from Bacillus subtilis is the smallest member of the LuxR-FixJ family. Its 74-amino-acid sequence is similar over its entire length to the DNA binding region of this protein family, including the HTH motif. Therefore, GerE provides a simple model for studies of DNA binding and transcription activation by the LuxR-FixJ family. GerE is expressed during endospore formation in B. subtilis, where it activates or represses ςK-associated RNA polymerase-dependent transcription of several genes. DNase I footprints have indicated that GerE binds to the promoter region of several ςK-dependent genes (31, 32), though it is not known how GerE stimulates promoter activity. The location of the GerE binding site(s) on GerE-dependent promoters can vary with respect to the transcription start site (TSS), indicating that the mechanism of transcription activation by GerE may be different depending on the specific promoter to which it binds. For example, GerE binds to three locations on the cotC promoter (32) and two sites on the cotX promoter (31) to activate transcription. GerE binds at the TSS of the sigK promoter to repress transcription (18).
To test the model that the amino acids of the putative HTH motif in GerE determine the specificity of DNA binding, we sought to identify amino acid substitutions in GerE that changed its specificity for binding DNA. A DNA consensus sequence for GerE target sites has been described based on the alignment of the regions protected by GerE on promoter DNA (Fig. 1). However, the role of this consensus sequence in signaling binding of GerE has not been tested. As the first step toward the definition of the determinants of GerE binding and specificity, we examined the effects on GerE binding of several single base pair substitutions in a target site. We identified specific base pair substitutions that reduced the apparent affinity of GerE for the mutant DNA sites. We next examined the effects on binding to wild-type or mutant target sites of single alanine substitutions at two positions in the HTH region of GerE. We found that the substitution of an alanine for the threonine at position 42 of GerE produced a protein that binds with equal affinity to two sites that differ by 1 bp, whereas wild-type GerE binds with different affinities to these two sites. These results provide evidence that the amino acyl residues in or near the putative HTH region of GerE and potentially other members of the LuxR-FixJ family determine the specificity of DNA binding.
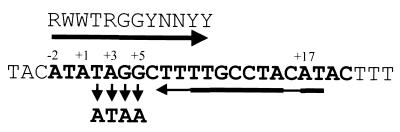
FIG. 1.
GerE binding site on the sigK promoter. Shown is the sequence of the nontranscribed strand of the GerE binding site on the sigK promoter. Numbers indicate nucleotide positions relative to the TSS, indicated as position +1. The GerE binding consensus sequence is shown at the top. Single-letter code, R, A or G; Y, C or T; W, A or T; N, A, C, G, or T. The thick horizontal arrows represent the nucleotide matches to the GerE binding consensus sequence, whereas the thin portions of the arrow denote deviations from the consensus. The single base pair substitutions described in the text are indicated by the downward-pointing arrows.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains used in this study are the E. coli strains DH5α (Gibco BRL, Grand Island, N.Y.), used for routine cloning, and BL21(DE3)(pLysS) (Stratagene, La Jolla, Calif.), used for protein overexpression. Plasmids used include pKW19 and pGerE-EX (29) and their mutant derivatives (Table 1). Plasmid pKW19 was constructed by inserting into pCR2.1-TOPO (Invitrogen, Carlsbad, Calif.) a 226-bp region of the sigK promoter that was amplified by using the primers sigKUP and sigK250-REV.
TABLE 1.
Plasmids used in this study
| Name | Contentsa | Source |
|---|---|---|
| pKW19 | PsigK PCR in pCR2.1-TOPO (Invitrogen) | K. Wade |
| psigK+2A | pKW19 with sigK+2A mutation | This study |
| psigK+3T | pKW19 with sigK+3T mutation | This study |
| psigK+4A | pKW19 with sigK+4A mutation | This study |
| psigK+5A | pKW19 with sigK+5A mutation | This study |
| pGerE-EX | GerE coding sequence in pET26b (Novagen) | J. Brannigan |
| pGerE-T42A | T42A-substituted GerE in pGerE-EX | This study |
| pGerE-R44A | R44A-substituted GerE in pGerE-EX | This study |
See Materials and Methods for the method of construction of each plasmid.
Site-directed PCR mutagenesis and DNA sequencing.
Oligonucleotide-directed base pair substitutions were made in the sigK promoter and in the coding region of gerE using the QuickChange kit from Stratagene. pKW19 or pGerE-EX was used to make changes in the sigK promoter or specific amino acids of GerE, respectively. The high-fidelity DNA polymerase used in these reactions was Pfu Turbo (Stratagene), and the presence of the mutations was confirmed by DNA sequence analysis.
