Abstract
Transcription factors (TFs) with the basic leucin zipper domain are key elements of the stress response pathways in filamentous fungi. In this study, we functionally characterized the two bZIP type TFs AtfA and AtfB by deletion (Δ) and overexpression (OE) of their encoding genes in all combination: ΔatfA, ΔatfB, ΔatfAΔatfB, ΔatfAatfBOE, ΔatfBatfAOE, atfAOE, atfBOE and atfAOEatfBOE in Aspergillus nidulans. Based on our previous studies, ΔatfA increased the sensitivity of the fungus to oxidative stress mediated by menadione sodium bisulfite (MSB) and tert-butylhydroperoxide (tBOOH), while ΔatfB was not sensitive to any oxidative stress generating agents, namely MSB, tBOOH and diamide at all. Contrarily, the ΔatfB mutant was sensitive to NaCl, but tolerant to sorbitol. Overexpression of atfB was able to compensate the MSB sensitivity of the ΔatfA mutant. Heavy metal stress elicited by CdCl2 reduced diameter of the atfBOE and atfAOEatfBOE mutant colonies to about 50% of control colony, while the cell wall stress generating agent CongoRed increased the tolerance of the ΔatfA mutant. When we tested the heat stress sensitivity of the asexual spores (conidiospores) of the mutants, we found that conidiospores of ΔatfAatfBOE and ΔatfBatfAOE showed nearly 100% tolerance to heat stress. Asexual development was negatively affected by ΔatfA, while atfAOE and atfAOE coupled with ΔatfB increased the number of conidiospores of the fungus approximately 150% compared to the control. Overexpression of atfB led to a 25% reduction in the number of conidiospores, but increased levels of abaA mRNA and size of conidiospores. Sexual fruiting body (cleistothecium) formation was diminished in the ΔatfA and the ΔatfAΔatfB mutants, while relatively elevated in the ΔatfB and the ΔatfBatfAOE mutants. Production of the mycotoxin sterigmatocystin (ST) was decreased to undetectable levels in the ΔatfA mutant, yet ST production was restored in the ΔatfAΔatfB mutant, suggesting that ΔatfB can suppress ST production defect caused by ΔatfA. Levels of ST were also significantly decreased in the ΔatfAatfBOE, ΔatfBatfAOE and atfAOEatfBOE mutants.
Keywords: Aspergillus nidulans, environmental stress, conidiospore, cleistothecium, sterigmatocystin
Introduction
Basic-region leucine zipper (bZIP)-type transcription factors contribute to a complex regulatory network to organize differentiation, maintenance of cell types as well as stress responses of eukaryotic organisms. By forming homo-or heterodimers with other bZIP-type transcription factors they coordinate a great variety of cellular processes (Jindrich and Degnan, 2016; Leiter et al., 2021). The Schizosaccharomyces pombe Atf1 ortholog bZIP-type transcription factor AtfA regulates several processes including stress tolerance, secondary metabolism and development in vegetative hyphae of numerous filamentous fungi, e.g., Aspergillus nidulans (Lara-Rojas et al., 2011), Neurospora crassa (Yamashita et al., 2008), Magnaporthe oryzae (Guo et al., 2010), Botrytis cinerea (Temme et al., 2012), Fusarium verticillioides (Szabó et al., 2020). Moreover, AtfA is also involved in the virulence of the human pathogenic Aspergillus fumigatus (Silva et al., 2017) and also in the infection of hosts by plant pathogenic fungi, e.g., Claviceps purpurea (Nathues et al., 2004), Magnaporthe oryzae (Guo et al., 2010), Botrytis cinerea (Temme et al., 2012), Fusarium graminearum (Nguyen et al., 2013). In Aspergillus nidulans the Atf1 ortholog AtfA has been thoroughly characterized (Balázs et al., 2010; Emri et al., 2015; Orosz et al., 2017). AtfA contributes to the vegetative growth and conidiospore formation and also to the tolerance of the fungus to oxidative stress reagents such as menadione sodium bisulfite (MSB) and tert-butylhydroperoxide (tBOOH) (Balázs et al., 2010; Emri et al., 2015). Conidia of the ΔatfA mutant was also sensitive to osmotic, fungicide and heat stress (Hagiwara et al., 2008, 2009) and their viability were reduced after storage at 4°C (Balázs et al., 2010). Transcriptome based data confirmed that AtfA is important in the regulation of many stress-related and stress-unrelated genes (Emri et al., 2015; Antal et al., 2020) and likely to be involved in the regulation of numerous genes indirectly (Orosz et al., 2017; Antal et al., 2020). Formation of heterodimer of AtfA with other bZIP-type transcription factors, e.g., AtfB was first indicated by Lara-Rojas et al. (2011) in A. nidulans. In Aspergillus oryzae a transcriptome based study found a set of genes co-regulated by AtfA and AtfB, but AtfA seems to be more important in the regulation of the oxidative stress in this fungus (Sakamoto et al., 2009). In Aspergillus oryzae conidia of the ΔatfA mutant were more sensitive to oxidative stress than that of the ΔatfB mutant (Sakamoto et al., 2009). In Aspergillus fumigatus AtfA interacts with AtfB-D transcription factors and coordinate the stress response pathway and virulence of this human pathogenic fungus (Silva et al., 2021). According to the phenotype of the single or double deletion mutants of atfA and atfB in the presence of different environmental stress agents resulted in either epistatic, additive and suppression interaction of AtfA and AtfB suggests a versatile function of these bZIP transcription factors in A. fumigatus (Silva et al., 2021).
