Abstract
The proteins involved in the utilization of l-arabinose by Bacillus subtilis are encoded by the araABDLMNPQ-abfA metabolic operon and by the araE/araR divergent unit. Transcription from the ara operon, araE transport gene, and araR regulatory gene is induced by l-arabinose and negatively controlled by AraR. The purified AraR protein binds cooperatively to two in-phase operators within the araABDLMNPQ-abfA (ORA1 and ORA2) and araE (ORE1 and ORE2) promoters and noncooperatively to a single operator in the araR (ORR3) promoter region. Here, we have investigated how AraR controls transcription from the ara regulon in vivo. A deletion analysis of the ara promoters region showed that the five AraR binding sites are the key cis-acting regulatory elements of their corresponding genes. Furthermore, ORE1-ORE2 and ORR3 are auxiliary operators for the autoregulation of araR and the repression of araE, respectively. Analysis of mutations designed to prevent cooperative binding of AraR showed that in vivo repression of the ara operon requires communication between repressor molecules bound to two properly spaced operators. This communication implicates the formation of a small loop by the intervening DNA. In an in vitro transcription system, AraR alone sufficed to abolish transcription from the araABDLMNPQ-abfA operon and araE promoters, strongly suggesting that it is the major protein involved in the repression mechanism of l-arabinose-inducible expression in vivo. The ara regulon is an example of how the architecture of the promoters is adapted to respond to the particular characteristics of the system, resulting in a tight and flexible control.
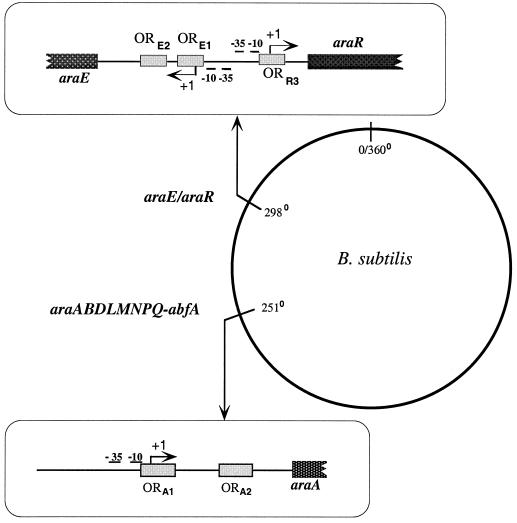
Bacillus subtilis is able to grow on l-arabinose as the sole carbon and energy source. The genes characterized so far involved in the utilization of l-arabinose in B. subtilis are those belonging to the araABDLMNPQ-abfA operon (41) and to the divergently arranged araE/araR genes (40, 42), located in distinct regions of the B. subtilis chromosome (Fig. 1). The first three genes from the l-arabinose metabolic operon, araA, araB, and araD, encode l-arabinose isomerase, l-ribulokinase, and l-ribulose-5-phosphate 4-epimerase (39), respectively, which are the enzymes required for the intracellular conversion of l-arabinose into d-xylulose-5-phosphate (21). d-Xylulose-5-phosphate is further catabolized through the pentose phosphate pathway. The product of the araE gene is a permease, the main transporter of l-arabinose into the cell (42). The araR gene encodes the regulatory protein of the l-arabinose metabolism in B. subtilis, negatively controlling the expression from the l-arabinose-inducible ςA-like promoters of the ara regulon (40–42). Additionally, this transcription factor controls the utilization of d-xylose and of d-galactose, since the AraE protein is a nonspecific permease also responsible for the transport of those carbohydrates into the cell (17). Therefore, AraR is a central element in the regulation of carbon catabolism in B. subtilis.
FIG. 1.
Localization of the ara genes on the B. subtilis chromosome. The promoter region of the araE/araR divergent unit (top left) and the araABDLMNPQ-abfA operon (bottom left) are depicted and drawn to scale. The −10 and −35 regions and the transcriptional start point (+1) of the araA, araE, and araR genes are indicated. The AraR binding sites identified in vitro (ORE1, ORE2, and ORR3 [top left] ORA1 and ORA2 [bottom left]) are represented as shaded boxes.
The amino acid sequence of AraR exhibits significant similarity to proteins from the LacI family of bacterial regulators (50), but the helix-turn-helix motif found in the N-terminal region is identical to the consensus signature of the GntR family of regulatory proteins (13). Purified AraR protein binds to two sequences within the araABDLMNPQ-abfA operon (ORA1 and ORA2) and araE (ORE1 and ORE2) promoters and to one sequence (ORR3) in the araR promoter, as detected by DNase I footprinting (Fig. 1). The repressor target sequences are palindromic, which suggests that the protein binds a single operator as a homodimer, and share high identity. The AraR protein binds cooperatively to ORA1-ORA2 and to ORE1-ORE2 and noncooperatively to ORR3. The duplicate operators in the catabolic operon and transport gene promoters are located on the same side of the DNA helix, separated by 42 and 43 bp, respectively, counting from the centers of symmetry, whereas ORR3 is in an opposite side of the DNA helix relative to ORE1 and ORE2. The cooperative binding is associated with a pattern of enhanced and diminished DNase I cleavage between the duplicate operators that indicates DNA bending. In spite of the similar affinity of AraR for all its operator sequences, the repression exerted by the cooperative binding in the metabolic operon and transport gene promoters is more efficient than the noncooperative binding of the transcrition factor to its own promoter (25).
Interestingly, despite the identity in the pathways of l-arabinose utilization (8, 21) and the functional homology between the structural genes (39) in B. subtilis and Escherichia coli, there is no similarity between the two regulatory proteins, AraR and AraC (7, 10), and their modes of action are distinct. Whereas the AraC protein acts as an activator and as a repressor that regulates transcription of the genes required for the uptake and catabolism of l-arabinose in E. coli (reviewed in references 44 and 45), the AraR protein functions solely as a repressor. Our knowledge of the mode of action of AraR has relied mainly on in vitro experiments; therefore, we have investigated here how AraR regulates the expression of the ara regulon in vivo. The AraR operators are shown to represent the key cis-acting regulatory elements of the ara regulon, and a complex interplay between the operators in the araE/araR divergent unit was observed. Communication between AraR molecules bound to two properly spaced operators is shown to be crucial for an efficient repression, and this implicates the formation of a DNA loop. AraR is the major protein directly involved in the control of the ara regulon since in an in vitro transcription system using purified components, the repressor sufficed to abolish transcription from the ara operon and araE promoters.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. coli strains DH5α (Gibco-BRL) or TOP10 One Shot Cells (Invitrogen) were used for routine molecular cloning work, and BL21(λDE3)/pLysS (47) was used for overexpression of the native AraR and AraR-His6 proteins. Transformation of E. coli strains was carried out according to the method of Hanahan (12). B. subtilis 168T+ (prototroph [F. E. Young]) or B. subtilis IQB215 (ΔaraR::km [40]) strains were transformed by the method of Anagnostopoulos and Spizizen (4) with promoter-lacZ transcriptional fusions. All the B. subtilis strains used to measure the β-galactosidase activity of promoter-lacZ fusions are listed in Fig. 1 and Tables 2 and 3. The B. subtilis PolHis strain [trpC2 pheA1 rpoC::pPolHis1(rpoC′ spc); G. Schyns and C. P. Moran, Jr.] bears a His tag at the 3′ terminus of the rpoC gene, which encodes β′ subunit of the B. subtilis RNA polymerase, and was used for the purification of ςA RNA polymerase. E. coli strains were grown on Luria-Bertani (LB) medium (23). Ampicillin (100 μg ml−1), chloramphenicol (30 μg ml−1), kanamycin (20 μg ml−1), X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 40 μg ml−1), and IPTG (isopropyl-β-d-galactopyranoside; 1 mM) were added as appropriate. The B. subtilis strains were grown on LB medium (23), Difco Sporulation Medium (DSM), or C minimal medium (30). Chloramphenical (5 μg ml−1). kanamycin (15 μg ml−1), and spectinomycin (50 μg ml−1) were added when appropriate. Solid medium was made with LB medium or DSM containing 1.6% (wt/vol) agar.
TABLE 2.
