Highlights
-
•
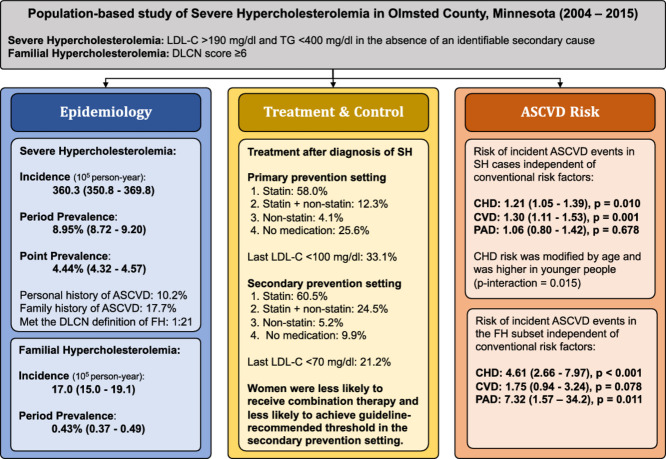
The age- and sex-adjusted point prevalence of severe hypercholesterolemia (SH, defined as LDL- C ≥190 mg/dl) in the US was 4.44%, and period prevalence was double at 8.95%.
-
•
1 in 21 SH patients (1 in 233 US adults) met clinical criteria for familial hypercholesterolemia.
-
•
Awareness and control were low with only 33.1% and 21.2% at goal LDL-C level in the primary and secondary prevention settings, respectively; with less women overall achieving the target than men.
Keywords: Severe hypercholesterolemia, Familial hypercholesterolemia, Atherosclerotic cardiovascular disease, Statin, Epidemiology
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; CVD, cerebrovascular disease; DLCN, dutch lipid clinic network; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; LLT, lipid lowering therapy; PAD, peripheral artery disease; SH, severe hypercholesterolemia
Abstract
Background
Contemporary prevalence, awareness, and control of severe hypercholesterolemia (SH) and familial hypercholesterolemia (FH) and the associated atherosclerotic cardiovascular disease risk in the US are unknown.
Method
Using electronic health records, we assessed the burden of SH and FH in Olmsted County, Minnesota, US, between 2004 and 2015. We defined SH as low-density lipoprotein cholesterol (LDL-C) level ≥190 mg/dl without secondary causes of hypercholesterolemia and FH as a Dutch Lipid Clinic Network score ≥6. Controls were age- and sex-matched individuals with LDL-C level <190 mg/dl.
Results
The age- and sex-adjusted point and period prevalence (age-recursive method) of SH was 4.44% and 8.95%, respectively; 1 in 21 had FH (∼1:233 adults), and 46.2% had a recorded diagnosis. Guideline recommended targets (LDL-C <100 mg/dl and <70 mg/dl in the primary and secondary prevention settings, respectively) were achieved in 33.1% and 21.2% of SH cases, with less women overall achieving the target than men (18.6% vs. 23.7%, p=0.022). After adjustment for conventional risk factors, the hazard ratio for incident coronary heart disease (CHD) in those with SH was 1.21 (1.05-1.39; p=0.010), in those with SH and a family history of CHD was 2.16 (1.57-2.96; p<0.001) and in those with FH was 4.61 (2.66-7.97; p<0.001). The association of SH with CHD was modified by age (p-interaction = 0.015), such that the risk was greater at younger ages.
Conclusions
SH was prevalent and an independent risk factor for incident CHD. Awareness and control were low, highlighting a treatment gap (more prominent in women) that needs to be addressed.
Graphical abstract
1. Introduction
Elevated low-density cholesterol (LDL-C) is the primary causal risk factor for atherosclerotic cardiovascular disease (ASCVD) and accounted for 4.3 million deaths world-wide in 2017 [1]. Within the spectrum of elevated LDL-C, severe hypercholesterolemia (SH) is defined as LDL-C ≥190 mg/dl. The American Heart Association/American College of Cardiology (AHA/ACC) guideline recommends statin therapy for SH, regardless of the estimated 10-year risk of ASCVD [2]. However, contemporary data on prevalence, awareness, and control of SH in a population-based setting in the US is not available and such knowledge is necessary to reduce the burden of ASCVD.
Epidemiologic studies provide important measures of disease/risk factor prevalence, awareness, detection, and control, thereby informing public health measures to reduce risk. The Framingham Heart Study, for example, identified cholesterol as a key risk factor for coronary heart disease (CHD), motivating population measures as well as drug therapy to lower cholesterol levels, and leading to a ∼40% reduction in CHD mortality over the ensuing 50 years [3]. Another example is the North Karelia project, a comprehensive community-based prevention program in Finland that reduced CHD mortality by 84% from 1972 to 2014, largely due to risk factor modification [4]. An assessment of prevalence awareness, detection and control of SH in the contemporary era is important to inform preventive strategies [5].
Prior reports of prevalence of SH in the general population vary considerably (6% to 13%) [6], [7], [8]. Both conventional surveys in population-based settings and electronic-health record (EHR) studies in primary-care settings have attempted to estimate the burden of SH in the US. Cross-sectional surveys, by their nature, only provide estimates at a point in time [6,7] whereas EHR-based studies have longitudinal data but may be affected by referral bias, variability in defining cases, and inclusion of secondary causes of hypercholesterolemia.
We therefore conducted the present study using longitudinal EHR data to provide population-based estimates of the epidemiology of SH including its incidence and prevalence, awareness, treatment, and control as well as the associated ASCVD risk. We excluded secondary causes associated with hypercholesterolemia and assessed treatment and control status in primary and secondary prevention settings separately. Additionally, we estimated the proportion of SH cases who met the clinical definition of familial hypercholesterolemia (FH) based on the Dutch Lipid Clinic Network (DLCN) criteria.
2. Methods
2.1. Study design and data sources
This study was conducted in Olmsted County, Minnesota, with an estimated population of 144,248 based on the 2010 census using Rochester Epidemiology Project (REP). Given its relative isolation from other metropolitan areas, Olmsted County is ideal to study disease epidemiology in a population-based setting. Medical care within this county is provided by Mayo Clinic, Olmsted Medical Center, and their affiliated hospitals, as well as the Rochester Family Medicine Clinics. These health care systems are connected to a unique medical records-linkage system, the REP, which covers nearly all residents of Olmsted County. During any given 3-year period, >90% of Olmsted residents see a local provider at least once. Although we did not perform population-based sampling, the REP provides population-based estimates in Olmsted County [9,10]. The EHR data used included both claims-based data (diagnosis and procedure codes), as well as clinical notes, medication prescriptions, and laboratory testing results. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards; patients who had authorized the use of their medical records for research were included in the analyses.
2.2. Study population
We ascertained fasting serum lipid levels including triglycerides, total cholesterol, and high-density lipoprotein cholesterol (HDL-C), tobacco use, body mass index (BMI), and lipid lowering treatment (LLT, including both statin and non-statin medications) using REP resources. Low-density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald equation [11].
Cases were identified as having fasting LDL-C ≥190 mg/dl or ≥140 mg/dl while on a statin, between January 1, 2004, to December 31, 2015. We estimated the untreated LDL-C level for those taking a statin by multiplying the LDL-C level by 1.33. Statin treatment was ascertained based on prescription data and medication reconciliation at medical visits. The earliest LDL-C measurement that exceeded the threshold was considered as the index LDL-C level and the corresponding date was recorded as the index date. Those with concomitant hypertriglyceridemia (defined as triglyceride level ≥400 mg/dl), or a secondary cause of hypercholesterolemia, noted within a 1-year window before the index date, were excluded. Secondary causes were identified using REP resources, and included hypothyroidism, significant liver disease, significant kidney disease, uncontrolled diabetes, and pregnancy (Supplementary Table 1).
