Abstract
Emerging evidence suggests that fasting could play a key role in cancer treatment by fostering conditions that limit cancer cells' adaptability, survival, and growth. Fasting could increase the effectiveness of cancer treatments and limit adverse events. Yet, we lack an integrated mechanistic model for how these two complicated systems interact, limiting our ability to understand, prevent, and treat cancer using fasting. Here, we review recent findings at the interface of oncology and fasting metabolism, with an emphasis on human clinical studies of intermittent fasting. We recommend combining prolonged periodic fasting with a standard conventional therapeutic approach to promote cancer‐free survival, treatment efficacy and reduce side effects in cancer patients.
Keywords: cancer, diet, fasting, intermittent fasting, oncology, review
Emerging evidence suggests that fasting could play a key role in cancer treatment by fostering conditions that limit cancer cells' adaptability, survival, and growth. Fasting could increase the effectiveness of cancer treatments and limit adverse events. Yet, we lack an integrated mechanistic model for how these two complicated systems interact, limiting our ability to understand, prevent, and treat cancer using fasting. Here, we review recent findings at the interface of oncology and fasting metabolism, with an emphasis on human clinical studies of intermittent fasting. We recommend combining prolonged periodic fasting with a standard conventional therapeutic approach to promote cancer‐free survival, treatment efficacy, and reduce side effects in cancer patients.
Abbreviations
- FGF21
Fibroblast growth factor 21
- STAT5
Signal transducer and activator of transcription
- IGF1
Insulin‐like growth factor 1
- IGFBP1
Insulin‐like growth factor‐binding protein 1
- NAD+
Nicotinamide adenine dinucleotide
- NAMPT
Nicotinamide Phosphoribosyltransferase
- SIRT3
Sirtuin 3
- SITR4
Sirtuin 4
- ROS
Reactive oxygen species
- PAX5
B cell specific activator protein
- IKZF1
IKAROS Family Zinc Finger 1
- B‐ALL
B cell Acute lymphoblastic leukemia
- DNA
Deoxyribonucleic acid
- CD8+
Cytotoxic T Lymphocytes
- IGFBP1
Insulin‐like growth factor binding protein 1
- SHBG
Sex hormone binding globulin
- RAS
Reticular activating system
- AKT
Protein Kinase B
- mTOR
Mechanistic target of rapamycin
- TKIs
Tyrosine Kinase inhibiters
1. BACKGROUND
Cancer, one of the most dreadful diseases, causes increased morbidity and mortality worldwide regardless of human development. Cancer is characterized by the uncontrolled growth of aberrant cells. It afflicted 19.3 million people and caused almost 10 million people deaths globally in 2020. 1 Surgical tumor removal, radiation, and chemotherapy, together with recently discovered immunotherapy, have become routine treatment protocols for cancer patients as a result of incredible advancements in oncology; yet, patients' overall survival time and prognosis remain unsatisfactory. 1 , 2 Furthermore, patients must endure various side effects when undergoing standard therapy, with the risk of illness recurrence, which affects patients on several levels, including physically, emotionally, cognitively, financially, and socially. Emerging evidence suggests that intermittent fasting might help prevent and treat cancer by boosting the efficacy and tolerance of anticancer drugs and improving cancer patients' quality of life through various adaptability biological mechanisms caused by intermittent fasting. 3 , 4 , 5 , 6 Studies have shown that fasting can benefit a person's general health throughout their entire life by lowering oxidative stress and inflammation, slowing the aging process and increasing longevity, initiating cellular repair mechanisms, and boosting brain and heart function. Till now, human studies of fasting on cancer have only focused on small groups of young individuals for short periods. This review examines the published and continuing clinical trials that have used intermittent fasting to treat cancer patients, focusing on improving cancer outcomes and decreasing adverse events. The biological underpinnings for adopting fasting along with intermittent fasting to prevent and treat cancer are also discussed.
2. FASTING, INTERMITTENT FASTING, AND ITS MECHANISM: FOCUSING ON CANCER
Fasting, a period of voluntary abstention from all foods or specific food products, has a long history steeped in religious traditions. For thousands of years, distinct types of fasting have been advocated and followed by different religions based on their own beliefs and norms. Intermittent fasting is an eating regimen that alternates between a brief period of fasting, with either no food or a considerable calorie restriction, and periods of unrestricted eating, focusing on when you eat rather than what you eat. Fasting on alternate days, short‐term intermittent fasting (16–8 h of daily fasting), prolonged intermittent fasting, entire day fasting with a particular frequency per week, or fasting within a predetermined time frame (i.e., 1–2 days per week of total fasting or meeting up to 25% of daily calorie needs with no dietary restriction on the other days) are all common variations of intermittent fasting. Fluids (without calories) are often ingested in sufficient quantities to fulfill hydration and physiological needs. Intermittent fasting can improve the body's overall function and help alleviate various diseases, such as obesity, diabetes, neurogenerative diseases, and cancer. 4 , 7
Fasting causes alterations in the activity of several metabolic pathways as the body transitions to a mode that generates energy and metabolites predominantly from adipose tissue and, to a lesser extent, muscle. 6 Normal cells are protected from chemotherapeutic assaults by changes in circulating hormones and metabolites, resulting in decreased cell differentiation and activity in the metabolism. Cancer cells may react differently to normal cells, making them more susceptible to chemotherapy and other cancer treatments if they violate the anti‐growth directives required by these fasting environments.