Deletion analysis of the araABDLMNPQ-abfA operon promoter
| Promoter fusiona (Plasmid/strain) | Constructb | β-Galactosidase activity (Miller units)c
|
Repression factord | |
|---|---|---|---|---|
| −l-Arabinose | +l-Arabinose | |||
| araA′-lacZ | 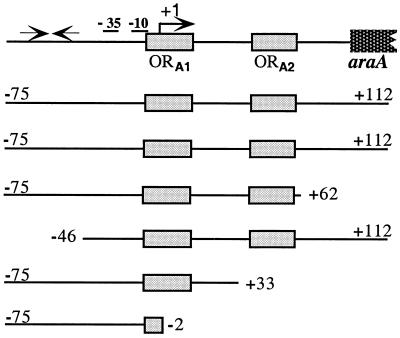 |
|||
| pLM32/IQB300 | 40.4 ± 0.4 | 1,806.6 ± 73.3 | 44.2 ± 1.8 | |
| pLM32/IQB327 (AraR−) | 3,233.7 ± 159.2 | 2,224.3 ± 150.0 | 0.7 ± 0.1 | |
| pLM33/IQB301 | 6.7 ± 0.2 | 312.7 ± 11.0 | 46.9 ± 2.4 | |
| pLS13/IQB304 | 39.0 ± 2.1 | 1,582.9 ± 73.3 | 40.6 ± 2.9 | |
| pLM34/IQB302 | 484.7 ± 23.1 | 608.1 ± 20.2 | 1.2 ± 0.1 | |
| pLM35/IQB303 | 936.0 ± 35.8 | 839.9 ± 18.0 | 0.9 ± 0.0 | |
Plasmids containing different araA′-lacZ promoter fusions were integrated at the amyE locus of the B. subtilis 168T+ wild-type or B. subtillis IQB215 (AraR−) chromosome (see Materials and Methods).
The promoter region of the araABDLMNPQ-abfA operon is depicted and drawn to scale. The −10 and −35 regions and transcriptional start point (+1) of the araA gene are indicated. The AraR binding sites identified in vitro (ORA1 and ORA2 [25]) are represented by gray boxes. The two convergent black arrows represent a putative hairpin loop structure previously identified by sequence analysis (41). Below the scheme the sizes of the various araA promoters are indicated. The start and end of each DNA fragment is labeled relative to the transcriptional start site.
The strains containing the various truncated araA promoters were grown on C minimal medium supplemented with casein hydrolysate 1% (wt/vol) in the absence or presence of 0.4% (wt/vol) l-arabinose. The levels of accumulated β-galactosidase activity were measured 2 h after induction.
AraR repression was calculated as the ratio between the level of expression (Miller units) obtained in the presence of l-arabinose, and the value was determined in the absence of inducer.
TABLE 3.
Deletion analysis of the araE/araR promoters
| Promoter fusiona (Plasmid/strain) | Constructb | β-Galactosidase activity (Miller units)c
|
Repression factord | |
|---|---|---|---|---|
| −l-Arabinose | +l-Arabinose | |||
| araE′-lacZ | 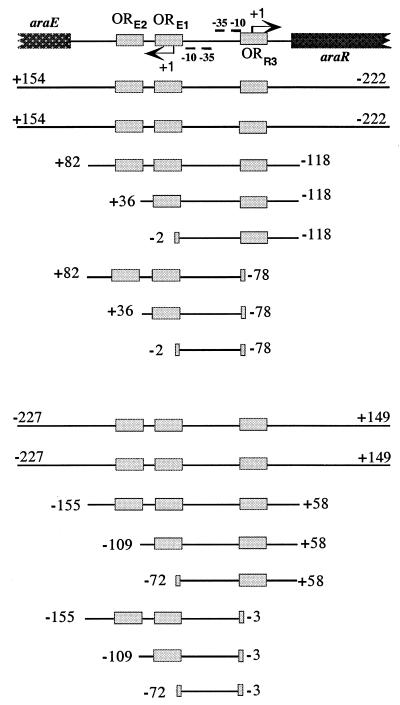 |
|||
| pSN34/IQB258 | 12.3 ± 0.3 | 752.2 ± 24.0 | 61.3 ± 2.5 | |
| pSN34/IQB325 (AraR−) | 1,423.3 ± 71.9 | 1,045.4 ± 147.4 | 0.7 ± 0.1 | |
| pLS14/IQB313 | 1.9 ± 0.2 | 123.4 ± 5.8 | 64.6 ± 7.4 | |
| pLM39/IQB315 | 213.6 ± 6.9 | 677.3 ± 51.2 | 3.2 ± 0.3 | |
| pLS3/IQB316 | 1,101.3 ± 61.9 | 947.5 ± 54.0 | 0.9 ± 0.1 | |
| pLS12/IQB317 | 4.4 ± 0.9 | 115.2 ± 3.6 | 26.2 ± 5.2 | |
| pLM38/IQB319 | 439.7 ± 8.6 | 727.9 ± 6.2 | 1.6 ± 0.0 | |
| pLS2/IQB320 | 1,235.8 ± 30.6 | 1,027.8 ± 13.9 | 0.8 ± 0.0 | |
| araR′-lacZ | ||||
| pLS29/IQB322 | 9.6 ± 0.4 | 38.0 ± 3.1 | 4.0 ± 0.4 | |
| pLS29/IQB323 (AraR−) | 44.7 ± 5.8 | 41.7 ± 3.4 | 0.9 ± 0.1 | |
| pLS8/IQB305 | 8.9 ± 0.4 | 31.7 ± 1.3 | 3.6 ± 0.2 | |
| pLS5/IQB307 | 12.0 ± 1.0 | 27.0 ± 1.7 | 2.3 ± 0.2 | |
| pLS10/IQB308 | 15.7 ± 0.8 | 25.2 ± 1.1 | 1.6 ± 0.1 | |
| pLS11/IQB309 | 81.6 ± 5.1 | 70.6 ± 0.8 | 0.9 ± 0.1 | |
| pLS4/IQB311 | 78.6 ± 5.8 | 67.4 ± 3.9 | 0.9 ± 0.1 | |
| pLS9/IQB312 | 73.2 ± 8.2 | 66.8 ± 2.8 | 0.9 ± 0.1 | |
Plasmids containing different araE′-lacZ (top) and araR′-lacZ (bottom) promoter fusions were integrated at the amyE locus of the B. subtilis 168T+ wild-type or B. subtillis IQB215 (AraR−) chromosome (see Materials and Methods).
The region of the araE/araR divergent promoters is depicted and drawn to scale. The −10 and −35 regions and transcriptional start point (+1) of the araE (left) and araR (right) genes are indicated. The AraR binding sites identified in vitro (ORE1, ORE2, and ORR3 [25]) are represented by gray boxes. Below the scheme the size of the various araE (top) and araR (bottom) promoters are indicated. The start and end of each DNA fragment is labeled, relative to the transcriptional start site of the araE or araR genes, respectively.
See footnote c in Table 2.
See footnote d in Table 2.
DNA manipulation, PCR amplification, and sequencing.
DNA manipulations were carried out as described in Sambrook et al. (37). Restriction enzymes were purchased from MBI Fermentas, New England Biolabs, or Roche and used according to the manufacturer's instructions. DNA was eluted from agarose gels using the GeneClean II Kit (Bio 101) or the MERmaid kit (Bio 101). DNA sequencing was performed by the method of Sanger et al. (38) by using Sequenase version 2.0 kit (Amersham Pharmacia Biotech). All PCR amplifications were done using high-fidelity native Pfu DNA polymerase (Stratagene). PCR products were purified using QIAquick PCR purification kit (Qiagen).
Construction of plasmids and strains.
The plasmids bearing the promoter-lacZ transcriptional fusions, listed in Tables 2 and 3 and Fig. 2B, are all derivatives of pSN32 (25), and the oligonucleotides used in their construction are indicated in Table 1. Plasmids pLS4, pLS5, pLS8, pLS10, pLS11, pLS13, pLM32, and pLM34 were previously described (25). To construct plasmids pLM33 and pLM35, DNA fragments from the araABDLMNPQ-abfA operon promoter region were obtained by PCR using pLM9 (25) as the template. The DNA fragments obtained were digested with EcoRI-BamHI and inserted into those sites of pSN32. Plasmids pLM38, pLM39, pLS2, pLS3, pLS12, pLS14 (araE′-lacZ), and pLS9 (araR′-lacZ) were obtained after PCR amplification of different DNA fragments from the araE/araR promoters region using pLM7 (40) as a template. To construct the araE′-lacZ transcriptional fusions, the suitable DNA fragments were restricted with EcoRI-DraI, EcoRV or Scal and inserted between the EcoRI and SmaI sites of pSN32. The araR′-lacZ transcriptional fusion in pLS9 was obtained by digestion of the adequate DNA fragment with BamHI and ScaI, followed by its insertion into pSN32 BamHI-SmaI. Plasmid pSN34 was constructed by subcloning a 384-bp BamHI-EcoRI DNA fragment from pSN31 (42), carrying the divergent araE/araR promoters, into the same sites of pSN32. To construct plasmid pLS28, a 421-bp NaeI-HindIII DNA fragment from pLM3 (40) was inserted into the EcoRV and HindIII sites of pLITMUS28 (New England Biolabs). Plasmid pLS29 was then obtained by subcloning a 550-bp BglII-StuI DNA fragment from pLS28 into pSN32 digested with BamHI and SmaI.
FIG. 2.