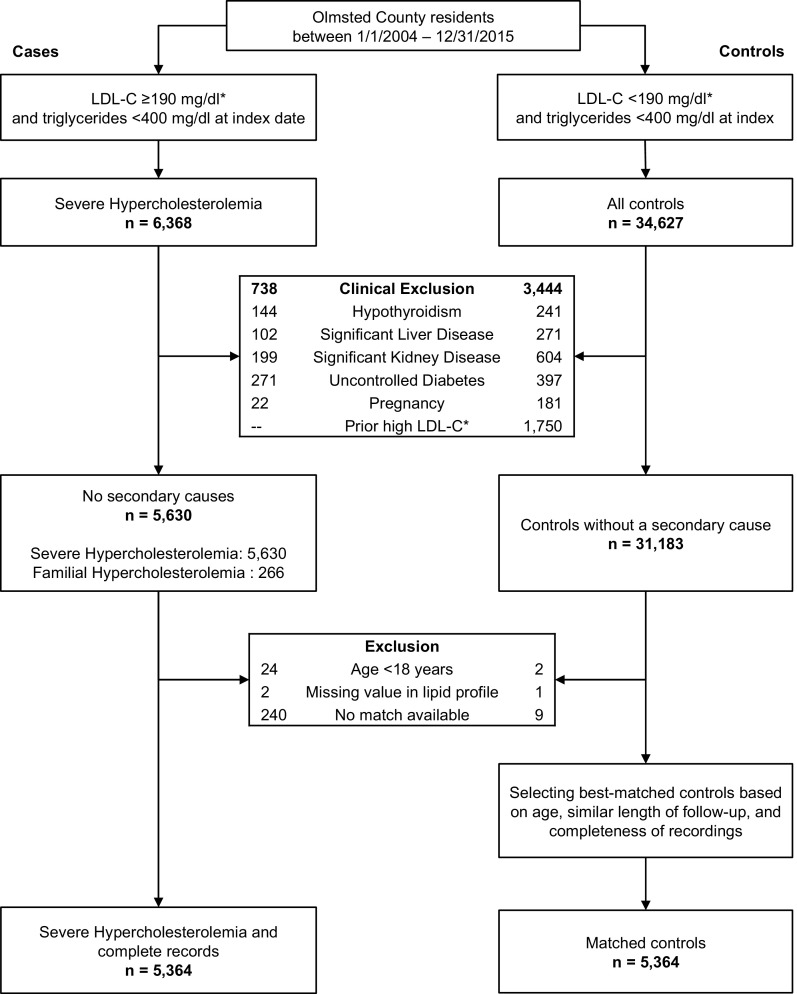
Potential controls were Olmsted County residents with an estimated untreated LDL-C <190 mg/dl, who did not have any of the conditions associated with secondary hypercholesterolemia and were matched based on age (±3 years) and sex to cases (Fig. 1). A single control was selected per case based on the closest match for age and length of follow-up and most complete data available for lipid values, tobacco use, and BMI. The LDL-C value obtained closest in time to the index date was selected for each control. Secondary causes of hypercholesterolemia were also excluded in controls. Study variables including demographics and conventional risk factors were extracted from the REP using previously validated electronic phenotyping algorithms available at www.PheKB.org [12].
Fig. 1.
Study design and case selection. LDL-C = low-density lipoprotein cholesterol. *For those on a statin, the untreated LDL-C was estimated by multiplying LDL-C by 1.33.
2.3. Definition of FH
We defined phenotypic FH using DLCN criteria. DLCN scores were calculated using a validated algorithm which uses both structured (i.e. ICD/CPT codes and lab values) and unstructured data (i.e. clinical notes) and those with DLCN score ≥6 were considered to have FH (Supplementary Table 2) [13]. The medical records of all cases who met criteria for FH were reviewed manually to ascertain the accuracy of electronic algorithm.
2.4. Family history of CHD
We used natural language processing (NLP) to extract family history of CHD from clinical notes. Gender, degree of relationship and age of the related family member at the time of ASCVD event were considered in the NLP algorithm [14]. Family history data was missing in 7.5% of cases and was imputed by the logistic regression method of Multivariate Imputation via Chained Equations (MICE) package.
2.5. Definition of ASCVD subtypes
The conditions of interest were incident CHD, cerebrovascular disease (CVD), peripheral artery disease (PAD), and the composite endpoint of these three. CHD was defined as cardiac angina, myocardial infarction, coronary atherosclerosis, acute ischemic heart disease, percutaneous coronary revascularization, or coronary artery bypass graft. CVD was defined as ischemic stroke, transient ischemic attack or carotid artery disease. PAD was defined as intermittent claudication, critical limb ischemia (i.e., rest pain, or gangrene), or other atherosclerosis of the extremities. Two unique diagnostic code at least 5 days apart or one procedural code were required to ascertain CHD events. CVD and PAD were ascertained based on one ICD or CPT code since more specific codes were available for these conditions. The ICD and CPT codes used to define each condition are listed in Supplementary Tables 3–5. The onset of each condition was assigned using the earliest date of corresponding diagnostic or procedural codes. The onset of the composite endpoint was defined as the earliest onset of CHD, CVD, or PAD. Prevalent conditions were defined as onset on or before the index date, and incident outcomes were defined as onset after the index date.
2.6. Cardiovascular risk
Cases and controls were followed until December 31, 2018, for incident CHD, CVD, PAD, and composite (ASCVD) outcomes. We assessed the risk of incident outcomes associated with SH after adjusting for demographic factors (age, sex, race/ethnicity) and conventional cardiovascular risk factors (hypertension, diabetes, tobacco use, BMI, triglyceride level, and HDL-C level). We also assessed whether age or sex modified the association of SH with incident ASCVD events, by including the appropriate interaction term in the multivariable regression models. Those noted to have prevalent disease at baseline were excluded from the analyses of the corresponding incident outcomes. The date of onset was considered as the endpoint; and those without outcomes were censored at the date of last visit or death. Kaplan-Meier curves were used to visualize time to event in cases and controls, while nested multivariable Cox models were constructed to obtain hazard of incident ASCVD events independent of demographic and risk factors.
2.7. Incidence and prevalence
To estimate the incidence of SH and the subset of these who met clinical criteria for FH, incident cases were defined as Olmsted County residents who met the criteria for the first time between 2004 and 2015. Incident cases who were <18 years old, had a missing value in the lipid profile, or did not have a matched control (n=266) were included for estimating incidence and prevalence, but were excluded from all other analyses. Denominators were based on annual REP population counts [15]. Prevalence of SH was estimated using two different approaches. The ‘point prevalence’ was estimated using a simple cross-sectional method whereby the person-years denominator was limited to the Olmsted population on July 1st of 2015, and the cases were limited to those with index date on or before that date who were still alive and resident in Olmsted County on July 1st of 2015. The ‘period prevalence’ was estimated using an age-recursive method, that incorporated estimates of age-specific incidence and relative survival as previously described [16] (details provided in the Supplementary Material). It represents the proportion of the population who met criteria for SH in the past. The prevalence of FH was estimated using the age-recursive model.
2.8. Awareness, treatment, and control
Awareness was estimated as the proportion of cases in whom the diagnosis of pure hypercholesterolemia (ICD-9 272.0), pure hypercholesterolemia (ICD-10 E78.00), familial hypercholesterolemia (ICD-10 E78.01) was noted in the REP diagnostic index. Lipid lowering therapy (LLT) was ascertained in three time periods: any LLT during the 18-months before the index date, 18-months after the index date, and 18-months before the last lipid measurement in those with at least 36 months of follow-up. We also investigated gender difference and temporal trends of statin use during the study period using logistic regression. The last LDL-C value during follow-up was obtained for those with ≥1 measurement at least 6 months after the index LDL-C. Control was defined based on the last LDL-C using three cutoffs: a) <130 mg/dl, b) <100 mg/dl, and c) the guideline-recommended goal as <70 mg/dl in those with secondary prevention and <100 mg/dl with primary prevention.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and p values <0.05 were considered statistically significant.