Mutant plasmid DNAs were subjected to DNA sequencing using the fmol sequencing kit (Promega, Madison, Wis.) following the specified protocol given by the manufacturer.
Preparation of end-labeled DNA and DNase I footprinting.
The oligonucleotide sigKUP (50 pmol) was labeled with [γ-32P]ATP using T4 polynucleotide kinase (Promega) as per the manufacturer's instructions. Unincorporated 32P was removed by a G-25 Microspin column (Amersham Pharmacia Biotech, Piscataway, N.J.). The labeled and purified oligonucleotide was then subjected to PCR using 36 pmol of the unlabeled sigK250-REV primer and the Herculase polymerase (Stratagene). The final PCR product was then cleaned with a G-50 Microspin column (Amersham Pharmacia Biotech) to remove any incorporated nucleotides, and the specific activity of the DNA probe was measured using a scintillation counter (LS-6500; Beckman). We routinely recovered greater than 90% of the probe with a typical specific activity of approximately 5 × 106 cpm/μg.
End-labeled DNA probes were subjected to DNase I footprinting reactions as described by Zheng et al. (32). Briefly, DNA fragments labeled at one end were incubated in separate reaction mixtures without protein or with various concentrations of purified GerE in a 42-μl reaction mixture containing 10 mM HEPES [pH 7.5], 50 mM NaCl, 1 mM EDTA [pH 8], 1 mM dithiothreitol, and 10% glycerol. Poly(dI-dC) was added to a final concentration of 1.2 μg/ml, and the reaction mixtures were incubated at 37°C for 10 min. DNase I (3 μl of 0.0004 mg/ml) was then added to the reaction mixtures, and after 1 min at 37°C, the digests were terminated by adding 50 μl of STOP buffer (100 mM Tris-HCl [pH 8], 50 mM EDTA, 200 μg of yeast tRNA/ml) and incubating them for 2 min at 65°C. The DNA in each reaction was then subjected to ethanol precipitation followed by electrophoresis in a 7 M urea-polyacrylamide gel containing 6% acrylamide.
Partial purification of wild-type and mutant forms of GerE.
BL21(DE3)(pLysS) cells containing either pGerE-EX or its mutant derivatives were grown at 37°C to an optical density at 600 nm of approximately 0.8 in Luria-Bertani medium containing chloramphenicol (25 μg/ml) and kanamycin (30 μg/ml). Expression of GerE was then induced with isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM at 37°C. After 30 min, rifampin was added to a final concentration of 200 μg/ml and the cells were incubated for another 2.5 h at 37°C. The cells were harvested and subjected to purification over a 5-ml HighTrap heparin column as described by Wade et al. (29) using an Acta Explorer 900 and version 3 of the Unicorn software (Amersham Pharmacia Biotech). The concentration of GerE present in each of the individual preparations was determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) per the manufacturer's instructions. The purity of the GerE protein was determined by Coomassie blue staining (Bio-Rad Laboratories) after electrophoresis into an 18% polyacrylamide gel containing sodium dodecyl sulfate. The active fraction of protein in each preparation was determined by titrating a DNase I footprint assay (see above) with various concentrations of cold specific competitor DNA.
Protein modeling.
The coordinates for the crystal structure of GerE (10) were provided by J. Brannigan (York University). RasMol version 2.6 (command language and program by Roger Sayle, Glaxo Wellcome, Stevenage, United Kingdom) was used to create the model of the GerE-GerE dimer.
RESULTS
Identification of the nucleotide sequence that signals recognition and binding by GerE.