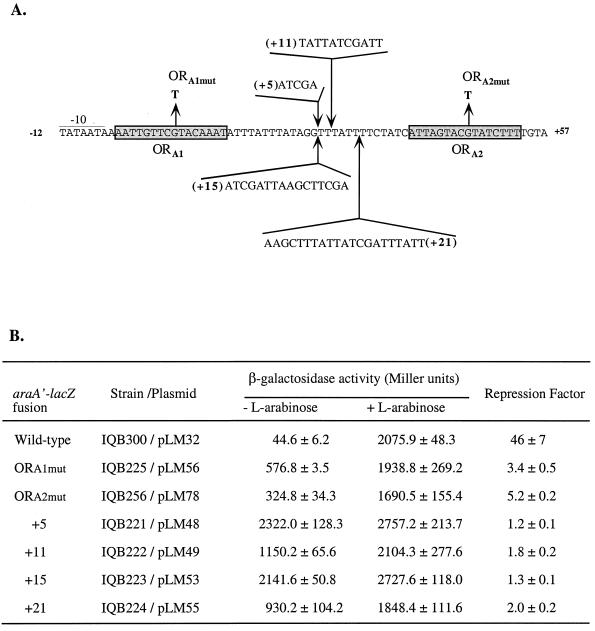
Site-directed mutagenesis of the araABDLMNPQ-abfA operon promoter. (A) The DNA sequence of the promoter is represented from position −12 to position +57, relative to the transcriptional start site of the araA gene. The −10 region is underlined and the AraR operators ORA1 and ORA2 are in shaded boxes. The single nucleotide changes introduced in ORA1 or ORA2 are shown. The sequence of the different DNA fragments and the position of its insertion between ORA1 and ORA2 are indicated. (B) Plasmids containing different araA-lacZ promoter fusions were integrated at the amyE locus of the B. subtilis 168T+ wild-type chromosome as described (see Materials and Methods). The strains containing the various mutated araA promoters were grown on C minimal medium supplemented with 1% (wt/vol) casein hydrolysate in the absence or presence of 0.4% (wt/vol) l-arabinose. The levels of accumulated β-galactosidase activity were measured 2 h after induction. AraR repression was calculated as the ratio between the level of expression (Miller units) obtained in the presence of l-arabinose and the value determined in absence of inducer.
TABLE 1.
Oligonucleotides used in this work
| Oligonucleotide | Sequence (5′→3′)a | Complementary sequence | Plasmid(s) constructed |
|---|---|---|---|
| ARA1 | −39TAAGGGTAACTATTGCCG−22 | pSN32 | pLM53, pLM55, pLM76 |
| ARA7 | +178CCTCTTCGCTATTACGCC+161 | pSN32 | pLM53, pLM55, pLM76 |
| ARA23 | +92CCCGGATATCGAGTAAAGCGTTTTCATTTAAACC+59 | araE | pLS12, pLS14 |
| ARA24 | +47GTACTTATTTTAAAATTTCG+26 | araE | pLM38, pLM39 |
| ARA25 | +8AGTAAGTACTAATATAGAATAATCTTGTTGC−23 | araE | pLS2, pLS3, pLS9 |
| ARA26 | +17CCAAAATGAATTCGGATCCATTTCAG−9 | araR | pLM38, pLS2, pLS9, pLS12 |
| ARA27 | +67GAACTGGATCCTTCTTTGAATTCCGCGTATTTTGG+34 | araR | pLM39, pLS3, pLS14 |
| ARA28 | +87CCTATTGAATTCAAAAGCCGG−67 | araA | pLM33, pLM34, pLM35, pLM48, pLM49 |
| ARA29 | +10TTGTAGGATCCATTTTATTATAGTAATTGAC−21 | araA | pLM35 |
| ARA30 | +47CGTACTAAAGATCTAAAATAAACC+24 | araA | pLM34 |
| ARA31 | +75AATAAAACGGATCCAAATAC+56 | araA | pLM33 |
| ARA32 | +124GAATTCAGGATCCTTTGTCTGAAGC+100 | araA | pLM48, pLM49 |
| ARA33 | +18TTTATCGATTTATTTTCTATC+38 | araA | pLM48, pLM49 |
| ARA34 | +43CTAATGATCGATAATAAAC+24 | araA | pLM49 |
| ARA35 | +37ATAGAAATCGATCCTATAAATAAATATTTGTACG+4 | araA | pLM48 |
| ARA50 | +16GGAGGAATGCATATGTTACC+35 | araR | pLS16 |
| ARA51 | +1128ACATTGCTTAAGCTTTTCATTCAGTTTTCGTGCGG+1094 | araR | pLS16 |
| ARA65 | +15TTATTTAAAGCTTTATT+32ATCG | araA | pLM55 |
| ARA66 | +34GAAAATAAA+26AGCTT+31AATAAACC+24 | araA | pLM55 |
| ARA67 | +13ATTTA+17AAGCTTCGA+26TTTATTTTCTATC+38 | araA | pLM53 |
| ARA68 | +39TGATAGAAGCTTAA+26TCGAT+25CC+24 | araA | pLM53 |
| ARA72 | +35AGTGTATCAACAAGCTGG+17 | pSN32 | − |
| ARA77 | +24GGTTTATTTTCTATCATTAGTACTTATCTTTTGTATTTG+62 | araA | pLM78 |
| ARA78 | +62CAAATACAAAAGATAAGTACTAATGATAGAAAATAAACC+24 | araA | pLM78 |
| ARA82 | −139TAATTTACCGAAACTTGCGG−120 | veg | pLM87 |
| ARA83 | +75CAGCCGTTAACCTAAATTCCC+55 | veg | pLM87 |
The number within the primers refers to the position of the sequence in the araABDLMNPQ-abfA operon, araE, araR, and veg relative to the transcription start point of each gene or in pSN32 relative to the EcoRI site (+1) in the multiple cloning site. Restriction sites used are underlined in the oligonucleotide sequences: BamHI (GGATCC), BglII (AGATCT), ClaI (ATCGAT), DraI (TTTAAA); EcoRI (GAATTC), EcoRV (GATATC), HincII (GTTAAC), Hind (AAGCTT), NdeI (CATATG), and ScaI (AGTACT).
The construction of pLM56, which carries a single-base-pair substitution in ORA1 was described previously (25). A single nucleotide substitution was made in ORA2 by using the QuikChange kit (Stratagene) and pLM51 (25) as the template DNA, according to the instructions of the manufacturer. The 204-bp BamHI-EcoRI DNA fragment from the plasmid obtained in the oligonucleotide-directed mutagenesis was subcloned into pSN32 digested with BamHI-EcoRI, yielding pLM78. Plasmids pLM48, pLM49, pLM53, and pLM55 bear DNA insertions of 5, 11, 15, and 21 bp, respectively, between ORA1 and ORA2. These plasmids were constructed by PCR amplification of different DNA fragments from the araABDLMNPQ-abfA operon promoter using specific pairs of oligonucleotides (Table 1). For the construction of pLM48 and pLM49, the template was chromosomal DNA from B. subtilis 168T+ strain, and to obtain pLM53 and pLM55 the templates were pLM48 and pLM49, respectively. Plasmid pLM48 was obtained by using oligonucleotides ARA32 and ARA33, yielding a 94-bp DNA fragment after BamHI-ClaI digestion, and by using oligonucleotides ARA28 and ARA35, which resulted in the recovery of a 109-bp ClaI-EcoRI DNA fragment. To construct pLM48, the two DNA fragments were mixed and ligated to pSN32 BamHI-EcoRI. Using oligonucleotides ARA28 and ARA34, a 115-bp DNA fragment was obtained after ClaI and EcoRI digestion. This 115-bp DNA fragment was mixed with the 94-bp BamHI-ClaI DNA fragment (see above) and ligated to pSN32 restricted with BamHI and EcoRI, resulting in plasmid pLM49. For pLM53 construction the oligonucleotides ARA67 and ARA7 were used and a 99-bp DNA fragment was recovered after BamHI-HindIII digestion. This DNA fragment and a 116-bp HindIII-EcoRI DNA fragment obtained after PCR with oligonucleotides ARA68 and ARA1 were ligated to pSN32 restricted with BamHI and EcoRI, to yield pLM53. In order to construct pLM55, by using oligonucleotides ARA65 and ARA7 a 105-bp DNA fragment was purified after BamHI and HindIII digestion, and by using oligonucleotides ARA66 and ARA1 a 116-bp HindIII-EcoRI DNA fragment was isolated. These two DNA fragments were ligated to pSN32 digested with BamHI and EcoRI, which yielded pLM55.
The plasmids with the promoter-lacZ transcriptional fusions were linearized with ScaI or PstI and the fusions integrated into the chromosome of B. subtilis 168T+ or IQB215 strains via double recombination with the amyE gene back and front sequences. Disruption of the amyE locus was confirmed as previously described (40).