3. Results
3.1. Participant characteristics
Using REP resources, we identified 6368 adults who were Olmsted County residents between 01/01/2004 to 12/31/2015 and had an LDL-C ≥190 mg/dl and triglycerides <400 mg/dl (Fig. 1). Secondary causes of high LDL-C level were found in 738 cases, leaving 5630 cases in the SH group for incidence and prevalence analysis; of these 266 (4.7%) met DLCN criteria for phenotypic FH. After excluding cases with age <18 years, missing lipid values, or no matched control available, a total of 5364 matched pairs of cases and controls remained, of whom 248 cases met the DLCN criteria for FH. The mean age of cases and controls were 54.6±13.2 and 54.4±13.3 years, respectively. The mean untreated LDL-C level was 208.5±23.3 mg/dl in SH cases, 240.6±57.8 mg/dl in FH cases, and 113.1±28.7 mg/dl in controls. The distributions of lipid profile parameters in cases and controls are depicted in Supplementary Fig. 1. Racial differences were also noted, and minorities were more often cases. While 3.3% and 4.4% of SH cases were Black and Asian, respectively, the proportions in controls were 2.5% and 2.8%, respectively. The frequencies of diabetes, hypertension, tobacco use, and obesity were higher in cases than controls (Table 1).
Table 1.
Baseline characteristics of SH and FH cases and controls.
| SH(n = 5364) | FH(n = 248) | Controls(n = 5364) | |
|---|---|---|---|
| Age, years | 54.6 ± 13.2 | 52.7 ± 10.3 | 54.4 ± 13.3 |
| Male sex | 2530 (47.2%) | 115 (46.4%) | 2530 (47.2%) |
| Race | |||
| White | 4677 (87.2%) | 219 (88.3%) | 4850 (90.4%) |
| Black | 178 (3.3%) | 9 (3.6%) | 132 (2.5%) |
| Asian | 236 (4.4%) | 11 (4.4%) | 152 (2.8%) |
| Other* | 273 (5.1%) | 9 (3.6%) | 230 (4.3%) |
| Hispanic ethnicity | 205 (3.8%) | 7 (2.8%) | 183 (3.4%) |
| Medical history | |||
| Diabetes | 1310 (24.4%) | 92 (37.1%) | 1123 (20.9%) |
| Hypertension | 3586 (66.9%) | 209 (84.3%) | 3270 (61.0%) |
| Tobacco use† | 2791 (52.0%) | 167 (67.3%) | 2730 (50.9%) |
| BMI, kg/m2 | 29.9 ± 5.8 | 31.3 ± 6.0 | 29.1 ± 6.4 |
| BMI categories† | |||
| Overweight (BMI 25 – 29.9) | 2132 (39.7%) | 76 (30.6%) | 1947 (36.3%) |
| Obesity (BMI ≥30) | 2269 (42.3%) | 135 (54.4%) | 1991 (37.1%) |
| Lipid profile (index) | |||
| Index LDL-C‡, mg/dl | 208.5 ± 23.3 | 240.6 ± 57.8 | 113.1 ± 28.7 |
| Total cholesterol, mg/dl | 265.5 ± 35.5 | 280.8 ± 60.8 | 189.7 ± 33.9 |
| HDL-C, mg/dL | 51.9 ± 14.2 | 49.1 ± 14.4 | 55.1 ± 16.8 |
| Triglyceride, mg/dl | 164.7 ± 69.6 | 184.6 ± 73.7 | 126.3 ± 64.8 |
| Documented diagnosis | 2478 (46.2%) | 133 (53.6%) | - |
| Lipid lowering medication | |||
| 18-months before the index date | |||
| Statin only | 2,499 (46.6%) | 144 (58.1%) | 780 (14.5%) |
| Non-statin only | 160 (3.0%) | 1 (0.4%) | 209 (3.9%) |
| Both statin and non-statin | 566 (10.6%) | 59 (23.8%) | 168 (3.1%) |
| 18-months after the index date | |||
| Statin only | 3133 (58.4%) | 140 (56.5%) | 938 (17.5%) |
| Non-statin only | 231 (4.3%) | 9 (3.6%) | 228 (4.3%) |
| Both statin and non-statin | 766 (14.3%) | 78 (31.5%) | 201 (3.7%) |
| 18-months before the last follow-up∥ | n = 4,828 | n = 232 | n = 4,823 |
| Statin only | 2734 (56.6%) | 123 (53.0%) | 1403 (29.1%) |
| Non-statin only | 178 (3.7%) | 8 (3.4%) | 305 (6.3%) |
| Both statin and non-statin | 697 (14.4%) | 70 (30.2%) | 331 (6.9%) |
| LDL-C level at last follow-up# | n = 5,223 | n = 246 | n = 5,199 |
| LDL-C ≥6 months after the index date | 4915 (94.1%) | 237 (96.3%) | 4534 (87.2%) |
| Last LDL-C, mg/dl | 115.7 ± 42.2 | 105.9 ± 55.3 | 100.3 ± 33.2 |
| Last LDL-C <130 mg/dl | 3283 (66.8%) | 178 (75.1%) | - |
| Last LDL-C <100 mg/dl | 1997 (40.6%) | 131 (55.3%) | - |
| Last LDL-C at target⁎⁎ | 1454 (29.6%) | 67 (28.3%) | - |
The numbers are given as mean ± SD, n (%). BMI = body mass index; FH = familial hypercholesterolemia; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; SH = severe hypercholesterolemia.
Other races include Hawaiian/Pacific islander, American Indian, mixed, or unknown. †The reference categories that are not shown include people with unknown values. ‡For those on a statin, the untreated LDL-C was estimated by multiplying the LDL-C by 1.33. ∥In people with at least 36 months of follow-up. #In people with at least 6 months of follow-up, people missing follow-up LDL altered percent calculation. ⁎⁎Last LDL-C <70 mg/dl in those with CHD, CVD, or PAD on or before the follow-up date (secondary prevention) and <100 mg/dl in those without CHD, CVD, or PAD on or before the follow-up date (primary prevention).