In this study, we analyzed the physiological functions of Aspergillus nidulans atfA and atfB through the construction of gene deletion and overexpression mutants in all combination. Stress sensitivity tests, conidiospore viability, sexual and asexual sporulation as well as sterigmatocystin (ST) production were involved in our phenotypic studies. Based on our observations AtfA seems to be more important in the stress response, conidiospore formation as well as mycotoxin production than AtfB and depending on the tested phenotype atfB overexpression can compensate the negative effect of the deletion of atfA.
Materials and methods
Strains, culture media
Aspergillus nidulans strains used in our study is summarized in Supplementary Table S1. All strains were maintained on Barratt’s nitrate minimal medium (NMM) with appropriate nutritional supplements (Barratt et al., 1965), and NMM agar plates were incubated at 37°C for 6 d (Balázs et al., 2010). Conidia harvested from these 6 days old plates were used in all further experiments.
Construction of gene deletion and overexpression strains
Gene deletion mutants were constructed by the Double-Joint PCR method of Yu et al. (2004) and Leiter et al. (2016) with primers listed in Supplementary Table S2. The amplified deletion cassettes were used to transform rJMP1.59 or TNJ36.1 strain using the Vinoflow FCE lysing enzyme (Szewczyk et al., 2006). Single copy transformants were selected after Southern blot analysis (Király et al., 2020a). To generate overexpression mutants ORFs were amplified with the primers presented in Supplementary Table S2. The amplicons were digested with restriction enzymes as indicated in Supplementary Table S2, and ligated between the niiA promoter and the trpC terminator in pHS11 (Leiter et al., 2016). Overexpression of the strains was confirmed by rRT-PCR method (Supplementary Figure S1; Király et al., 2020b).
Stress sensitivity studies
To study the stress sensitivity of the mutant strains, the agar plate assays of Balázs et al. (2010) were adapted. The following stress generating agents were tested: oxidative stress: 2.0 mM diamide (eliciting GSH/GSSG redox imbalance), 0.08 mM menadione sodium bisulfite (MSB, increasing intracellular superoxide level), 0.8 mM tert-butyl hydroperoxide (tBOOH, triggering lipid peroxidation; Emri et al., 1997; Pócsi et al., 2005); hyperosmotic stress: 1.5 M NaCl and 2.0 M sorbitol; heavy metal stress: 300 μm cadmium chloride (Leiter et al., 2016); cell wall integrity stress: 54 μm CongoRed (an agent known to alter cell wall polymer composition; Leiter et al., 2016). Plates were point-inoculated with 5 μl freshly made conidia suspension (2*107 conidia/ml) and were incubated at 37°C for 5 days (Balázs et al., 2010). In all stress sensitivity studies, the isogenic prototrophized THS30.3 strain was used as the control strain.
Conidiospore heat stress-sensitivity
To test the heat sensitivity of asexual spores, conidia were harvested from 6 days old colonies and suspended in physiological saline-0.01% Tween 80. Conidia in 105/ml concentration were incubated at 50°C for 10 min and, following that, were diluted and spread on NMM agar plates. The numbers of colonies representing successfully germinated conidia were counted after incubation for 2 days at 37°C. Conidia without any heat treatment were used as reference.
Sexual and asexual developments
To induce cleistothecium formation, 6 days old conidia were spread in agar at 105 conidia/plate and incubated at 37°C. After 24 h, plates were sealed with Parafilm and samples were taken with a cork borer after 14 days incubation and cleistothecia/cm2 were determined under a dissection microscope (Leiter et al., 2016).
The conidiospore forming capabilities of the A. nidulans strains were determined as published by Vargas-Pérez et al. (2007). Briefly, conidia (105) of the mutant and control strains were spotted onto NMM agar plates as described above, and were incubated and were allowed to sporulate at 37°C for 5 days. Conidia were washed, counted in a Burker chamber and spore numbers were expressed as number/cm2 of colony surface.
Evaluation of the size of conidiospores
5*105 conidia were spread onto NMM medium, then incubated at 37°C for 5 days. After incubation microscopic images were taken of the conidospores of the mutants and control strain in Burker chamber by ToupView image processing software. Correlated to the known length grid lines of Burker chamber, the size of conidiospores can be calculated. The size of conidiospores was also determined by SEM according to Springer and Yanofksy (1989). Briefly, point-inoculated 5 d old surface cultures were dehydrated stepwise by an ethanol series consisting of 30, 50, 70, 95, and 100% ethanol, 15 min per step. The samples were coated with gold, and observed under a scanning electron microscope (Hitachi S 4300, Schaumburg, United States).
Sterigmatocystin analysis
Levels of sterigmatocystin (ST) was determined from 5 days old surface cultures according to Yin et al. (2013). A 2 cm2 agar plug was removed of each plate culture and extracted with 800 μl by 70% (v/v) acetone. Metabolites were separated in the developing solvent toluene:ethyl acetate:acetic acid (TEA, 8:1:1) on silica coated thin-layer chromatography (TLC) plates and photographs were taken following exposure to UV radiation at 366 nm wavelengths.
