Highlights
-
•
Data of retinol and β-carotene in 333 and 271 foods prepared as consumed in Germany.
-
•
Highest levels of retinol were found in liver and liver-based food.
-
•
High β-carotene levels occurred in orange/green leafy vegetables and fruit nectars.
-
•
In some foods, levels varied significantly based on production type and seasonality.
-
•
Margarine represents a food often fortified voluntarily with retinol and β-carotene.
Chemical compounds: Retinol (PubChem CID445354), β-carotene (PubChem CID5280480)
Abbreviations: BfR, Bundesinstitut für Risikobewertung (German Federal Institute for Risk Assessment); BLE, Federal Office for Agriculture and Food; BLS, Bundeslebensmittelschlüssel (German Nutrient Database); BMEL, Federal Ministry of Food and Agriculture; BVL, Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (Federal Office of Consumer Protection and Food Safety); FLD, Florescence detection; g, Gram; HPLC, High-performance liquid chromatography; LOD, Limit of Quantification; LOQ, Limit of Detection; MEAL, Meals for Exposure Assessment and Analyses of Foods; mLB, Modified lower bound; MS, Mass spectrometry; n, Number; NP, Normal-phase; NVS, Nationale Verzehrsstudie (German National Nutrition Survey); RP, Reversed-phase; SD, Standard deviation; TDS, Total diet study; UB, Upper bound; VELS, Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern (Consumption survey of food intake among infants and young children); µ, micro
Keywords: Total diet study, BfR MEAL Study, (preformed) vitamin A, Retinol, β-carotene, Unprepared and prepared foods, Organic and conventional types of production, Seasonality
Abstract
This Total Diet Study (TDS) provides representative data on substance levels in foods, prepared as typically consumed by the population in Germany for future dietary exposure assessment. Vitamin A is essential and must be obtained from the diet, either as preformed vitamin A or as provitamin A carotenoids. Levels of retinol and β-carotene were analysed in 333 and 271 foods, respectively. Highest mean retinol levels were found in cod liver (25,000 µg∙100 g−1), followed by other animal livers, liver-based products, butter, eel and fortified margarine. In contrast, highest mean β-carotene levels were found in carrots (4,650 µg∙100 g−1), followed by other yellow-orange fruits and vegetables, green leafy vegetables and fortified fruit nectars. Sampling by production type and seasonality revealed differences in retinol and β-carotene levels in individual foods. This TDS expands the existing data for β-carotene and vitamin A extensively by providing representative data on most consumed foods.
Introduction
Through our diet, we are exposed to different substances that can be beneficial or potentially harmful to our health. To estimate the dietary intake and/or exposure to substances and to assess their potential health risks, it is necessary to assess their concentration in foods and match them with food consumption data. While data about food consumption in Germany are available, current data on vitamin A levels in foods are scarce. To investigate levels of substances in foods for exposure assessment, the most cost-effective study design is a Total Diet Study (TDS) (EFSA/FAO/WHO, 2011). Characteristic steps of a TDS are: i) selecting representative foods of a population's diet, ii) preparing of foods as typically consumed and iii) combining similar food items to one sample (pooling) (EFSA/FAO/WHO, 2011). Especially, for thermally labile analytes such as vitamins, the consideration of analytes losses due to food preparation can reduce uncertainties. In 2015, the German Federal Institute for Risk Assessment (BfR) started the first German TDS, the BfR MEAL Study (MEAL: meals for exposure assessment and analysis of foods).
The term vitamin A comprises several fat-soluble compounds with the biological activity of retinol such as all-trans-retinol (retinol) and related molecules (e.g. retinal, retinoic acid, retinyl esters), as well as provitamin A carotenoids such as β-carotene. In particular, β-carotene can be cleaved to retinol and functions as dietary precursor of vitamin A. In the human body, vitamin A compounds have many functions. They are essential for vision and gene regulation, contribute to growth, differentiation and proliferation of a wide range of epithelial tissues, as well as to bone growth, reproduction and embryonic development (Chea et al., 2021, EFSA, 2015). In addition, dietary carotenoids show the ability for radical quenching, can function as antioxidants, possess anticarcinogenic activities and are regulators for gene-expression. However, their physiological and nutritional relevance has not been conclusively proven and confirmed in humans (Böhm et al., 2021).
Vitamin A deficiency might cause impaired vision and affects other functions of the body such as reproduction and immunity. The latter is, however, often associated with multiple deficiencies, whereby the exact role of vitamin A remains to be ascertained. Overall, vitamin A deficiency is rare in Europe. However, intakes of excessive amounts of vitamin A may occur and result in hypervitaminosis A with adverse effects on health. Since vitamin A accumulates in the liver, hepatotoxicity is one of the most severe outcomes of chronically high intakes of vitamin A. Moreover, vitamin A is considered teratogenic if taken in excessive amounts during early pregnancy (Chea et al., 2021).
Consumption of foods naturally containing high β-carotene levels has not been associated with adverse health effects apart from carotenodermia, which is a harmless yellowish discoloration of the skin (IOM, 2000). However, long-term daily intake of high amounts of synthetic β-carotene (i.e. 20 or 30 mg/day by supplements and fortified foods) increased the risk of lung cancer in heavy smokers and asbestos workers (Albanes et al., 1996, Omenn et al., 1996). Major dietary sources of preformed vitamin A are foods of animal origin, in particular liver, meat, milk, dairy products and fish. Whereas provitamin A carotenoids are naturally present mainly in yellow, red and orange fruits and vegetables such as carrots, sweet potato, pumpkin, and broccoli (Weber & Grune, 2012). In addition to its natural presence in foods, β-carotene is permitted as food additive (E 160a (ii)) for colouring purposes (EC, 2008b). Both, preformed vitamin A (as retinol, retinyl acetate, retinyl palmitate) and β-carotene are authorised in the EU as additives for use in animal feed (EC, 2003, EU, 2015). In the past years, the use of retinol and its esters in feed often resulted in high levels of vitamin A in animal-derived foods, especially in pig, bovine and sheep liver and liver-based products (BVL, 2017). Other sources of preformed vitamin A and β-carotene are food supplements and fortified foods (EFSA, 2015). Foods most commonly fortified with β-carotene are fruit juices, nectars and non-alcoholic beverages (Tennant, Gedrick, Godfrey, & Davidson, 2004). Preformed vitamin A is rarely used to fortify common foods, with the exception of margarine sold as butter substitute, which may be fortified with up to 10 mg∙kg−1 retinol in Germany (LMvitV, 2017).
Levels of preformed vitamin A and β-carotene in representatively prepared foods are scarce (EFSA, 2008). Moreover, current data about levels of preformed vitamin A in animal-derived foods (specifically liver) and of β-carotene in plant-based foods, but also in foods to which β-carotene is added either for physiological or technological purposes is limited. To close this gap of information, the BfR MEAL Study will give a detailed overview on the realistic contents of preformed vitamin A and β-carotene in foods, typically prepared as consumed by German households. Furthermore, the impact of seasonality and type of production (organic and conventional farming) on vitamin A and β-carotene levels was investigated in this study. This dataset will help reducing the uncertainties in a later dietary exposure assessment in Germany.
Material and methods
All foods for retinol and β-carotene analysis were purchased and analysed from November 2016 to May 2019.