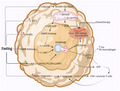
To fulfill the body's fuel demands during fasting, metabolic stress causes insulin levels to drop and glucagon levels to rise, increasing the breakdown of liver glycogen reserves (into glucose) and triglycerides (into glycerol and free fatty acids). As a result, in the long run, fasting benefits the brain, muscle, liver, and adipose tissue through various biological mechanisms (Figure 1). 3 , 6 , 8 The brain progressively adjusts using the ketone bodies in addition to glucose to satisfy its energy requirements, while other tissues use fatty acids for energy. Gluconeogenesis is fueled by ketone bodies created in the liver from fatty acids, fat‐derived glycerol, and amino acids during the ketogenic state. Therefore, glucose levels are maintained at around 70 mg per dL, which is used mainly by the brain. 9 , 10 Histone deacetylases are inhibited by ketone bodies, which may slow tumor development. 11 , 12 Furthermore, β‐hydroxybutyrate, a ketone body, works as an endogenous histone deacetylase inhibitor. This inhibitor inhibits the signals, which protects the body from oxidative stress. 13 Glucocorticoids and adrenaline are also involved in direct metabolic responses to fasting, assisting in maintaining blood sugar levels and increasing lipolysis. 14 FGF21 rises during intermittent fasting, and it plays a vital role in lowering IGF1 levels by inhibiting phosphorylated STAT5 in the liver. 15 In addition, increased insulin‐like growth factor‐binding protein 1 (IGFBP1) binds to circulating IGF1 and inhibits it from interacting with the appropriate cell surface receptor, limiting IGF1 biological activity under intermittent fasting conditions. 6 , 16 IGF1 downregulation is one of the fundamental mechanisms engaged in fasting to promote chemotherapy tolerance and minimize significant adverse effects. Increased IGF‐1 levels appear to be attributed to the colon, prostate colon, and breast cancer due to suppressed apoptosis, boosted cell proliferation, and genetic instability. 17 , 18 Finally, fasting lowers glucose, IFG1, insulin, and circulating leptin levels while raising adiponectin levels and altering the IGF‐1/mTOR signaling pathway. All of these effects are known to affect cancer etiology, resulting in anti‐tumor effects, decreased free radical production, and increased body resistance to stress (Figure 2). 3 , 4 , 5 , 6 In addition, lowered levels of IGF1 and insulin have the potential to protect healthy cells from side effects. 19
FIGURE 1.
Effect of prolonged fasting on different systems of the body on patients. During fasting, the breakdown of liver glycogen reserves (into glucose) and triglycerides (into glycerol and free fatty acids) occurs. The brain progressively adjusts using the ketone bodies in addition to glucose to satisfy its energy requirements, while other tissues use fatty acids for energy. Gluconeogenesis is fueled by ketone bodies created in the liver from fatty acids, fat‐derived glycerol, and amino acids during the ketogenic phase of fasting.
FIGURE 2.
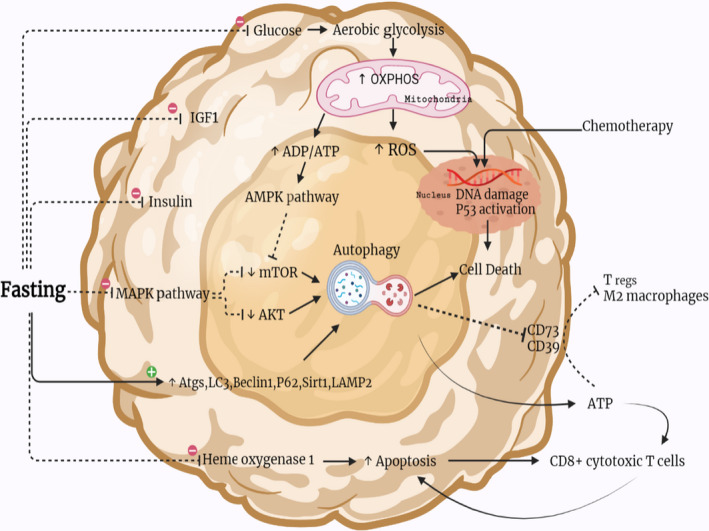
Mechanisms of fasting on cancer cells. Fasting suppresses glucose, IGF1, insulin, the MAPK pathway, and heme oxygenase 1 while increasing many autophagy‐regulating components (Atgs, LC3, Beclin1, p62, Sirt1, and LAMP2). Fasting causes cancer cells to release oxidative phosphorylation (OXPHOS) through aerobic glycolysis, which leads to an increase in reactive oxygen species (ROS), p53 activation, DNA damage, and cell death in response to chemotherapy. Fasting activates the autophagic process, which induces cell death through a variety of mechanisms. It also suppresses CD73 and CD39 expression and causes extracellular ATP accumulation, which inhibits Treg cells and the M2 phenotype while activating CD8+ cytotoxic T cells. Fasting also inhibits hemoxygenase 1. It accelerates cellular death and activates CD8+ cytotoxic T lymphocytes, which drive the apoptosis cycle once again.