The mycelial extracts were also subjected for HPLC analysis. Aliquots of 10 μl were injected into the chromatographic system which consisted of a Waters 2,695 Separations Module equipped with a thermostable autosampler (5°C) and column module (35°C). UV detection was applied by a Waters 2,996 photodiode array detector (254 nm). Separations were performed using an Agilent Zorbax SB-C18 (4.6 mm × 75 mm, 3.5 m) column with 1 ml/min flow rate. Isocratic elution was used where the mobile phase was methanol/acetonitrile/ water 50/15/35 (v/v), respectively (Yin et al., 2013).
rRT-PCR assays to determine abaA gene expression
Total RNA was isolated from surface cultures according to Chomczynski (1993) and rRT-PCR experiments were carried out as described previously (Emri et al., 2015). The applied primer pairs are summerized in Supplementary Table S2. Relative transcript levels were calculated by the ‘delta method’ where ΔCP = CP reference gene − CP abaA gene and CP stands for the rRT-PCR cycle numbers corresponding to the crossing points. For statistical analysis, the mean ± SD values were calculated from three independent experiments (Pfaffl, 2001). As reference gene, actA (AN6542) was used (Emri et al., 2015).
Statistical analysis of experimental data
All experiments were performed in three independent sets, and mean ± SD values were calculated and are presented. Statistical significances were calculated using Student’s t-test, and p-values less than 5% were considered as statistically significant.
Results
Stress sensitivity phenotypes of the mutants
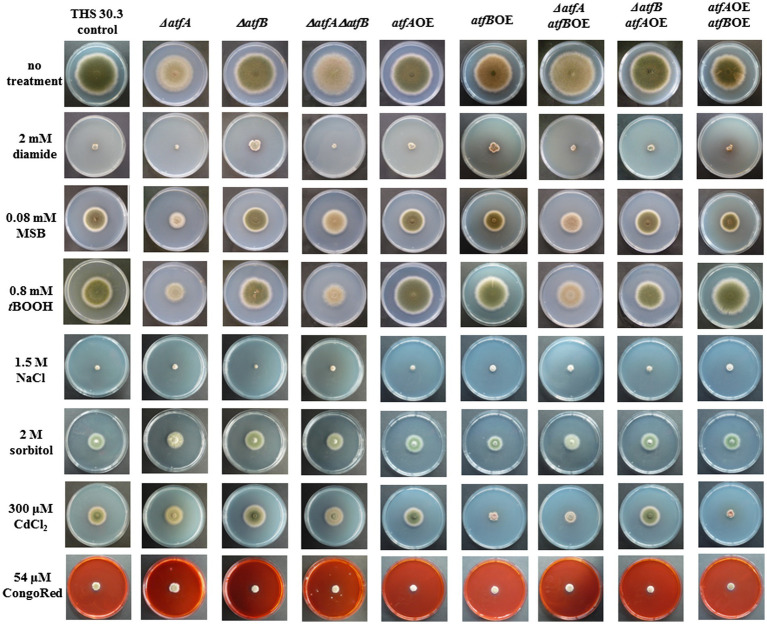
ΔatfA, atfBOE and atfAOEatfBOE strains showed reduced growth compared to the control strain on minimal medium at 37°C without any stress treatment. Increased sensitivity to oxidative stress inducing agent diamide was observed in the ΔatfAatfBOE and ΔatfAΔatfB as well as in the atfAOEatfBOE strains. Interestingly both the deletion and overexpression of atfB increased the diamide tolerance of the fungus. MSB sensitivity was detected only in the ΔatfA strain and atfBOE was able to compensate this stress sensitivity in the ΔatfAatfBOE mutant with approximately doubled colony growth compared to the control. Overexpression of atfA, atfB alone and together increased the tBOOH tolerance of the fungus with approximately 20%, while ΔatfA reduced the growth of A. nidulans to 50% in the presence of tBOOH. In the ΔatfB mutant compared to the control there was no difference in the tBOOH sensitivity, therefore the tBOOH sensitivity of the double deletion mutant is as a result of the deletion of atfA (Figure 1). To study the osmotic stress sensitivity we tested our mutants in the presence of 1.5 M NaCl and 2 M sorbitol. 1.5 M NaCl significantly reduced the growth of the ΔatfB mutant compared to the control, while the double overexpression mutant showed increased the tolerance to NaCl. Surprisingly, the ΔatfB mutant was the most tolerant while the atfBOE was the most sensitive to 2 M sorbitol compared to the other strains (Figure 1). The heavy metal stress sensitivity was tested in the presence of 300 μm CdCl2. The ΔatfAatfBOE mutant showed slightly reduced growth, while the diameter of the colony growth of atfBOE and atfAOEatfBOE mutant was half of that of the control strain exposed to CdCl2. Contrarily, the ΔatfB mutant was moderately tolerant to CdCl2 (Figure 1). Only the ΔatfA mutant was affected to the exposure to 54 μm CongoRed and showed moderate tolerance (Figure 1).
Figure 1.
Stress sensitivities of the THS30.3 (control) and the atfA and atfB gene deletion (Δ) and overexpression (OE) mutant strains exposed to various types of stress reagents. Stress sensitivities observed in surface cultures on NMM agar plates are shown. NMM agar plates were incubated at 37°C for 5 days.
