Abstract
In many microorganisms the first step for alkane degradation is the terminal oxidation of the molecule by an alkane hydroxylase. We report the characterization of a gene coding for an alkane hydroxylase in a Burkholderia cepacia strain isolated from an oil-contaminated site. The protein encoded showed similarity to other known or predicted bacterial alkane hydroxylases, although it clustered on a separate branch together with the predicted alkane hydroxylase of a Mycobacterium tuberculosis strain. Introduction of the cloned B. cepacia gene into an alkane hydroxylase knockout mutant of Pseudomonas fluorescens CHAO restored its ability to grow on alkanes, which confirms that the gene analyzed encodes a functional alkane hydroxylase. The gene, which was named alkB, is not linked to other genes of the alkane oxidation pathway. Its promoter was identified, and its expression was analyzed under different growth conditions. Transcription was induced by alkanes of chain lengths containing 12 to at least 30 carbon atoms as well as by alkanols. Although the gene was efficiently expressed during exponential growth, transcription increased about fivefold when cells approached stationary phase, a characteristic not shared by the few alkane degraders whose regulation has been studied. Expression of the alkB gene was under carbon catabolite repression when cells were cultured in the presence of several organic acids and sugars or in a complex (rich) medium. The catabolic repression process showed several characteristics that are clearly different from what has been observed in other alkane degradation pathways.
Alkanes are major components of crude oil and are also present in many organisms, such as green plants (43, 54). It has long been recognized that many microorganisms can use medium- or long-chain n-alkanes as sources of carbon and energy (27), which has stimulated many studies on the usefulness of these organisms in the bioremediation of oil spills and contaminated sites (reviewed in references 2 and 44). In addition, the enzymes involved in alkane degradation have proven to be useful and versatile biocatalysts, opening the possibility of their use in the industrial production of fine chemicals (16, 57, 59).
Alkane degradation by bacteria usually occurs through the sequential oxidation of one or both terminal methyl groups of the molecule, first to an alcohol, then to an aldehyde, and finally to a fatty acid, which is assimilated through the β-oxidation pathway (reviewed in references 7 and 43). The best-characterized alkane degradation pathway is that encoded by the OCT plasmid of Pseudomonas putida (Pseudomonas oleovorans) GPo1 (reviewed in references 56 and 58). In this case, initial oxidation is performed by an alkane monooxygenase system composed of a membrane-bound nonheme iron monooxygenase (a hydroxylase) and two soluble proteins, rubredoxin and rubredoxin reductase, which act as electron carriers between NADH and the hydroxylase (40). The study of alkane hydroxylase diversity could provide useful information about the mechanism of action and about the regions involved in substrate specificity. Sequencing of small PCR products from several alkane-degrading bacteria suggested that alkane hydroxylases related to that of P. putida GPo1 are widely distributed (49). However, only a few bacterial alkane hydroxylases have been cloned and sequenced in addition to that of strain GPo1, namely those of P. putida P1 (49), Stenotrophomonas maltophilia (28), and some Acinetobacter spp. strains (41, 49, 53).
Regulation of the expression of alkane degradation enzymes has received much less attention. In the case of P. putida GPo1, the expression of alkane degradation genes is controlled by the AlkS protein (39), a transcriptional regulator that controls its own levels through a positive feedback mechanism (8). Expression of the pathway is also modulated by catabolite repression, depending on the carbon source being used (8, 20, 50, 62). In the case of Acinetobacter sp. ADP1, expression of the alkane hydroxylase gene is controlled by the transcriptional activator AlkR, a protein unrelated to AlkS (42). In Acinetobacter sp. strain M-1, which has two alkane hydroxylases of different substrate specificities, two regulators have been predicted (53). To our knowledge, regulation has not been studied in other alkane degradation pathways.