3.2. Incidence and prevalence of SH and FH
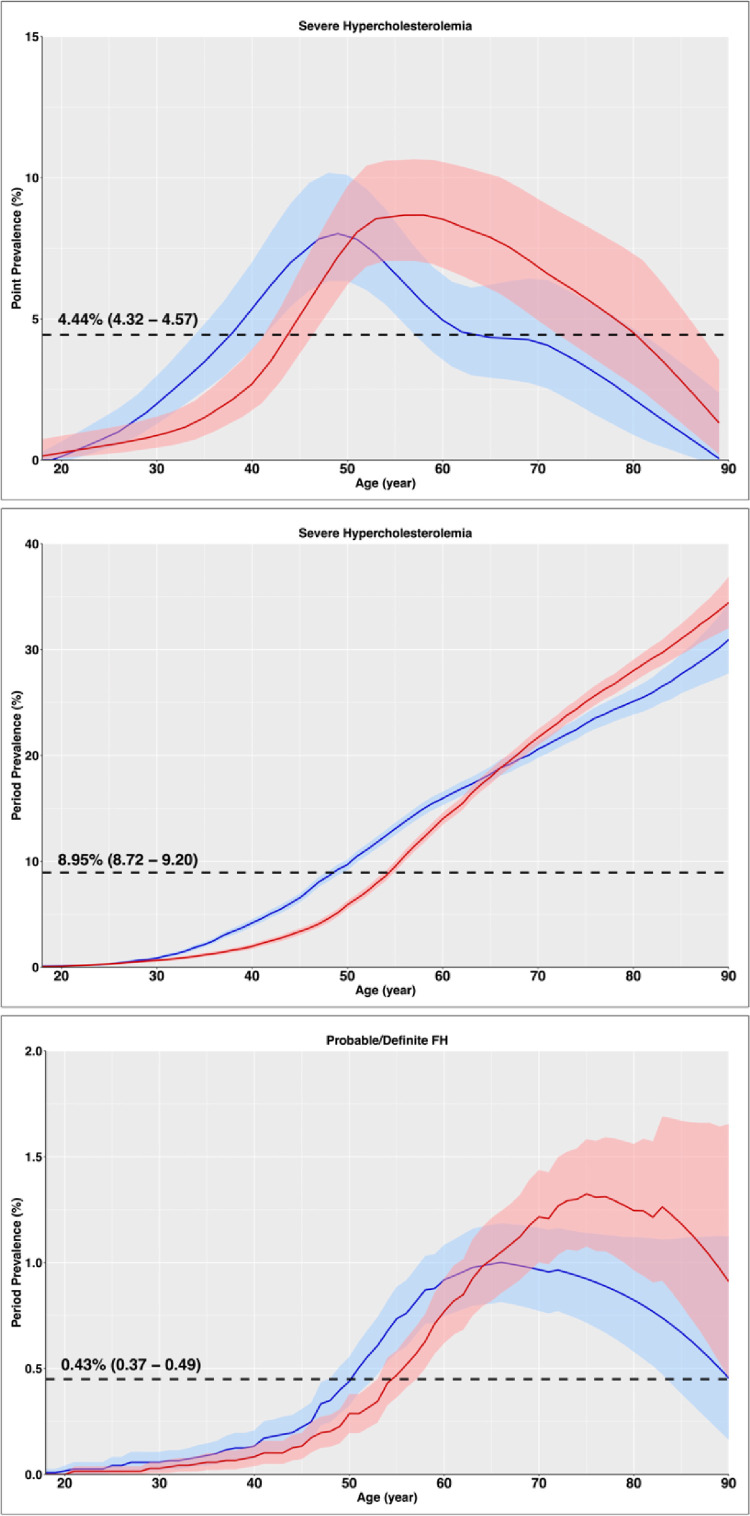
Adjusted to the US white 2010 population, the overall incidence rate of SH per 100,000 person-years was 360.3 (350.8 – 369.8) (Table 2). The point prevalence of SH was 4.44% (4.32–4.57) and period prevalence was 8.95% (8.72 – 9.20). As depicted in Fig. 2, the point prevalence increased by ages 50–60, reaching as high as 8.02% in men and 8.68% in women and then declined. However, the period prevalence continuously increased with age as patients will be considered cases for the rest of their lives whenever they have an LDL-C ≥190 mg/dl. SH was slightly more prevalent in men before age 65 and slightly more prevalent in women after this age. This led to a slightly greater prevalence of SH in men than women 9.16% (8.78 – 9.50) vs. 8.82% (8.51 – 9.13) despite the greater incidence rate in women 377.9 (364.5 – 391.4) vs. 336.3 (323.1 – 349.5) as men were identified at younger ages. Based on the current adult population of Olmsted County and US, there are estimated ∼11,000 and ∼22.8 million SH cases in Olmsted County and US, respectively. The prevalence of phenotypic FH (defined based on DLCN criteria) adjusted to the age and sex distribution of the US white 2010 population, was 0.43% (0.37 – 0.49), or 1 in 233 US adults (Table 2 and Fig. 2).
Table 2.
Sex-specific incidence rate and prevalence of SH and FH.
| Severe hypercholesterolemia | Total | Male | Female | P-value |
|---|---|---|---|---|
| Incidence Rate* (95% CI) | n = 5,630 | n = 2,557 | n = 3,073 | |
| Olmsted County | 316.5 (308.2 - 324.8) |
300.6 (289.1 - 312.5) |
331.0 (319.4 - 342.9) |
<0.001 |
| US‡ | 360.3 (350.8 - 369.8) |
336.3 (323.1 - 349.5) |
377.9 (364.5 - 391.4) |
<0.001 |
| Point Prevalence# (95% CI) | n = 4,891 | n = 2,204 | n = 2,687 | |
| Olmsted County | 4.18 (4.06 - 4.29) |
4.04 (3.87 - 4.21) |
4.30 (4.14 - 4.46) |
0.03 |
| US‡ | 4.44 (4.32 - 4.57) |
4.22 (4.04 - 4.40) |
4.59 (4.42 - 4.77) |
0.004 |
| Period Prevalence† (95% CI) | n = 5,630 | n = 2,557 | n = 3,073 | |
| Olmsted County | 7.79% (7.59 - 8.01) |
8.26% (7.91 - 8.57) |
7.39% (7.12 - 7.65) |
<0.001 |
| US‡ | 8.95% (8.72 - 9.20) |
9.16% (8.78 - 9.50) |
8.82% (8.51 - 9.13) |
0.16 |
| Familial hypercholesterolemia∥ | Total | Male | Female | P-value |
| Incidence Rate* (95% CI) | (n = 266) | (n = 118) | (n = 148) | |
| Olmsted County | 15.0 (13.2 - 16.9) |
13.9 (11.5 - 16.6) |
16.0 (13.5 - 18.7) |
0.26 |
| US‡ | 17.0 (15.0 - 19.1) |
15.3 (12.5 - 18.1) |
18.4 (15.4 - 21.4) |
0.13 |
| Period Prevalence† (95% CI) | (n = 266) | (n = 118) | (n = 148) | |
| Olmsted County | 0.37% (0.32 - 0.43) |
0.38% (0.31 - 0.45) |
0.36% (0.30 - 0.43) |
0.68 |
| US‡ | 0.43% (0.37 - 0.49) |
0.42% (0.34 - 0.51) |
0.43% (0.36 - 0.51) |
0.86 |
ASCVD = atherosclerotic cardiovascular disease; FH = familial hypercholesterolemia; LDL-C = low-density lipoprotein cholesterol; OC = Olmsted County; SH = severe hypercholesterolemia.
Incidence rates for 100,000 person-years. Incidence rates were measured by 850,276 person-years of follow-up in males and 928,154 in females. The denominator for incidence calculations is Olmsted County population defined by the REP from 2004 through 2015. The values are 1,778,430 including 850,276 male and 928,154 female.
Period prevalence rates were calculated as the mean in adults (18-89 years), weighted to the population counts of whites in Olmsted County or the United States from 2010 census estimates. Confidence limits are calculated using the 2.5th and 97.5th percentile of all prevalence rates across 1,000 bootstrapped samples (within each sample, the prevalence rate is the mean rate across all ages, as above). Since the period prevalence estimation relies on an age-recursive model, and there is no relevant denominator for prevalence.
Overall incidence rates were adjusted to the age and sex distribution of the US white population from 2010.
The denominator when calculating point prevalence was Olmsted County population on July 1, 2015. The values are 117,143 including 54,612 males and 62,531 females.
Dutch Lipid Clinic Network definition of FH.
Fig. 2.
Age-specific Point and Period Prevalence Rate of Severe Hypercholesterolemia (top and middle) and Period Prevalence Rate of Familial Hypercholesterolemia (bottom). The prevalence rates were adjusted to the US white population from 2010. Males are depicted in blue and females in red. Dashed lines represent mean prevalence values for adults (age 18–89 years). Point prevalence is depicted based on the smoothed data of year 2015.
3.3. Awareness, treatment, and control
The diagnosis of pure hypercholesterolemia or FH was recorded in 2478 (46.2%) and 133 (53.6%) of SH and FH cases, respectively. The use of lipid-lowering medications differed in the primary prevention vs. secondary prevention settings. In the primary prevention setting, 54.7% of SH cases were on LLT on or before the index date, increasing to 74.4% during 18-month interval after the index date (Table 3). The rate of treatment was higher in secondary prevention setting: 88.1% and 90.1% in the aforementioned timeframes. Younger patients were more often untreated (Supplementary Table 6). In the secondary prevention setting, women were less likely than men to be on LLT at last follow-up (80.4% vs. 89.0% p <0.001).