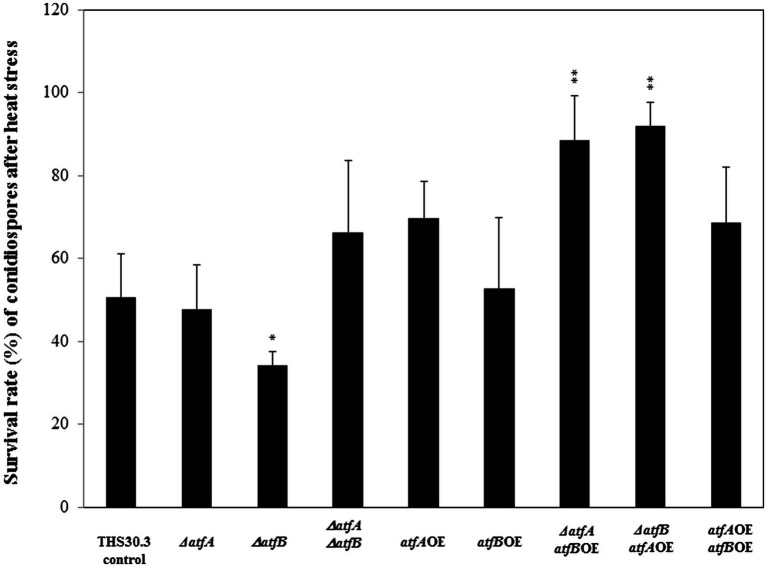
Heat stress-sensitivity of the conidiospores
We tested the viability of the conidiospores under heat stress. Incubation of conidiospores of the ΔatfAatfBOE and ΔatfBatfAOE mutant at 50°C for 10 min resulted in increased viability - survival rates of the conidiospores were nearly 100% -, meanwhile the asexual spores of the ΔatfB showed reduced viability after heat stress compared to the control strain (Figure 2). We did not find any differences in the heat stress sensitivity of the other mutants and the control strain.
Figure 2.
Heat stress sensitivity of conidiospores of the control and mutant strains. Conidia without heat treatment (50°C for 10 min) were used as control. Data are presented as mean ± SD values calculated from three independent experiments. Significant differences between control and mutant cultures (*p < 5%, **p < 1%) are indicated.
Sexual and asexual developments
We also quantified cleistothecia formation and conidiospore production in all mutants. Deletion of atfA and atfA, atfB together inhibited the cleistothecium formation, while in ΔatfB and ΔatfBatfAOE mutants approximately one and the half times higher fruiting body formation was observed compared to the control (Figure 3A).
Figure 3.
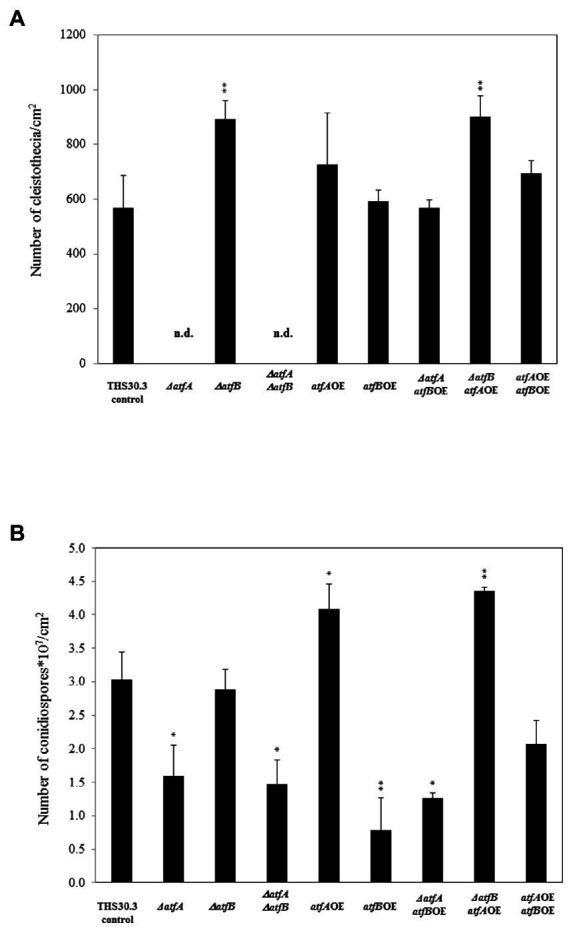
Cleistothecia and conidiospore productions of the control and mutant strains. (A) Cleistothecia productions observed after 14 days incubations. (B) Conidiospore productions. Data are presented as mean ± SD values calculated from three independent experiments. Significant differences between control and mutant cultures (*p < 5%, **p < 1%) are presented. n.d., not detected.
Deletion of atfA significantly decreased the number of conidiospores both in the ΔatfA as well as in the ΔatfAΔatfB mutants. In the atfBOE and ΔatfAatfBOE mutants also reduced conidiospore formation was observed. The overexpression of atfA alone and in the ΔatfB background increased the number of asexual spores of the fungus with nearly one and the half times (Figure 3B).
Evaluation of the size of conidiospores and abaA expression
We determined the size of conidiospores by light microscopy and SEM. We found that atfBOE mutant produced significantly larger conidiospores compared to the control strain (Figure 4A). We did not find any differences in the size of asexual spores in the rest of the mutants compared to the control strain. We also evaluated the abaA (element of the central regulatory pathway of conidiogenesis) gene expression of the surface cultures of the mutants. abaA was upregulated in the atfBOE mutant compared to the control, but there was no significant differences in the abaA expression between the control and ΔatfB gene deletion mutant (Figure 4B).
Figure 4.