The isolation and characterization of a number of bacterial strains which can grow at the expense of residues obtained as end products of crude oil processing has recently been described (63). These residues show a considerable toxicity because they contain large amounts of high-molecular-mass polyaromatic compounds and heavy metals. The isolated strains did not degrade the polyaromatic compounds but rather degraded the high-molecular-mass alkanes present in the residue. We describe here the characterization of a gene coding for an alkane hydroxylase in one of these strains, Burkholderia cepacia RR10. Strain RR10 is interesting because it can grow at the expense of alkanes containing 12 to at least 30 carbon atoms (63), a range that is broader than that reported for most bacterial isolates (43, 58). The alkane hydroxylase gene, which was named alkB, is related to that of P. putida GPo1. Its promoter was identified, and its expression was analyzed under different growth conditions. The regulation pattern showed both differences and similarities to that of other alkane hydroxylases characterized so far.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used are described in Table 1. Plasmid pRR10 derives from pGEM7-Zf(+) by insertion between its SacI and EcoRI restriction sites of a 550-bp DNA fragment from B. cepacia RR10, which was obtained by PCR using degenerated oligonucleotides designed to amplify conserved regions of alkane hydroxylases related to the P. putida GPo1 AlkB protein, as described previously (49). Plasmids pALK7 and pALK8 were selected from a gene bank of B. cepacia RR10 total DNA in the cosmid vector pLAFR3 on the basis of their positive hybridization to the 550-bp DNA fragment cloned into pRR10. They contain overlapping DNA inserts of about 22 and 20 kbp, respectively. Plasmid pALK12 was constructed by cloning a 3.4-kbp PstI DNA segment from pALK8, hybridizing with the 550-bp probe, in the PstI site of pUC18. This DNA fragment includes the 5′ end of the B. cepacia RR10 alkB gene. A 3.2-kbp BamHI fragment from plasmid pALK7, including the 3′ end of the B. cepacia alkB gene, was cloned into the BamHI site of pUC18 to produce plasmid pALK14. To obtain a transcriptional fusion of the promoter for the alkB gene to the lacZ reporter gene, a 624-bp DNA fragment including positions −574 to +51 relative to the alkB transcription start site (the alkB translation start is located at position +117) was inserted between the EcoRI and BamHI sites of plasmid pUJ8. The fragment was obtained by PCR using plasmid pALK12 as template and oligonucleotides 5′-aaggtcgaattcatgcaggc and 5′-ttttgtgcggatcctcgtcg as primers, which include restriction sites for EcoRI or BamHI. The resulting plasmid was named pALK31. Subsequently, the 4.9-kbp NotI fragment with the PalkB::lacZ fusion was cut from plasmid pALK31 and inserted in the NotI site of Mini-Tn5-Tc, obtaining plasmid pALK32. Plasmid pALK301 contains the complete B. cepacia alkB gene obtained by PCR using total B. cepacia DNA as template; the DNA fragment obtained was digested with EcoRI and cloned into the EcoRI site of plasmid pNM185.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description or relevant phenotype | Reference or source |
|---|---|---|
| Bacterial strains | ||
| E. coli CC118(λpir) | CC118 lysogenized with λpir phage | 23 |
| E. coli HB101 | Host for transfection with cosmid pLAFR3 | 6 |
| E. coli TG1 | Host for DNA manipulations | 45 |
| B. cepacia RR10 | Degrades alkanes containing 12 to 30 carbon atoms | 63 |
| B. cepacia CPBC2 | Strain RR10 with a PalkB::lacZ fusion in the chromosome | This work |
| P. putida KT2442 | hsdR, Rif; host for DNA manipulations | 15 |
| P. fluorescens KOB2Δ1 | P. fluorescens CHAO with a deletion in the alkB gene | J. B. van Beilen |