Fasting has been demonstrated to have significant restorative benefits through molecular mechanisms, such as enhanced autophagy and sirtuin activity (Figure 2). Autophagy is a naturally preserved process that uses a lysosome‐dependent controlled mechanism to deconstruct superfluous or malfunctioning cellular components, allowing for orderly breakdown and recycling. 20 , 21 Both procarcinogenic and anticarcinogenic mechanisms have been connected to autophagy, which regulates oncogenes and tumor suppressor genes and has a complicated relationship with cancer. It has several functions in healthy cells that all work together to prevent malignant transformation, including maintaining adequate energy and the oxidative metabolism, eliminating highly unsafe and mutagenic and carcinogenic chemicals, battling against cancer‐causing infections, and maintaining healthy stem cellular organelles. 21 , 22 , 23 Two protein kinases, AMPK and mTOR, inhibit ULK1 and ULK2, which control autophagy. 21 , 22 , 23 Furthermore, autophagy‐related genes (Atgs), Beclin‐1, LC3, P62, Sirt1, LAMP2, and the class III PI3K pathway all play essential roles in autophagy regulation (Figure 2). 21 , 22 , 23 Autophagy might also alter cancer treatments since it is known that autophagy makes cancer cells more sensitive to chemotherapy. 24 Enhanced autophagy, in contrast, may play a role in cancer therapy resistance. 23 It is unclear if intermittent fasting boosts autophagic processes in people, and further study is needed to better understand the advantages and risks of fasting‐induced autophagy in cancer patients. Moreover, in model organisms, Sirtuins, which function as NAD + ‐dependent deacetylases, have protective and lifespan‐extending benefits. 25 , 26 During nutrient deprivation, amplified intracellular NAD+ levels promote the mitochondrial activity of sirtuins, especially SIRT3 and SIRT4, and thus safeguard the cells from chemotherapeutics. 25 , 26 Overall, fasting causes normal healthy cells to adopt a slower cell division process, protecting them from toxic shocks induced by anticancer medications while sensitizing cancer cells to these treatments.
By lowering glucose intake and boosting fatty acid oxidation, fasting can induce a transition from aerobic glycolysis to mitochondrial oxidative phosphorylation in cancerous cells, resulting in increased ROS. Thus, enhanced activity of mitochondrial respiratory and cellular redox potential might be reduced due to a decrease in the glutathione synthesis resulting from the pentose phosphate and glycolysis mechanism (Figure 2). 5 , 6 , 8 , 27 The combined impact of increased ROS and decreased antioxidant defense increases oxidative stress in cancerous cells and enhances chemotherapeutic action. Moreover, fasting induces SUMO2‐ and/or SUMO3‐mediated REV1 alteration, resulting in enhanced p53‐mediated transcription of pro‐apoptotic genes and, eventually, death of cancerous cells. 3 , 5 , 6 , 28 Fasting also improves the efficacy of TKIs to limit cancerous cell growth and death by enhancing MAPK signaling inhibition. 3 , 28 , 29 Finally, via the protein PR/SET domain 1 (PRDM1), fasting might regulate the leptin receptor, suppressing and reversing the course of B cell and T cell acute lymphoblastic leukemia. 3 , 5 , 6 , 16 , 29
As per differential stress sensitization (DSS) assertion, many types of cancer cells cannot implement adjustments that would allow them to survive in the nutrient‐deficient hazardous environment created by fasting and chemotherapy; thus, incorporating fasting with other treatments is far more promising. 29 Periodic fasting cycles have the potential to limit the growth of several cancerous cells and make cancerous cells more susceptible to chemotherapeutics, radiation, and tyrosine kinase inhibitors (TKIs). 5 , 16 , 29 Interestingly, the transcription factors PAX5 and IKZF1 impose a prolonged limitation in glucose and energy sources on B cell progenitors. 30 Genetic encoding mutations of PAX5 and IKZF1 are seen in more than two‐thirds of pre‐B cell ALL cases, which enhance glucose absorption and the production of ATP. Reconstituting PAX5 and IKZF1 in pre‐B‐ALL cells, in contrast, results in an energy crisis and cell death. Altogether, it is observed that ALL might have more sensitivity towards the nutritional and energy limitation enforced by the fasting state, thus making it an excellent clinical candidate for determining the efficiency rate of fasting.
Fasting has been shown to have a direct impact on the gut microbial community's constitution, function, and interaction with the host, which is the complex and diverse microbial population that lives in the intestine. The interaction between the host and the gut microbiota results in a homeostatic balance of bacteria that is advantageous to the host and boosts immunity. Dysbiosis has been linked to a variety of illnesses, including cancer. The gut microbiota is undoubtedly one of the key mechanisms through which fasting produces metabolic or cellular benefits (inducing beige formation). 31 Fasting, for example, caused a change in the gut microbiota composition, boosting Firmicutes while lowering most other phyla and enhancing short‐chain fatty acid synthesis compared to control animals fed ad libitum. Fasting also reduces the number of potentially harmful Proteobacteria while boosting the levels of Akkermansia muciniphila. 32 Donohoe et al. (2012) discovered that when butyrate metabolism is disrupted in colon cancer cells, it accumulates in the nucleus, enhancing its action as a class I and II histone deacetylase inhibitor (HDACi), which inhibits cell development and causes cell death. 33 Anti‐PD1 treatment is effective in patients with metastatic melanoma and high levels of A. muciniphila, suggesting that microbiota may play a role in immunotherapy as well. 34 Fasting generates an anti‐Warburg effect in colon cancer models, which increases oxygen demand but decreases ATP production, indicating an increase in mitochondrial uncoupling. 35