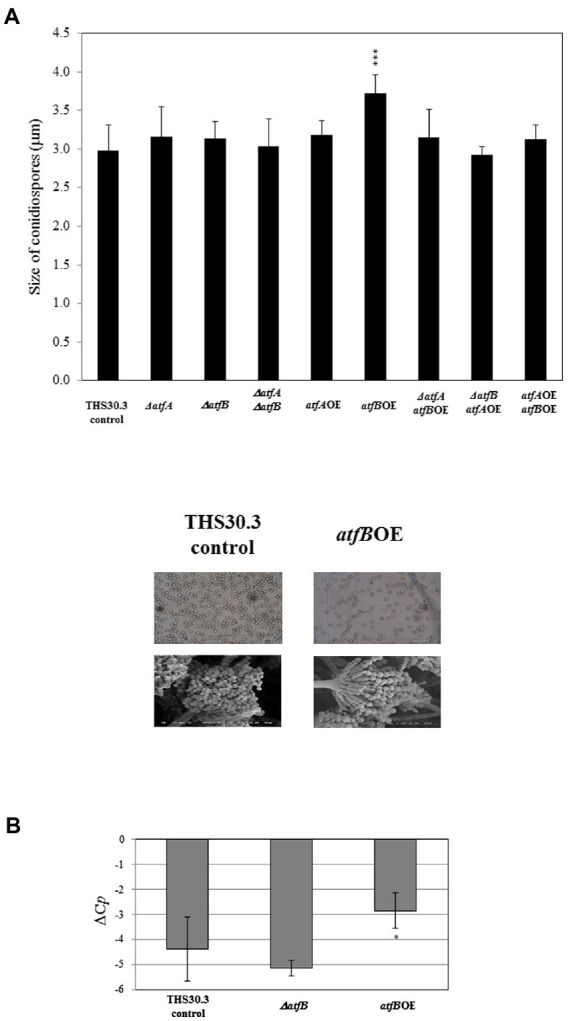
Determination of the size of conidiospores of the control and mutant strains (A). Evaluation of abaA gene expression in the control, ΔatfB and atfBOE mutants (B). Data are presented as mean ± SD values calculated from three independent experiments. Significant differences between control and mutant cultures (*p < 5% and ***p < 0.1%) are presented.
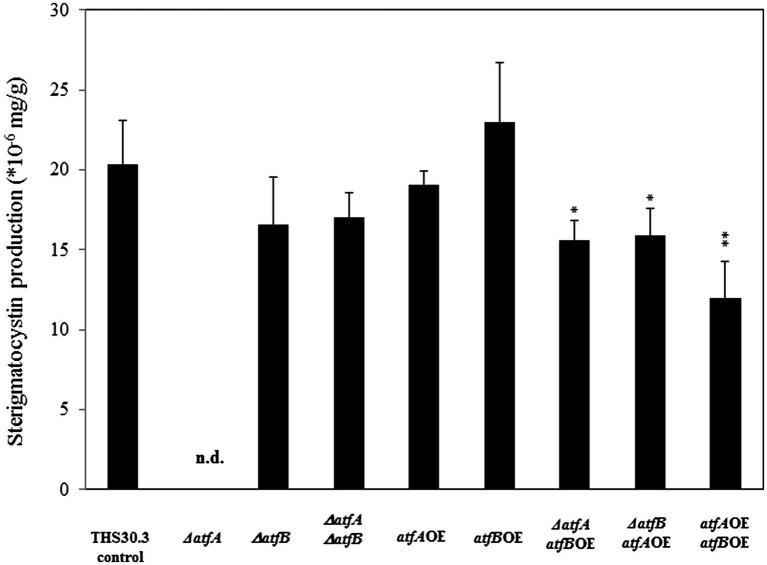
Sterigmatocystin determination
Sterigmatocystin production was determined from 5 days old surface cultures. Deletion of atfA resulted in a remarkable reduction of sterigmatocystin production (Figure 5). Interestingly deletion of both atfA and atfB together did not affect the sterigmatocystin biosynthesis compared to the control. We found decreased sterigmatocystin level in the ΔatfAatfBOE and ΔatfBatfAOE mutants, and also in the atfAOEatfBOE mutant where sterigmatocystin concentration was approximately half of that of the control strain.
Figure 5.
Sterigmatocystin production of the control and mutant strains. Data are presented as mean ± SD values calculated from three independent experiments. Significant differences between control and mutant cultures (*p < 5%, **p < 1%) are indicated. n.d., not detected.
Discussion
It is well known that bZIP type transcription factors are important elements of the stress signaling pathway, reproduction and secondary metabolite production in filamentous fungi (Bayram et al., 2008; Jindrich and Degnan, 2016; Leiter et al., 2021). In this study we constructed a series of gene deletion and overexpression mutants of atfA and atfB either alone or in combination to understand how these bZIP-type transcription factors regulates the stress tolerance, sexual and asexual reproduction and sterigmatocystin production in Aspergillus nidulans.
We managed to confirm previous observations that AtfA is involved in the oxidative stress defense system of Aspergillus nidulans (Figure 1; Hagiwara et al., 2008, 2009; Balázs et al., 2010; Emri et al., 2015). Deletion of atfA resulted in reduced growth in the presence of oxidative stress generating agents, e.g., diamide, tBOOH and menadione (Figure 1). In this work further functions of AtfA were unfolded. Deletion of atfA inhibited the cleistothecia production completely (Figure 3A) suggesting the outstanding role of AtfA in sexual reproduction of Aspergillus nidulans. It is well known that bZIP transcription factors play crucial role in the sexual development of filamentous fungi (Bayram et al., 2008; Yin et al., 2013). For example, Yin et al. (2013) confirmed that overexpression of rsmA (restorer of secondary metabolism A), a Yap-like bZIP showed near loss of ascospore production. In fungi sexual reproduction is coupled with secondary metabolism by the Velvet Complex (Bayram et al., 2008). For example, overexpression of rsmA increased the ST production with 100 fold in A. nidulans (Yin et al., 2013). Relation of secondary metabolism and sexual development was also described in the napA overexpression mutant (Yin et al., 2013). NapA similarly to AtfA and AtfB belongs to the Yap-family proteins (Yin et al., 2013). This correlation was also verified in our study since the ΔatfA mutant showed failure in fruting body formation and also loss of sterigmatocystion production (Figure 5). Similar phenotype was also observed in F. verticillioides, where the deletion of FvatfA inhibited fumonisin production (Szabó et al., 2020).