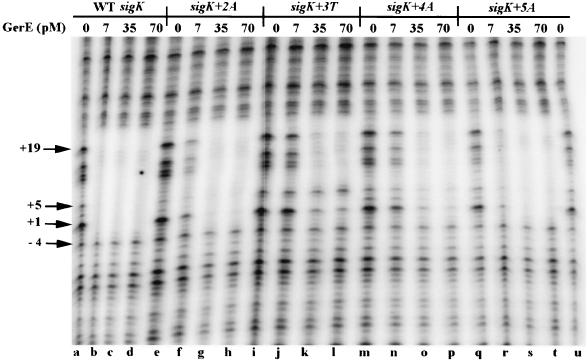
The GerE binding site in the sigK promoter contains an inverted repeat of sequences that are similar to the consensus sequence derived from a comparison of the sequences from several GerE binding sites (Fig. 1). One repeat perfectly matches the consensus sequence (Fig. 1). To test the role of the consensus sequence in GerE binding, we examined the effects of single base pair substitutions in the sigK promoter on binding of GerE. We used site-directed mutagenesis to create single base pair changes at positions +2, +3, +4, and +5 relative to the TSS of sigK (Fig. 1). These positions are in the center of the GerE consensus sequence and appear to be the most highly conserved. We then measured the apparent affinity of GerE for these DNA sites by determining the amount of GerE required to protect these sites in DNase I footprinting experiments (Fig. 2). The wild-type site was protected with 7 pM GerE (Fig. 2, lane b). The sites having single base pair substitutions at positions +2 and +4 were not completely protected by 7 pM GerE (lanes f and n), but both were protected by 35 pM GerE (lanes g and o), indicating that these base pair substitutions reduced the apparent affinity of GerE for these sites. The site with a single base pair substitution at +3 was also not completely protected by 7 pM GerE but was protected by 35 pM GerE. However, we also noted that nucleotides at +1 and +5 of this site were not fully protected even with 70 pM GerE (Fig. 2, lanes k and l). Evidently, the substitution at +3 not only reduced the apparent affinity of GerE for this site but may also have changed the way that GerE binds to this site. The substitution at position +5 had the smallest effect on GerE binding, reducing the apparent affinity of GerE for this site by less than fivefold.
FIG. 2.
DNase I footprint analysis of PsigK with wild-type (WT) GerE. PCR fragments representing wild-type or mutant GerE binding sites end labeled on the nontranscribed strand of the sigK promoter were incubated in separate reactions with 0 (lanes a, e, i, m, q, and u), 7 (lanes b, f, j, n, and r), 35 (lanes c, g, k, o, and s), or 70 (lanes d, h, l, p, and t) pM wild-type GerE and subjected to DNase I digestion. The specific promoter mutation is indicated at the top. The arrows at the left represent the respective positions relative to the TSS.
Mutagenesis of the DNA binding region of GerE.
The amino acid sequence of GerE is similar to an HTH region of sigma factors that interacts with specific nucleotides within the −35 regions of their cognate promoters (Fig. 3). Single amino acid substitutions in several sigma factors have been shown to change the specificity of their interaction with promoters (Fig. 3). For example, a substitution of a histidine for the tyrosine at position 219 (Y219H) in ςE specifically suppressed the effect of a single base pair substitution at position −33 in the ςE-dependent promoter of spoIIID (26). These and other results strongly supported the model in which the amino acyl residues in this HTH of the sigma factors contact base pairs in the −35 regions of their cognate promoters. The structure of NarL, another member of the LuxR-FixJ family, has been determined by X-ray crystallography (3). This structure indicates that some of the amino acid side chains in the putative HTH region are exposed on the surface. Therefore, the homologous residues in GerE may be available to interact with DNA.
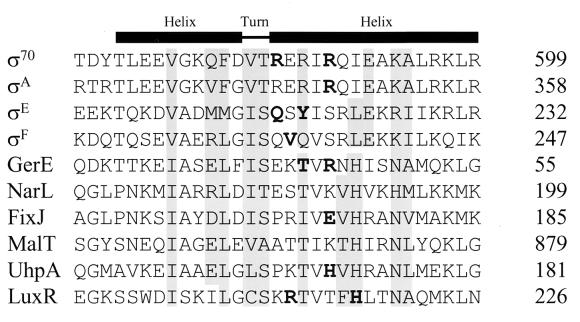
FIG. 3.
Alignment of partial amino acid sequences from the −35 binding regions of several sigma factors and representatives of the LuxR-FixJ family. The numbers at the right indicate the positions of the amino acid sequences in each protein. The boldface indicates the amino acids that have been shown elsewhere to be important for the specificity of binding by ς70 (16), ςA (20), and ςE (26). The boldface amino acids in GerE indicate the positions at which single alanine substitutions were made. The boldface amino acids in FixJ (19), UhpA (30), and LuxR (11, 27) have also been implicated in DNA binding. Sequences and their references include ς70 (21), ςA (21), ςE (21), ςF (21), GerE (5), NarL (17), FixJ (7), MalT (4), UhpA (14), and LuxR (8, 13).