Cancerous cell types are likely to develop resilience by avoiding the cellular alterations induced by fasting, and the metabolic heterogeneity that characterizes various tumors adds to this probability. 36 As a result, identifying the cancers most sensitive to these dietary regimens through biomarkers will be a significant focus in future days to come. Identifying which cancers are the ideal prospects for fasting benefits will be a critical future problem. Even with cancerous cells that are less sensitive to fasting, it might be feasible to discover the causes of resilience and assist with medication that can reverse the resilience. Fasting, in contrast, has just a few times led in the development of resilience when combined with standard therapies in cancer preclinical studies. Resilience to fasting combined with chemotherapy is even more rare in vitro research studies, thus stressing the necessity of discovering therapies that result in significant cytotoxic actions on cancerous cells with negligible adverse effects on normal healthy cells. 3 , 5 , 6 , 24 , 37 , 38
3. INTERMITTENT FASTING IN CLINICAL STUDIES OF CANCER
Clinical studies have been carried out to measure the relevance of various modalities of intermittent fasting on metabolic and hormonal endpoints correlated to cancer development and prognosis, given their practicality and the beneficial weight reduction effect in overweight/obese people (Tables 1 and 2). Intermittent fasting has been demonstrated to improve cancer risk variables in several short‐term randomized clinical studies, including lowered levels of glucose, insulin, leptin, and higher adiponectin; which are linked to cancer etiology. 39 Long‐term intermittent fasting, however, has failed to show a substantial increase in the sensitiveness of insulin or C reactive protein. 40 , 41 , 42 Time‐restricted feeding has been reported to cause substantial alterations in biochemical indicators linked to weight, including insulin sensitivity and oxidative stress in small and underpowered investigations. 43 , 44 Nonetheless, in one major study (including 116 obesity diagnosed patients) on time‐limited feeding, no significant change in weight, fasting insulin, or fasting glucose level was reported. 45 Furthermore, evidence shows that, unlike rats, people require weight loss to optimize metabolic health. Whether weight reduction has a causal function in lowering cancer risk and improving prognosis without considerable food composition changes remains a critical but unresolved subject.
TABLE 1.
Intermittent fasting on cancer patients
| Author and Year | Type of cancer | Enrolled patient details | Fasting regime | Results |
|---|---|---|---|---|
| Schreck et al. 46 | 2–4 grade astrocytoma | A total of 25 patients were recruited in an 8‐week glioma Atkins diet. | Two fasting days (calories <20% calculated estimated needs) interleaved between 5 modified Atkins diet days (net carbohydrates ≤20 g/d) each week. |
|
| de Groot et al. 47 | HER2‐negative breast cancer, Stage II/III | In total, 131 individuals received neoadjuvant chemotherapy. | Fasting mimicking diet 3 days before and during neoadjuvant chemotherapy |
|
| Zorn et al. 48 | Gynecological cancers at any stage | Thirty patients receiving (neo)‐adjuvant chemotherapy for a minimum of four cycles of the same treatment | Ninety‐six‐hour fasting for half of scheduled chemotherapy cycles, followed by a regular diet for the remaining cycles |
|
| Bauersfeld et al. 49 | Breast and ovarian cancer at any stage | A total of 34 individuals are undergoing chemotherapy. | Patients were randomly assigned to either a short‐term fasting diet followed by a normal caloric diet or a normal caloric diet followed by a short‐term fasting diet in the first half of chemotherapy. |
|
| Marinac et al. 50 | Breast cancer at an early stage | At baseline, year 1 and year 4, 2413 patients completed 24‐h dietary recalls. | Dietary recalls were utilized to calculate the length of time spent fasting at night. |
|
| Dorff et al. 51 | Breast, ovarian, and uterine cancer at any stage | Twenty individuals are being treated with platinum‐based chemotherapy. | Fasting for 24 hours, 48 hours, or 72 hours before chemotherapy |
|
| De Groot et al. 52 | HER2‐negative breast cancer II/III | Thirteen patients are being treated with (neo)‐adjuvant chemotherapy. | Before and after chemotherapy, patients were randomly assigned to either a 24‐hour fast or a diet that followed appropriate dietary standards. |
|
| Badar et al. 53 | Breast, non‐Hodgkin lymphoma, acute myeloid leukemia, nasopharynx, ovarian, and colon | Chemotherapy is given to 11 patients. | During Ramadan, patients received chemotherapy. |
|
| Safdie et al. 32 | Breast, prostate, ovarian, uterine, non‐small cell lung, and esophagus | Ten patients who are undergoing chemotherapy | Fasting was required before (48–140 hours) and/or after (5–56 hour) treatment. |
|
TABLE 2.