Based on our results AtfB seems to be more important in the heat stress sensitivity, CdCl2 sensitivity (Figure 1) and number (Figure 3B) and size of conidiospores than AtfA (Figure 4). Overexpression of atfB decreased the tolerance of the fungus to CdCl2 (Figure 1). Genome wide expression study by Emri et al. (2021) in A. nidulans confirmed that exposure to CdCl2 downregulates atfB gene expression in the control strain, while no alteration of the atfB expression was observed in the ΔatfA mutant (transcriptome data accession number: GSE166128). Overexpression of atfB decreased the number and increased the size of asexual spores (Figures 3B, 4) and also increased the abaA gene expression (Adams et al., 1998). In Beauveria bassiana, a filamentous entomopathogen deletion of wetA resulted in 90% repression of abaA gene expression and concomitantly smaller size of conidia (Li et al., 2015). In Fusarium graminearum overexpression of abaA caused in pleiotropic defects such as impaired sexual and asexual development, delayed conidium germination, and decreased trichothecene production (Son et al., 2013). In Aspergillus fumigatus overexpression of AfuabaA resulted in autolysis and cell death (Tao and Yu, 2011). Similarly, in Aspergillus oryzae AtfB is also important in the production of conidia (Sakamoto et al., 2008). Under osmotic stress conditions ΔatfB produced less conidia in A. oryzae suggesting the role of atfB in the development of conidiospores as well (Sakamoto et al., 2008, 2009). In our study, ΔatfB was sensitive to heat stress similarly to the ΔatfB in A. oryzae (Sakamoto et al., 2008; Figure 2).
Analysis of the phenotype of the mutants where both atfA and atfB were manipulated genetically indicates that some of the physiological functions of Aspergillus nidulans are coordinated by both of the bZIPs. For example, we observed the highest heat stress tolerance in the ΔatfAatfBOE and ΔatfBatfAOE strains compared to those of the rest of the mutants and the control strain (Figure 2). No fruiting body formation was observed in the ΔatfAΔatfB double deletion mutant, but more cleistothecia were produced in the ΔatfBatfAOE strain compared to the control, but the number of cleistothecia of ΔatfBatfAOE and ΔatfB was similar (Figure 3A). Surprisingly, when atfB was also deleted in the ΔatfA mutant sterigmatocystin production was similar to that of the control strain and in the atfAOEatfBOE mutant we observed less toxin production than in the atfAOE and atfBOE mutants (Figure 5) suggesting that toxin production is likely under the control of both bZIPs in A. nidulans.
bZIP transcription factors can form homodimers with themselves and heterodimers with other bZIPs and may also interact physically with stress signaling proteins as well (Lara-Rojas et al., 2011; Silva et al., 2021). For example, in A. fumigatus AtfA physically interacts with other three bZIP transcription factors, namely AtfB, AtfC and AtfD as well as with the MAPK SakA to coordinate stress response (Silva et al., 2021). In contrast to our observation the double deletion mutant ΔatfAΔatfB was as sensitive as MSB as the corresponding single mutants in A. fumigatus. Both ΔatfA and ΔatfB was as sensitive as ΔatfAΔatfB to the cell wall stress generating agents calcofluor white (CFW) and CongoRed (Silva et al., 2021).
Based on our results AtfA and AtfB may interact with each other to coordinate expressions of genes involved in the stress tolerance, sexual and asexual development as well as secondary metabolite production in A. nidulans. To confirm this hypothesis further studies, e.g., BiFC experiments are in progress in our laboratory.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
J-HY, IP, and ÉL: conceptualization and writing. BK, M-KL, TN, LD, and GB: methodology. All authors discussed the review and contributed to the final manuscript.