To test the model that the region of GerE that is most similar to the −35 binding region of sigma factors also interacts with specific base pairs in its target DNA, we examined the effects of alanine replacements of threonine at position 42 or arginine at position 44 (Fig. 3) on binding to both wild-type and mutated target sequences. T42 of GerE is homologous to the tyrosine at position 219 in ςE that has been shown elsewhere to be important for a specific interaction with promoter DNA (26). In addition, R44 of GerE is homologous to the arginine at position 347 of ςA that has been shown to affect its specificity for the DNA interaction as well (20).
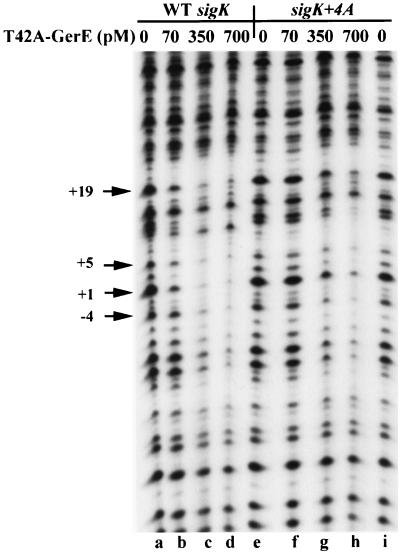
Site-directed mutagenesis was used to create the alanine substitutions at T42 or R44 of GerE. The mutant forms of GerE were then expressed in E. coli and purified by heparin chromatography. The apparent affinity of each protein for wild-type and mutant binding sites on the sigK promoter was estimated by determining the amount of protein required to protect the binding site from DNase I in protection assays. The concentration of both mutant proteins (T42A and R44A) required to bind to the wild-type (consensus) binding site in the sigK promoter was at least 10-fold higher than that required of wild-type GerE (Fig. 4 and data not shown). Base pair substitutions at positions +2, +4, and +5 relative to the TSS further reduced the apparent affinity of GerE T42A and GerE R44A (Fig. 4 and data not shown). For example, 350 pM T42A-substituted GerE did not protect the GerE binding site on the sigK+4A promoter to the same degree as it did on the wild-type sigK promoter (Fig. 4, lanes f to h and lanes b to d). This result shows that DNA binding by the T42A-substituted form of GerE, like wild-type GerE, involves a base-pair-specific interaction at position +4.
FIG. 4.
DNase I footprint analysis of T42A-GerE on wild-type (WT) and sigK+4A sites. PCR fragments representing wild-type (lanes a to d) or mutant (lanes e to i) GerE binding sites end labeled on the nontranscribed strand of the sigK promoter were incubated in separate reactions with 0 (lanes a, e, and i), 70 (lanes b and f), 350 (lanes c and g), or 700 (lanes d and h) pM T42A-substituted GerE and subjected to DNase I digestion. Specific promoter mutations are indicated at the top. The numbers with arrows represent nucleotide positions relative to the TSS.
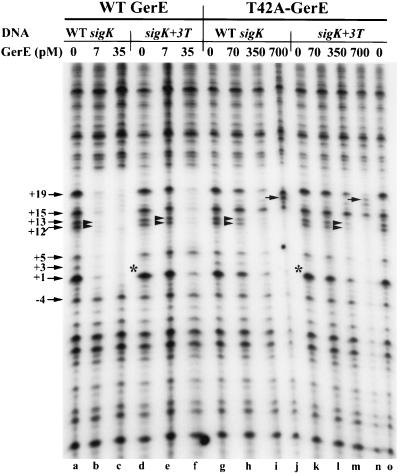
In contrast, one base-pair-specific interaction that affected the apparent affinity of binding by the wild-type GerE did not play a role in binding by the T42A-substituted GerE. The base pair substitution of T:A for the A:T at position +3 relative to the TSS (indicated as “sigK+3T” in Fig. 5), which reduced binding by the wild-type form of GerE (Fig. 5, lanes e and f) and R44A-substituted GerE (data not shown), did not further reduce the apparent affinity of the T42A-substituted GerE for this site (Fig. 5, lanes h to j and l to n). For example, 7 pM wild-type GerE protected positions +12 and +13 in the wild-type DNA, whereas 35 pM wild-type GerE was needed to protect the +3T-substituted sigK DNA, indicating a fivefold difference in the apparent affinities of the protein for these two sites. However, 350 pM T42A-substituted GerE protected the +12 and +13 bands on either the wild-type or the +3T-substituted sigK DNA. Taken together, these data indicated that the wild-type GerE bound with different affinities to the wild-type and +3T binding sites, whereas the T42A-substituted GerE was unable to recognize these sites as different and bound with an equal affinity to both.