Selection, purchasing and preparation of foods
Study design, food selection and purchasing. The BfR MEAL Study follows the design of a TDS as described elsewhere (Kolbaum et al., 2022, Sarvan et al., 2017, Sarvan et al., 2021). More than 90 % of foods typically consumed in the German diet as well as foods known to possibly contain high levels were included in the analyses. Similar food items (such as “Pizza Diavolo”, “Pizza Salami” and “Pizza Hawaii”) are aggregated to one pooled sample with the generic term MEAL food (here: “Pizza with meat”). The selection of MEAL foods and the implementation of the MEAL food list were based on the German consumption data sets for children (VELS: Consumption survey of food intake among infants and young children; covering age groups 0.5 to < 5 years) and for adults (NVS II: National consumption survey II, covering age groups 14 to < 80 years). For each substance investigated within the study, the MEAL food list was adjusted to sample and analyse only relevant foods. Some MEAL foods were sampled according to type of production (conventional and organic) and/or the seasonality (season 1 and 2) and resulting in two to four MEAL food pools per MEAL food. Due to global food supply, foods may originate from geographically different areas or be affected by various production conditions such as climate, soil and animal husbandry (e.g. pasture versus stable feeding) during the year. Therefore, seasonally sampled foods were purchased at two different times of the year to reflect the products as closely as possible. Sampling points for both seasons were defined individually for each MEAL food. For retinol, 522 MEAL food pools of 333 MEAL foods were investigated, of which 98 foods were differentiated in terms of type of production, and 58 were purchased at two different dates of the year. For β-carotene, 436 MEAL food pools of 271 MEAL foods were analysed, and a differentiation according to type of production and seasonality was considered for 88 and 51 MEAL foods, respectively. Every MEAL food pool consisted of at least 15 to 20 single food items/products (subsamples) (Supplementary A, Table S2,S3), with the exception of boletus and edible offal, which are composed of fewer subsamples due to limited market availability. The composition of a MEAL food pool was based on consumption as well as market share data and included subsamples with or without fortification of preformed vitamin A or β-carotene. Documentation of the composition of MEAL food pools and individual subsamples was performed with the software FoodCASE (Premotec GmbH, Switzerland, version 7.0.1). Sample collection, food preparation as well as homogenisation and storage was performed as described in Sarvan et al. 2021, and further information can be found in Supplementary B.
Determination of retinol and β-carotene
With respect to preformed vitamin A compounds, all–trans–retinol and all–trans–retinyl esters were quantified together as sum after saponification and extraction as all–trans–retinol by florescence detection (FLD). Quantification of β-carotene was performed after saponification and extraction with a diode array detection (DAD) system at the wavelength 450 nm. All samples were analysed in duplicate and a blank control was included. For retinol, the limit of detection (LOD) was determined at 0.2 µg∙100 g−1 and the limit of quantification (LOQ) at 0.5 µg∙100 g−1, with the exception of rapeseed oil, linseeds, vegetarian sandwich spread and buckwheat (Supplementary A, Table S1). For β-carotene, 3 µg∙100 g−1 and 10 µg∙100 g−1 were determined as LOD and LOQ, respectively. The calibration functions for retinol and β-carotene exhibit good linearities with regression coefficients of R2 ≥ 0.99. The expanded measurement uncertainty (k = 2) was 20 % for retinol and 25 % for β-carotene, respectively (Supplementary A, Table S1).
Chemicals and laboratory equipment. Further information on chemicals and laboratory equipment is provided in Supplementary B.
Retino
Saponification and extraction. Samples were prepared following DIN EN 12823–1: Dry sample materials were dissolved in max. 20 mL of water prior to saponification. For preparation of dry sample materials, such as powdered foods, 20 g of powder were mixed with water to a total weight of 200 g. 30 g of this mixture were then transferred into a round-bottomed flask for saponification. For all other samples, 1–10 g of sample material were weighted directly into a round-bottomed flask. For saponification, 100 mL ethanol (with 0.05 % (w/v) 2,6-Di-tert-butyl-p-cresol (BHT)), 1 g sodium l-ascorbate, 2 mL sodium sulphide hydrate solution (61.5 g/L) and 25 mL potassium hydroxide solution (50 % (w/v)) were added. The solution was coated with a nitrogen layer and saponified at 65 °C for 45 min in an ultrasonic bath (Sonorex RK 1028CH, Bandelin, Berlin, Germany). After cooling to room temperature, the solution was extracted with 80 mL and then two times with 50 mL hexane/MTBE (90:10, v/v). The hexane extracts were washed with water until a neutral pH was reached and filled up with hexane to 200 mL. Of this extract solution, 20–25 mL were evaporated to dryness by means of a rotary evaporator (Laborata 4002 control, Heidolph Instruments, Schwabach, Germany) and dissolved in 1 mL hexane for further analysis.
Chromatographic separation and detection. Samples were analysed by normal-phase (NP)–high-performance liquid chromatography (HPLC) with FLD. For detailed HPLC and FLD parameters, see Supplementary B. In case of high matrix interferences, a validation by NP–HPLC coupled to tandem mass spectrometry (MS/MS) was performed in addition to NP–HPLC–FLD analysis. Furthermore, the levels of retinol in spices were determined by HPLC–MS/MS using quantification by standard addition (Supplementary B).
Quantification. Quantification was performed using a 4-point external calibration in the concentration range of 25 to 500 ng∙mL−1 by injecting different volumes of a retinol standard solution into the HPLC–FLD system. For further information about retinol standard solution preparation, see Supplementary B.
β-Carotene
Saponification and extraction: sample matrices with low fat content. Samples were prepared according to ASU L 00.00–149. Sample material (5 to 10 g) was weighted into a 200 mL round-bottomed flask and 200 mg BHT, 0.5 mL Protex 6L stock solution and max. 12 mL water, depending on the water content of the sample, were added. For saponification, the sample solution was placed in an ultrasonic bath (Sonorex RK 1028CH, Bandelin) for 30 to 45 min at 50 °C and stirred occasionally. Ethanol was added to the warm sample solution and placed for another 10 min in the ultrasonic bath. After addition of 95 mL dichloromethane, the mixture was shaken vigorously, tempered to room temperature and filled up with dichloromethane to 200 mL. The mixture was shaken vigorously again and a commercial syringe filter with polytetrafluoroethylene (PTFE) membrane (0.45 µm, 25 mm, Opti-Flow, Wicom, Heppenheim, Germany) was used for extraction. If necessary, the mixture was further diluted prior to HPLC analysis with eluent.
Saponification and extraction: sample matrices with high fat content. Samples were prepared according to DIN EN 12823–2: Sample preparation (saponification and extraction) was performed together with the sample preparation for retinol analysis (DIN EN 12823). From 200 mL extract solution, 4 mL were evaporated to dryness with a nitrogen steam (Barkey Evaporator, Leopoldshöhe, Germany) and dissolved in 1 mL eluent. The eluent composition is described in Supplementary B (see section “β-Carotene; Chromatographic separation and detection”). Depending on the analyte level, the amount of eluent added was adjusted.
Chromatographic separation and detection. The extract was analysed by reversed-phase (RP)–HPLC with UV detection. Chromatographic separation and UV detection parameters are provided in detail in Supplementary B.
Quantification. β-Carotene was quantified by HPLC–DAD using 4–point external calibration in the concentration range of 25 to 500 ng∙mL−1 by injecting different volumes of a β-carotene standard solution. For further information about standard solution preparation, see Supplementary B.
Statistical methods
For handling left-censored data (results reported below LOD and/or LOQ), two scenarios were applied: (1) the modified lower bound approach (mLB), by replacing results below LOD by zero and results below LOQ by the respective LOD and (2) the upper bound (UB) approach, by replacing results below LOD by the respective LOD and results below LOQ and above LOD by the respective LOQ. Both scenarios provide an uncertainty range in which the actual substance level is located.