| Plasmids | ||
| pALK12 | Apr, contains a 3.4-kbp PstI DNA segment from pALK8 including the 5′ end of the B. cepacia alkB gene | This work |
| pALK14 | Apr, contains a 3.2-kbp BamHI fragment from pALK7 cloned into the BamHI site of pUC18; contains the 3′ end of the alkB gene | This work |
| pALK301 | Kmr, contains the B. cepacia RR10 alkB gene in pNM185 | This work |
| pALK31 | Apr, contains a PalkB::lacZ transcriptional fusion; derives from pUJ8 | This work |
| pALK32 | Apr Tcr, contains a PalkB::lacZ fusion into mini-Tn5Tc | This work |
| pALK7 | Tcr, contains a 22-kbp DNA segment including the B. cepacia alkB gene | This work |
| pALK8 | Tcr, contains a 20-kbp DNA segment including the 5′ end of the B. cepacia alkB gene | This work |
| pGEM7-Zf(+) | Apr, cloning vector | Promega |
| pLAFR3 | Tcr, broad-host-range cosmid vector | 51 |
| pNM185 | Kmr, Mob+, broad-host-range expression vector | 36 |
| pRK2013 | Kmr, Mob+, Tra+, donor of transfer functions | 14 |
| pRR10 | Apr, contains a 550-bp DNA segment of the B. cepacia alkB gene | This work |
| pUC18 | Apr, cloning vector | 61 |
| pUJ8 | Apr, vector to make transcriptional fusions to lacZ | 13 |
| pUT-mini-Tn5Tc | Apr Tcr, mini-Tn5 suicide donor plasmid | 13 |
Media and culture conditions.
Cells were grown at 37°C in Luria-Bertani (LB) medium or in minimal salts M9 medium (45), the latter supplemented with trace elements (4) and a carbon source (30 mM for organic acids or sugars and 1% [vol/vol] for alkanes, alkanols, or fatty acids). To prepare spent LB medium (46), B. cepacia RR10 was grown in fresh LB to stationary phase; cells were eliminated by centrifugation and subsequent filtration through a 0.45-μm-pore-size filter, the pH was adjusted to 7.0, and the medium was filter sterilized. Antibiotics were used at the following concentrations (in μg/ml): ampicillin, 100; kanamycin, 50; tetracycline, 12; streptomycin, 50.
Construction of a gene bank of B. cepacia chromosomal DNA.
Total DNA from B. cepacia RR10, obtained as previously described (3), was partially digested with endonuclease Sau3AI and fractionated on a 10 to 40% linear sucrose gradient. DNA fragments 15 to 30 kbp in length were cloned into the BamHI site of cosmid pLAFR3, using Escherichia coli HB101 as the host for transfection. About 7,000 independent clones were obtained, which were pooled and stored at −70°C in LB medium containing 30% glycerol.
Recombinant DNA techniques.
General methods for DNA manipulation and Southern blots were performed as described previously (45). DNA was sequenced on both strands with an Applied Biosystems DNA sequencer by the S.I.D.I-U.A.M. Sequencing Service. The primers used to PCR amplify a 550-bp internal region of the B. cepacia alkane hydroxylase as well as PCR conditions have been described previously (49). All other amplifications were performed using standard protocols (annealing temperature, 55 to 65°C; elongation temperature, 70°C; 30 cycles). Plasmids were introduced into B. cepacia RR10 by conjugation using plasmid pRK2013 as the donor of transfer functions in triparental matings (13). In the case of Pseudomonas fluorescens, plasmids were introduced by electroporation as described previously (25).
Assay for β-galactosidase.
An overnight culture of a B. cepacia strain harboring a PalkB::lacZ transcriptional fusion was diluted to a final turbidity of about 0.04 either in LB medium (fresh or spent, as indicated) or in minimal salts M9 medium supplemented with the specified carbon source and in the absence or presence of the indicated inducer. Cultures were grown at 37°C, and at different cell densities aliquots were taken and β-galactosidase activity was measured as described by Miller (37). At least three independent assays were performed in each case.
S1 nuclease analyses of mRNAs.