FIG. 5.
DNase I footprint analysis of wild-type (WT) or T42A-substituted GerE on wild-type and sigK+3T sites. PCR fragments representing wild-type (lanes a to c and g to j) or mutant (lanes d to f and k to o) GerE binding sites end labeled on the nontranscribed strand of the sigK promoter were incubated in separate reactions with 0 (lanes a and d), 7 (lanes b and e), or 35 (lanes c and f) pM wild-type GerE and subjected to DNase I digestion. The same DNA fragments were incubated in separate reactions with 0 (lanes g, k, and o), 70 (lanes h and l), 350 (lanes i and m), or 700 (lanes j and n) pM T42A-substituted GerE and subjected to DNase I digestion. Specific promoter mutations are indicated at the top. The numbers with arrows represent nucleotide positions relative to the TSS. The double arrowheads indicate positions +12 and +13, which are consistently protected by GerE. The single arrows in the gel point to the +18 band, which is present only in the T42A-substituted GerE reactions. The asterisks denote the absence of the +3 band in the sigK+3T promoter.
Several other differences were observed in analyzing the binding patterns of the two different forms of GerE on the wild type and the +3 derivative of the sigK promoter (Fig. 5). Position +15 is protected from DNase I cleavage by wild-type GerE (lanes c and f) but not by T42A-substituted GerE (lanes i, j, m, and n). We also noted that the band produced by cleavage at position +18 on the sigK DNA was not visible when wild-type GerE was bound to either the wild-type or +3T-substituted sigK DNA. However, when the T42A-substituted GerE was bound to either wild-type or +3-substituted sigK DNA, cleavage at position +18 was enhanced. These results indicated that the T42A-substituted GerE may be positioned on the DNA differently from wild-type GerE, thus allowing DNase I to produce a different cleavage pattern.
We also examined the effect of a C:G substitution for the A:T base pair at position +3 in the sigK promoter (+3C site) on binding by wild-type and the T42A-substituted GerE. A 70 pM amount of wild-type GerE was required to protect the +3C site from DNase I (data not shown), whereas 7 pM wild-type GerE protected the wild-type site. Therefore, the +3C substitution reduced the apparent affinity of wild-type GerE for sigK+3C. A 350 pM amount of T42A-substituted GerE was required to protect both the wild-type and +3C-substituted sites (data not shown). Evidently, T42A-substituted GerE has similar affinities for the two sites. Unlike wild-type GerE, the T42A-substituted GerE bound equally well to three sites that differed by single base pair substitutions at position +3 (+3A, +3T, and +3C). However, the T42A-substituted GerE bound less tightly to sites having substitutions at site +2, +4, or +5. Therefore, the effect of the T42A substitution in GerE on binding to the mutant sites was specific to one position in the binding site on the sigK promoter.
DISCUSSION
In this study, we sought to determine if the HTH motif in GerE was required for the specificity of binding to ςK-dependent promoters. As the first step toward the identification of the determinants of GerE binding and specificity, we identified several specific nucleotides in the GerE binding consensus sequence that were important for the binding affinity of GerE. The results suggested that specific nucleotides in the center of the consensus sequence are necessary for a high-affinity interaction with GerE. However, we found that a substitution at a position highly conserved in the consensus (+5) had the smallest effect of the substitutions examined, whereas the change at a weakly conserved position (+3) had the greatest effect on binding. It is not known whether the optimal sequence for GerE binding differs from the consensus generated from the comparison of a few sites, or whether the effects of these mutations are dependent on the surrounding DNA sequences. The GerE binding site on sigK is complicated because there are inverted repeats of the consensus, and the stoichiometry of GerE binding to this region is not known. In addition, it is not known whether binding to these two consensus-like sites is cooperative or independent.