A fusion of the C terminus of AraR to six histidines in the pET30a(+) plasmid (Novagen) was engineered, placing araR under the control of a T7 promoter. The araR gene was amplified from chromosomal DNA of the B. subtilis 168T+ strain using oligonucleotides ARA51 and ARA50. The resulting 1,112-bp PCR product was separately digested with AvaI-NdeI and AvaI-HindIII, resulting in 282 and 805-bp DNA fragments, respectively, that were mixed and ligated into pET30a(+) restricted with NdeI and HindIII, thus yielding pLS16. The veg promoter was amplified from chromosomal DNA of the B. subtilis 168T+ strain using oligonucleotides ARA82 and ARA83. The 213-bp DNA fragment obtained was inserted into pCR2.1-TOPO (Invitrogen), yielding pLM87. The accuracy of the nucleotide sequence from all the inserts obtained after PCR amplification was confirmed by DNA sequencing.
β-Galactosidase assays.
Strains of B. subtilis harboring transcriptional lacZ fusions were grown in C minimal medium supplemented with 1% (wt/vol) casein hydrolysate in the presence or in the absence of l-arabinose 0.4% (wt/vol) as previously reported (41). Samples of cell culture were collected and analyzed 2 h after the addition of l-arabinose. The ratio of β-galactosidase activity, determined as described by Miller (23), from cultures grown for 2 h in the presence or absence of inducer was taken as a measure of AraR repression in each strain analyzed (repression factor).
Protein purification.
The AraR native protein used in this work was purified as described previously (25). For the purification of the fusion protein AraR-His6, E. coli BL21 (λDE3)/pLysS (47) cells transformed with pLS16 were grown at 37°C to an optical density at 600 nm (OD600) of 0.6 in 500 ml of LB medium, at which time expression of AraR-His6 was induced by the addition of IPTG to 5 mM. The cells were grown for an additional 2 h at 37°C. All subsequent steps were carried out at 4°C. The harvested cells were resuspended in 7 ml of lysis buffer containing 20 mM sodium phosphate buffer (pH 7.4), 500 mM NaCl, 10% (wt/vol) glycerol, 100 mM imidazole, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Cells were lyzed by passing twice through a French pressure cell and centrifuged for 1 h at 15,000 × g. Proteins from the supernatant were loaded onto a 1-ml HisTrap column (Amersham Pharmacia Biotech). The bound proteins were eluted by a discontinuous imidazole gradient of 200, 300, and 500 mM. The different fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and those containing AraR-His6 that were more than 95% pure were separately dialyzed overnight against storage buffer containing 20 mM sodium phosphate buffer (pH 7.4), 500 mM NaCl, 1 mM EDTA, 1 mM dithioerythritol (DTE), and 10% (wt/vol) glycerol. The protein fractions were tested for DNA binding activity, separated into aliquotes and stored at −80°C.
The B. subtilis PolHis strain was grown in 2 liters of LB medium, and the cells were collected in the exponential-growth phase, at an OD600 of 0.9, to ensure the predominant recovery of ςA RNA polymerase. The pelleted cells were resuspended in 10 ml of a buffer containing 10 mM Tris-HCl (pH 8), 100 mM NaCl, 5% (wt/vol) glycerol, 1 mM β-mercaptoethanol, 1 mM PMSF, and 2.5 mM imidazole. The His-tagged RNA polymerase was then purified and its activity was tested as described by Wade et al. (49). Protein concentrations were determined by the method of Bradford (5) using bovine serum albumin (BSA) as a standard.
In vitro transcription.
In vitro transcription reactions were performed essentially as described by Wade et al. (49). Plasmids pLM32 (araABDLMNPQ-abfA wild type), pLM48 (+5), pLM49 (+11), pLM56 (ORA1mut), pLM78 (ORA2mut), and pSN34 (araE) digested with BamHI and plasmid pLM87 (veg) digested with HincII were used as templates for in vitro transcription reactions. Specific transcription from the araABDLMNPQ-abfA wild-type, ORA1mut, and ORA2mut promoters was expected to produce nucleotide transcripts of 118 bp. Specific transcription from +5, +11, araE, and veg promoters was expected to produce nucleotide transcripts of 123, 129, 170, and 70 bp, respectively. Both templates (2 to 4 nM concentrations of the linearized plasmids), RNA polymerase (1.2 μg of protein), and AraR-His6 in the indicated amounts when necessary were preincubated for 15 min at 37°C in a 50-μl volume of AraR-His6 binding buffer (100 mM NaCl; 30 mM KCI; 16 mM Hepes-KOH, pH 7.6; 10 mM sodium phosphate buffer, pH 7.4; 10 mM MgCl2; 2 mM EDTA; 1 mM DTE; 10% [wt/vol] glycerol, 0.1% [wt/vol] BSA) in the presence of 500 ng of poly(dI-dC). l-Arabinose and d-xylose were added to a final concentration of 15 mM to the transcription mixture when necessary. Ribonucleotides (500 μM final concentration of ATP, GTP, and UTP [Amersham Pharmacia Biotech] and 2 μCi of [γ-32P]CTP [400 Ci/mmol; Amersham Pharmacia Biotech]) were added for 1 min before reinitiation was prevented by the addition of 10 μg of Heparin (Sigma). Ten minutes later unlabeled CTP (500 μM final concentration [Amersham Pharmacia Biotech]) was added for an additional 5-min incubation. Reactions were stopped by the addition of sodium acetate (0.3 M final concentration) and ethanol precipitation of nucleic acids. Before being loaded on a 6% polyacrylamide–7 M urea sequencing gel, nucleic acids were resuspended in 8 μl of a urea sequencing dye. Transcripts were quantified by densitometry with the ImageQuant software coupled to a Storm 860 (Molecular Dynamics). The veg promoter was used in all in vitro transcriptions as an internal control for quantification.
DNase I footprinting.
The labeling of the target DNA fragments and the DNase I footprinting experiments were conducted as previously described by Mota et al. (25). Plasmids pLM48 and pLM49 (see above) were used as templates with oligonucleotides ARA1 (radiolabeled with [γ-32P]ATP using polynucleotide kinase) and ARA72 (see Table 1), yielding 263- and 269-bp DNA fragments, respectively, bearing mutant araABDLMNPQ-abfA operon promoters radiolabeled in the coding strand.
RESULTS
Deletion analysis of the promoter region of the araABDLMNPQ-abfA operon.
Previous studies of AraR-DNA interaction in vitro have shown that this transcription factor binds to two distinct sequences (ORA1 and ORA2) within the araABDLMNPQ-abfA operon promoter (25). To define the relevant regions for the mechanism of transcription regulation mediated by AraR, a set of DNA fragments covering different domains of the araA promoter were synthesized and fused with a promoterless reporter lacZ gene of E. coli (Table 2). The araA′-lacZ transcriptional fusions were integrated in a single copy at the amyE locus of the wild-type B. subtilis 168T+ chromosome or of the araR-null mutant strain IQB215. The level of accumulated β-galactosidase activity of the resulting strains was examined in vivo in the presence or in the absence of the inducer l-arabinose, and the results are shown in Table 2. The degree of AraR repression (i.e., the repression factor) was determined indirectly by the ratio between the values obtained in the induced and noninduced cultures.
The level of promoter activity from the different araA′-lacZ fusions measured in the presence of l-arabinose in a wild-type background varies between 300 and 1,800 Miller units. The plasmid used to construct these transcriptional fusions, pSN32, is a derivative of pDH32 (31), considered a promoter probe plasmid, in which expression of the reporter gene should depend on the strength of the fused promoter. This is assured by successive stop codons in all three reading frames located 49 to 36 bp upstream from the spoVG-lacZ start codon in the plasmid (33). However, by DNA sequencing of this region in pSN32, we confirmed a deletion of 2 bp, previously reported for pDH32 (15), that affects one of the stop codons and creates an extended reading frame, which terminates at position +14 in the spoVG-lacZ reading frame. This might explain the sixfold difference in the levels of accumulated β-galactosidase activity measured in strains IQB300 (or IQB304) and IQB301 since, in the araA′-lacZ fusion present in IQB300 and IQB304, the stop site for araA-induced translation is 14 bp downstream from the spoVG start codon and in strain IQB301 the araA translational start site is not present. Thus, the higher expression observed in strain IQB300 relative to IQB301 might be due to translational coupling of araA and spoVG-lacZ, as previously observed with xylA-lacZ fusions (15). The reason for the 2- to 2.5-fold increase in β-galactosidase activity measured in strains IQB302 and IQB303 compared to IQB301 is unknown but may be caused by differences in translation or transcription efficiency or a combination of both. Although we cannot use the absolute levels of β-galactosidase activity from the various fusions to measure promoter strengths, the differences observed do not interfere with the determination of AraR repression since we are comparing the levels of expression in the presence and absence of l-arabinose.