Table 3.
Lipid lowering medications among SH cases with complete records in the 18-month period before the index date, after the index date, and before the last follow-up, in primary and secondary prevention settings.
| Time Period and Type of Prevention | All | Males | Females | p value |
|---|---|---|---|---|
| 18-month period before the index date | ||||
| Primary prevention, n* | 4,493 | 2,049 | 2,444 | 0.54 |
| Both statin and non-statin | 375 (8.3%) | 169 (8.2%) | 206 (8.4%) | |
| Statin | 1,935 (43.1%) | 890 (43.4%) | 1,045 (42.8%) | |
| Non-statin | 148 (3.3%) | 59 (2.9%) | 89 (3.6%) | |
| No medication | 2,035 (45.3%) | 931 (45.4%) | 1,104 (45.2%) | |
| Secondary prevention, n† | 871 | 481 | 390 | 0.07 |
| Both statin and non-statin | 191 (21.9%) | 118 (24.5%) | 73 (18.7%) | |
| Statin | 564 (64.8%) | 309 (64.2%) | 255 (65.4%) | |
| Non-statin | 12 (1.4%) | 5 (1.0%) | 7 (1.8%) | |
| No medication | 104 (11.9%) | 49 (10.2%) | 55 (14.1%) | |
| 18-month period after the index date | ||||
| Primary prevention, n* | 4,493 | 2,049 | 2,444 | 0.07 |
| Both statin and non-statin | 553 (12.3%) | 267 (13.0%) | 286 (11.7%) | |
| Statin | 2,606 (58.0%) | 1,171 (57.1%) | 1,435 (58.7%) | |
| Non-statin | 186 (4.1%) | 71 (3.5%) | 115 (4.7%) | |
| No medication | 1,148 (25.6%) | 540 (26.4%) | 608 (24.9%) | |
| Secondary prevention, n† | 871 | 481 | 390 | 0.20 |
| Both statin and non-statin | 213 (24.5%) | 131 (27.2%) | 82 (21.0%) | |
| Statin | 527 (60.5%) | 279 (58.0%) | 248 (63.6%) | |
| Non-statin | 45 (5.2%) | 25 (5.2%) | 20 (5.1%) | |
| No medication | 86 (9.9%) | 46 (9.6%) | 40 (10.3%) | |
| 18-month period before the last follow-up date‡ | ||||
| Primary prevention, n* | 3,429 | 1,523 | 1,906 | 0.09 |
| Both statin and non-statin | 414 (12.1%) | 174 (11.4%) | 240 (12.6%) | |
| Statin | 1,878 (54.8%) | 864 (56.7%) | 1,014 (53.2%) | |
| Non-statin | 131 (3.8%) | 48 (3.2%) | 83 (4.4%) | |
| No medication | 1,006 (29.3%) | 437 (28.7%) | 569 (29.9%) | |
| Secondary prevention, n† | 1,399 | 709 | 690 | <0.001 |
| Both statin and non-statin | 283 (20.2%) | 157 (22.1%) | 126 (18.3%) | |
| Statin | 856 (61.2%) | 454 (64.0%) | 402 (58.3%) | |
| Non-statin | 47 (3.4%) | 20 (2.8%) | 27 (3.9%) | |
| No medication | 213 (15.2%) | 78 (11.0%) | 135 (19.6%) | |
SH = severe hypercholesterolemia.
In people with at least 36 months of follow-up.
Primary prevention setting is defined as SH cases with no coronary heart disease, cerebrovascular disease, or peripheral artery disease at the index date for the first two time periods, and with no such disease at the follow-up date for the last time period.
Secondary prevention setting was defined as SH cases with coronary heart disease, or cerebrovascular disease, or peripheral artery disease at the index date for the first two time periods, and with any such disease at the follow-up date for the last time period.
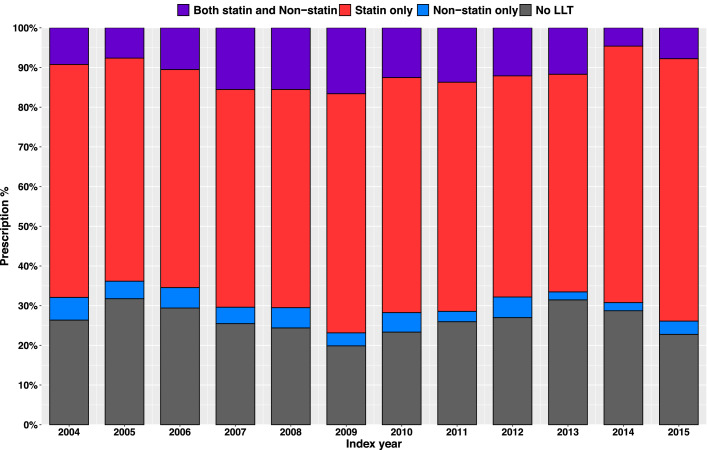
There was a non-significant trend toward increasing statin use (p = 0.059) over the study period (2004 – 2015) (Fig. 3). Among 4915 SH cases with LDL-C measured at least 6 months after index, the last LDL-C level was <130 mg/dl, or <100 mg/dl in 66.8% and 40.6% of cases, respectively. The magnitude of LDL-C reduction was greater in the secondary prevention setting than in the primary prevention setting. The guideline-recommended LDL-C levels (i.e., <70 mg/dl in the secondary prevention setting and <100 mg/dl in the primary prevention setting) were achieved only in 33.1% of primary prevention cases and only 21.2% of secondary prevention cases [2] (Table 4, Supplementary Figs. 2 and 3). However, if more recent LDL-C treatment goals of <55 mg/dL for secondary prevention and <70 for high-risk primary prevention are considered, then the control status would be even worse [17]. The treatment gap between men and women in the secondary prevention setting was reflected in the control status. While the last LDL-C in men was <100 mg/dl in 63.7% and <70 mg/dl in 23.7% of cases, these figures in women were 53.5% and 18.6%, respectively (p <0.001 and p = 0.02).
Fig. 3.
Lipid-lowering Treatment for Primary Prevention in the 18-month Period After Detection of High LDL-C Level Among SH Cases with Complete Records. LDL-C = low-density cholesterol; LLT = lipid-lowering treatment; SH = severe hypercholesterolemia.
Table 4.
Degree of control in primary and secondary prevention settings among SH cases.
| Type of Prevention and Measure of Control | All | Males | Females | p value |
|---|---|---|---|---|
| Overall, n | 4,915 | 2,276 | 2,639 | |
| Last LDL-C <130 mg/dl | 3,283 (66.8%) | 1,547 (68.0%) | 1,736 (65.8%) | 0.10 |
| Last LDL-C <100 mg/dl | 1,997 (40.6%) | 992 (43.6%) | 1,005 (38.1%) | <0.001 |
| Last LDL-C at target* | 1,454 (29.6%) | 696 (30.6%) | 758 (28.7%) | 0.15 |
| Primary prevention, n† | 3,470 | 1,537 | 1,933 | |
| Last LDL-C <130 mg/dl | 2,123 (61.2%) | 938 (61.0%) | 1,185 (61.3%) | 0.87 |
| Last LDL-C <100 mg/dl | 1,148 (33.1%) | 521 (33.9%) | 627 (32.4%) | 0.36 |
| Last LDL-C at target* | 1,148 (33.1%) | 521 (33.9%) | 627 (32.4%) | 0.36 |
| Secondary prevention, n‡ | 1,445 | 739 | 706 | |
| Last LDL-C <130 mg/dl | 1,160 (80.3%) | 609 (82.4%) | 551 (78.0%) | 0.04 |
| Last LDL-C <100 mg/dl | 849 (58.8%) | 471 (63.7%) | 378 (53.5%) | <0.001 |
| Last LDL-C at target* | 306 (21.2%) | 175 (23.7%) | 131 (18.6%) | 0.02 |
LDL-C, low-density lipoprotein cholesterol.
Primary prevention setting was defined as cases with no coronary heart disease, cerebrovascular disease, or peripheral artery disease on or before the follow-up date.
Secondary prevention setting was defined as cases with coronary heart disease, or cerebrovascular disease, or peripheral artery disease on or before the follow-up date.
LDL-C <70 mg/dl in those with secondary prevention and <100 mg/dl in those with primary prevention.
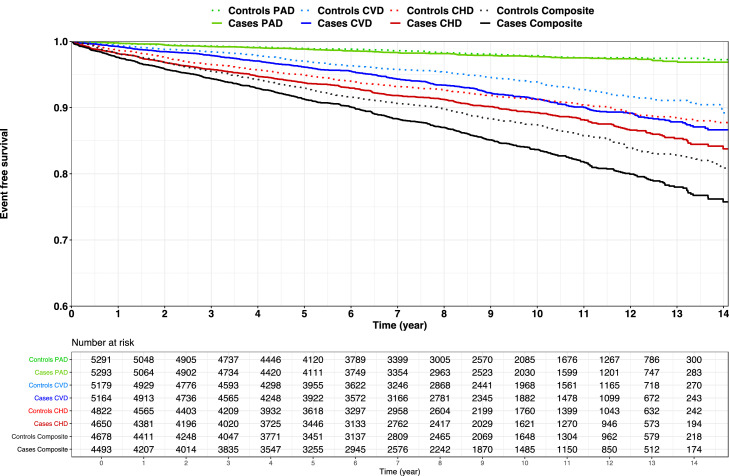
3.4. Cardiovascular risk
The rates of baseline and incident events in SH cases and controls are shown in Supplementary Table 7. Kaplan-Meier curves for event-free survival comparing SH vs. controls are depicted in Fig. 4. In a fully adjusted model, SH was associated with a history of CHD [OR: 1.47; 95% CI: 1.29 to 1.68; p <0.001], but not with CVD [OR: 1.10; 95% CI: 0.88 to 1.36; p = 0.400] nor with PAD [OR: 0.95; 95% CI: 0.67 to 1.35; p = 0.785]. During follow-up, SH cases without prevalent ASCVD were at an increased risk of new CHD events [HR: 1.21; 95% CI: 1.05 to 1.39; p = 0.010], and CVD [HR: 1.30; 95% CI: 1.11 to 1.53; p = 0.001], but not PAD [HR: 1.06; 95% CI: 0.80 to 1.42; p = 0.678] (Table 5). The association of SH with incident CHD (but not CVD or PAD) was modified by age (p interaction = 0.015) such that the association of SH with incident CHD events was greater at younger ages. After inclusion of SH-age interaction term in the model, the HR for CHD associated with SH decreased from 2.10 at age 20 to 1.07 at age 70. The complete set of coefficients for logistic regression models and Cox proportional hazard models is presented in the Supplementary Material (Supplementary Tables 8, 9). Kaplan-Meier curves for event-free survival comparing SH cases with a family history of premature ASCVD vs. their matched controls are depicted in Supplementary Fig. 4.
Fig. 4.
Kaplan-Meier Curves for Survival Free of ASCVD Outcomes. PAD is shown in green (p value: 0.438), CVD is shown in blue (p value: <0.001), CHD is shown in red (p value: 0.002), and the composite endpoint is shown in black (p value: <0.001). The numbers of at-risk SH cases and the corresponding controls are displayed. The solid lines represent cases, and the dotted lines represent controls. ASCVD = atherosclerotic cardiovascular disease; CHD = coronary heart disease; CVD = cerebrovascular disease; PAD = peripheral artery disease; SH = severe hypercholesterolemia.
Table 5.
Multivariable logistic regression models and cox proportional hazards regression models for the hazard of prevalent and incident CHD, CVD, and PAD events, as well as composite endpoint in SH and FH cases in comparison with matched controls.
| Baseline(Prevalent) Events | New(Incident) Events | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p value | HR (95% CI) | p value | Follow-up (years) ‡ | |
| CHD | |||||
| SH | 1.47 (1.29 - 1.68) | <0.001 | 1.21 (1.05 - 1.39) | 0.010 | 8.9 (5.6 - 11.8) |
| SH + family history* | 3.81 (2.78 - 5.22) | <0.001 | 2.16 (1.57 - 2.96) | <0.001 | 9.3 (6.0 - 12.2) |
| FH† | - | - | 4.61 (2.66 - 7.97) | <0.001 | 9.8 (6.0 - 12.9) |
| CVD | |||||
| SH | 1.10 (0.88 - 1.36) | 0.400 | 1.30 (1.11 - 1.53) | 0.001 | 8.9 (5.6 - 11.9) |
| SH + family history* | 1.64 (0.92 - 2.90) | 0.092 | 2.00 (1.40 - 2.85) | <0.001 | 9.4 (6.1 - 12.3) |
| FH† | - | - | 1.75 (0.94 - 3.24) | 0.078 | 9.9 (6.3 - 12.7) |
| PAD | |||||
| SH | 0.95 (0.67 - 1.35) | 0.785 | 1.06 (0.80 - 1.42) | 0.678 | 8.9 (5.5 - 11.9) |
| SH + family history* | 4.22 (1.53 – 11.7) | 0.006 | 1.89 (0.96 - 3.72) | 0.067 | 9.3 (6.0 - 12.2) |
| FH† | - | - | 7.32 (1.57 – 34.2) | 0.011 | 9.9 (6.2 - 12.7) |
| Composite | |||||
| SH | 1.43 (1.26 - 1.62) | <0.001 | 1.24 (1.10 - 1.39) | <0.001 | 8.9 (5.7 - 11.9) |
| SH + family history* | 3.57 (2.65 - 4.81) | <0.001 | 2.15 (1.65 - 2.80) | <0.001 | 9.3 (6.0 - 12.2) |
| FH† | - | - | 5.10 (3.08 - 8.47) | <0.001 | 9.8 (6.1 - 12.9) |
The models are adjusted for age, sex, race, hypertension, diabetes, tobacco use, body mass index, triglyceride level ≥150 mg/dl, and low HDL-C level (<40 mg/dl for males, <50 mg/dl for females).
CHD = coronary heart disease; CI = confidence interval; CVD = cerebrovascular disease; FH = familial hypercholesterolemia; HDL-C = high-density lipoprotein cholesterol; HR = hazard ratio; OR = odds ratio; PAD = peripheral artery disease; SH = severe hypercholesterolemia.
Family history of premature atherosclerotic cardiovascular disease.
ORs are not calculated since baseline CHD, CVD, and PAD were used in defining the FH group.
Follow-up time is presented as median and interquartile range.
SH individuals who had family history of premature ASCVD were at increased risk of incident CHD [HR: 2.16; 95% CI: 1.57 to 2.96; p <0.001], CVD [HR: 2.00; 95% CI: 1.40 to 2.85; p <0.001] and tended to be at increased risk of PAD [HR: 1.89; 95% CI: 0.96 to 3.72; p = 0.067] compared to the matched controls. Since ASCVD is one of the criteria to define FH patients, we did not estimate the odds of prevalent ASCVD events in FH. FH patients without CHD events at baseline were at greatly increased risk of CHD during the follow-up period [HR: 4.61; 95% CI: 2.66 to 7.97; p <0.001] (Table 5).
4. Discussion
The main findings of the present study were: (1) the prevalence of SH was high (point prevalence: 4.44%, period prevalence: 8.95%); (2) the diagnosis was documented in 46.2% of cases, 74.4% of cases were on LLT for primary prevention within 18 months following detection of elevated LDL-C level but only 33.1% achieved guideline recommended goal. These figures were 90.1% and 21.2% in the secondary prevention setting; (3) women were less likely than men to be on LLT and to reach goal LDL-C; and 4) SH was an independent risk factor for incident CHD and CVD and the association of SH with CHD was modified by age, such that the risk was stronger at younger ages. The high prevalence, low awareness and control highlight the significant burden of SH in the community and the need to address treatment gaps to reduce the burden of ASCVD.
4.1. Incidence and prevalence
The point prevalence of SH in adults in our study was 4.44% and the period prevalence was 8.95%. Previous studies reported a wide range for the prevalence of SH ranging from 6% -13% [6], [7], [8]. The differences can be due to study design, different sampling methods and selection bias, and different approaches to ascertaining LLT. Cross-sectional surveys capture high LDL-C level at a point in time. However, the longitudinal data in the REP allowed us to capture LDL-C levels over time. The point prevalence of SH estimated by the cross-sectional method was about half the period prevalence. The latter better reflects the burden of SH because a) other studies and our results demonstrate that only a small percentage of SH cases will achieve normal LDL-C levels in the future [18]; and (b) the cumulative exposure model for LDL-C suggests that elevated early-life cholesterol levels increase future cardiovascular risk independent of midlife cholesterol levels [19], [20], [21]. Among SH cases, ∼5% had clinically defined FH, 10% had personal history of premature ASCVD, and nearly 18% had family history of ASCVD. The prevalence of FH was 0.43% (∼1:233) similar to previous national surveys [6,7] but higher than a recently published meta-analysis (∼1:310) [22,23]. We also noticed sex differences in the prevalence of SH. Despite higher incidence of SH in women, the prevalence of SH was greater in men because men were identified at younger ages.
4.2. Awareness, treatment, and control
A diagnosis of hypercholesterolemia was recorded in 46.2% of SH cases and 53.6% of FH cases. In NHANES, the rate of statin prescription for SH was 37% on average, with a higher rate (52%) in those with a clinical FH diagnosis [7]. In the Copenhagen Heart Study, 48% of FH cases were on a statin [24]. In our cohort 60.1% of SH cases and 82.3% of FH cases were on LLT in the 18-month period before the index date, increasing to 77.0% and 91.5%, respectively, in an 18-month period after the index date. We noted a modest trend towards increased statin use over time. Our results are comparable to the CASCADE registry findings of 75% statin use in a cohort of 1295 FH cases from 11 lipid clinics [25]; 24.7% of cases achieved an LDL-C <100 mg/dL compared to 40.6% in the SH group and 55.3% in the FH group in our study. However, it should be noted that only 21.2% of SH cases in the secondary prevention setting met the guideline-recommended goal LDL-C (<70 mg/dl), highlighting a treatment gap that needs to be addressed. This treatment gap was wider in women in the secondary prevention setting since they met the guideline-recommended threshold less often.
4.3. Cardiovascular risk
SH was associated with higher odds of prevalent CHD as well as hazard of incident CHD. The association was modified by age such that SH was associated with a greater risk of incident CHD in younger individuals (i.e., relative risk of CHD from SH was greater at younger ages). Perak et.al., based on pooled data from population-based cohorts, reported that in SH individuals 50-59 years old (corresponding to the mean age of our study), the HR of CHD was 2.0 (1.7–2.3) in comparison to a control group with LDL-C <130 mg/dL [26]; an effect modification by age was not reported. In our cohort, family history of ASCVD nearly doubled the risk of incident CHD events in SH cases and increased the risk of CVD by 54%. The highest risk for CHD was noted in subset of SH cases with FH [HR: 4.61; 95% CI: 2.66 – 7.97; p <0.001].
SH was associated with increased hazard of incident CVD in SH cases, consistent with prior reports [27,28]; of note genetically defined FH has not been reported to be associated with increased risk of CVD [29,30]. We did not find a statistically significant relationship between SH and PAD probably due to a lack of power given the limited number of incident PAD events. However, findings from other studies suggest that LDL-C may be a weaker risk factor for PAD than for CHD [31,32]
4.4. Implications for population health
ASCVD is the leading cause of mortality and morbidity and a major contributor to health care costs in the US [5]. Our results highlight potential strategies to reduce the burden of ASCVD from SH: at the individual level, increasing awareness of the risk from SH, and adopting healthy lifestyle choices (AHA's Simple 7) [5]; at the provider level, such as by clinical decision support that encourages achievement of target LDL-C levels [33]; and at the population level, such as the Million Hearts initiative [34]. In the subset of patients with FH, cascade testing of family members may enable early detection and treatment [35,36]. An important finding of our study is the relatively low proportion of individuals reaching goal LDL-C levels despite relatively high use of LLT. This could be due to poor adherence, since prior studies suggest that 1 year after starting a statin medication, only ∼50-60% remained adherent [37]. Provider education and the use of a team approach (including nurse, dietician, pharmacist and physician) to manage SH in order to achieve to target LDL-C levels, should be also considered [38].
4.5. Strength and limitations
We used EHR data from REP for population-based estimates, counting cases among residents of a defined geographical region and excluding cases among non-residents while excluded secondary causes of hypercholesterolemia and had minimal missing data. We combined an EHR-based algorithm with manual review to ascertain family history of ASCVD in clinically defined FH cases. The longitudinal data in the REP allowed us to capture the cases when they met the case definition. Our study population was a contemporary US cohort, representative of current real-world practice and care and provided epidemiologic data related to incidence, prevalence, awareness, and control of SH.
Limitations of our study include a relatively low number of non-white individuals. Our findings may not be generalizable to the entire US. However, given the diversity of the US, no single cohort is completely representative of the entire country and our study cohort represents a large segment of the US population. Although REP resources have been proven as a reliable source of epidemiology studies, caution should be taken in extrapolating data regarding the prescription of medication and the status of control. We ascertained FH based on clinical criteria as genetic testing data were not available for the study population. In prior studies, clinical and genetic FH have only modest overlap [16,39]. Our estimates are based on available lipid test results, and we cannot ascertain SH in the absence of lipid testing. During our study period, approximately 33.0% of adults in Olmsted County did not have lipid levels available. For those on LLT, the potency and dose of LLT were not taken into account for imputation of untreated LDL-C. We also did not have data on drug compliance in those who were on LLT but did not meet the treatment goal.
5. Conclusion
Our results profile the contemporary burden, awareness, and control of SH in a population-based sample in the US. Despite knowledge of LDL-C as the key causal factor in ASCVD and multiple programs to improve detection and control, our study demonstrates that cross-sectional methods underestimate the burden of SH and reveals a high period prevalence of SH (8.95%; 95% CI 8.72-9.20). SH was independently associated with an increased risk of CHD and CVD. Only 33.1% in the primary prevention setting and 21.2% in the secondary prevention setting met guideline-recommended LDL-C goal and in the latter setting women achieved goal LDL-C less often than men. Further efforts are needed to address this treatment gap. Our results should inform the design of initiatives at the population as well as individual levels, to increase awareness, detection, and control of SH.
Funding
This study was funded by grants R01 HL135879 and K24 HL137010 from the National Heart Lung and Blood Institute. The study used the resources of the Rochester Epidemiology Project which is supported by the National Institute on Aging of the National Institutes of Health under award number R01AG034676.
CRediT authorship contribution statement
Seyedmohammad Saadatagah: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Lubna Alhalabi: Data curation, Project administration, Writing – review & editing. Medhat Farwati: Data curation, Validation, Writing – original draft, Writing – review & editing. Magdi Zordok: Data curation, Validation, Project administration, Writing – review & editing. Ashwini Bhat: Validation, Writing – original draft, Writing – review & editing. Carin Y. Smith: Data curation, Formal analysis, Methodology, Visualization, Writing – review & editing. Christina M. Wood-Wentz: Data curation, Formal analysis, Methodology, Visualization, Project administration, Writing – review & editing. Kent R. Bailey: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing. Iftikhar J. Kullo: Conceptualization, Project administration, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2022.100393.
Appendix. Supplementary materials
References
- 1.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/s0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Dawber T.R., Meadors G., Moore F. Epidemiological approaches to heart disease: the framingham study. Am J Public Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vartiainen E. The North karelia project: cardiovascular disease prevention in Finland. Glob Cardiol Sci Pract. 2018;2018(2):13. doi: 10.21542/gcsp.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/cir.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 6.de Ferranti S.D., Rodday A.M., Mendelson M.M., Wong J.B., Leslie L.K., Sheldrick R.C. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States national health and nutrition examination surveys (NHANES) Circulation. 2016;133(11):1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 7.Bucholz E.M., Rodday A.M., Kolor K., Khoury M.J., de Ferranti S.D. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999-2014) Circulation. 2018;137(21):2218–2230. doi: 10.1161/circulationaha.117.032321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel P., Hu Y., Kolinovsky A., Geng Z., Ruhl J., Krishnamurthy S., et al. Hidden burden of electronic health record-identified familial hypercholesterolemia: clinical outcomes and cost of medical care. J Am Heart Assoc. 2019;8(13) doi: 10.1161/JAHA.118.011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver J.L., Grossardt B.R., Leibson C.L., Yawn B.P., Melton L.J., 3rd, Rocca W.A. Generalizability of epidemiological findings and public health decisions: an illustration from the rochester epidemiology project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca W.A., Yawn B.P., St Sauver J.L., Grossardt B.R., Melton L.J. History of the Rochester epidemiology project: half a century of medical records linkage in a US population. Mayo Clinic Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 12.Kirby J.C., Speltz P., Rasmussen L.V., Basford M., Gottesman O., Peissig P.L., et al. PheKB: a catalog and workflow for creating electronic phenotype algorithms for transportability. J Am Med Inform Assoc. 2016;23(6):1046–1052. doi: 10.1093/jamia/ocv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordestgaard B.G., Chapman M.J., Humphries S.E., Ginsberg H.N., Masana L., Descamps O.S., et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European atherosclerosis society. Eur Heart J. 2013;34(45):3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safarova M.S., Liu H., Kullo I.J. Rapid identification of familial hypercholesterolemia from electronic health records: the SEARCH study. J Clin Lipidol. 2016;10(5):1230–1239. doi: 10.1016/j.jacl.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Sauver J.L., Grossardt B.R., Yawn B.P., Melton L.J., 3rd, Rocca W.A. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saadatagah S., Pasha A.K., Alhalabi L., Sandhyavenu H., Farwati M., Smith C.Y., et al. Coronary heart disease risk associated with primary isolated hypertriglyceridemia; a population-based study. J Am Heart Assoc. 2021;10(11) doi: 10.1161/jaha.120.019343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., et al. 2019ss ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 18.Newton S.L., Hoffmann A.P., Yu Z., Haidermota S., Natarajan P., Honigberg M.C. Management of severe and moderate hypercholesterolemia in young women and men. JAMA Cardiol. 2022;7(2):227–230. doi: 10.1001/jamacardio.2021.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Pletcher M.J., Vittinghoff E., Clemons A.M., Jacobs D.R., Jr., Allen N.B., et al. Association between cumulative low-density lipoprotein cholesterol exposure during young adulthood and middle age and risk of cardiovascular events. JAMA Cardiol. 2021;6(12):1406–1413. doi: 10.1001/jamacardio.2021.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domanski M.J., Tian X., Wu C.O., Reis J.P., Dey A.K., Gu Y., et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76(13):1507–1516. doi: 10.1016/j.jacc.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 21.Ference B.A., Graham I., Tokgozoglu L., Catapano A.L. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol. 2018;72(10):1141–1156. doi: 10.1016/j.jacc.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 22.Hu P., Dharmayat K.I., Stevens C.A.T., Sharabiani M.T.A., Jones R.S., Watts G.F., et al. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease. Circulation. 2020;141(22):1742–1759. doi: 10.1161/CIRCULATIONAHA.119.044795. [DOI] [PubMed] [Google Scholar]
- 23.Beheshti S.O., Madsen C.M., Varbo A., Nordestgaard B.G. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol. 2020;75(20):2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 24.Benn M., Watts G.F., Tybjaerg-Hansen A., Nordestgaard B.G. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97(11):3956–3964. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 25.deGoma E.M., Ahmad Z.S., O'Brien E.C., Kindt I., Shrader P., Newman C.B., et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE-FH registry. Circ Cardiovasc Genet. 2016;9(3):240–249. doi: 10.1161/CIRCGENETICS.116.001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perak A.M., Ning H., de Ferranti S.D., Gooding H.C., Wilkins J.T., Lloyd-Jones D.M. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134(1):9–19. doi: 10.1161/circulationaha.116.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurth T., Everett B.M., Buring J.E., Kase C.S., Ridker P.M., Gaziano J.M. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68(8):556–562. doi: 10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura T., Doi Y., Arima H., Yonemoto K., Hata J., Kubo M., et al. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke. 2009;40(2):382–388. doi: 10.1161/strokeaha.108.529537. [DOI] [PubMed] [Google Scholar]
- 29.Beheshti S., Madsen C.M., Varbo A., Benn M., Nordestgaard B.G. Relationship of familial hypercholesterolemia and high low-density lipoprotein cholesterol to ischemic stroke: Copenhagen general population study. Circulation. 2018;138(6):578–589. doi: 10.1161/circulationaha.118.033470. [DOI] [PubMed] [Google Scholar]
- 30.Akioyamen L.E., Tu J.V., Genest J., Ko D.T., Coutin A.J.S., Shan S.D., et al. Risk of ischemic stroke and peripheral arterial disease in heterozygous familial hypercholesterolemia: a meta-analysis. Angiology. 2019;70(8):726–736. doi: 10.1177/0003319719835433. [DOI] [PubMed] [Google Scholar]
- 31.Joosten M.M., Pai J.K., Bertoia M.L., Rimm E.B., Spiegelman D., Mittleman M.A., et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308(16):1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fowkes F.G.R., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 33.Bangash H., Sutton J., Gundelach J.H., Pencille L., Makkawy A., Elsekaily O., et al. Deploying clinical decision support for familial hypercholesterolemia. ACI Open. 2020;4(2) doi: 10.1055/s-0040-1721489. e-157-e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frieden T.R., Berwick D.M.The. Million hearts" initiative–preventing heart attacks and strokes. N Engl J Med. 2011;365(13):e27. doi: 10.1056/NEJMp1110421. [DOI] [PubMed] [Google Scholar]
- 35.Saadatagah S., Jose M., Dikilitas O., Alhalabi L., Miller A.A., Fan X., et al. Genetic basis of hypercholesterolemia in adults. NPJ Genom Med. 2021;6(1):28. doi: 10.1038/s41525-021-00190-z. [Erratum in: NPJ Genom Med. 2021 Jun 2029;2026(2021):2056] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee C., Rivera-Valerio M., Bangash H., Prokop L., Kullo I.J. New case detection by cascade testing in familial hypercholesterolemia: a systematic review of the literature. Circ Genom Precis Med. 2019;12(11) doi: 10.1161/circgen.119.002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colantonio L.D., Rosenson R.S., Deng L., Monda K.L., Dai Y., Farkouh M.E., et al. Adherence to statin therapy among US adults Between 2007 and 2014. J Am Heart Assoc. 2019;8(1) doi: 10.1161/jaha.118.010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pape G.A., Hunt J.S., Butler K.L., Siemienczuk J., LeBlanc B.H., Gillanders W., et al. Team-based care approach to cholesterol management in diabetes mellitus: two-year cluster randomized controlled trial. Arch Intern Med. 2011;171(16):1480–1486. doi: 10.1001/archinternmed.2011.417. [DOI] [PubMed] [Google Scholar]
- 39.Khera A.V., Won H.H., Peloso G.M., Lawson K.S., Bartz T.M., Deng X., et al. Diagnostic yield of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67(22):2578–2589. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.