We next examined the effects of alanine substitutions in the HTH region of GerE on its ability to bind to wild-type or mutant target sites on the sigK promoter. The T42A-substituted GerE binds with similar apparent affinities to two DNA sequences that differ by a single specific base pair, whereas wild-type GerE binds with different affinities to the two sequences. Therefore, the T42A-substituted GerE has lost its ability to discriminate between these two DNA sequences. The T42A-substituted GerE retained its ability to discriminate between the sites with other single base pair substitutions (i.e., +4 [Fig. 4]). Recently, Ducros et al. (9, 10) determined the crystal structure of GerE at 2.05 Å. In their structure, threonine at position 42 is located on the surface of the protein near the amino-terminal end of the putative recognition helix of the HTH motif (10). Therefore, a substitution of alanine at this position is not likely to grossly affect the structure of GerE. Since the T42A substitution affects binding specificity but is unlikely to have long-range effects on the structure of the protein, the threonine at position 42 of GerE probably is located within or near the amino acids that make sequence-specific contacts with DNA.
We have also considered an alternative class of models in which the effects of the T42A substitution would be explained by suggesting that T42A affects the interaction between adjacent GerE molecules bound to the sigK site. We have rejected these models because the crystal structure of GerE predicts that T42 is located far from the dimer interface; therefore, T42A is not likely to affect dimer formation. Furthermore, models in which T42A affects adjacent dimers cannot be reconciled with the result that binding of T42A-substituted GerE is unaffected by the +3 nucleotide substitution on the sigK promoter, whereas its binding is reduced by the +4 substitution, which lies in the same half of the consensus binding site. We therefore suggest that the effects of the T42A substitution on DNA binding cannot be explained by models in which T42 is involved in protein-protein interactions.
Although we suggest that T42 of GerE lies in close proximity to DNA in GerE-DNA complexes and near other amino acids of GerE that contact DNA, it is not known whether T42 interacts directly with a base pair in the DNA. The T42A-substituted GerE failed to recognize base pair changes at position +3 of sigK (i.e., its binding to the sigK promoter was affected by changes at this position). However, T42 of GerE may interact with a region of the DNA site that is different from position +3. The loss of an interaction with DNA caused by the T42A substitution may have affected other interactions between GerE and the DNA so that the interaction between GerE and position +3 contributed little to the binding energy. The sigK promoter contains two regions that are similar to the consensus GerE binding site (Fig. 1). Since the stoichiometry of binding to this site is not known, we have not attempted to build high-resolution models of GerE bound to DNA. Nevertheless, we can imagine models in which T42A affects the specificity of binding but T42 in wild-type GerE does not directly interact with position +3 of the sigK site. For example, in one model T42 in each subunit of the GerE dimer would be involved in binding the distal ends of the DNA site so that the DNA would be bent around GerE. In this model, the T42A-GerE binds without bending the DNA. In this complex of T42A-GerE and DNA, an interaction between the base pair at +3 and a residue in GerE may be absent because the +3 position of the DNA is not held in close proximity to GerE. Therefore, interaction between the +3 position of the DNA and an amino acid in GerE at a position different from 42 would contribute nothing to the binding energy. In contrast, the interaction between position +3 in the bent DNA complex and a residue in the wild-type protein would contribute to the binding energy. Evidence that the T42A form of GerE is positioned differently on DNA complexes than is wild-type GerE can be seen in the DNase I footprinting experiments. For example, position +15, which is located near the end of the binding site, is not protected from DNase by bound T42A-GerE, whereas this position is protected from DNase by the wild-type GerE (Fig. 5). Therefore, the T42A substitution affects DNA binding both by reducing affinity for the DNA and by altering the positioning of GerE on the site. Thus, we cannot determine how T42 interacts with DNA, rather only that it probably lies in close proximity to the DNA.
We have studied the effect of only one other alanine substitution within this region of GerE. The R44A substitution in GerE reduced binding of GerE; however, we did not observe a change in its specificity for binding to the DNA. The R44A substitution would be a good candidate for further exploration with other mutant DNA sites since it is homologous to the residue of ς70 that makes a sequence-specific interaction with promoter DNA (15, 25) (Fig. 3). Furthermore, R44 of GerE aligns with position E174 of FixJ (Fig. 3), which was shown elsewhere to be important for the transcription activation of PnifA (19). Kahn and Ditta suggested that this position of the HTH may be involved in the specificity of promoter recognition (19). It is possible that R44 in GerE affects the specificity of its interaction with a base pair in the target sequence that we have not yet tested.
The model that the T42A substitution defines the surface of GerE that binds DNA has important implications but also raises a number of questions. Most members of the LuxR-FixJ family of transcription activator proteins bind DNA as homomultimers. This idea is supported by the crystal structure of NarL (3), in which NarL was purified as a dimer (2). Although GerE was crystallized as a dimer (9, 10), it is unknown whether a dimer binds the DNA, or how the dimer is positioned over the consensus sequence.