Clinical trials of cancer using intermittent fasting
| Clinical trial | Study title | Trial phase/total participants | Status | Primary outcome measure | Remarks |
|---|---|---|---|---|---|
| NCT05023967 | Metformin and Nightly Fasting in Women with Early Breast Cancer | Phase 2/120 | Not yet recruiting |
|
Age: 18 years and older Gender: Female Study start: April 1, 2022 Study completion: November 30, 2024 |
| NCT04626843 | Intermittent Fasting and CLL/SLL | Phase 1/20 | Recruiting |
|
Age: 19–85 years Gender: All Study start: February 3, 2021 Study completion: December 2021 |
| NCT04560439 | Diabetes Prevention Program (METFIT) in Reducing Insulin Resistance in Stage I‐III Breast Cancer Survivors | NA/25 | Recruiting |
|
Age: 18–75 years Gender: Female Study start: June 15, 2022 Study completion: June 15, 2023 |
| NCT02710721 | Fasting and Nutritional Therapy in Patients with Advanced Metastatic Prostate Cancer | NA/60 | Active, not recruiting |
|
Age: 25–89 years Gender: Male Study start: April 2016 Study completion: December 2021 |
| NCT05114798 | Time‐restricted Eating Versus Daily Continuous Calorie Restriction on Body Weight and Colorectal Cancer Risk Markers | NA/255 | Not yet recruiting |
|
Age: 25–89 years Gender: All Study start: April 2022 Study completion: September 2026 |
| NCT03523377 | Overnight Fasting After Completion of Therapy: The OnFACT Study | NA/40 | Recruiting |
|
Age: 18 years and older Years Gender: All Study start: April 27, 2018 Study completion: April 2024 |
| NCT04387084 | Short‐term Fasting Prior to PD‐1/PD‐L1 Inhibitor Therapy for of Advanced or Metastatic Skin malignancy | Phase 1 /16 | Recruiting |
|
Age: 18 years and older Gender: All Study start: August 12, 2020 Study Completion: August 12, 2023 |
| NCT02286167 | Glioma Modified Atkins‐based Diet in Patients with Glioblastoma | NA/25 | Completed |
|
Age: 18 years and older Gender: All Study start: November 2014 Study completion: July 12, 2019 |
| NCT03151161 | Intermittent and Maintenance of Icotinib in Combination with Pemetrexed/Carboplatin Compared With Icotinib Single Drug in IIIb/IV Non‐Small Cell Lung Cancer With Epidermal Growth Factor Receptor (EGFR) Mutation | Phase 2/118 | Unknown | Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 |
Age: 18–75 years Gender: All Study Start: December 2015 Study completion: May 2019 |
| NCT02066038 | Intermittent and Maintenance of Erlotinib in Combination With Pemetrexed/Carboplatin in IIIb/IV Non Small Cell Lung Cancer With Epidermal Growth Factor Receptor (EGFR) Mutation | Phase 2/60 | Unknown | Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 |
Age: 18–75 years Gender: All Study Start: January 2014 Study completion: March 2016 |
| NCT04691999 | The Effect of Intermittent Fasting on Body Composition in Women With Breast Cancer | NA/0 | Withdrawn |
|
Age: 20–70 years Gender: Female Study start: December 2, 2021 Study completion: August 2023 |
| NCT04288336 | Daily, Long‐Term Intermittent Fasting for the Prevention of PSA‐Recurrence in Patients with Localized Prostate Cancer After Radical Prostatectomy | Early Phase 1/25 | Recruiting |
|
Age: 18 years and older Gender: Male Study start: January 8, 2020 Study completion: June 1, 2022 |
| NCT03162289 | Intermittent Fasting Accompanying Chemotherapy in Gynecological Cancers | NA/150 | Recruiting |
|
Age: 18–75 years Gender: Female Study start: May 10, 2017 Study completion: June 10, 2022 |
| NCT05083416 | Effect of Prolonged Nightly Fasting on Immunotherapy Outcomes in HNSCC ‐ Role of Gut Microbiome | NA/52 | Recruiting |
|
Age: 18 years and older Gender: All Study start: September 28, 2021 Study completion: August 2022 |
| NCT04708860 | Feasibility of Fasting & Exercise in Pts With HR+ MBC | NA/30 | Recruiting |
|
Age: 18 years and older Gender: Female Study start: January 15, 2021 Study completion: July 31, 2023 |
| NCT04557540 | Weight Loss Interventions for Black Adults of Faith | NA/60 | Recruiting |
|
Age: 18 years and older Gender: All Study start: August 25, 2020 Study completion: September 15, 2023 |
| NCT04330339 | Prolonged Nightly Fasting in Breast Cancer Survivors | NA/40 | Active, not recruiting |
|
Age: 18 years and older Gender: female Study start: July 24,2020 Study completion: August 31, 2021 |
| NCT04345978 | Effects of Fasting Strategies on Postoperative Recovery and Long‐term Prognosis in Patients with Colorectal Cancer | NA/2400 | Active, not recruiting |
|
Age: 20–70 years Gender: All Study start: January 1,2020 Study completion: September 30,2023 |
| NCT04461938 | Characterization of Metabolic Changes in the Glioma Tumor Tissue Induced by Transient Fasting (ERGO3) | NA/15 | Recruiting |
|
Age: 18 years and older Gender: All Study start: August 19, 2020 Study completion: March 2022 |
| NCT03700437 | Fasting‐mimicking Diet With Chemo‐immunotherapy in Non‐small Cell Lung Cancer (NSCLC) | NA/10 | Recruiting |
|
Age: 18–70 years Gender: All Study start: November 2, 2018 Study completion: December 2024 |
A few studies have used intermittent fasting in patients as one of the regimens (Tables 1 and 2). 32 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 One recently published clinical trial of glioma patients observed a significant decrease in HbA1c, insulin, and fat, while lean body weight and ketone bodies (in the brain) were increased with a well‐tolerated ketogenic intermittent fasting diet. 46 Another study on patients with a high risk of breast cancer reported that 1 month of intermittent resulted in a 4.8% weight loss, 8% fat loss, and an improvement in insulin resistance. 54 Furthermore, a study that enrolled stage II breast cancer patients suggested that the fasting regime is well tolerated, with a lower rate of withdrawals from adverse events. 52 In addition, fasting for a brief time was observed to minimize hematologic damage in female cancer patients receiving chemotherapy, with the fasting group having considerably increased erythrocyte and thrombocyte levels following treatment. Following chemotherapeutic treatments, patients in the control group exhibited higher DNA damage indicators and lowered circulating IGF1 levels than those who had fasted and had lower IGF1 levels. 52 Fasting may have aided in the healing process of chemotherapy‐induced DNA damage. In another clinical study of diverse tumor types, fasting for 24, 48, or 72 hours before treatment was determined to be possible and safe, with only minor adverse effects, such as weariness, headache, and dizziness. 51 Patients fasting for 48–72 h before the chemotherapeutic treatment had a nonsignificant tendency towards a lower incidence of neutropenia and neuropathy than those who fasted for 24 h before treatment. 51 In peripheral blood mononuclear cells, markers of DNA damage rose in all groups, although less so in the extended fasting group.