Because results of mLB and UB approach did not differ substantially, results are presented and discussed according to the mLB approach. Results according to the UB approach are available in Supplementary A (Table S2, S3). Levels resulting from multiple analysis for each single MEAL food pool were averaged arithmetically to obtain an mLB and UB result for each MEAL food pool (Table S2, S3). For each MEAL food, results of one to four MEAL food pools were available to calculate statistical parameters for MEAL foods sampled according to season or production type. To calculate mean levels of main food groups, firstly, all MEAL food pools of the same food were arithmetically averaged, and secondly, the mean levels of the single MEAL foods in the respective main food group were arithmetically averaged. Hence, standard deviations (SD) determined on main food group level reflect the variability between different MEAL foods of the respective group and do not reflect the variation between duplicate analysis or between several pooled samples of one MEAL food. Microsoft Excel 2016 was used to calculate mean values, median (50th percentile, P50) as well as minimum (Min.) and maximum (Max.) levels. Further statistical analyses were performed using IBM® SPSS® Statistics (version 26.0). The Mann-Whitney U test (significance level of 0.05) was used to test on differences between organically and conventional production. The test was applied over all MEAL foods where both production types were considered, using mean values for each MEAL food. Statistical differences by production type between main food groups were not investigated due to limited sample size.
Results
Retinol
Retinol was quantified in 348 out of 522 pooled samples (67 %) of 333 MEAL foods included in this study (Table1). In the four main food groups “meat and meat products”, “fish, seafood and invertebrates”, “milk and dairy products”, “eggs and egg products”, with foods of mainly animal origin, all samples contained quantifiable levels of retinol. In contrast, in samples of the main food groups “fruit and vegetable juices and nectars” and “fruit and fruit products”, no retinol levels were quantified (Table1, Supplementary A, Table S2). In the remaining main food groups, the quantifiable retinol levels varied depending on the proportion of animal-derived ingredients or on the amount of fortification.
Table 1.
Levels of retinol in the main food groups (mLB) [µg∙100 g−1].
| Main food group | Pooled samples (n) | MEAL Foods (n) | <LOD/LOQ (%) | Mean* | SD* | Median* | Min.* | Max.* |
|---|---|---|---|---|---|---|---|---|
| Grains and grain-based products | 58 | 40 | 48 | 28.6 | 47.6 | 0.79 | 0 | 160 |
| Vegetable and vegetable products | 67 | 33 | 43 | 5.04 | 7.46 | 2.15 | 0 | 32.0 |
| Starchy roots or tubers and products thereof | 19 | 8 | 58 | 8.83 | 17.3 | 0.100 | 0 | 53.3 |
| Legumes, nuts, oilseeds and spices | 23 | 20 | 74 | 0.626 | 1.56 | 0 | 0 | 7.15 |
| Fruit and fruit products | 40 | 22 | 98 | 0.030 | 0.137 | 0 | 0 | 0.660 |
| Meat and meat productsa | 59 | 35 | 0 | 2023 | 4,732 | 15.3 | 2.10 | 17,000 |
| Fish, seafood and invertebratesa | 30 | 30 | 0 | 918 | 4478 | 15.6 | 3.15 | 25,000 |
| Milk and dairy products | 36 | 23 | 0 | 125 | 99.6 | 97.5 | 4.70 | 318 |
| Eggs and egg products | 4 | 2 | 0 | 168 | 27.5 | 168 | 140 | 195 |
| Sugar, confectionery and water-based sweet desserts | 18 | 15 | 56 | 12.1 | 16.5 | 0.100 | 0 | 50.5 |
| Animal and vegetable fats and oils | 13 | 8 | 46 | 297 | 349 | 96.8 | 0 | 915 |
| Fruit and vegetable juices and nectars | 12 | 10 | 100 | 0 | 0 | 0 | 0 | 0 |
| Coffee, cocoa, tea and infusions | 4 | 3 | 75 | 16.0 | 22.6 | 0 | 0 | 48.0 |
| Food products for infants and toddlers | 13 | 10 | 38 | 81.2 | 152.5 | 2.26 | 0 | 410 |
| Products for non-standard diet and food imitates | 8 | 7 | 75 | 0.821 | 1.30 | 0 | 0 | 3.00 |
| Composite dishes | 100 | 52 | 4 | 60.5 | 259 | 15.3 | 0 | 1,900 |
| Seasoning, sauces and condiments | 18 | 15 | 22 | 52.4 | 68.6 | 20.5 | 0 | 220 |
| Total | 522 | 333 |
*Left-censored data were calculated using the modified lower bound (mLB) approach. Results below LOD were replaced by zero and results above LOD and below LOQ were set at the value reported as LOD.
including offals.
The highest mean levels of retinol were found in the main food group “meat and meat products” (2,023 µg∙100 g−1), followed by the groups “fish, seafood and invertebrates” and “animal and vegetable fats and oils” with considerably lower mean retinol levels of 918 µg∙100 g−1 and 297 µg∙100 g−1, respectively (Table 1). Among the other main food groups, mean retinol levels ranged from 168 µg∙100 g−1 (“eggs and egg products”) to 0.63 µg∙100 g−1 (“legumes, nuts, oilseeds and spices”).
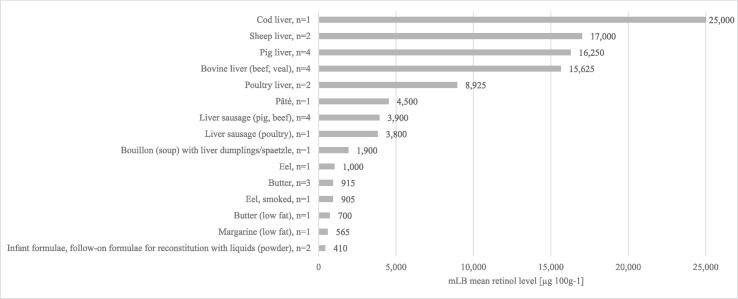
The 15 MEAL foods with highest retinol levels are shown in Fig. 1. All those foods are either assigned to the main food groups with highest contents, are containing liver such as bouillon (soup) with liver dumplings/spaetzle (1,900 µg∙100 g−1) or are fortified such as infant and follow-up formula for reconstitution with liquids (powder) (410 µg∙100 g−1). The highest retinol levels were measured in cod liver (25,000 µg∙100 g−1), followed by sheep liver, pig liver, bovine liver and poultry liver (17,000; 16,250; 15,626; 8,925 µg∙100 g−1, respectively; Fig. 1). High retinol levels were also found in various liver-based foods including pâté and liver sausages made out of pig, beef or poultry liver. In fish, highest retinol levels were detected in eel and smoked eel with 1,000 and 905 µg∙100 g−1, respectively. Additionally, high retinol levels were found for butter, butter with reduced fat content and margarine with reduced fat content.
Fig. 1.
Highest mean levels of retinol in 15 out of 333 MEAL foods analysed (mLB) [µg∙100 g−1], n = number of MEAL food pools (including 15 to 20 subsamples) per MEAL food.