Finally, we emphasize that T42 of GerE is located in a position that is highly conserved among the LuxR-FixJ family members (Fig. 3). It has recently been suggested that R212 of LuxR is important for its interaction with DNA (11, 27). This amino acid corresponds to K41 of GerE (Fig. 3), the amino acid adjacent to T42. Therefore, it seems likely that the amino acid at this position on the other members of the LuxR-FixJ family makes sequence-specific contacts with DNA or that it is located near the residue(s) that directly interacts with DNA.
ACKNOWLEDGMENTS
We gratefully acknowledge Jim Brannigan and his collaborators at York University for providing us with the GerE crystal structure information, as well as thoughtful comments throughout this study, and L. Kroos, J. Boss, and G. Munson for critical comments on the manuscript.
This work was supported by grant MCB-9727722 to C.P.M. from the National Science Foundation.
REFERENCES
- 1.Arico B, Miller J F, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baikalov I, Schroder I, Kaczor-Grzeskowiak M, Cascio D, Gunsalus R P, Dickerson R E. NarL dimerization? Suggestive evidence from a new crystal form. Biochemistry. 1998;37:3665–3676. doi: 10.1021/bi972365a. [DOI] [PubMed] [Google Scholar]
- 3.Baikalov I, Schroder I, Kaczor-Grzeskowiak M, Grzeskowiak K, Gunsalus R P, Dickerson R E. Structure of the Escherichia coli response regulator NarL. Biochemistry. 1996;35:11053–11061. doi: 10.1021/bi960919o. [DOI] [PubMed] [Google Scholar]
- 4.Cole S T, Raibaud O. The nucleotide sequence of the malT gene encoding the positive regulator of the Escherichia coli maltose regulon. Gene. 1986;42:201–208. doi: 10.1016/0378-1119(86)90297-0. [DOI] [PubMed] [Google Scholar]
- 5.Cutting S, Panzer S, Losick R. Regulatory studies on the promoter for a gene governing synthesis and assembly of the spore coat in Bacillus subtilis. J Mol Biol. 1989;207:393–404. doi: 10.1016/0022-2836(89)90262-3. [DOI] [PubMed] [Google Scholar]
- 6.Danot O, Vidal-Ingigliardi D, Raibaud O. Two amino acid residues from the DNA-binding domain of MalT play a crucial role in transcriptional activation. J Mol Biol. 1996;262:1–11. doi: 10.1006/jmbi.1996.0493. [DOI] [PubMed] [Google Scholar]
- 7.David M, Daveran M L, Batut J, Dedieu A, Domergue O, Ghai J, Hertig C, Boistard P, Kahn D. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988;54:671–683. doi: 10.1016/s0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- 8.Devine J H, Countryman C, Baldwin T O. Nucleotide sequence of the luxR and luxI genes and the structure of the primary regulatory region of the lux regulon of Vibrio fischeri ATCC 7744. Biochemistry. 1988;27:837–842. [Google Scholar]
- 9.Ducros V M, Brannigan J A, Lewis R J, Wilkinson A J. Bacillus subtilis regulatory protein GerE. Acta Crystallogr D Biol Crystallogr. 1998;54:1453–1455. doi: 10.1107/s0907444998004892. [DOI] [PubMed] [Google Scholar]
- 10.Ducros V M, Lewis R J, Verma C S, Dodson E J, Leonard G, Turkenburg J P, Murshudov G N, Wilkinson A J, Brannigan J A. Crystal structure of GerE, the ultimate transcriptional regulator of spore formation in Bacillus subtilis. J Mol Biol. 2001;306:759–771. doi: 10.1006/jmbi.2001.4443. [DOI] [PubMed] [Google Scholar]
- 11.Egland K A, Greenberg E P. Quorum sensing in Vibrio fischeri: analysis of the LuxR DNA binding region by alanine-scanning mutagenesis. J Bacteriol. 2001;183:382–386. doi: 10.1128/JB.183.1.382-386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egland K A, Greenberg E P. Quorum sensing in Vibrio fischeri: elements of the luxI promoter. Mol Microbiol. 1999;31:1197–1204. doi: 10.1046/j.1365-2958.1999.01261.x. [DOI] [PubMed] [Google Scholar]