Despite initial concerns about weight loss in cancer patients receiving chemotherapy, no trials have revealed substantial loss of body weight (lean body mass) or malnutrition due to fasting. 3 Fasting was deemed safe and tolerable in a study of patients with diverse cancers undergoing treatment during Ramadan. 53 In a case series, fasting before and after chemotherapy was deemed secure and well‐received in 10 patients with numerous types of cancer. 32 In Bauersfeld et al., cancer patients were assigned to either a short‐term fasting diet followed by a standard caloric diet or a standard caloric diet followed by a short‐term fasting diet in a clinical trial of patients undergoing chemotherapy. 49 Those patients fasted for 36 h before treatment and 24 h thereafter, having a total of 350 calories per day. Within 8 days of chemotherapy, no substantial weight loss was recorded, although there was an improvement in quality of life and weariness. Such benefits, in contrast, were not seen while having a regular diet.
Although clinical evidence is limited, several studies are under way in the advanced cancer scenario. For instance, intermittent fasting clinical trials for metastatic prostate cancer (NCT02710721), early breast cancer patients (NCT05023967) and metastatic breast cancer (NCT04708860), gynecological cancers (NCT03162289), breast cancer survivors (NCT04330339, NCT04691999, NCT04560439), head and neck cancer (NCT05083416), prostate cancer survivors (NCT04288336), colon cancer survivors (NCT04345978, NCT05114798), obesity‐related malignant neoplasm, chronic lymphocytic leukemia/small lymphocytic lymphoma survivors (NCT04626843), gliomas survivors (NCT04461938, NCT02286167), melanoma (NCT04387084), childhood cancer survivors (NCT03523377), and non‐small cell lung cancer (NCT03700437, NCT03151161, NCT02066038) are currently underway (Table 2). However, the information on the nutritional interventions carried out in clinical trials is incomplete/inconsistent, including nutrition guidelines and supervision, meal quality, and food items included in the diets.
4. FASTING, INTERMITTENT FASTING, AND CANCER PREVENTION/TREATMENT
Emerging evidence suggests that intermittent fasting might lower cancer risk in people by creating healthy eating habits and lifestyle changes. However, a well‐tolerated fasting regimen with excellent clinical efficacy and few adverse effects has yet to be found and adopted in cancer patients. Calorie restriction, one type of fasting, has been shown in rats to be effective in lowering tumor development, including breast malignancies and lymphomas. 5 , 7 , 38 , 55 Recently, a 30% calorie‐restricted diet resulted in a 50% reduction in spontaneous cancer incidence in rhesus monkeys with a nearly identical genome to humans compared to ad libitum‐fed animals. 56 , 57 Human trials have demonstrated similar results. After a median 20 follow up, Carlsson et al. discovered a 29% reduction in cancer incidence and a 20% reduction in cancer death. 58 The lower cancer incidence was linked to a lower risk of malignancies, especially cancers related to women, with a more significant benefit in individuals with higher baseline blood insulin levels who underwent bariatric surgery. 58 , 59
The preventive benefits of a fasting, calorie‐restricted diet on cancer formation and progression are due to several interconnected processes. Energy restriction resulted in major metabolic and hormonal adaptations referenced to lower cancer risk, including lower insulin levels and enhanced sensitiveness of insulin sensitivity, higher levels of IGFBP1 and SHBG, lower levels of testosterone and estrogen, and lower oxidative and inflammatory processes. 3 , 5 , 6 , 9 , 16 Long‐term calorie restriction diets, a form of fasting, stimulate repair in the DNA, autophagy, antioxidant, and heat‐shock protein chaperone pathways at the molecular level while reducing cell growth and senescence indicators. 3 , 5 , 6 , 8 , 21 , 50 Reduced synthesis of various growth factors and reactive oxygen and improved anticancer immunity are other processes. 55
Fasting for more than 1 day regularly might be beneficial to health through shielding healthy normal cells from the toxicity of chemotherapy and radiation. Cancerous cells, but not healthy normal surrounding cells, may be prevented from converting into a stress resilience state in fasting by genetic abnormalities such as RAS, AKT, and mTOR. 5 , 22 , 28 , 29 Fasting could preserve normal tissue against the harmful side effects of chemotherapeutic drugs while enhancing cancerous cell therapeutic outcomes, using a process known as the differential stress response. 24 , 29 , 37 Furthermore, cancerous cells have an aberrant metabolic activity predominantly based on glycolysis, resulting in greater glucose absorption and glucose degradation to lactate, a phenomenon known as the Warburg effect. 27 , 60 As a result, glucose deprivation may sensitize cancerous cells to the harmful side effects of chemotherapeutic drugs and increase apoptosis, as shown in extended fasting and ketogenic diets. 18 , 24 , 29 , 37 , 38 A decrease in IGF‐1 due to prolonged fasting enhanced cancer cells' sensitization to chemotherapy, promoting hematopoietic stem cell‐based regeneration and reversing immunosuppression action. 61 , 62 This long‐term fasting practice was correlated with low insulin and IGF‐1 levels, as well as a reduction in headaches, weakness, and stomatitis. Additionally, during the fasting periods, the overall toxicity score was dramatically lowered, and there were much fewer treatment delays.