Funding
The research was supported by the European Union and the European Social Fund through the project EFOP-3.6.1-16-2016-00022, Thematic Excellence Programme (TKP2021-EGA-20; Biotechnology) of the Ministry for Innovation and Technology in Hungary, and National Research, Development and Innovation Office with the grants NKFIH K119494 and NN125671. The work at UW-Madison was supported by the UW Food Research Institute.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1003709/full#supplementary-material
References
- Adams T. H., Wieser J. K., Yu J. H. (1998). Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62, 35–54. doi: 10.1128/MMBR.62.1.35-54.1998, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal K., Gila B. C., Pócsi I., Emri T. (2020). General stress response or adaptation to rapid growth in Aspergillus nidulans?. Fungal Biol. 124, 376–386. doi: 10.1016/j.funbio.2019.10.009 [DOI] [PubMed] [Google Scholar]
- Balázs A., Pócsi I., Hamari Z., Leiter É., Emri T., Miskei M., et al. (2010). AtfA BZIP-type transcription factor regulates oxidative and osmotic stress responses in Aspergillus nidulans. Mol. Gen. Genomics 283, 289–303. doi: 10.1007/s00438-010-0513-z, PMID: [DOI] [PubMed] [Google Scholar]
- Barratt R. W., Johnson G. B., Ogata W. N. (1965). Wild-type and mutant stocks of Aspergillus nidulans. Genetics 52, 233–246. doi: 10.1093/genetics/52.1.233, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram O., Krappmann S., Ni M., Bok J. W., Helmstaedt K., Valerius O., et al. (2008). VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320, 1504–1506. doi: 10.1126/science.1155888, PMID: [DOI] [PubMed] [Google Scholar]
- Chomczynski P. (1993). A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 15, 532–4–536–7. [PubMed] [Google Scholar]
- Emri T., Gila B., Antal K., Fekete F., Moon H., Yu J.-H., et al. (2021). AtfA-independent adaptation to the toxic heavy metal cadmium an Aspergillus nidulans. Microorganisms 9:1433. doi: 10.3390/microorganisms9071433, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emri T., Pócsi I., Szentirmai A. (1997). Glutathione metabolism and protection against oxidative stress caused by peroxides in Penicillium chrysogenum. Free Radic. Biol. Med. 23, 809–814. doi: 10.1016/s0891-5849(97)00065-8, PMID: [DOI] [PubMed] [Google Scholar]
- Emri T., Szarvas V., Orosz E., Antal K., Park H., Han K. H., et al. (2015). Core oxidative stress response in Aspergillus nidulans. BMC Genomics 16:478. doi: 10.1186/s12864-015-1705-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Guo W., Chen Y., Dong S., Zhang X., Zhang H., et al. (2010). The basic leucine zipper transcription factor Moatf1 mediates oxidative stress responses and is necessary for full virulence of the rice blast fungus Magnaporthe oryzae. Mol. Plant-Microbe Interact. 23, 1053–1068. doi: 10.1094/MPMI-23-8-1053, PMID: [DOI] [PubMed] [Google Scholar]
- Hagiwara D., Asano Y., Marui J., Yoshimi A., Mizuno T., Abe K. (2009). Transcriptional profiling for Aspergillus nidulans HogA MAPK signaling pathway in response to fludioxonil and osmotic stress. Fungal Genet. Biol. 46, 868–878. doi: 10.1016/j.fgb.2009.07.003, PMID: [DOI] [PubMed] [Google Scholar]
- Hagiwara D., Asano Y., Yamashino T., Mizuno T. (2008). Characterization of bZip-type transcription factor AtfA with reference to stress responses of conidia of Aspergillus nidulans. Biosci. Biotechnol. Biochem. 72, 2756–2760. doi: 10.1271/bbb.80001, PMID: [DOI] [PubMed] [Google Scholar]
- Jindrich K., Degnan B. M. (2016). The diversification of the basic leucine zipper family in eukaryotes correlates with the evolution of multicellularity. BMC Evol. Biol. 16:28. doi: 10.1186/s12862-016-0598-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Király A., Hámori C., Gyémánt G., Kövér E. K., Pócsi I., Leiter É. (2020a). Characterization of gfdb, putatively encoding a glycerol 3-phosphate dehydrogenase in Aspergillus nidulans. Fungal Biol. 124, 352–360. doi: 10.1016/j.funbio.2019.09.011, PMID: [DOI] [PubMed] [Google Scholar]
- Király A., Szabó I. G., Emri T., Leiter É., Pócsi I. (2020b). Supplementation of Aspergillus glaucus with gfdB gene encoding a glycerol 3-phosphate dehydrogenase in Aspergillus nidulans. J. Basic Microbiol. 60, 691–698. doi: 10.1002/jobm.202000067, PMID: [DOI] [PubMed] [Google Scholar]
- Kong Q., Wang L., Liu Z., Kwon N.-J., Kim S. C., Yu J.-H. (2013). Gβ-like CpcB plays a crucial role for growth and development of Aspergillus nidulans and Aspergillus fumigatus. PLoS One 8:e70355. doi: 10.1371/journal.pone.0070355, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Rojas F., Sanchez O., Kawasaki L., Aguirre J. (2011). Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Mol. Microbiol. 80, 436–454. doi: 10.1111/j.1365-2958.2011.07581.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter É., Emri T., Pákozdi K., Hornok L., Pócsi I. (2021). The impact of bZIP Atf1 ortholog global regulators in fungi. Appl. Microbiol. Biotechnol. 105, 5769–5783. doi: 10.1007/s00253-021-11431-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter É., Park H.-S., Kwon N.-J., Han K.-H., Emri T., Oláh V., et al. (2016). Characterization of the aodA, dnmA, mnSOD and pimA genes in Aspergillus nidulans. Sci. Rep. 6:20523. doi: 10.1038/srep20523, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Shi H.-Q., Ying S.-H., Feng M.-G. (2015). WetA and VosA are distinct regulators of conidiation capacity, conidial quality, and biological control potential of a fungal insect pathogen. Appl. Microbiol. Biotechnol. 99, 10069–10081. doi: 10.1007/s00253-015-6823-7, PMID: [DOI] [PubMed] [Google Scholar]