- 13.Engebrecht J, Silverman M. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic Acids Res. 1987;15:10455–10467. doi: 10.1093/nar/15.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich M J, Kadner R J. Nucleotide sequence of the uhp region of Escherichia coli. J Bacteriol. 1987;169:3556–3563. doi: 10.1128/jb.169.8.3556-3563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardella T, Moyle H, Susskind M M. A mutant Escherichia coli sigma 70 subunit of RNA polymerase with altered promoter specificity. J Mol Biol. 1989;206:579–590. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 16.Gross R, Arico B, Rappuoli R. Families of bacterial signal-transducing proteins. Mol Microbiol. 1989;3:1661–1667. doi: 10.1111/j.1365-2958.1989.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 17.Gunsalus R P, Kalman L V, Stewart R R. Nucleotide sequence of the narL gene that is involved in global regulation of nitrate controlled respiratory genes in Escherichia coli. Nucleic Acids Res. 1989;17:1965–1975. doi: 10.1093/nar/17.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichikawa H, Halberg R, Kroos L. Negative regulation by the Bacillus subtilis GerE protein. J Biol Chem. 1999;274:8322–8327. doi: 10.1074/jbc.274.12.8322. [DOI] [PubMed] [Google Scholar]
- 19.Kahn D, Ditta G. Modular structure of FixJ: homology of the transcriptional activator domain with the −35 binding domain of sigma factors. Mol Microbiol. 1991;5:987–997. doi: 10.1111/j.1365-2958.1991.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 20.Kenney T J, Moran C P., Jr Genetic evidence for interaction of ςA with two promoters in Bacillus subtilis. J Bacteriol. 1991;173:3282–3290. doi: 10.1128/jb.173.11.3282-3290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonetto M, Gribskov M, Gross C A. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkel T J, Nelson D M, Brauer C L, Kadner R J. Promoter elements required for positive control of transcription of the Escherichia coli uphT gene. J Bacteriol. 1992;174:2763–2770. doi: 10.1128/jb.174.9.2763-2770.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pao G M, Saier M H., Jr Response regulators of bacterial transduction systems: selective domain shuffling during evolution. J Mol Evol. 1995;40:136–154. doi: 10.1007/BF00167109. [DOI] [PubMed] [Google Scholar]
- 24.Pao G M, Tam R, Lipschitz L S, Saier M H., Jr Response regulators: structure, function and evolution. Res Microbiol. 1994;145:356–362. doi: 10.1016/0923-2508(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 25.Siegele D A, Hu J C, Walter W A, Gross C A. Altered promoter recognition by mutant forms of the sigma 70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989;206:591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- 26.Tatti K M, Shuler M F, Moran C P., Jr Sequence-specific interactions between promoter DNA and the RNA polymerase sigma factor E. J Mol Biol. 1995;253:8–16. doi: 10.1006/jmbi.1995.0531. [DOI] [PubMed] [Google Scholar]
- 27.Trott A E, Stevens A M. Amino acid residues in LuxR critical for its mechanism of transcriptional activation during quorum sensing in Vibrio fischeri. J Bacteriol. 2001;183:387–392. doi: 10.1128/JB.183.1.387-392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal-Ingigliardi D, Richet E, Danot O, Raibaud O. A small C-terminal region of the Escherichia coli MalT protein contains the DNA binding domain. J Biol Chem. 1993;268:24527–24530. [PubMed] [Google Scholar]
- 29.Wade K H, Schyns G, Opdyke J A, Moran C P., Jr A region of ςK involved in promoter activation by GerE in Bacillus subtilis. J Bacteriol. 1999;181:4365–4373. doi: 10.1128/jb.181.14.4365-4373.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webber C A, Kadner R J. Involvement of the amino-terminal phosphorylation module of UhpA in activation of uphT transcription in Escherichia coli. Mol Microbiol. 1997;24:1039–1048. doi: 10.1046/j.1365-2958.1997.4021765.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Ichikawa H, Halberg R, Kroos L, Aronson A I. Regulation of the transcription of a cluster of Bacillus subtilis spore coat genes. J Mol Biol. 1994;240:405–415. doi: 10.1006/jmbi.1994.1456. [DOI] [PubMed] [Google Scholar]
- 32.Zheng L, Halberg R, Roels S, Ichikawa H, Kroos L, Losick R. Sporulation regulatory protein GerE from Bacillus subtilis binds to and can activate or repress transcription from promoters for mother-cell-specific genes. J Mol Biol. 1992;226:1037–1050. doi: 10.1016/0022-2836(92)91051-p. [DOI] [PubMed] [Google Scholar]