β-Carotene
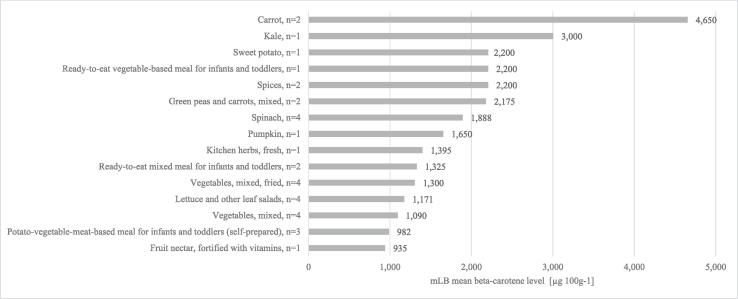
β-Carotene was quantified in 327 out of 436 pooled samples (75 %) of 271 MEAL foods included in this study (Table 2). In the main food group “composite dishes” as well as “sugar, confectionary and water-based sweet desserts”, all samples (n = 97 and n = 1, respectively) contained quantifiable levels of β-carotene. In the remaining main food groups, the amount of quantifiable levels of β-carotene varied depending on the proportion of plant-derived foods or the use of β-carotene as food additive or fortificant. Highest mean levels of β-carotene were found in the main food groups “vegetable and vegetable products” (671 µg∙100 g−1) followed by the groups “food products for infants and toddlers” (448 µg∙100 g−1) and “starchy roots or tubers and products thereof” (309 µg∙100 g−1). For the other main food groups, levels ranged from 307 µg∙100 g−1 (“composite dishes”) to 6.33 µg∙100 g−1 (“coffee, cocoa, tea and infusions”). The 15 MEAL foods with highest β-carotene levels (Fig. 2) comprise mainly vegetables and processed vegetable dishes, with the exception of spices (dry) (2,200 µg∙100 g−1) and fruit nectars fortified with vitamins (935 µg∙100 g−1). The highest level of β-carotene was measured in carrots (4,650 µg∙100 g−1). High β-carotene levels were also found in other vegetables including kale, sweet potato, spinach, pumpkin, lettuce and other leaf salads as well as kitchen herbs (fresh), with levels ranging from 3,000 µg∙100 g−1 to 1,171 µg∙100 g−1. Additionally, high β-carotene levels were found in two ready-to-eat meals for infants and toddlers as well as in a self-prepared meal for infants and toddlers.
Table 2.
Levels of β-carotene in the main food groups (mLB) [µg∙100 g−1].
| Main food group | Pooled samples (n) | MEAL Foods (n) | <LOD/LOQ (%) | Mean* | SD* | Median* | Min.* | Max.* |
|---|---|---|---|---|---|---|---|---|
| Grains and grain-based products | 57 | 40 | 58 | 19.9 | 26.1 | 3.00 | 0 | 95 |
| Vegetables and vegetable products | 66 | 33 | 15 | 671 | 1,011 | 238 | 0 | 4,650 |
| Starchy roots or tubers and products thereof | 19 | 8 | 53 | 309 | 717 | 14.1 | 0 | 2,200 |
| Legumes, nuts, oilseeds and spices | 24 | 20 | 50 | 151 | 477 | 5.75 | 1.50 | 2,200 |
| Fruit and fruit products | 40 | 22 | 18 | 64.5 | 83.3 | 39.3 | 0 | 370 |
| Meat and meat productsa | 19 | 13 | 37 | 14.3 | 12.8 | 12.5 | 0 | 45.5 |
| Fish, seafood and invertebratesa | 5 | 5 | 20 | 55.6 | 30.2 | 63.5 | 0 | 91.5 |
| Milk and dairy products | 37 | 23 | 11 | 71.8 | 61.8 | 58.7 | 0 | 205 |
| Eggs and egg products | 4 | 2 | 50 | 7.25 | 5.75 | 7.25 | 1.50 | 13.5 |
| Sugar, confectionery and water-based sweet desserts | 1 | 1 | 0 | 150 | 0 | 150 | 150 | 150 |
| Animal and vegetable fats and oils | 13 | 8 | 15 | 250 | 214 | 258 | 0 | 610 |
| Fruit and vegetable juices and nectars | 12 | 10 | 58 | 205 | 312 | 5.00 | 0 | 935 |
| Coffee, cocoa, tea and infusions | 4 | 3 | 75 | 6.33 | 8.96 | 0 | 0 | 19.0 |
| Food products for infants and toddlers | 13 | 10 | 40 | 448 | 728 | 24.5 | 0 | 2,200 |
| Products for non-standard diet and food imitates | 8 | 7 | 50 | 31.6 | 57.1 | 7.50 | 0 | 170 |
| Composite dishes | 97 | 52 | 0 | 307 | 299 | 218 | 7.75 | 1,300 |
| Seasoning, sauces and condiments | 17 | 14 | 6 | 221 | 265 | 91.0 | 0 | 858 |
| Total | 436 | 271 |
*Left-censored data were calculated using the modified lower bound (mLB) approach. Results below LOD were replaced by zero and results above LOD and below LOQ were set at the value reported as LOD.
including offals.
Fig. 2.
Highest mean levels of β-carotene in 15 out of 271 MEAL foods analysed (mLB) [µg∙100 g−1], n = number of MEAL food pools (including 15 to 20 subsamples) per MEAL food.
Levels of retinol and β-carotene in MEAL foods sampled according to type of production (organic or conventional) and seasonality
Overall, 98 and 88 MEAL foods were sampled and analysed, respectively, for retinol and β-carotene with distinction between organic and conventional type of production (Supplementary A, Table S2,S3). These MEAL foods are assigned to 15 and 14 main food groups of the BfR MEAL Study, respectively (Figure S1 A-B). Considering all MEAL foods differentiated by type of production, no significant differences in retinol and β-carotene levels were found for this study (p = 0.898).
Although no significant differences were found over all MEAL foods, significant differences for individual foods are possible. Varying levels of retinol and β-carotene between organic and conventional foods were found in the main food group “animal and vegetable fats and oils”. In this main food group, conventionally produced margarine had more than 200-fold higher retinol levels (385 versus 1.60 µg∙100 g−1) and more than 4.7-fold higher β-carotene levels (575 versus 120 µg∙100 g−1) than organically produced margarine. In the main food group “legumes, nuts, oilseeds and spices”, differences were mainly attributable to the MEAL food olives. In conventionally compared to organically produced olives, 3.3-fold higher β-carotene levels were found (315 versus 96.5 µg∙100 g−1). For the main food group “meat and meat products”, conventionally produced MEAL foods showed a tendency of higher mean retinol levels compared to organically produced foods. Three pooled samples of livers (poultry, bovine and pig liver) as well as liver sausage (pig, bovine) had the greatest impact on these differences.
For the other main food groups, minor or no differences were found with regard to production types, except for a few specific MEAL foods, such as e.g. green peas and carrots (mixed) and red cabbage (Supplementary A, Table S2, S3). Among others, higher differences of β-carotene levels were e.g. found in organically and conventionally produced cereal crackers (puffed) and fresh kitchen herbs.
In addition, 58 and 51 MEAL foods were sampled seasonally for retinol and β-carotene, respectively (Table S2, S3). Because the sampling times of season 1 and season 2 are specific for each MEAL food, retinol and β-carotene levels were not compared at the main food group level. Nevertheless, in the main food group “composite dishes”, differences in retinol levels between seasons were reported for goulash (5.8-fold), beef roulade (1.8-fold), tomato soup (3.5–fold) and potato-vegetable-meat-based meal for infants and toddlers (2.9-fold). In two sauces (vegetable and tomato sauce) and in fennel (bulb), retinol levels differed by a factor of 1.8- to 2.0. For organically produced milk and butter, lower retinol levels were found in milk and butter sampled in season 1 compared to foods sampled in season 2 (25 µg∙100 g−1 versus 30.5 µg∙100 g−1 and 756 µg∙100 g−1 versus 1,000 µg∙100 g−1, respectively). Different β-carotene levels for two seasons were found in three MEAL foods of the main food group “vegetable and vegetable products” including apples, sweet pepper, lettuce and other leaf salads (factor 1.6 to 2.5). Additionally, 1.8 to 2.0-fold differences in β-carotene levels were found between seasons in three potato-based MEAL foods including pan fried potatoes, potato salad and potato-vegetable-meat-based meal for infants and toddlers.
Discussion
Levels of retinol in MEAL foods
Highest levels of retinol were found in livers of cod, sheep, pig, bovine, poultry and liver-based products such as liver sausage and liver pâté. Liver is known to be rich in retinol, as it is the main storage organ for vitamin A in both animals and humans (EFSA, 2008, EFSA, 2015).