Total RNA was isolated from bacterial cultures as described earlier (38). S1 nuclease reactions were performed as described previously (3), using 25 μg of total RNA and an excess of a 5′-end-labeled single-stranded DNA (ssDNA) hybridizing to the 5′ region of the mRNA. The ssDNA probe was generated by linear PCR (62) using plasmid pALK12 cut with endonuclease NotI as substrate. This plasmid contains the 5′ end of the B. cepacia RR10 alkB gene and about 2.3 kbp upstream from it; the NotI target is located 213 nucleotides upstream of the RR10 PalkB translation start site.
Nucleotide sequence accession number.
The nucleotide sequence of the 6,237-bp B. cepacia RR10 chromosomal region containing the alkane hydroxylase gene was submitted to the EMBL data bank under accession no. AJ293306.
RESULTS AND DISCUSSION
Identification of a gene coding for an alkane hydroxylase in B. cepacia RR10.
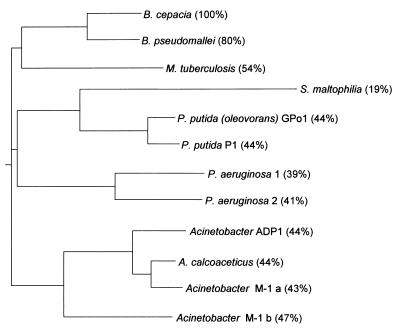
A set of degenerated oligonucleotides has been described which allows the PCR amplification of a small internal region of genes related to the P. putida GPo1 and Acinetobacter sp. ADP1 alkane hydroxylases (49). Using these oligonucleotides, a 550-bp DNA fragment was obtained from B. cepacia RR10 coding for a polypeptide which shows 53% identity (71% similarity) to the corresponding internal region of the P. putida GPo1 alkane hydroxylase. The amplified DNA fragment was used as a probe to screen a gene bank of total B. cepacia RR10 DNA in cosmid pLAFR3. Two clones, named pALK7 and pALK8, yielded a positive signal and contained partially overlapping DNA inserts more than 20 kbp in length. A 3.4-kbp PstI DNA segment from pALK8 and a 3.2-kbp BamHI DNA segment from pALK7, both hybridizing to the probe, were cloned in pUC18, obtaining plasmids pALK12 and pALK14, respectively. The inserts in these two plasmids were sequenced. They were found to contain partially overlapping sequences spanning a 6,237-bp contig. A BLASTX (1, 19) analysis of this sequence revealed the presence of a 1,158-bp open reading frame (ORF) coding for a polypeptide showing high similarity scores to known or predicted alkane hydroxylases. The highest scores were obtained for the putative alkane hydroxylase from Mycobacterium tuberculosis H37Rv (54% identity), followed by the alkane hydroxylases from P. putida GPo1 (44% identity), P. putida F1 (44% identity), Acinetobacter sp. ADP1 (44% identity), and S. maltophilia (22% identity). A BLASTX search on the Pseudomonas aeruginosa PAO1 genome (http://www.pseudomonas.com) showed the presence of two putative alkane hydroxylases with 39 and 41% identity, respectively, to the B. cepacia RR10 predicted alkane hydroxylase. A similar search at the unfinished sequencing project of the Burkholderia pseudomallei genome (http://www.sanger.ac.uk) showed the presence of a polypeptide with 80% identity to the B. cepacia RR10 protein. A phylogenetic tree showing the relationships among these proteins is shown in Fig. 1. The B. cepacia RR10 alkane hydroxylase clustered in a separate group together with the predicted hydroxylases of M. tuberculosis and B. pseudomallei. The presence of the M. tuberculosis alkane hydroxylase in this group is surprising, considering that gram-positive bacteria are evolutionarily very distant from the Burkholderia group. The similarity between the different alkane hydroxylases was distributed throughout the entire polypeptide (not shown), being particularly strong at a series of invariant histidine boxes which have been found to be important and highly conserved in integral membrane nonheme iron proteins such as hydrocarbon hydroxylases and desaturases (26, 48, 49). The size (386 amino acids) and molecular weight (43,953) of the polypeptide are also similar to those of known alkane hydroxylases. Therefore, the cloned ORF most likely corresponds to an alkane hydroxylase and was named alkB, following the nomenclature used for P. putida GPo1, the most thoroughly characterized alkane hydroxylase. It is worth noting that among the reported PCR-amplified fragments of genes related to P. putida GPo1 alkane hydroxylase obtained from several alkane-degrading bacteria (49), the B. cepacia RR10 alkB gene showed highest similarity to that of B. cepacia ATCC 25416 (92.3% identity over the 182 amino acids in the PCR fragment).
FIG. 1.
Similarity of the B. cepacia RR10 alkane hydroxylase to other known or predicted bacterial alkane hydroxylases. Proteins were aligned with ClustalW (55) at the European Bioinformatics Institute web site (http://www2.ebi.ac.uk). The data obtained were used to generate a phylogenetic tree with the Phylodendron application at the IUBio Archive for Biology web site (http://iubio.bio.indiana.edu). The identity of each alkane hydroxylase to that of B. cepacia RR10 is indicated in parentheses.
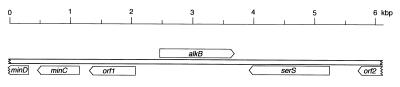
A BLASTX analysis of the regions sequenced upstream and downstream of the B. cepacia RR10 alkB gene did not reveal the presence of other ORFs showing similarity to genes known to play a role in alkane oxidation. A detailed description is presented in Fig. 2. An inverted repeat (15-bp stem, 4-bp loop) followed by a run of T's, similar to a Rho-independent terminator, was found 19 bp downstream from the stop codon of the B. cepacia alkB gene, suggesting that transcription of alkB is not coupled to that of any other ORF located downstream of it. Altogether, these observations indicate that the gene coding for the membrane component of the B. cepacia RR10 alkane hydroxylase is not clustered with other genes required for alkane degradation. Present knowledge suggests that there is a large variability in the clustering of alkane degradation genes in bacteria. For example, the genes coding for alkane oxidation in the OCT plasmid of P. putida GPo1 are grouped in two clusters (58). In S. maltophilia a gene coding for a protein with similarity to rubredoxins but named rubredoxin reductase is located adjacent to that coding for the hydroxylase (28), while the two analyzed Acinetobacter sp. alkane hydroxylase genes are not linked to those encoding the rubredoxin and rubredoxin reductases (17, 18, 41, 53). Finally, the two putative alkane hydroxylases of P. aeruginosa PAO1 are located on separate sites of the chromosome and are not clustered with other genes of the alkane degradation pathway (unpublished observations).
FIG. 2.
Analysis of the ORFs surrounding the B. cepacia RR10 alkane hydroxylase gene. The 6,237-bp B. cepacia DNA region sequenced was analyzed for the presence of ORFs homologous to described genes of known function at the website services of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) and of the European Bioinformatics Institute (http://www2.ebi.ac.uk) using the program BLASTX (19). The 1,158-bp ORF coding for a polypeptide showing high similarity scores to known or predicted alkane hydroxylases was named alkB (see the text). A polypeptide showing 30% identity to the Methanococcus jannaschii hypothetical protein MJ1207, which belongs to the acetyltransferase family of proteins, was found upstream of alkB; it is indicated as orf1. Downstream of orf1, an ORF was present encoding a protein 43% identical to E. coli MinC, followed by part of another ORF coding for a polypeptide homologous to E. coli MinD (80% identity in the region analyzed, which covers just the 112 N-terminal residues). E. coli MinC and MinD are known to play an important role in the process of cell division (reviewed in reference 31). Downstream of alkB and oriented in the opposite direction, an ORF was present encoding a protein 61% identical to the E. coli seryl-tRNA synthetase (serS gene). Upstream from serS and in the same orientation, there is an ORF coding for a polypeptide homologous to part of an E. coli hypothetical protein named YcaJ (84% identity in the region analyzed, which covers only the C-terminal half of the protein), the gene of which is also located upstream of serS in E. coli. It is indicated as orf2. The nucleotide sequence of the analyzed segment was deposited at the EMBL data bank under accession no. AJ293306.
Expression of the B. cepacia RR10 alkB gene in a heterologous host.
To show that the cloned alkB gene indeed encodes a functional alkane hydroxylase, the gene was transferred to P. fluorescens KOB2Δ1, a derivative of the alkane-degrading strain P. fluorescens CHAO in which part of the alkane hydroxylase gene has been deleted (T. H. M. Smits, S. Balada, B. Witholt, and J. B. van Beilen, unpublished data). For this purpose, the B. cepacia RR10 alkB gene was cloned into the broad-host-range expression vector pNM185 under the influence of the Pm promoter. This promoter is activated by the plasmid-encoded XylS activator in the presence of an appropriate inducer, such as 3-chlorobenzoate, a compound that is not used by P. fluorescens KOB2Δ1 as a carbon source. Transfer of the resulting plasmid, named pALK301, to P. fluorescens KOB2Δ1 restored the ability of this strain to grow on alkanes. Growth was faster in the presence of the inducer 3-chlorobenzoate (doubling time of about 40 h, compared to about 24 h for P. fluorescens CHAO), although it was also evident in its absence (doubling time of about 70 h), probably because of a low basal expression of the B. cepacia alkB gene in the absence of the inducer. The recombinant strain-degraded alkanes have between 12 and 22 carbon atoms, a range similar to that observed for the parental strain P. fluorescens CHAO in parallel control assays. All these results show that the B. cepacia alkB gene encodes a functional alkane hydroxylase.
Characterization of the promoter of the B. cepacia RR10 alkane hydroxylase gene.
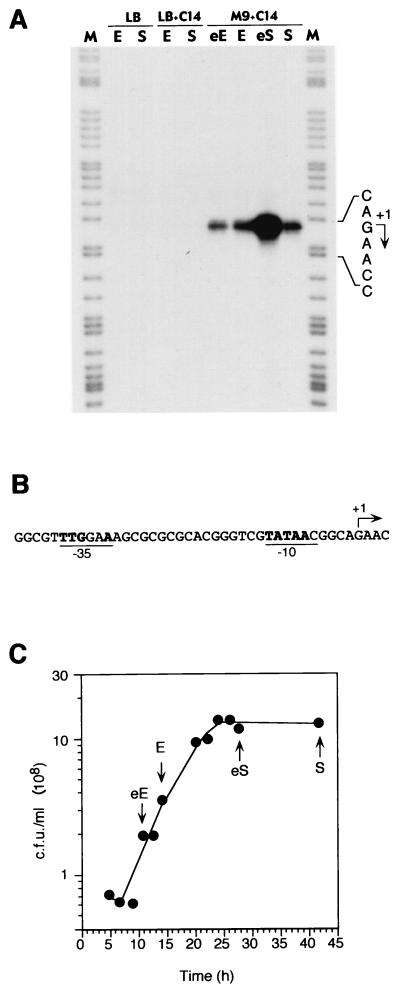
To analyze the expression of the B. cepacia RR10 alkB gene in its original host, total RNA was purified from B. cepacia RR10 cells grown in minimal salts medium using tetradecane as the carbon source. The transcription initiation site as well as the levels of expression at different stages of the culture growth were determined by S1 nuclease protection assays. As shown in Fig. 3, a single transcription start site was detected, located 118 bp upstream of the alkB translation initiation codon. A clear −10 recognition sequence for the vegetative RNA polymerase (RNAP) was present upstream of the start site (Fig. 3B). A moderately conserved −35 box for the vegetative RNAP could also be recognized 17 bp upstream of the 5′ end of the −10 box. The amount of transcripts detected was reproducibly higher in cells collected at the start of the stationary phase than in cells collected in the exponential or in the late stationary phase. This behavior, which has not been observed in the few alkane degraders whose regulation has been studied to date, is reminiscent of the E. coli promoters recognized by ςS-RNAP (21, 22, 30). However, the consensus for this form of RNAP in E. coli (5), which is probably conserved in other gram-negative bacteria as well (9), is not present in the PalkB promoter. Alternatively, promoter activity could be under the control of a quorum-sensing mechanism which is known to be present in B. cepacia (29). Despite several attempts, however, ethyl-acetate extracts obtained from tetradecane-grown stationary-phase cultures failed to stimulate expression of the PalkB promoter in exponential phase. Addition to a fresh culture of up to 10% (vol/vol) of a spent M9 medium obtained from tetradecane-grown cells did not have a positive effect either. The behavior of the B. cepacia PalkB promoter could be explained by assuming that it is regulated by a factor that is more active or that is present at higher levels in stationary-phase cells. In fact, when the PalkB::lacZ fusion was transferred to E. coli CC118(λpir) or to P. putida KT2442, only low β-galactosidase levels were observed (in the range of 150 to 200 Miller units), which were constant under all growth conditions. It is likely, therefore, that this promoter requires a transcriptional activator for induction.
FIG. 3.
Identification of the promoter for the B. cepacia alkane hydroxylase gene. (A) B. cepacia RR10 was grown in either M9 minimal salts medium in the presence of tetradecane (indicated as C14) or in LB medium in the absence or presence of tetradecane. At different time points (early exponential phase, eE; exponential phase, E; early stationary phase, eS; or stationary phase, S), samples were collected and processed to obtain total RNA. Transcripts originating upstream of alkB were analyzed by S1 nuclease protection assays using equal amounts of total RNA and an excess of ssDNA probe in all cases. Samples were electrophoresed in parallel with a DNA size ladder obtained by chemical sequencing (35) of the same ssDNA used as a probe (lane M). The transcription start site is indicated by an arrow. (B) Sequence of the promoter for the alkB gene. The transcription start site observed in the S1 nuclease protection assay is indicated with an arrow. Positions at the −10 and −35 regions identical to those recognized by the vegetative RNA polymerase at promoters in most eubacteria (60) are highlighted in bold face. (C) The growth curve of B. cepacia RR10 grown in M9 minimal salts medium with tetradecane. Arrows indicate the time points when samples were collected to obtain the total RNA used for the S1 nuclease protection assays described for panel A. Growth was followed by measuring the increase in CFU on LB plates.
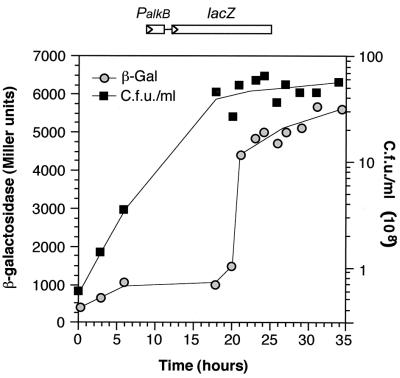
To gain further insight into the regulation of the PalkB promoter, its expression was investigated in cells of strain RR10 growing either in a rich LB medium or in a minimal salts medium using citrate as a carbon source, in the absence or presence of tetradecane. No expression of the promoter could be detected in LB medium independent of the absence or presence of tetradecane and of the growth phase (Fig. 3A). Similar results were found with cells grown in minimal salts medium using citrate as a carbon source or using a mixture of citrate and tetradecane (not shown). This indicates that expression of alkB is regulated. Furthermore, these results suggest that alkanes are not preferred carbon sources for B. cepacia, so that when other more favorable carbon sources are used, expression of the PalkB promoter is downregulated by a strong catabolite repression. To further investigate this possibility, a transcriptional fusion of promoter PalkB to the lacZ reporter gene was cloned into the suicide donor plasmid pUT-mini-Tn5Tc and transferred to the B. cepacia RR10 chromosome. A representative transconjugant, named CPCB2, was selected for further analyses. Measurements of turbidity values in cultures of B. cepacia RR10 growing on alkanes proved to be unreliable due to the fine emulsification of the alkane, a problem which impaired a confident determination of β-galactosidase activities. However, we observed that the PalkB::lacZ fusion was efficiently induced by tetradecanol, which is a good growth substrate for B. cepacia RR10 and does not present the emulsification problem. Therefore, expression of the PalkB::lacZ fusion was monitored by measuring β-galactosidase activity throughout the growth curve in cells of strain CPCB2 grown in minimal salts medium containing tetradecanol as a carbon source. As shown in Fig. 4, β-galactosidase activity remained at moderate levels (about 1,000 Miller units) during the exponential phase of growth and rapidly increased as cells entered into stationary phase, eventually reaching about sixfold higher values. Higher expression of the PalkB promoter in stationary-phase tetradecanol-grown cells agrees with the PalkB activity values observed in tetradecane-grown cells with S1 nuclease protection assays (Fig. 3).
FIG. 4.
Expression of the B. cepacia RR10 PalkB promoter in cells growing in a minimal salts medium using tetradecanol as the carbon source. B. cepacia strain CPBC2, a derivative of RR10 containing a PalkB::lacZ transcriptional fusion integrated into the chromosome, was grown in minimal salts medium containing tetradecanol as the sole carbon source. Samples were taken at different times, and the amount of β-galactosidase present in the cells was measured (represented as gray circles). Growth was followed in parallel by counting CFU on solid media (dark rectangles).
Having established the behavior of the PalkB::lacZ fusion, we proceeded to test the effect of a number of carbon sources on the expression of the PalkB promoter. Promoter activity in cells grown in LB medium in the presence of tetradecanol was low and very similar to that of cells grown in its absence (Table 2). In exponentially growing cells, β-galactosidase levels in minimal salts cultures where tetradecanol was the sole source of carbon were about 12-fold higher than those in LB cultures in the presence of tetradecanol, a value that increased to 63-fold in stationary-phase cultures. These results agree with the repressing effect of the LB medium observed by the S1 nuclease protection assays in cells growing in the presence of tetradecane (Fig. 3A). A similar repressing effect of the LB medium has been observed in the expression of the alkane hydroxylase of P. putida GPo1, as well as in other catabolic pathways for hydrocarbons (24, 33, 52), and is probably due to the amino acids in LB (8, 62). However, the repression effect on the P. putida GPo1 pathway vanishes when cells reach the stationary phase of growth. The behavior of the B. cepacia PalkB promoter was different. As shown in Table 2, stationary-phase cultures of strain CPBC2 grown in LB medium containing tetradecanol as the inducer showed essentially the same β-galactosidase levels as exponential cultures. This agrees with the transcription levels of the parental strain when grown in LB medium in the presence of tetradecane (Fig. 3). Another interesting characteristic of the catabolic repression effect of LB medium on the promoters of the P. putida GPo1 alkane degradation pathway is that repression is no longer observed when cells are grown on spent LB medium (a medium that has already supported cell growth) (62). Again, the behavior of the B. cepacia PalkB promoter was different, since cells grown in a spent LB medium did not show an increase in promoter activity in the presence of an inducer (Table 2). This distinct behavior suggests mechanistic differences in the way the metabolic status of the cell is coupled to the expression of the alkane hydroxylase in P. putida GPo1 and in B. cepacia RR10.
TABLE 2.
Expression of the B. cepacia PalkB promoter in strain CPBC2 grown at the expense of different carbon sources
| