Abstract
Purpose
Proton beam radiation therapy (PBRT) is a treatment option for advanced retinoblastoma (RB) resistant to chemotherapy and focal ophthalmic treatment. Here we report a case of RB with phthisis bulbi following PBRT.
Observations
A 16-day-old boy with a family history of RB was referred to our institution. Initial examination revealed an extensive white mass in the right eye and a small tumor near the optic disk of the left eye. The patient was diagnosed with bilateral RB and treated with chemotherapy and focal ophthalmic therapy. The right eye showed shrinkage in the treatment course. The tumor control was not achieved bilaterally, and, therefore, PBRT was performed to preserve the eyes. However, the right eye became significantly phthisical following PBRT and ultimately required enucleation.
Conclusions and importance
PBRT for RB may result in phthisis bulbi. Further investigations of its role and possible complications are warranted.
Keywords: Retinoblastoma, Proton beam radiation therapy, Phthisis bulbi
1. Introduction
Retinoblastoma (RB) is the most common intraocular malignancy in childhood. The incidence is estimated at approximately 1 in every 16,000 live births, with sporadic (60%) and hereditary (40%) forms.1 Although uncommon and atypical, the incidence of patients with RB who presented with primary phthisis bulbi was reported to be approximately 3%.2,3 Currently, proton beam radiation therapy (PBRT) is one of the final treatment options for eye-preserving therapy with progressive diseases resistant to chemotherapy and focal treatment.4 Protons are physically advantageous over photons in sparing normal tissues.5 This reduction in orbital irradiation is clinically important, which potentially decreases the late occurrence of secondary malignancies and retardation of orbital bone growth. Reports that analyzed the complication of PBRT for RB are limited. Here we present a clinical course of RB that led to phthisis bulbi and eventually required enucleation.
2. Case report
A 16-day-old boy with a family history of bilateral RB (father and elder brother) visited our institution for an eye evaluation and was diagnosed with bilateral tumors. Color fundus performed using a RetCam3® (Clarity Medical System, Pleasanton, CA, USA) showed RB with focal subretinal seeding and fluid in the right eye and juxtapapillary RB in the left eye. Computed tomography (CT) scan revealed that the tumor was occupying half of the right eyeball (Fig. 1). According to the International Classification for Intraocular Retinoblastoma staging system,6 the disease stage was group E and B in the right and left eye, respectively. Based on the American Joint Committee on Cancer staging 8th edition,7 the tumors were categorized as cT2b and cT1b in the right and left eye, respectively. No intracranial abnormality (trilateral RB8) was observed at the initial imaging.
Fig. 1.
Fundus photography and CT findings at baseline.
A and B, fundus images of OU. Extensive mass with local subretinal seeding and serous retinal detachment in OD. Small mass located near the optic nerve in OS.
C, Representative axial CT slice, the tumor occupying >50% of OD.
Chemotherapy with vincristine, etoposide, and carboplatin was administered. After six cycles of the regimen, additional focal treatments were administered. Local therapy included five consecutive intra-arterial melphalan injections (2 mg and 5 mg/m2/body surface area)9 with transpupillary thermotherapy for both eyes, two intravitreal melphalan injections (16 μg in 0.05 mL solution)10 for the right eye, and cutting-cryotherapy for the juxtapapillary tumor in the left eye. The tumors regressed first, but the right eye showed recurrence with diffuse subretinal and vitreous seeding, and the tumor near the optic disk persisted in the left eye. In this case, the juxtapapillary tumor of the left eye was refractory to chemotherapy and multiple focal treatments. At that time, no useful vision would have been possible in the right eye as it was already shrinking. Enucleation of the right eye or the use of simultaneous bilateral irradiation was carefully considered. When making the medical decision, the parents wished for immediate treatment for the left eye and preservation of the right eye if possible. Therefore, we deemed radiotherapy as a viable treatment option to salvage the affected eye as retinoblastomas are highly sensitive to radiation.
PBRT is a widely used treatment modality in pediatric and ocular tumors such as pediatric brain tumors11 or uveal melanomas12; however, the application of PBRT on RB is limited. Compared to external beam radiation therapy (EBRT), PBRT has unique physical properties by using photons, which reduces not only ocular side effects, such as cataract development, orbital fat atrophy, and bone growth abnormalities, but also the incidence of subsequent primary malignancies, as the size of the target volume in PBRT is smaller.11 Therefore, PBRT was decided as a reasonable treatment option to preserve both eyes. Consequently, bilateral PBRT was performed at 11 months. Radiation covered the whole retina behind the lens of the right eye and was distributed to the retina around the optic disk of the left eye, with a total dose of 45 Gy delivered in 25 fractions (Fig. 2). One month after the end of the PBRT, laser photocoagulation and intravitreal chemotherapy were additionally performed. After 3 months, monitoring with examination under anesthesia (EUA) showed inactive and calcified tumors in the right and left eyes, respectively. After completing the PBRT, the right eye was evidently phthisical at the age of 1 year and 11 months. With a poor prognosis, the right eye was enucleated.
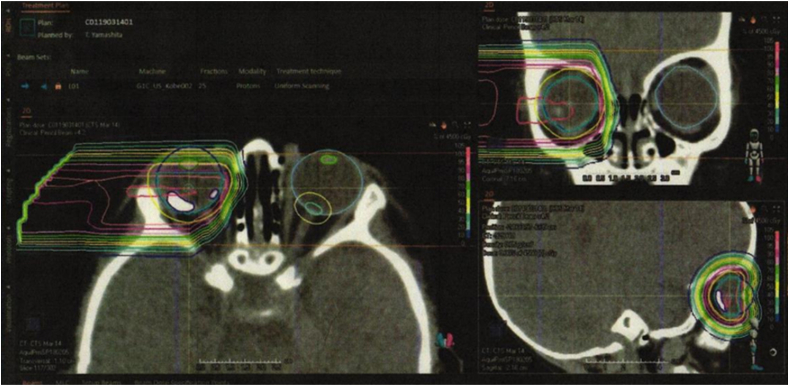
Fig. 2.
Proton dose distribution plan with lateral beam, covering a target volume confined to the tumors in the posterior retina, sparing the lens.
The patient underwent EUA before the enucleation surgery, and fundus photos were obtained (Fig. 3). During EUA, a phthisical right eye with lens opacity and hypotony was observed. Fluorescein angiography revealed no neovascularization into the anterior chamber. The posterior pole was not visible due to cataract and shrunken globe. The left eye showed a normal anterior chamber and a regressed tumor adjacent to the optic disk.
Fig. 3.
EUA images before enucleation.
A and B, External photograph showing lens opacity and fluorescein angiogram of the iris showing no neovascularization in OD.
C and D, Fundus photography showing juxtapapillary retinoblastoma with calcification, depicted as hypofluorescence by fluorescein angiography in OS.
Histopathological analysis of the enucleated eye (Fig. 4) showed a dysplastic retina with almost complete tumor necrosis. Microscopic foci of choroidal invasion without viable tumor cells were also observed. However, high-risk histopathological features for metastasis,13,14 such as anterior structure, massive choroidal, and optic nerve invasions and scleral infiltration, were not observed.
Fig. 4.
Pathology of the enucleated eye.
A, Gross photograph of the enucleated eye. A residual tumor fills half of the posterior segment.
B, Histopathology of the whole eye (hematoxylin-eosin stain).
C, Photomicrograph showing calcification and a cluster of inviable tumor cells (hematoxylin-eosin stain, original magnification × 20).
Follow-up brain magnetic resonance imaging was performed regularly for screening and monitoring the optic nerve invasion. The patient is alive at the time of writing this report and did not develop a recurrence or metastatic disease during the 4-year follow-up period. The intraocular condition of the left eye was stable at the last follow-up.
3. Discussion
RB is highly radiosensitive and EBRT has been the first-line and major treatment modality for this condition for more than a century.15 However, the use of EBRT has significantly decreased because of radiation-induced side effects, particularly orbital bone growth abnormalities and secondary malignancies.1,16 Therefore, modern management strategies were replaced by intravenous chemotherapy to reduce the tumor size (chemoreduction) combined with local therapy, such as cryotherapy, laser photocoagulation, plaque radiotherapy, intra-arterial chemotherapy,9 and intravitreal chemotherapy.10,17
Advancement in radiation therapy resulted in the development of the novel PBRT technique.11 Compared with conventional photon-based radiotherapy, PBRT minimizes damage to normal adjacent structures18 and may potentially reduce the risk for long-term adverse events.16,19,20 Well-known PBRT-induced adverse events include cataracts, radiation retinopathy, neovascular glaucoma, optic neuropathy, and vitreous hemorrhage, though phthisis bulbi is rarely reported.18,21 To the best of our knowledge, only one case with ciliary body failure, which was highly similar to phthisis bulbi after PBRT, has been reported.4 PBRT is also the treatment of choice in patients with uveal melanoma, which is the most common type of primary ocular malignancy in adults. Phthisis bulbi after PBRT in patients with melanoma is rare. In a large case-control study, 5% of juvenile patients and 4% of adult patients developed phthisis bulbi.12
In this report, we present a case of phthisis bulbi after PBRT for RB that shrunk in the course of preceding treatments. However, the exact mechanism of phthisis bulbi was unclear. We postulated that the eye with inadequate blood supply caused by the tumor and vascular toxicities triggered by the intra-arterial chemotherapy22 began to shrink before PBRT and ultimately progressed to phthisis bulbi with ciliary body failure induced by irradiation.23
4. Conclusions
It is unclear which cases of PBRT performed for RB result in phthisis bulbi, as the number of such cases reported in the literature is small. Further studies are required to better understand the complication profile and overall role of PBRT in the context of RB management.
Patient consent
The patient's family consented to publication of the case in writing.
Funding
This research was supported by AMED under Grant Number JP20gk0110034(T. Morimoto) and a grant from Ministry of Health, Labour and Welfare, Japan (K20FC1057b).
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
The following authors have no financial disclosures: NN, TM, TM, SS, HS, and KN.
Acknowledgments and Disclosures
The authors thank Enago (www.enago.jp) for the English language review.
References
- 1.The Committee for the National Registry of Retinoblastoma The National registry of retinoblastoma in Japan (1983–2014) Jpn J Ophthalmol. 2018;62(4):409–423. doi: 10.1007/s10384-018-0597-2. [DOI] [PubMed] [Google Scholar]
- 2.Kashyap S., Meel R., Pushker N., et al. Phthisis bulbi in retinoblastoma. Clin Exp Ophthalmol. 2011;39(2):105–110. doi: 10.1111/j.1442-9071.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 3.Mullaney P.B., Karcioglu Z.A., Al-Mesfer S., Abboud E.B. Presentation of retinoblastoma as phthisis bulbi. Eye. 1997;11(3):403–408. doi: 10.1038/eye.1997.85. [DOI] [PubMed] [Google Scholar]
- 4.Biewald E., Kiefer T., Geismar D., et al. Feasibility of proton beam therapy as a rescue therapy in heavily pre-treated retinoblastoma eyes. Cancers (Basel) 2021;13(8):1–11. doi: 10.3390/cancers13081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C.T., Bilton S.D., Famiglietti R.M., et al. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys. 2005;63(2):362–372. doi: 10.1016/j.ijrobp.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 6.Shields C.L., Mashayekhi A., Au A.K., et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–2280. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Mallipatna A.C., Gallie B.L., Chevez-Barrios P., et al. Retinoblastoma . In: AJCC Cancer Staging Manual. eighth ed. Amin M.B., Edge S.B., Greene F.L., et al., editors. Springer International Publishing; New York: 2017. pp. 819–831. [Google Scholar]
- 8.Qureshi S., Francis J.H., Haque S.S., et al. Magnetic resonance imaging screening for trilateral retinoblastoma: the memorial Sloan Kettering Cancer Center experience 2006–2016. Ophthalmol Retin. 2020;4(3):327–335. doi: 10.1016/j.oret.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S., Yamane T., Mohri M., Kaneko A. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology. 2011;118(10):2081–2087. doi: 10.1016/j.ophtha.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S., Aihara Y., Fujiwara M., Sano S., Kaneko A. Intravitreal injection of melphalan for intraocular retinoblastoma. Jpn J Ophthalmol. 2015;59(3):164–172. doi: 10.1007/s10384-015-0378-0. [DOI] [PubMed] [Google Scholar]
- 11.Thomas H., Timmermann B. Paediatric proton therapy. Br J Radiol. 2020;93(1107) doi: 10.1259/bjr.20190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrovic A., Bergin C., Schalenbourg A., Goitein G., Zografos L. Proton therapy for uveal melanoma in 43 juvenile patients: long-term results. Ophthalmology. 2014;121(4):898–904. doi: 10.1016/j.ophtha.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 13.De Jong M.C., De Graaf P., Noij D.P., et al. Diagnostic performance of magnetic resonance imaging and computed tomography for advanced retinoblastoma: a systematic review and meta-analysis. Ophthalmology. 2014;121(5):1109–1118. doi: 10.1016/j.ophtha.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Kaliki S., Shields C.L., Rojanaporn D., et al. High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120(5):997–1003. doi: 10.1016/j.ophtha.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Shields C.L., Honavar S.G., Meadows A.T., et al. Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol. 2002;133(5):657–664. doi: 10.1016/s0002-9394(02)01348-x. [DOI] [PubMed] [Google Scholar]
- 16.Mouw K.W., Yeap B.Y., Caruso P., et al. Analysis of patient outcomes following proton radiation therapy for retinoblastoma. Adv Radiat Oncol. 2017;2(1):44–52. doi: 10.1016/j.adro.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ancona-Lezama D., Dalvin L., Shields C. Modern treatment of retinoblastoma: a 2020 review. Indian J Ophthalmol. 2020;68(11):2356. doi: 10.4103/ijo.IJO_721_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouw K.W., Sethi R.V. Yeap BY, et al. Proton radiation therapy for the treatment of retinoblastoma. Int J Radiat Oncol Biol Phys. 2014;90(4):863–869. doi: 10.1016/j.ijrobp.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethi R.V., Shih H.A., Yeap B.Y., et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014;120(1):126–133. doi: 10.1002/cncr.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroy R., Benahmed N., Hulstaert F., Van Damme N., De Ruysscher D. Proton therapy in children: a systematic review of clinical effectiveness in 15 pediatric cancers. Int J Radiat Oncol Biol Phys. 2016;95(1):267–278. doi: 10.1016/j.ijrobp.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Jung E.H., Kim J.H., Kim J.Y., Jo D.H., Yu Y.S. Outcomes of proton beam radiation therapy for retinoblastoma with vitreous seeds. J Pediatr Hematol Oncol. 2018;40(8):569–573. doi: 10.1097/MPH.0000000000001176. [DOI] [PubMed] [Google Scholar]
- 22.Steinle J.J., Zhang Q., Thompson K.E., et al. Intra-ophthalmic artery chemotherapy triggers vascular toxicity through endothelial cell inflammation and leukostasis. Invest. Ophthalmol Vis Sci. 2012;53(4):2439–2445. doi: 10.1167/iovs.12-9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripathy K., Chawla R., Temkar S., et al. Phthisis bulbi—a clinicopathological perspective. Semin Ophthalmol. 2018;33(6):788–803. doi: 10.1080/08820538.2018.1477966. [DOI] [PubMed] [Google Scholar]