In a wild-type background, the araA′-lacZ′ fusions that bear simultaneously the two AraR binding sites identified in the l-arabinose metabolic operon showed no significant differences in the levels of repression (strains IQB300, IQB301, and IQB304). The deletion of the inverted repeat sequence identified upstream from the −35 region (IQB304), a putative terminator of the abnA gene located upstream from the metabolic operon promoter (18, 41), or of the entire DNA region positioned 4 bp downstream from the boundary of ORA2 (IQB301) caused no effect on the regulation by AraR. These results restrained the AraR regulatory region to a DNA fragment of 108 bp, from position −46 to +62 relative to the transcriptional start site of araA. Significantly, an araA-lacZ fusion with only ORA2 deleted showed a dramatic 37-fold decrease in the repression level (IQB302) relative to the full-length promoter fusion in a wild-type context (IQB300). When both operators are absent the negative effect on repression increased 49-fold (IQB303), a result similar to that observed with the full-length fusion analyzed in an AraR− background (IQB327). In conclusion, the two AraR binding sites identified in vitro are very likely to be fundamental cis-acting elements for the regulation of the l-arabinose metabolic operon promoter by AraR in vivo. Furthermore, the almost complete derepression observed in a promoter with just one of the operators present supports a regulatory mechanism dependent on cooperative binding of the regulatory protein to its DNA targets.
Deletion analysis of the araE/araR divergent promoters region.
DNase I protection assays have shown that the AraR repressor binds to two sequences within the araE (ORE1 and ORE2) promoter and to one sequence (ORR3) in the araR promoter (25). The important regions for the regulatory mechanism mediated by AraR in this araE/araR divergent transcription unit were defined by deletion analysis as described above for the metabolic operon promoter. B. subtilis strains bearing different truncated araE′-lacZ or araR′-lacZ promoter fusions were analyzed, and the results are shown in Table 3.
The levels of promoter activity from the different araE′-lacZ and araR′-lacZ fusions measured in the presence of l-arabinose in a wild-type background range from 115 to 1,027 and from 25 to 75 Miller units, respectively. However, this does not affect the measurement of AraR repression as discussed above for the araA′-lacZ fusions. Promoter-lacZ fusions bearing the three AraR binding sites in a wild-type background display similar levels of AraR repression of the araE (strains IQB258 and IQB313) or araR (IQB322 and IQB305) promoters. Thus, the deletion of the DNA 27-bp downstream from ORE2 and 43-bp downstream from ORR3 caused no effect on the repression by AraR in both promoters. These results confined the AraR regulatory region within this transcriptional divergent unit to a 200-bp DNA fragment.
An araE′-lacZ fusion with only ORE2 absent showed a 19-fold decrease in the level of repression (IQB315) relative to the full-length araE′-lacZ promoter fusion (IQB258). When both ORE1 and ORE2 were deleted, a 68-fold deregulation was observed (IQB316) and further deletion of ORR3 did not affect the level of repression (IQB320). This degree of derepression is similar to the one observed with a full-length promoter in an araR-null mutant (IQB325). Interestingly, in a wild-type background an araE′-lacZ promoter fusion with ORR3 truncated and both ORE1 and ORE2 present resulted in a twofold decrease of repression (IQB317) compared to the full-length promoter (IQB258). A promoter fusion with only ORE1 present (IQB319) showed a 38-fold deregulation relative to the full-length promoter (IQB258) and a 2-fold deregulation relative to the araE promoter with both ORE1 and ORR3 present (IQB315). Taken together, the data indicated that the role of ORE1 and ORE2 in the araE promoter parallel to that observed with ORA1 and ORA2 in the metabolic operon promoter. The contribution of ORR3 for the repression of the transcription from the araE promoter by AraR was the same if ORE2 and ORE1 were present or if only ORE1 was intact, but ORR3 showed no influence if both ORE1 and ORE2 were deleted. These results suggested that the presence of both ORE1 and ORE2 is fundamental for in vivo regulation of transcription from this promoter by AraR, which supports the crucial involvement of cooperativity for its regulation, but full repression is only achieved with the contribution of ORR3.
In a wild-type background, an araR′-lacZ fusion with ORR3 deleted, even if ORE1 and ORE2 were present (IQB309), showed a fourfold reduction in the level of repression relative to the full-length araR′-lacZ promoter (IQB322). Further deletion of ORE2 and ORE1 did not affect repression (IQB311 and IQB312). This fourfold derepression level is identical to the one measured in the strain bearing a full-length araR′-lacZ promoter in an AraR− context (IQB323). However, in a wild-type background, an araR′-lacZ fusion with ORR3 intact showed a 2- or a 2.5-fold decrease in autorepression due to the deletion of ORE2 (B. subtilis IQB307) or of both ORE2 and ORE1 (IQB308), respectively, compared to the full-length promoter (IQB322). The data revealed that ORR3 is most probably the key cis-regulatory element in the autorepression of araR transcription, but it is not sufficient for the necessary level of regulation, which is only achieved if the two AraR binding sites identified in the neighboring araE promoter are present.
Effect of mutations designed to prevent communication between AraR molecules on in vivo repression of the araABDLMNPQ-abfA operon promoter.
In order to understand in more detail how AraR controls transcription of the ara regulon in vivo, we focused our attention on the metabolic operon promoter as a model of study. To test the formation of a DNA loop in vivo, we have designed mutations that are predicted to prevent the communication between bound AraR molecules in the promoter. First, we tested the effect of a single-base-pair alteration in ORA1 or ORA2. Previous studies have shown that the binding affinity of AraR to both operators is dramatically reduced in a DNA fragment carrying a single nucleotide substitution in a highly conserved position of ORA1 and that the DNase I hypersensibility pattern associated with cooperativity is almost completely lost (25). Here, we have introduced the same mutation in an identical position of ORA2 (Fig. 2A). The two ara operon mutant promoters carrying the point mutation. ORA1mut or ORA2mut (Fig. 2A), were fused to the lacZ gene of E. coli and analyzed in a B. subtilis wild-type background as described above. Both mutations resulted on significant derepression levels, 13-fold in ORA1mut and 9-fold in ORA2mut, relative to the wild-type promoter (Fig. 2B). These data indicated that just one base pair change in one of the two operators is sufficient to affect significantly the repression level and strongly suggested that the guanine in the center of the palindromic operators is an important element for the specific recognition of the DNA by AraR.
Since the duplicate AraR operators in the araABDLMNPQ-abfA operon promoter are located on the same side of the DNA helix (25), we have engineered DNA insertions between them that either destroyed or restored the phasing. As observed in similar systems involving DNA loop formation (43), we reasoned that DNA insertions destroying the phasing (+5 or +15) would strongly affect repression because AraR molecules would be on different sides of the DNA helix impairing communication. It was expected to recover this cross talking between repressor molecules by restoring the phasing (+11 or +21). We have made random 5-, 11-, 15-, and 21-bp insertions, here referred to as “+5,” “+11,” “+15,” and “+21” promoters (Fig. 2A). In the +5 and +15 promoters the AraR repression was indeed reduced, about 40-fold (Fig. 2B), to a level similar to that observed with the wild-type promoter in an AraR− background (IQB327; Table 2). However, in the +11, and +21 promoters regulation was not restored at all and only a small, but reproducible, 1.5-fold positive effect on repression was observed in both cases compared to the +5 and +15 mutations (Fig. 2B). Taken together, these results suggested that a maximum or a precise distance of 42 or 43 bp between the centers of AraR duplicate operators is necessary for the occurrence of cooperative interactions that lead to an efficient repression in vivo. The communication between AraR molecules implicates DNA loop formation.
Mutations affecting AraR repression in vivo also affect cooperative binding of AraR in vitro.
To understand the molecular basis for the loss of repression in the promoters with insertions between ORA1 and ORA2, we analyzed DNA fragments bearing the +5 and +11 promoters by quantitative DNase I footprinting over a wide range of AraR concentrations (Fig. 3). The relative binding affinity of AraR to ORA1 and ORA2 was reduced (Fig. 3) compared to the wild-type promoter values: 34 nM for ORA1 and 47 nM for ORA2 (25). In the DNA fragments bearing the +5 and +11 promoters, binding to the ORA2 site was not detected but AraR still showed a measurable affinity for ORA1, 195 and 107 nM, respectively (Fig. 3). In both cases the characteristic pattern of DNase I hypersensibility bands detected in the wild-type araABDLMNPQ-abfA promoter (25) was not observed, which confirmed that cooperative binding and DNA bending were lost.
FIG. 3.
Quantitative DNase I footprinting of DNA fragments bearing mutated araABDLMNPQ-abfA operon promoters. DNA fragments of 263 and 269 bp carrying the ara metabolic operon promoter region with 5-bp (A) and 11-bp (B) insertions, respectively, between ORA1 and ORA2 were end labeled with [γ-32P]ATP and used as target DNA in the assays. Only the coding strand was radiolabeled, as described in Materials and Methods. AraR concentrations were calculated by reference to a pure dimeric protein. Lane 1, no protein; lane 2, 25 nM AraR; lane 3, 50 nM AraR; lane 4, 100 nM AraR; lane 5, 150 nM AraR; lane 6, 200 nM AraR; lane 7, 250 nM AraR; lane 8, 250 nM AraR plus 15 mM l-arabinose. DNA sequencing reactions were run side by side with the DNase I footprinting reactions (not shown), and the localization of the AraR binding sites in the wild-type promoter (ORA1 and ORA2) are indicated in the auradiographs by brackets. A schematic representation of the DNA fragments used in the experiments, with the DNA insertions made between ORA1 and ORA2 represented by white triangles, is shown above the autoradiographs: AraR binding sites are represented as gray boxes, DNA from the araABDLMNPQ-abfA operon promoter is shown as white boxes, and heterologous DNA (from plasmid pSN32 [see Materials and Methods]) is shown as dotted boxes. The total repressor concentration at which the half-maximal site occupancy is achieved (a value that represents the apparent affinity of AraR for each site) is indicated within parentheses for each operator in the two DNA fragments.
Effect of AraR on the in vitro transcription of the araABDLMNPQ-abfA wild-type and mutant promoters and of the araE gene promoter.
We have developed an in vitro transcription system to confirm that the mutations analyzed in vivo are affecting the AraR repressing action rather than some unknown factor in the physiology of the cell. In these experiments we used an AraR protein with six histidines fused to its carboxyl terminus purified to more than 95% homogeneity by affinity chromatography, a His-tagged ςA RNA polymerase, and linearized plasmid DNA templates carrying the promoters in study. The fusion protein, AraR-His6 was shown to bind to the araABDLMNPQ-abfA and araE/araR promoters DNA as the native AraR protein by DNase I footprintings (data not shown). In the in vitro transcription reactions the veg promoter, which is strongly transcribed by ςA RNA polymerase (24), was used as an internal control to normalize the results when the AraR repression was quantified.
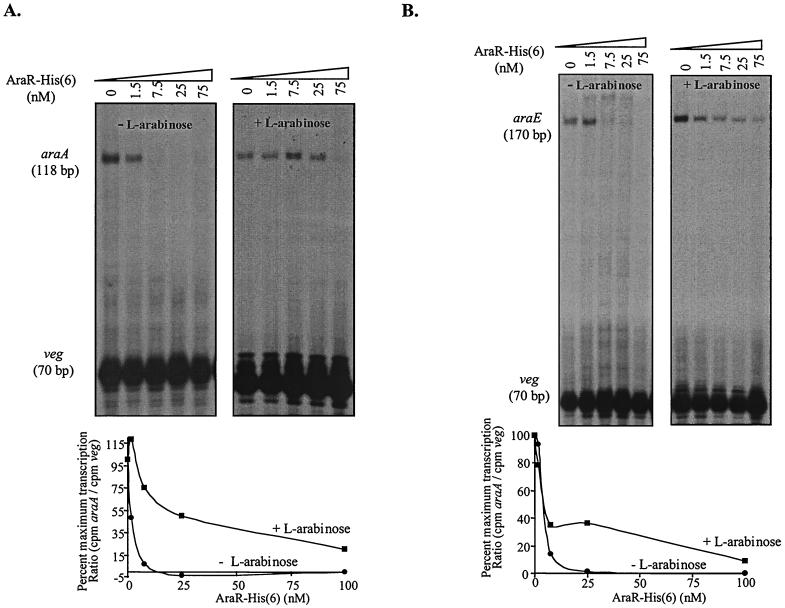
First, we investigated if AraR protein alone was able to repress the transcription from the araABDLMNPQ-abfA and araE/araR promoters. In the absence of AraR the expected specific runoff transcripts of 118 bp for araA, 170 bp for araE, and 70 bp for veg were observed (Fig. 4A and B). Increasing concentrations of AraR-His6 completely blocked in vitro transcription from the l-arabinose metabolic operon promoter and araE transport gene promoter, whereas having no detectable effect on the amount of veg transcript (Fig. 4). Identical results were obtained in related experiments without the veg promoter (data not shown). In the experimental conditions used we were unable to observe a specific runoff transcript from the araR promoter even in the absence of the veg promoter (data not shown). This was probably caused by competition with the araE promoter for the polymerase. In the presence of l-arabinose, the repressing action of AraR was inhibited but a reduction in the amount of araA and araE transcripts was still observed upon increasing AraR concentrations (Fig. 4). No effect on AraR action was observed when d-xylose was added instead to the transcription mixture (Fig. 5 and data not shown), which indicated that the observed l-arabinose inhibition is specific. These results strongly suggested that in the absence of l-arabinose AraR is the major protein necessary to repress in vivo the initiation of transcription from the metabolic operon and araE promoters.
FIG. 4.
Effect of AraR-His6 on the in vitro transcription of the araABDLMNPQ-abfA operon (A) and araE (B) promoters with purified His-tag B. subtilis RNA polymerase. Linear plasmid DNA (2 to 4 nM) carrying the araABDLMNPQ-abfA, araE, or veg promoter were the templates in the experiments. All reactions were carried out in the presence of 1.2 μg of purified His-tag B. subtilis RNA polymerase as described in Materials and Methods. The increasing amounts of AraR-His6 added to each reaction are indicated at the top of each autoradiograph. The AraR-His6 concentration was calculated considering a pure dimeric protein. l-Arabinose was added to a final concentration of 15 mM. The positions of the runoff transcripts of the expected sizes are indicated, as judged by the migration of DNA sequencing reactions. The densitometric quantification of the data of the in vitro transcriptions is shown below the respective autoradiograph. The araA and araE signals were standardized by division with the veg signal in the same lane. The values in the graphics are the average of two independent experiments and are represented as the percentage of maximum transcription relative to the reactions made in the absence of AraR.
FIG. 5.
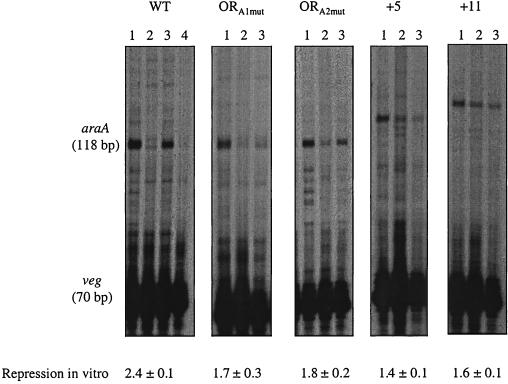
In vitro transcription of araABDLMNPQ-abfA operon wild-type versus mutant promoters. Linear plasmid DNA (2 to 4 nM) carrying the araABDLMNPQ-abfA wild-type (WT) or the ORA1mut, ORA2mut, +5, and +11 mutant promoters and the veg promoter were the templates in the experiments. All reactions were carried out in the presence of 1.2 μg of purified His-tag B. subtilis RNA polymerase as described in Materials and Methods. Lanes 1, no AraR added; lanes 2, 25 nM AraR, lanes 3, 25 nM AraR plus 15 mM l-arabinose; lane 4, for the WT promoter only, 25 nM AraR plus 15 mM d-xylose. The AraR-His6 concentration was calculated considering a pure dimeric protein. The positions of the runoff transcripts of the expected sizes are indicated, as judged by the migration of DNA sequencing reactions. To calculate repression in vitro by AraR, the amount of transcript detected by densitometric analysis in the absence of l-arabinose is divided by the amount of transcript detected in the presence of l-arabinose, both standardized with the veg signal in the same lane. The values of repression in vitro are the average ± the standard deviation of two independent experiments except for the wild-type promoter value, which is the result of four independent experiments.
Linearized plasmids carrying the mutant promoters ORA1mut, ORA2mut, +5, and +11 and also the wild-type promoter present in strain IQB300 were used as templates using the in vitro transcription system described above. To measure repression in vitro by AraR, the amount of transcript in the presence or absence of l-arabinose was quantified by densitometric analysis, using the veg transcript as a normalizing factor and the repression factor obtained as described above for in vivo repression. When AraR was absent, transcripts of the expected size were observed in all cases, indicating that there was no alteration of the transcription start site as a consequence of the mutations introduced in the araABDLMNPQ-abfA operon promoter (Fig. 5). The effect of the mutations on AraR repression in vitro was not so dramatic as it was observed to be in vivo, but a specific transcript was always detected in the absence of l-arabinose in all mutants analyzed (compare lanes 2 in Fig. 5). Furthermore, the levels of repression measured in vitro correlated to those observed in vivo and followed the order +5 < +11 < ORA1mut < ORA2mut (compare Fig. 2B and Fig. 5). These results confirmed that the mutations analyzed are directly affecting the mechanism of repression by AraR.
DISCUSSION
We have previously shown that binding of AraR in vitro to the in-phase duplicate operators in the araABDLMNPQ-abfA operon and araE promoters is cooperative and results in DNA bending, whereas the binding of AraR to the single site in its own promoter is noncooperative with no detectable distortion of the DNA helix (25). In this work, an in vivo deletion analysis of the ara operon and araE/araR promoters region strongly suggests that the AraR binding sites identified in vitro are the key cis-acting regulatory elements of their corresponding genes. Furthermore, the study of mutations designed to prevent the communication between repressor molecules bound to ORA1 and ORA2 in the l-arabinose metabolic operon promoter provided evidence that the cooperative binding of AraR is a requirement for efficient repression in vivo. This was supported by analyzing the effect of the mutations in an in vitro transcription system, which showed that the mutations are directly affecting the mechanism of repression mediated by AraR. The communication between AraR molecules implicates the formation of a small 42-bp DNA loop, an idea which is also supported by our previous observation of cooperative binding of AraR concomitant with DNA bending to the operators within the ara operon promoter.
In the lac (29), gal (11), or deo (3) systems in E. coli and in the catabolite repression of xyl in B. megaterium (9), DNA loop formation involves additional operator sites, i.e., sites other than those that fall nearby (or overlap) the promoter, called auxilliary operators (27). In the l-arabinose metabolic operon ORA1 and ORA2 are both essential for repression by AraR as shown by the similar effect on repression observed by an identical point mutation in each operator. However, we cannot rule out the possibility that other regulatory elements outside of the 204-bp DNA fragment used to construct the full-length araA′-lacZ fusion can contribute to an even tighter repression because when araA′-lacZ fusions are analyzed in the araA locus of the B. subtilis chromosome repression by AraR is two- to three-fold higher (41) compared to the fusions analyzed here in the amyE locus. Two potential auxillary operators located within the promoter and coding region of the abnA gene, ca. 1,200 and 330 bp upstream from ORA1, respectively, were found by sequence analysis (25). Despite this hypothesis, which is currently under investigation, the operators in the ara operon promoter are the key cis-acting regulatory elements in the control of the l-arabinose metabolic operon transcription by AraR.
The in vivo deletion analysis of the araE/araR promoter indicates that the operators in the araE promoter have identical functions to those of the ara operon promoter, and similar mechanisms of repression by AraR are expected. This is supported by the identical relative localization of the operators in the two promoters and by our previous DNase I footprinting analysis (25). However, in this case the divergently located araR promoter and its single operator provide a higher degree of complexity. The in vivo analysis described here indicates that the AraR binding sites ORE1-ORE2 and ORR3, besides being the key cis-acting regulatory elements controlling transcription of their respective genes, are auxiliary operators for the autoregulation of araR and repression of araE, respectively. In the araE/araR promoter, all regulatory sequences required for AraR control seem to be contained within the 384-bp DNA fragment used to construct the full-length araE′-lacZ and araR′-lacZ fusions. Similar levels of repression are observed when these promoter fusions are analyzed in the araE (42) or araR (40) loci of the B. subtilis chromosome.
DNA insertions between ORA1 and ORA2 placing the operators in opposite sides of the DNA helix result in almost complete derepression in vivo and the absence of cooperative binding in vitro. Interestingly, however, repression and cooperative binding are not restored by DNA insertions that restore the phasing. This is different from the DNA looping promoted by several other proteins showing phasing dependence in vivo or in vitro such as the E. coli regulatory proteins AraC (20), LacI (16, 26), the λ repressor (14), or the B. subtilis response regulator ComA (28, 36). DNA loop formation is energetically disfavored if the binding sites are separated by 30 to 140 nucleotides, as in the case of ORA1 and ORA2, due to a limited flexibility of DNA fragments of this range. In some of these cases, bringing the proteins in close contact sometimes requires a special composition of the intervening DNA, being already bent or favoring the bending of the DNA, or the use of architectural (DNA bending) proteins (reviewed in reference 32). Nevertheless, quite often the energetic burden of bending the DNA is compensated for by the protein-protein interactions, as observed in artificial situations with the λ repressor or the AraC protein that have been shown to form 52- and 32-bp loops, respectively (14, 20). Apparently, ComA also forms by itself a 44-bp DNA loop (28, 36). Clearly, and in contrast with these systems, in the ara operon promoter other factors besides the phasing between ORA1 and ORA2 and AraR are crucial for the formation of the small 42-bp DNA loop. An intrinsically bent intervening sequence has been shown to be required for the contacts at a 20-bp distance between the p4 protein of B. subtilis phage φ29 and RNA polymerase that mediate transcription activation (46) and also for the formation of the 93-bp DNA loop by the E. coli NagC repressor (34, 35). It is plausible that the insertions made between the AraR binding sites in the metabolic operon promoter may have destroyed an unidentified special sequence motif of the interoperator region that favors the bending of the DNA and/or the interactions between AraR dimers that lead to DNA loop formation may be rather weak. However, the promoters carrying the different insertions here analyzed do not show a distinct structure compared to the wild-type promoter, as judged by migration of the respective DNA fragments on native polyacrylamide gels (data not shown). The possibility that the insertion destroyed the binding site for an architectural protein that assists DNA looping, resembling the role of IHF, HU, or CAP in E. coli (1, 2, 32), seems unlikely, since the distortion of the DNA helix is detected in vitro by DNase I footprinting with just purified AraR and the target DNA present (25).
By analogy to other systems involving DNA loop (reviewed in references 6, 22, and 43), there are two models by which looping can bring out transcription repression of the ara operon. First, DNA looping plays a passive role, just increasing the local concentration of AraR within the promoter region. In this case, and since repression can be increased by a higher rate of promoter clearance (19), the explanation for the tighter repression of the ara operon relative to the autoregulation of araR might be a lower clearance rate of the araR promoter. In a second hypothesis, DNA looping plays an active role by altering the structure of the promoter region in such a way that transcription initiation is no longer possible. In both cases, the promoter characteristics, either the clearance rates and/or the number and localization of the repressor binding sites/determine the efficiency of repression. The AraR protein is the first member from the GntR family of bacterial regulators to which DNA looping is proposed for the mechanism of repression. Although this mechanism is a common theme in the regulation of bacterial gene expression, in each case, the number of operators, their relative affinities, location and distances separating them are diverse and were selected to respond to the characteristics of a particular system (6, 22, 43). This is well illustrated by the ara regulon, where the design of the AraR target promoters, with precisely spaced duplicate operators and/or a special interoperator sequence, is required for the formation of the small DNA loop that is crucial for a tight and flexible control of the ara regulon. It is possible that this organization of the operators has been selected to account for weak interactions between AraR dimers.
AraR is the major protein involved in the repression of the ara promoters in vivo upon l-arabinose depletion, since in an in vitro transcription system it suffices to abolish transcription from the araABDLMNPQ-abfA operon and araE promoters. However, in addition to the negative control by AraR, the metabolic operon and transport gene promoters are also subject to catabolite repression (40, 42). In particular physiological conditions AraR and CcpA, a protein that mediates carbon catabolite repression in B. subtilis (48), will be closely bound in the same promoter DNA region. It will thus be interesting to study to what extent these two proteins cooperate in the negative control of the ara regulon.
ACKNOWLEDGMENTS
We thank P. Tavares, T. A. Trautner, H. de Lencastre, and A. O. Henriques for their constant interest in this work and many helpful discussions.
This work was partially supported by grant no. PRAXIS XXI Bio/0033/96 to 1.S.-N. from Fundação para a Ciência e Tecnologia (FCT). L.J.M was a recipient of fellowship no. PRAXIS XXI BD/5689/95, and L.M.S. was a recipient of fellowship no. BIC/14707/97 from the FCT.
REFERENCES
- 1.Akl T, Adhya S. Repressor induced site-specific binding of HU for transcriptional regulation. EMBO J. 1997;16:3666–3674. doi: 10.1093/emboj/16.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aki T, Choy H E, Adhya S. Histone-like protein HU as a specific transcriptional regulator: co-factor role in repression of gal transcription by Gal repressor. Genes Cells. 1996;1:179–188. doi: 10.1046/j.1365-2443.1996.d01-236.x. [DOI] [PubMed] [Google Scholar]
- 3.Amouyal M, Mortensen L, Buc H, Hammer K. Single and double loop formation when deoR repressor binds to its natural operator sites. Cell. 1989;58:545–551. doi: 10.1016/0092-8674(89)90435-2. [DOI] [PubMed] [Google Scholar]
- 4.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–253. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 6.Choy H, Adhya S. Negative control. In: Neidhart F C, Curtiss III R, Ingraham J L, Lin E E C, Low K, Maganasik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1287–1299. [Google Scholar]
- 7.Engelsberg E, Irr J, Power J, Lee N. Positive control of enzyme synthesis by gene C in the l-arabinose system. J Bacteriol. 1965;90:946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englesberg E, Anderson R, Weinberg R, Lee N, Hoffee P, Huttenhauer G, Boyer H. l-arabinose sensitive, l-ribulose-5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962;90:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gösseringer R, Küster E, Galinier A, Deutscher J, Hillen W. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J Mol Biol. 1997;266:665–676. doi: 10.1006/jmbi.1996.0820. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt J, Schielf R. Regulation of the l-arabinose operon in vitro. Nat New Biol. 1971;233:166–170. doi: 10.1038/newbio233166a0. [DOI] [PubMed] [Google Scholar]
- 11.Haber R, Adhya S. Interaction of spacially separated protein-DNA complexes for control of gene expression: operator conversions. Proc Natl Acad Sci USA. 1988;85:9683–9687. doi: 10.1073/pnas.85.24.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D. Techniques for transformation of E. coli. In: Glover D M, editor. DNA cloning II: a practical approach. Washington, D.C.: IRL Press; 1985. pp. 109–135. [Google Scholar]
- 13.Haydon D J, Guest J R. A new family of bacterial regulatory proteins. FEMS Microbiol Lett. 1991;79:291–296. doi: 10.1016/0378-1097(91)90101-f. [DOI] [PubMed] [Google Scholar]
- 14.Hochschild A, Ptashne M. Cooperative binding of λ repressors to sites separated by integral turns of the DNA helix. Cell. 1986;44:681–687. doi: 10.1016/0092-8674(86)90833-0. [DOI] [PubMed] [Google Scholar]
- 15.Jacob S, Allmansberger R, Gärtner D, Hillen W. Catabolite repression of the operon for xylose utilization from Bacillus subtilis W23 is mediated at the level of transcription and depends on a cis site in the xylA reading frame. Mol Gen Genet. 1991;229:189–196. doi: 10.1007/BF00272155. [DOI] [PubMed] [Google Scholar]
- 16.Krämer H, Niemolier M, Amouyal M, Revet B, von Wilcken-Bergmann B, Müller-Hill B. lac repressor forms loops with linear DNA carrying two suitably spaced lac operators. EMBO J. 1987;6:1481–1491. doi: 10.1002/j.1460-2075.1987.tb02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krispin O, Allmansberg R. The Bacillus subtilis AraE protein displays a broad substrate specificity for several different sugars. J Bacteriol. 1998;180:3250–3252. doi: 10.1128/jb.180.12.3250-3252.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 19.Lanzer M, Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci USA. 1988;85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee D, Schleif R F. In vivo DNA loops in araCBAD: size limits and helical repeats. Proc Natl Acad Sci USA. 1989;86:476–480. doi: 10.1073/pnas.86.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepesant J A, Dedonder R. Metabolism du l-arabinose chez Bacillus subtilis Marburg Ind− 168. C R Acad Sci Ser D. 1967;264:2683–2686. [PubMed] [Google Scholar]
- 22.Matthews K S. DNA looping. Microbiol Rev. 1992;56:123–136. doi: 10.1128/mr.56.1.123-136.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 24.Moran C P, Jr, Lang N, Le Grice S F J, Lee G, Stephens M, Sonensheim A L, Pero J, Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186:339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- 25.Mota L J, Tavares P, Nogueira I S. Mode of action of AraR, the key regulator of l-arabinose metabolism in Bacillus subtilis. Mol Microbiol. 1999;33:476–489. doi: 10.1046/j.1365-2958.1999.01484.x. [DOI] [PubMed] [Google Scholar]
- 26.Müller J, Oehler S, Müller-Hill B. Repression of lac promoter as a function of distance, phase and quality of an auxiliary lac operator. J Mol Biol. 1996;257:21–29. doi: 10.1006/jmbi.1996.0143. [DOI] [PubMed] [Google Scholar]
- 27.Müller-Hill B. The function of auxiliary operators. Mol Microbiol. 1998;29:13–18. doi: 10.1046/j.1365-2958.1998.00870.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakano M M, Zuber P. Mutational analysis of the regulatory region of the srfA operon in Bacillus subtilis. J Bacteriol. 1993;175:3188–3191. doi: 10.1128/jb.175.10.3188-3191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oehler S, Eismann E R, Krämer H, Müller-Hill B. The three operators of the lac operon cooperate in repression. EMBO J. 1990;9:973–979. doi: 10.1002/j.1460-2075.1990.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascal M, Kunst F, Lepesant J A, Dedonder R. Characterization of two sucrase activities in Bacillus subtilis Marburg. Biochimie. 1971;53:1059–1066. doi: 10.1016/s0300-9084(71)80193-1. [DOI] [PubMed] [Google Scholar]
- 31.Perego M. Integrational vectors for genetic manipulation in Bacillus subtilis. In: Sonensheim A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- 32.Pérez-Martín J, de Lorenzo V. Clues and consequences of DNA bending in transcription. Annu Rev Microbiol. 1997;51:593–628. doi: 10.1146/annurev.micro.51.1.593. [DOI] [PubMed] [Google Scholar]
- 33.Perkins J B, Youngman P J. Construction and properties of Tn917-lac, a transposon derivative that mediates transcriptional gene fusions in Bacillus subtilis. Proc Natl Acad Sci USA. 1986;83:140–144. doi: 10.1073/pnas.83.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plumbridge J, Kolb A. DNA bending and expression of the divergent nagE-nagB operons. Nucleic Acids Res. 1998;26:1254–1260. doi: 10.1093/nar/26.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plumbridge J, Kolb A. DNA loop formation between Nag repressor molecules bound to its operator sites is necessary for repression of the nag regulon of Escherichia coli in vivo. Mol Microbiol. 1993;10:973–981. doi: 10.1111/j.1365-2958.1993.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 36.Roggiani M, Dubnau D. ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA. J Bacteriol. 1993;175:3182–3187. doi: 10.1128/jb.175.10.3182-3187.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-termination inhibition. Proc Natl Acad Sci USA. 1977;74:140–144. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sá-Nogueira I, Lencastre H. Cloning and characterization of araA, araB, and araD, the structural genes for l-arabinose utilization in Bacillus subtilis. J Bacteriol. 1989;171:4088–4091. doi: 10.1128/jb.171.7.4088-4091.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sá-Nogueira I, Mota L J. Negative regulation of l-arabinose metabolism in Bacillus subtilis: characterization of the araR (araC) gene. J Bacteriol. 1997;179:1598–1608. doi: 10.1128/jb.179.5.1598-1608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sá-Nogueira I, Nogueira T V, Soares S, Lencastre H. The Bacillus subtilisl-arabinose (ara) operon: nucleotide sequence, genetic organization and expression. Microbiology. 1997;143:957–969. doi: 10.1099/00221287-143-3-957. [DOI] [PubMed] [Google Scholar]
- 42.Sá-Nogueira I, Ramos S. Cloning, functional analysis, and transcriptional regulation of the Bacillus subtilis araE gene involved in l-arabinose utilization. J Bacteriol. 1997;179:7705–7711. doi: 10.1128/jb.179.24.7705-7711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schleif R. DNA looping. Annu Rev Biochem. 1992;61:199–223. doi: 10.1146/annurev.bi.61.070192.001215. [DOI] [PubMed] [Google Scholar]
- 44.Schleif R. Regulation of the l-arabinose operon of Escherichia coli. Trends Genet. 2000;16:559–565. doi: 10.1016/s0168-9525(00)02153-3. [DOI] [PubMed] [Google Scholar]
- 45.Schleif R. Two positively regulated systems, ara and mal. In: Neidhart F C, Curtiss III R, Ingraham J L, Lin E E C, Low K, Maganasik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1300–1309. [Google Scholar]
- 46.Serrano M, Barthelmy I, Salas M. Transcription activation at a distance by phage φ29 protein p4. Effect of bent and non-bent intervening DNA sequences. J Mol Biol. 1991;219:403–414. doi: 10.1016/0022-2836(91)90182-6. [DOI] [PubMed] [Google Scholar]
- 47.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 48.Stülke J, Hillen W. Regulation of carbon catabolism in Bacillus species. Annu Rev Microbiol. 2000;54:849–880. doi: 10.1146/annurev.micro.54.1.849. [DOI] [PubMed] [Google Scholar]
- 49.Wade K H, Schyns G, Opdyke J A, Moran C P., Jr A region of ςK involved in promoter activation by GerE in Bacillus subtilis. J Bacteriol. 1999;181:4365–4373. doi: 10.1128/jb.181.14.4365-4373.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weickert M J, Adhya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992;267:15869–15874. [PubMed] [Google Scholar]