The risk that this dietary regimen might trigger malnutrition, sarcopenia, and cachexia in susceptible or fragile individuals is a major issue in the studies of periodic fasting in cancer. 63 However, in the clinical investigations of fasting in conjunction with chemotherapeutic drugs published so far, no cases of severe weight loss or malnutrition have been documented with a discernible effect. Nonetheless, clinical trials include periodic anorexia and nutritional status checks, and any nutritional deficiency that occurs as a result of fasting is quickly rectified.
5. CONCLUSIONS
Because intermittent fasting efficiently decreases body mass and has a tremendous impact on cancerous pathophysiology through its associated metabolic, biochemical, and immunologic abnormalities, research into the function of intermittent fasting in the prevention and treatment of cancer is continuing. Human studies examining the effects of intermittent fasting on insulin‐stimulated growth and other relevant hormonal and inflammatory indicators of carcinogenesis, in contrast, appear to be clinically unimportant thus far. Due to limited clinical research, the effects of intermittent fasting on clinically relevant cancer‐related effects remain unclear. Despite the information gaps and problems involved with modifying human dietary habits, intermittent fasting remains an appealing modality to investigate in a research environment since it has few side effects, is inexpensive, and is likely tumor agnostic.
Several studies posit that prolonged periodic fasting may be acceptable, viable, and able to potentiate the chemoradiotherapy and TKIs, triggering anticancer immunity and curbing chemo‐related hazards and tumorigenesis in certain cancer patients undergoing chemotherapy. In particular, extended periodic fasting would presumably have slight effectiveness against existing cancers if it used alone without any adjunct treatments. Fasting, in fact, has a comparable effect on the course of a variety of malignancies in mice as chemotherapy. However, when used alone, they seldom achieve the same level of success as when used in conjunction with cancer medications, resulting in cancer‐free survival. As a corollary, we advocate for the adoption of prolonged periodic fasting and conventional therapies.
There is presently little evidence that intermittent fasting, without any reduction in body weight and proper balanced diet and exercise, can enhance cancer outcomes. Fasting's risks and benefits must be discussed with patients, just like any other prospective treatment option. Patients who are frail or malnourished or who are in danger of malnutrition should not be included in fasting clinical trials, and patients' overall physical and mental health ought to be closely monitored during the clinical research studies. The advantages of fasting will be maximized while patients are protected from malnutrition with this multimodal dietary strategy. Before suggesting fasting in the care of cancer patients, further research is needed to see if and how patients would benefit from fasting in the long run. Overall, evidence suggests that if done properly under the supervision of a dietician/physician, intermittent fasting is not hazardous to cancer patients physically or emotionally and, hence, may be added to standard anticancer therapies to maximize their benefit while minimizing adverse effects.
AUTHOR CONTRIBUTIONS
Sagun Tiwari and Namrata Sapkota contributed substantially to the conception and design of the study. Sagun Tiwari drafted and revised the manuscript. Namrata Sapkota and Zhenxiang Han provided critical revision of the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ETHICAL APPROVAL
N/A
ACKNOWLEDGMENTS
N/A
Tiwari S, Sapkota N, Han Z. Effect of fasting on cancer: A narrative review of scientific evidence. Cancer Sci. 2022;113:3291‐3302. doi: 10.1111/cas.15492
Sagun Tiwari and Namrata Sapkota contributed equally.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Miranda‐Filho A, Bray F, Charvat H, Rajaraman S, Soerjomataram I. The world cancer patient population (WCPP): an updated standard for international comparisons of population‐based survival. Cancer Epidemiol. 2020;69:101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. 2018;18:707‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clifton KK, Ma CX, Fontana L, Peterson LL. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J Clin. 2021;71:527‐546. [DOI] [PubMed] [Google Scholar]
- 6. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371‐393. [DOI] [PubMed] [Google Scholar]
- 7. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381:2541‐2551. [DOI] [PubMed] [Google Scholar]
- 8. Antoni R, Johnston KL, Collins AL, Robertson MD. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc. 2017;76:361‐368. [DOI] [PubMed] [Google Scholar]
- 9. Hall JE, Hall ME. Guyton and Hall Textbook of Medical Physiology e‐Book. Elsevier Health Sciences; 2020. [Google Scholar]
- 10. Nelson DL, Lehninger AL, Cox MM. Lehninger Principles of Biochemistry. Macmillan; 2008. [Google Scholar]
- 11. Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends in Endocrinology & Metabolism. 2014;25:42‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmermann S, Kiefer F, Prudenziati M, et al. Reduced body size and decreased intestinal tumor rates in HDAC2‐mutant mice. Cancer Res. 2007;67:9047‐9054. [DOI] [PubMed] [Google Scholar]
- 13. Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by β‐hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell JE, Peckett AJ, D'Souza AM, Hawke TJ, Riddell MC. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am J Physiol Cell Physiol. 2011;300:C198‐C209. [DOI] [PubMed] [Google Scholar]
- 15. Byun S, Seok S, Kim YC, et al. Fasting‐induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat Commun. 2020;11:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salvadori G, Mirisola MG, Longo VD. Intermittent and periodic fasting, hormones, and cancer prevention. Cancers (Basel). 2021;13:4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rigiracciolo DC, Nohata N, Lappano R, et al. IGF‐1/IGF‐1R/FAK/YAP transduction signaling prompts growth effects in triple‐negative breast cancer (TNBC) cells. Cells. 2020;9:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bowers LW, Rossi EL, O'Flanagan CH, deGraffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance‐cancer link. Front Endocrinol. 2015;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564‐1576. [DOI] [PubMed] [Google Scholar]