- Nathues E., Joshi S., Tenberge K. B., von den Driesch M., Oeser B., Bäumer N., et al. (2004). CPTF1, a CREB-like transcription factor, is involved in the oxidative stress response in the phytopathogen Claviceps purpurea and modulates ROS level in its host Secale cereale. Mol. Plant-Microbe Interact. 17, 383–393. doi: 10.1094/MPMI.2004.17.4.383, PMID: [DOI] [PubMed] [Google Scholar]
- Nguyen T. V., Kröger C., Bönnighausen J., Schäfer W., Jörg B. (2013). The ATF/CREB transcription factor Atf1 is essential for full virulence, deoxynivalenol production, and stress tolerance in the cereal pathogen Fusarium graminearum. Mol. Plant-Microbe Interact. 26, 1378–1394. doi: 10.1094/MPMI-04-13-0125-R, PMID: [DOI] [PubMed] [Google Scholar]
- Orosz E., Antal K., Gazdag Z., Szabó Z., Han K. H., Yu J.-H., et al. (2017). Transcriptome-based modeling reveals that oxidative stress induces modulation of the AtfA-dependent signaling networks in Aspergillus nidulans. Int. J. Genom. 2017:6923849. doi: 10.1155/2017/6923849, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. doi: 10.1093/nar/29.9.e45, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pócsi I., Miskei M., Karányi Z., Emri T., Ayoubi P., Pusztahelyi T., et al. (2005). Comparison of gene expression signatures of diamide, H2O2 and menadione exposed Aspergillus nidulans cultures-linking genome-wide transcriptional changes to cellular physiology. BMC Genomics 6:182. doi: 10.1186/1471-2164-6-182, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Arima T. H., Iwashita K., Yamada O., Gomi K., Akita O. (2008). Aspergillus oryzae atfB encodes a transcription factor required for stress tolerance in conidia. Fungal Genet. Biol. 45, 922–932. doi: 10.1016/j.fgb.2008.03.009, PMID: [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Iwashita K., Yamada O., Kobayashi K., Mizuno A., Akita O., et al. (2009). Aspergillus oryzae atfA controls conidial germination and stress tolerance. Fungal Genet. Biol. 46, 887–897. doi: 10.1016/j.fgb.2009.09.004, PMID: [DOI] [PubMed] [Google Scholar]
- Silva L. P., Alves de Castro P., Reis T. F., Paziani M. H., Von Zeska Kress M. R., Riaño-Pachón D. M., et al. (2017). Genome-wide transcriptome analysis of Aspergillus fumigatus exposed to osmotic stress reveals regulators of osmotic and cell wall stresses that are SakAHOG1 and MpkC dependent. Cell. Microbiol. 19. doi: 10.1111/cmi.12681, PMID: [DOI] [PubMed] [Google Scholar]
- Silva L. P., Horta M. A. C., Goldman G. H. (2021). Genetic interactions between aspergillus fumigatus basic leucine zipper (bZIP) transcription factors AtfA, AtfB, AtfC and AtfD. Front. Fungal Biol. 2:632048. doi: 10.3389/ffunb.2021.632048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H., Kim M. G., Min K., Seo Y. S., Lim J. Y., Choi G. J., et al. (2013). AbaA regulates conidiogenesis in the ascomycete fungus Fusarium graminearum. PLoS One 8:e72915. doi: 10.1371/journal.pone.0072915, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. L., Yanofsky C. (1989). A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 3, 559–571. doi: 10.1101/gad.3.4.559, PMID: [DOI] [PubMed] [Google Scholar]
- Szabó Z., Pákozdi K., Murvai K., Pusztahelyi T., Kecskeméti Á., Gáspár A., et al. (2020). FvatfA regulates growth, stress tolerance as well as mycotoxin and pigment productions in Fusarium verticillioides. Appl. Microbiol. Biotechnol. 104, 7879–7899. doi: 10.1007/s00253-020-10717-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E., Nayak T., Oakley C. E., Edgerton H., Xiong Y., Taheri-Talesh N., et al. (2006). Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 1, 3111–3120. doi: 10.1038/nprot.2006.405, PMID: [DOI] [PubMed] [Google Scholar]
- Tao L., Yu J. H. (2011). AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology 157, 313–326. doi: 10.1099/mic.0.044271-0, PMID: [DOI] [PubMed] [Google Scholar]
- Temme N., Oeser B., Massaroli M., Heller J., Simon A., Collado I. G., et al. (2012). BcAtf1, a global regulator, controls various differentiation processes and phytotoxin production in Botrytis cinerea. Mol. Plant-Microbe Interact. 13, 704–718. doi: 10.1111/j.1364-3703.2011.00778.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Pérez I., Sánchez O., Kawasaki L., Georgellis D., Aguirre J. (2007). Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot. Cell 6, 1570–1583. doi: 10.1128/EC.00085-07, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Shiozawa A., Watanabe S., Fukumori F., Kimura M., Fujimura M. (2008). ATF-1 transcription factor regulates the expression of ccg-1 and cat-1 genes in response to fludioxonil under OS-2 MAP kinase in Neurospora crassa. Fungal Genet. Biol. 45, 1562–1569. doi: 10.1016/j.fgb.2008.09.012, PMID: [DOI] [PubMed] [Google Scholar]
- Yin W. B., Reinke A. W., Szilágyi M., Emri T., Chiang Y. M., Keating A. E., et al. (2013). bZIP transcription factors affecting secondary metabolism, sexual development and stress responses in Aspergillus nidulans. Microbiology 159, 77–88. doi: 10.1099/mic.0.063370-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.-H., Hamari Z., Han K. H., Seo J. A., Reyes-Dominguez Y., Scazzocchio C. (2004). Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–981. doi: 10.1016/j.fgb.2004.08.001, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.