In the present study, cod liver had by far the highest retinol levels in all foods (Fig. 1). Compared to levels reported by a food database from France (ANSES, 2021), the retinol levels of cod liver were 6–fold higher in the present study. Cod liver is mainly available on the market as canned product in its own oil, since fresh fish liver perishes quickly (BfR, 2007). For tinning, cod liver is usually salted and sterilised, causing leaching of oil from the liver. The sampled cod liver contained exclusively subsamples of canned cod liver in its own oil. The lower retinol levels in the French database (4,170 µg∙100 g−1) might be explained by different handling of samples before analysis. In the French database, the excess of oil from cod liver was drained before analysis, whereas cod liver and its oil were included in the MEAL food samples. Both eating behaviours are common. Retinol levels of cod liver oil of up to 30,000 µg∙100 g−1 were reported in literature (ANSES, 2021), which could explain the higher retinol levels in cod liver detected in the present study.
Retinol levels determined in the BfR MEAL Study in liver samples (Fig. 1) were similar to values reported in literature with sheep liver of 16,039 µg∙100 g−1 (BLS, 2014), pig liver of 14,100 µg∙100 g−1,bovine liver of 15,500 µg∙100 g−1 (DTU, 2021) and poultry liver of 8,490 µg∙100 g−1 ANSES, 2021).. Due to concerns of high vitamin A levels caused by accumulation, especially in pig and bovine liver (EFSA, 2008), a new implementing regulation was adopted in the EU in 2015. The regulation stipulates new, and in part considerably reduced, maximum levels for vitamin A (retinyl acetate, retinyl palmitate and retinyl propionate) use as feed additive. For “pigs for fattening” e.g. 6,500 IU instead of 13,500 IU per kg are adopted, to prevent adverse effects on animal and human health or the environment by the reduction of vitamin A transfer from feed to foodstuffs of animal origin (EU, 2015). Results of the present study show 1.3 to 1.8-fold lower retinol levels in liver of sheep (25,100 μg∙100 g−1), pig (29,000 μg∙100 g−1) and bovine (beef 20,800 μg∙100 g−1, veal 16,800 μg∙100 g−1) than in a food monitoring project in Germany, in 2016 (BVL 2017). In the monitoring project, no poultry liver was analysed. Although the new EU regulation had already become effective, animal feed produced after the old regulation were allowed on the market till stocks were exhausted. The sampling of foods for the monitoring project and partly for the present study were conducted in the transitional period (EU, 2015), and might explain higher levels obtained in the monitoring project. Besides animal feed, breed and age of animals can influence the vitamin A levels stored in the liver (Majchrzak, Fabian, & Elmadfa, 2006).Those data are not available for either the BfR MEAL Study or the monitoring project and their influence could therefore not be assessed. As animals are slaugthered for food production at a similar age, this factor might not influence the vitamin A content. Furthermore, no information of the type of production is available for samples of the monitoring project and different production types could have led to diverging levels (BVL, 2017).
Foods in the monitoring project were analysed on commodity level i.e. raw for liver, while foods investigated in TDSs are analysed as typically consumed, taking different preparation techniques into account. Losses of vitamin A during food processing and or preparation are known but difficult to quantify, because information on levels of nutrients are often lacking for prepared foods (EFSA, 2008). Although compared to other vitamins, vitamin A is often reported as stable during cooking over a wide temperature–time range, losses of up to 30 % have been reported in processed foods of animal origin (Ottaway, 2010, USDA, 2007). Moreover, lower retinol levels in the MEAL food livers could be explained by the addition of other ingredients like spices, oils or flour during food preparation, causing possible dilution of levels in the prepared foods.
For liver-based products such as liver pâté, liver sausage (pork and beef, poultry) as well as bouillon with liver dumpling/spaetzle similar levels were reported in the German Nutrient Data Base (BLS, 2014).
In the present study, high retinol levels were measured in eel and smoked eel (1000 and 905 µg∙100 g−1, respectively) (Fig. 1), which were comparable with those reported in the German Nutrient Data Base (998 and 940 µg∙100 g−1, respectively) (BLS, 2014). In contrast, Salma and Hechmi (2013) reported 2–fold lower levels in eel and smoked eel (468 and 429 µg∙100 g−1, respectively). The differences between the studies might be caused by different fishing grounds. Eel analysed by Salma and Hechmi (2013) originated from a lagoon in Tunisia, whereas most of the samples in the present study originated from aquaculture in Germany and the Netherlands. In line with our results, Salma and Hechmi (2013) reported only a slight reduction of retinol in smoked eel due to the hot smoking process.
High retinol levels were obtained in full and low fat butter (Fig. 1), which were comparable with levels from international food databases with retinol levels ranging from 542 to 912 µg∙100 g−1 (ANSES, 2021, DTU, 2021, USDA, 2021). Since vitamin A levels are correlated with the fat content, relatively lower retinol levels were found in low fat butter (with an average fat content of 56 %).
In the present study, high retinol levels were detected in low fat margarine (Fig. 1). Margarine is made from fats or oils of vegetable or animal origin or a mixture of both (EU, 2013). Although subsamples of the MEAL food low fat margarine partly contained ingredients of animal origin, e.g. buttermilk, skimmed milk or yoghurt, the high retinol levels can be mainly attributed to an addition of retinol (as retinyl esters) in 11 out of 15 subsamples with 800 to 900 µg∙100 g−1. The fortification of margarine and fat spreads with retinol (as retinyl esters) is voluntary in Germany and is permitted up to a maximum of 1,000 µg∙100 g−1 (LMvitV, 2017). The maximum permitted level of vitamin A was not exceeded in any of the three MEAL food pools of margarines included in this study.
Similarly, in infant formulae (IF) and follow-on formulae (FOF) for reconstitution with liquids, the observed retinol levels can be explained by regulatory compositional requirements for preformed vitamin A, which are based on scientific advise by EFSA (2014) and stipulated in the Commission Delegated Regulation (EU) 2016/127 (EU, 2016). The samples of IF/FOF were analysed as powders in the BfR MEAL Study and revealed adequate vitamin A levels, calculated on the basis of instructions for reconstitution with water provided by the manufacturers, with 86.3 and 75.9 µg∙100 kcal−1 for organic and conventional IF/FOF products, respectively. No provisions for the addition of β-carotene to IF/FOF as carotenoids are considered as a source of vitamin A due to the unknown bioconversion of carotenoids in infants (EFSA, 2014).
Levels of β-carotene in MEAL foods
β-Carotene was primarily detected in plant-derived MEAL foods whereas retinol was primarily found in foods of animal origin (Table S2, S3).
The highest β-carotene levels were found in the MEAL food carrot (Fig. 2). Carrots are known to contain high β-carotene levels, causing their characteristic orange colour. In the present study, β-carotene levels in raw and cooked carrots were comparable with levels reported in the USDA food database (raw 1,990–21,000 µg∙100 g−1, cooked 4,330–10,800 µg∙100 g−1) (USDA, 2021). Furthermore, high β-carotene levels were found in green leafy vegetables such as kale, spinach, lettuce and other leaf salads as well as in fresh herbs (Fig. 2). For the MEAL foods kale and spinach, β-carotene levels were within the range reported in international food databases (kale raw 2,160–3,830 µg∙100 g−1 and spinach raw 1,610–5,750 µg∙100 g−1) (DTU, 2021, USDA, 2021).
Numerous factors influence the content of carotenoids in fruits and vegetables. Apart from the genotype, several environmental factors can affect the composition and levels of carotenoids in varieties and cultivars, e.g. geographic location, climate (season), cultivation conditions, ripening stage when harvested as well as post-harvest handling, industrial/home processing and storage conditions (Dias et al., 2021, Meléndez-Martínez et al., 2021, Rodriguez-Amaya et al., 2008). In addition, differences of analytical methods may also lead to variations in levels reported (Rodriguez-Amaya et al., 2008). Since many factors influence β-carotene levels in fruits and vegetables, levels vary widely and it is difficult to determine the influence of a particular factor (Dias et al., 2021).
Besides vegetables, high levels of β-carotene were found in spices (dry) (Fig. 2). The MEAL food pools of spices consisted of 15 subsamples i.e. pepper (n = 5), paprika (n = 3), and others (each n = 1; curry, garlic powder, cinnamon, caraway seed, nutmeg, salad herbs, meat spice mixture). The high β-carotene levels in the spices analysed as MEAL food were most likely attributable to paprika powder, as red peppers contain high levels of β-carotene (Rodríguez-Rodríguez, Sánchez-Prieto, & Olmedilla-Alonso, 2020). Levels of β-carotene were reported of 26,200 µg∙100 g−1 in paprika powder in the US (USDA, 2021).
Additionally, high levels of β-carotene were found in the MEAL food fruit nectar fortified with vitamins (Fig. 2). According to the declaration on the nutritional label and the list of ingredients of each subsample, the high β-carotene levels in the MEAL food category of fruit nectars are attributable to fortification (120 to 300 µg∙100 mL−1) or to the addition of natural ingredients rich in β-carotene such as carrot juice and acerola. Based on the labeled vitamin A levels and bioconversion into β-carotene (by use of the bioconversion factor by EFSA (2015) of 1 µg retinol = 6 µg β-carotene), the converted β-carotene levels (918 µg∙100 g−1) are in agreement with the β-carotene levels determined in this study.
Levels of retinol and β-carotene in MEAL foods sampled according to organic and conventional type of production
In general, differences of retinol and β-carotene levels for organically and conventionally produced MEAL foods might be caused by differences in food production and processing along the food chain (e.g. fertilisation, supplementation of animal feed, food fortification, the availability of products on the market, food preparation, food processing as well as recipe variations).
For the MEAL food margarine, different levels of β-carotene and retinol comparing organically and conventionally produced MEAL foods can be attributed to fortification. In conventionally produced margarine, 240- and 4.7-fold higher levels of retinol and β-carotene were found compared to organically produced margarine, respectively. For retinol, high levels in the conventionally produced pooled sample can be explained by a fortification of 8 out of 15 subsamples with vitamin A (fortification ranged from 450 to 900 µg∙100 g−1) as declared on the packaging whereas organic margarine subsamples were not fortified with vitamin A. In Germany, it is not allowed to fortify organic margarine with vitamin A (EC, 2008a, LMvitV, 2017). As declared on the packaging, β-carotene was used as a food additive in all 15 conventional margarine subsamples to imitate the typical yellowish colour of butter. In contrast, in organically produced foods only the addition of colouring organic ingredients such as carrot juice or carrot juice concentrate are allowed and could lead to lower levels of carotenoids (used in 14 out of 15 subsamples in this study) (EC, 2008a).
In the present study, a tendency towards higher mean levels of retinol were found in livers of conventionally compared to organically produced pig, bovine and poultry as well as in liver sausages sourced from pig and bovine. Different retinol levels might be explained by the supplementation of animal feed with retinyl esters. The addition of vitamin A to animal feed is authorised in the EU for use in organic as well as conventional farming. However, the EU regulations for the production of organic products stipulate a more conscious usage of feed additives, which might explain the lower vitamin A levels in organically produced foods (EC, 2008a). In addition, the routine use of retinyl esters as feed additives might be more inconsistent in organic farming as compared to conventional farming and furthermore may depend on production type (beef vs milk production).
For some MEAL foods, different levels of β-carotene and retinol can be attributed to the composition of pooled samples, depending on market share data and commercial availability. For olives e.g., 3.3-fold higher β-carotene levels were found in conventionally compared to organically produced olives (Supplementary A, Table S3). In the conventionally pooled sample, some subsamples contained marinated olives with herbs or peppers and the higher β-carotene levels might be attributed to these ingredients. With respect to cereal crackers (puffed), levels of β-carotene were 5.3-fold higher in organically than in conventionally produced products (Supplementary A, Table S3). Due to commercial accessibility, organically produced cereal crackers (puffed) are based on maize, rice, spelt and millet as ingredients, whereas the conventional products were exclusively available on maize basis. maize contains higher average levels of β-carotene in contrast to rice, millet and spelt and might cause higher β-carotene levels in conventional cereal crackers (puffed) (ANSES, 2021).
In addition, recipes, ingredients used and preparation methods can influence β-carotene and retinol levels in foods. Different retinol levels might be attributed to different types and quantities of ingredients of animal origin such as butter, cream or margarine fortified with retinol. As retinol is derived from foods of animal origin, differences between organically and conventionally prepared MEAL foods of plant origin such as red cabbage, green peas and carrot (mixed) can presumably be attributed to different quantities of ingredients.
In the present study, different levels of β-carotene and retinol in conventionally and organically produced foods could be explained by different plant food production or cultivation procedures. For example, 7.2-fold higher β-carotene levels were found in conventionally compared to organically produced kitchen herbs (Supplementary A, Table S2). Pooled samples of both production types were composed of similar herbs. Four subsamples of herbal mixtures contained different composition of herbs due to market availability. Organic herbs are subject to EU regulation EC 834/2007 and are cultivated with organic fertilisers (manure and compost) and often with lower levels of nitrogen fertilisation compared to conventional herbs (EC, 2008a). As the synthesis of carotenoidsis positively correlated with the nitrogen supply to plants (Reif, Arrigoni, Neuweiler, Baumgartner, Nyström, & Hurrell, 2012) and could explain higher β-carotene levels in conventionally produced herbs. Hallmann and Sabala (2020) also reported significantly higher levels of carotenoids, including β-carotene, in conventional compared to organic dry herbs. Besides the extent of nitrogen fertilisation, differences in β-carotene levels between conventionally and organically produced herbs might also be attributed to (i) genetic variations of the plants (ii) further differences in cultivation conditions, post-harvest handling and storage conditions and (iii) different composition of the four subsamples of herbal mixtures, based on market data.
Levels of retinol and β-carotene in MEAL foods sampled according to seasonality
Comparing mean retinol and β-carotene levels in MEAL foods sampled in season 1 and season 2, differences were found in some of the MEAL foods (Supplementary A, Table S2, S3).
Seasonally differences in retinol levels were particularly found in MEAL foods of the main food group “composite dishes” (goulash, beef roulade, tomato soup, potato-vegetable-meat-based meal for infants and toddlers) as well as in plant-derived MEAL foods (fennel, vegetable and tomato soup). The latter can be explained by the ingredients used, as retinol is only found in animal food sources. For β-carotene, different levels between MEAL foods sampled seasonally were observed in three potato-based MEAL foods, including pan-fried potatoes, potato salad and potato-vegetable-meat-based meal for infants and toddlers (1.8- to 2.0-fold difference) but not in peeled and unpeeled potatoes. The seasonal differences in retinol and β-carotene levels for the MEAL foods mentioned above might be caused by the following reasons: (i) losses during food preparation (ii) different composition of the pooled samples, especially in subsamples of convenience foods, and (iii) recipe variations of convenience foods as well as household recipes, including the use of different quantities and types of animal ingredients particularly animal fats or margarine fortified with vitamin A.
In the present study, approximately 20 % lower retinol levels were observed in milk and butter sampled in February (season 1) compared to milk and butter sampled in August (season 2). Only organically produced milk and organically produced dairy products were sampled seasonally. In contrast, no differences in β-carotene levels were observed in milk and butter. In the Netherlands, retinol and β-carotene levels of milk and butter sampled in winter contained approximately 20 % less retinol and β-carotene compared to samples from summer. Seasonal differences in the animal feeding practices might be the main cause for this observation (Hulshof, van Roekel-Jansen, van de Bovenkamp, & West, 2006).
Seasonal differences in β-carotene levels were found in the MEAL foods apple, sweet pepper and lettuce and other leaf salads (1.6- to 2.5-fold difference). Levels were higher in these foods sampled during spring and winter in comparison to those sampled in summer. This could be explained with the origin of foods sampled in winter and spring as these were mainly imported from countries with suitable climatic conditions during the European winter season, which might have caused higher β-carotene levels. Higher temperatures and greater exposure to sunlight are factors increasing carotenogenesis in fruits and vegetables (Dias et al., 2021). Besides seasonal influences, β-carotene levels could have been influenced by cultivation methods (greenhouse or open field), maturation stage, plant genetics, soil type, effect of agrochemicals, and distribution within the plant as well as storage and processing conditions of the food (Dias et al., 2021, Meléndez-Martínez et al., 2021, Rodriguez-Amaya et al., 2008). In summary, the varying levels of β-carotene and retinol in MEAL foods sampled seasonally depend on many factors, which can be partly attributed to natural variations.
Limitations and uncertainties
The efficiency of the TDS design entails that similar foods are pooled to one sample before analysis. Therefore, levels of substances detected in a TDS cannot be assigned to single foods or brand products, to different cooking methods or specific recipes in the case of prepared foods or to specific varieties or cultivars and cultivation conditions, e.g. for fruit and vegetables. However, the analytical data generated by a TDS represent average levels, providing a realistic approach for exposure assessment and are representative of the foods consumed within a population (EFSA/FAO/WHO, 2011).
Within this study, the implementation of the MEAL food list is based on consumption data sets for children from 2002 (VELS) and for adults from 2006 (NVS II) covering the age groups from 0.5 to < 5 years (VELS) and 14 to 80 years (NVS II). Therefore, consumption habits of 5 to 13 year old children and adolescents were not explicitly considered when compiling the food list. According to the follow-up study “German National Nutrition Monitoring” (NEMONIT), which was conducted from 2008 to 2012/2013 in Germany, no substantial deviations in the eating behaviour were observed over time (Gose, Krems, Heuer, & Hoffmann, 2016) but recent trends in food consumption might not be captured by the present study. As the consumption data are based on two-days 24 h recalls for adults (NVS II) and food records over six days for children (VELS), rarely consumed foods might be underrepresented (Sarvan et al., 2017).
If differences regarding the type of production were expected, foods were sampled according to market share data representative for Germany. As the market share data as well as availability is sometimes limited to only a few products (e.g. specific brands or product types e.g. in case of flavours), identical individual products were sampled several times to depict the market situation and ensure that each pooled sample consisted of 15 subsamples. Therefore the composition of organically and conventionally pooled samples may differ, but represents the current market availability. Furthermore, all ingredients with < 5 % (w/w) were food items from conventional type of production and were used for conventional and organic MEAL food pools. The statistical test applied on differences between organic and conventional food has the following limitations: (i) the test does not consider that each MEAL food pool is already representing a mean level of the subsamples with an unknown variability (ii) the test might not be able to detect significant differences if there are significant differences in only some foods (iii) or if the direction of the differences is not the same. Further details could be investigated in the future through specific fitted statistical tests.
Due to the limited storage stability of the analytes, foods for retinol and β-carotene analysis were only sampled in the area of Berlin and no regional sampling was performed.
Conclusion
The determination of retinol and β-carotene in foods of the BfR MEAL Study provides a detailed data basis to estimate dietary exposure of vitamin A for the population in Germany. Retinol and β-carotene levels were analysed in 333 and 271 MEAL foods of the BfR MEAL Study, respectively. For relevant food, additional samples were taken according to production type and seasonality, which resulted in a total of 522 and 436 pooled samples for retinol and β-carotene analyses, respectively. All foods were prepared as commonly consumed. As expected, highest mean levels of retinol were found in foods of animal origin, in particular in liver, liver-based products (e.g. liver sausage), butter and eel as well as margarine fortified with vitamin A. With respect to β-carotene, highest mean levels were found in foods of plant origin, including orange and yellow vegetables such as carrot, pumpkin and sweet potato, green leafy vegetables such as kale and lettuce and other salads as well as fruit nectars. Levels of retinol and β-carotene determined in the BfR MEAL Study might be caused by natural origin and/or the addition for either technological or nutritional purposes. The sampling by production type and seasonality showed for some MEAL foods differences in retinol and β-carotene levels, thus improving the understanding of the variability for levels of retinol and β-carotene in foods. The present study provides representative data on preformed vitamin A and β-carotene levels in mostly consumed foods and expands the data basis for chronic dietary exposure and nutritional assessment in Germany.
CRediT authorship contribution statement
Sophia Schendel: Investigation, Formal analysis, Validation, Visualization, Writing – original draft. Tanja Berg: Investigation, Visualization, Writing – review & editing. Maria Scherfling: Investigation, Validation. Carina Drößer: Methodology, Investigation. Sebastian Ptok: Validation, Writing – review & editing. Anke Weißenborn: Validation, Writing – review & editing. Oliver Lindtner: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. Irmela Sarvan: Conceptualization, Project administration, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The project is supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support program (grant number: BfR-EXPO-08-1393-02). Experimental analysis were carried out by Institute Kirchhoff GmbH. We would like to thank the expert group for nutrients of the BfR MEAL Study for supporting us with their substance-specific expertise and the international advisory board of the BfR MEAL Study for advising the study in terms of the TDS design. We would also like to thank our colleagues from the purchasing as well homogenisation, kitchen and documentation team for their great support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100458.
Contributor Information
Sophia Schendel, Email: sophia.schendel@bfr.bund.de.
Tanja Berg, Email: tanja.berg@bfr.bund.de.
Maria Scherfling, Email: maria.scherfling@bfr.bund.de.
Sebastian Ptok, Email: sebastian.ptok@bfr.bund.de.
Anke Weißenborn, Email: anke.weissenborn@bfr.bund.de.
Oliver Lindtner, Email: oliver.lindtner@bfr.bund.de.
Irmela Sarvan, Email: irmela.sarvan@bfr.bund.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Albanes D., Heinonen O.P., Taylor P.R., Virtamo J., Edwards B.K., Rautalahti M.…Huttunen J.K. α-Tocopherol and β-Carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: Effects of base-line characteristics and study compliance. JNCI: Journal of the National Cancer Institute. 1996;88(21):1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- ANSES, French Agency for Food, Environmental and Occupational Health & Safety. (2021). French food composition table. Retrieved from: https://ciqual.anses.fr/ Accessed 2021-09-21.
- BfR, Bundesinstitut für Risikobewertung (2007). BfR rät zu regionalen Verzehrsempfehlungen für frische Dorschleber, Stellungnahme Nr. 038/2007 des BfR vom 13. Juli 2007. Berlin, DE: Bundesinstitut für Risikobewertung.
- BLS, Bundeslebensmittelschlüssel. German Nutrient Database version 2014 3.02.
- Böhm V., Lietz G., Olmedilla-Alonso B., Phelan D., Reboul E., Bánati D.…Bohn T. From carotenoid intake to carotenoid blood and tissue concentrations – implications for dietary intake recommendations. Nutrition Reviews. 2021;79(5):544–573. doi: 10.1093/nutrit/nuaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BVL, Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. (2017). BVL-Report 12.4 Berichte zur Lebensmittelsicherheit - Monitoring 2016. https://www.bvl.bund.de/DE/Home/home_node.html.
- Chea, E. P., Lopez, M. J., & Milstein, H. (2021). Vitamin A. Treasure Island (FL): StatPearls Publishing, Copyright © 2021, StatPearls Publishing LLC.
- Dias M.G., Borge G.I.A., Kljak K., Mandić A.I., Mapelli-Brahm P., Olmedilla-Alonso B.…Meléndez-Martínez A.J. European Database of Carotenoid Levels in Foods. Factors Affecting Carotenoid Content. Foods. 2021;10(5):912. doi: 10.3390/foods10050912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DTU, N. F. I., Technical University of Denmark. (2021). Frida Food Data. Retrieved from: https://frida.fooddata.dk/?lang=en Accessed 2021-09-21.
- EC, European Community. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Official Journal of the European Union. 2003;268:29–43. [Google Scholar]
- EC, European Community. (2008a). Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control. Official Journal of the European Union, 1-84.
- EC, European Community. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Official Journal of the European Union. 2008;354:16–33. [Google Scholar]
- EFSA, European Food Safety Authority Consequences for the consumer of the use of vitamin A in animal nutrition. EFSA Journal. 2008;7(2):873. doi: 10.2903/j.efsa.2009.873. [DOI] [Google Scholar]
- EFSA, European Food Safety Authority Scientific Opinion on the essential composition of infant and follow-on formulae, EFSA Panel on Dietetic Products. Nutrition and Allergies. EFSA Journal. 2014;12(7):3760. doi: 10.2903/j.efsa.2014.3760. [DOI] [Google Scholar]
- EFSA, European Food Safety Authority Scientific Opinion on Dietary Reference Values for vitamin A, EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA)S. EFSA Journal. 2015;13(3):4028. doi: 10.2903/j.efsa.2015.4028. [DOI] [Google Scholar]
- EFSA/FAO/WHO, European Food Safety Authority, Food and Agriculture Organization of the United Nations, World Health Organization Towards a harmonised Total Diet Study approach: A guidance document. EFSA Journal. 2011;9(11):2450. [Google Scholar]
- EU, European Union. Regulation (EU) No. 1308/2013 of the European Parliament and of the Council of 17 December 2013 Establishing a Common Organisation of the Markets in agricultural Products and Repealing Council Regulations (EEC) No. 922/72,(EEC) No. 234/79,(EC) No. 1037/2001 and (EC) No. 1234/2007. Off J Euro Union. 2013;347:671–854. [Google Scholar]
- EU, European Union. (2015). Commission Implementing Regulation (EU) 2015/724 of 5 May 2015, concerning the authorisation of retinyl acetate, retinyl palmitate and retinyl propionate as feed additives for all animal species. Official Journal of the European Union.
- EU, European Union. Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding. OJEC. 2016;59:1–29. [Google Scholar]
- Gose M., Krems C., Heuer T., Hoffmann I. Trends in food consumption and nutrient intake in Germany between 2006 and 2012: Results of the German National Nutrition Monitoring (NEMONIT) British Journal of Nutrition. 2016;115(8):1498–1507. doi: 10.1017/S0007114516000544. [DOI] [PubMed] [Google Scholar]
- Hallmann E., Sabała P. Organic and Conventional Herbs Quality Reflected by Their Antioxidant Compounds Concentration. Applied Sciences. 2020;10(10):3468. doi: 10.3390/app10103468. [DOI] [Google Scholar]
- Hulshof P.J.M., van Roekel-Jansen T., van de Bovenkamp P., West C.E. Variation in retinol and carotenoid content of milk and milk products in The Netherlands. Journal of Food Composition and Analysis. 2006;19(1):67–75. doi: 10.1016/j.jfca.2005.04.005. [DOI] [Google Scholar]
- IOM, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E Selenium, and Carotenoids. 528 2000 https://doi.org/10.17226/9810. [PubMed]
- Kolbaum A.E., Jaeger A., Ptok S., Sarvan I., Greiner M., Lindtner O. Collection of occurrence data in foods – The value of the BfR MEAL study in addition to the national monitoring for dietary exposure assessment. Food Chemistry: X. 2022;13 doi: 10.1016/j.fochx.2022.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LMvitV, Verordnung über vitaminisierte Lebensmittel. (2017). Verordnung über vitaminisierte Lebensmittel in der im Bundesgesetzblatt Teil III, Gliederungsnummer 2125-4-23, veröffentlichten bereinigten Fassung, die zuletzt durch Artikel 24 der Verordnung vom 5. Juli 2017 (BGBl. I S. 2272) geändert worden ist. https://www.gesetze-im-internet.de/lmvitv/BJNR005380942.html.
- Majchrzak D., Fabian E., Elmadfa I. Vitamin A content (retinol and retinyl esters) in livers of different animals. Food Chemistry. 2006;98(4):704–710. doi: 10.1016/j.foodchem.2005.06.035. [DOI] [Google Scholar]
- Meléndez-Martínez A.J., Mandić A.I., Bantis F., Böhm V., Borge G.I.A., Brnčić M.…O’Brien N. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Critical Reviews in Food Science and Nutrition. 2021;1–51 doi: 10.1080/10408398.2020.1867959. [DOI] [PubMed] [Google Scholar]
- Omenn G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A.…Hammar S. Risk Factors for Lung Cancer and for Intervention Effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. JNCI: Journal of the National Cancer Institute. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- Ottaway, P. B. (2010). 19 - Stability of vitamins during food processing and storage. In L. H. Skibsted, J. Risbo & M. L. Andersen (Eds.), Chemical Deterioration and Physical Instability of Food and Beverages (pp. 539-560): Woodhead Publishing; 10.1533/9781845699260.3.539.
- Reif C., Arrigoni E., Neuweiler R., Baumgartner D., Nyström L., Hurrell R.F. Effect of Sulfur and Nitrogen Fertilization on the Content of Nutritionally Relevant Carotenoids in Spinach (Spinacia oleracea) Journal of Agricultural and Food Chemistry. 2012;60(23):5819–5824. doi: 10.1021/jf301114p. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Amaya D.B., Kimura M., Godoy H.T., Amaya-Farfan J. Updated Brazilian database on food carotenoids: Factors affecting carotenoid composition. Journal of Food Composition and Analysis. 2008;21(6):445–463. doi: 10.1016/j.jfca.2008.04.001. [DOI] [Google Scholar]
- Rodríguez-Rodríguez E., Sánchez-Prieto M., Olmedilla-Alonso B. Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD. Food Chemistry: X. 2020;6 doi: 10.1016/j.fochx.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salma E.O., Hechmi M. Oil Soluble Vitamins and Fatty Acids Profile of Smoked European Eel Fillets. Advances in Bioresearch. 2013;4(1):86–91. [Google Scholar]
- Sarvan, I., Bürgelt, M., Lindtner, O., & Greiner, M. (2017). Expositionsschätzung von Stoffen in Lebensmitteln: Die BfR-MEAL-Studie – die erste Total-Diet-Studie in Deutschland. Bundesgesundheitsblatt – Gesundheitsforschung – Gesundheitsschutz, 60(7: Kontaminanten in Lebensmitteln), 689–696. https://doi.org/10.1007/s00103-017-2566-1. [DOI] [PubMed]
- Sarvan I., Kolbaum A.E., Pabel U., Buhrke T., Greiner M., Lindtner O. Exposure assessment of methylmercury in samples of the BfR MEAL Study. Food and Chemical Toxicology. 2021;149 doi: 10.1016/j.fct.2021.112005. [DOI] [PubMed] [Google Scholar]
- D. Tennant K. Gedrick D. Godfrey J. Davidson Intakes of beta-carotene from its use as a food additive, fortificant and dietary supplement in France, Germany and the UK British Food Journal 106 6 2004 436 456 https://doi.org/https://doi.org/10.1108/00070700410539752.
- USDA, U.S. Department of Agriculture. (2007). USDA Table of Nutrient Retention Factors, Release 6 (2007).
- USDA, U.S. Department of Agriculture. (2021). FoodData central. Retrieved from: https://fdc.nal.usda.gov/index.html Accessed 2021-09-21.
- Weber D., Grune T. The contribution of β-carotene to vitamin A supply of humans. Molecular Nutrition and Food Research. 2012;56(2):251–258. doi: 10.1002/mnfr.201100230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.