- 21. Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting autophagy in cancer. Cancer. 2018;124:3307‐3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, He S, Ma B. Autophagy and autophagy‐related proteins in cancer. Mol Cancer. 2020;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 2019;9:1167‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadeghian M, Rahmani S, Khalesi S, Hejazi E. A review of fasting effects on the response of cancer to chemotherapy. Clin Nutr. 2021;40:1669‐1681. [DOI] [PubMed] [Google Scholar]
- 25. Aventaggiato M, Vernucci E, Barreca F, Russo MA, Tafani M. Sirtuins' control of autophagy and mitophagy in cancer. Pharmacol Ther. 2021;221:107748. [DOI] [PubMed] [Google Scholar]
- 26. Singh CK, Chhabra G, Ndiaye MA, Garcia‐Peterson LM, Mack NJ, Ahmad N. The role of Sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28:643‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Icard P, Ollivier L, Forgez P, et al. Perspective: do fasting, caloric restriction, and diets increase sensitivity to radiotherapy? A literature review. Adv Nutr. 2020;11:1089‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y, Karsli‐Uzunbas G, Poillet‐Perez L, et al. Autophagy promotes mammalian survival by suppressing oxidative stress and p53. Genes Dev. 2020;34:688‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Biase S, Longo VD. Fasting‐induced differential stress sensitization in cancer treatment. Mol Cell Oncol. 2016;3:e1117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan LN, Chen Z, Braas D, et al. Metabolic gatekeeper function of B‐lymphoid transcription factors. Nature. 2017;542:479‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li G, Xie C, Lu S, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26:672‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Safdie FM, Dorff T, Quinn D, et al. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY). 2009;1:988‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate‐mediated histone acetylation and cell proliferation. Mol Cell. 2012;48:612‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti–PD‐1 efficacy in metastatic melanoma patients. Science. 2018;359:104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bianchi G, Martella R, Ravera S, et al. Fasting induces anti‐Warburg effect that increases respiration but reduces ATP‐synthesis to promote apoptosis in colon cancer models. Oncotarget. 2015;6:11806‐11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strickaert A, Saiselet M, Dom G, et al. Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene. 2017;36:2637‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee C, Raffaghello L, Brandhorst S, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012; 4: 124ra127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gabel K, Cares K, Varady K, Gadi V, Tussing‐Humphreys L. Current evidence and directions for intermittent fasting during cancer chemotherapy. Adv Nutr. 2021;13:667‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho Y, Hong N, Kim KW, et al. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta‐analysis. J Clin Med. 2019;8:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sundfør TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1‐year trial. Nutr Metab Cardiovasc Dis. 2018;28:698‐706. [DOI] [PubMed] [Google Scholar]
- 41. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate‐day fasting on weight loss, weight maintenance, and Cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schübel R, Nattenmüller J, Sookthai D, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. 2018;108:933‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hutchison AT, Regmi P, Manoogian ENC, et al. Time‐restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27:724‐732. [DOI] [PubMed] [Google Scholar]
- 44. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time‐restricted feeding improves 24‐hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lowe DA, Wu N, Rohdin‐Bibby L, et al. Effects of time‐restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180:1491‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schreck KC, Hsu F‐C, Berrington A, et al. Feasibility and biological activity of a ketogenic/intermittent‐fasting diet in patients with glioma. Neurology. 2021;97:e953‐e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Groot S, Lugtenberg RT, Cohen D, et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat Commun. 2020;11:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zorn S, Ehret J, Schäuble R, et al. Impact of modified short‐term fasting and its combination with a fasting supportive diet during chemotherapy on the incidence and severity of chemotherapy‐induced toxicities in cancer patients ‐ a controlled cross‐over pilot study. BMC Cancer. 2020;20:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bauersfeld SP, Kessler CS, Wischnewsky M, et al. The effects of short‐term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross‐over pilot study. BMC Cancer. 2018;18:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marinac CR, Nelson SH, Breen CI, et al. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016;2:1049‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dorff TB, Groshen S, Garcia A, et al. Safety and feasibility of fasting in combination with platinum‐based chemotherapy. BMC Cancer. 2016;16:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Groot S, Vreeswijk MP, Welters MJ, et al. The effects of short‐term fasting on tolerance to (neo) adjuvant chemotherapy in HER2‐negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Badar T, Ismail A, AlShanqeeti A. Safety and feasability of Muslim fasting while receiving chemotherapy. IOSR Journal of Pharmacy. 2014;4:15‐20. [Google Scholar]
- 54. Harvie MN, Sims AH, Pegington M, et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res. 2016;18:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31:89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med. 2020;383:1535‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anveden Å, Taube M, Peltonen M, et al. Long‐term incidence of female‐specific cancer after bariatric surgery or usual care in the Swedish obese subjects study. Gynecol Oncol. 2017;145:224‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee C, Safdie FM, Raffaghello L, et al. Reduced levels of IGF‐I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheng CW, Adams GB, Perin L, et al. Prolonged fasting reduces IGF‐1/PKA to promote hematopoietic‐stem‐cell‐based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer‐related malnutrition. Clin Nutr. 2017;36:1187‐1196. [DOI] [PubMed] [Google Scholar]


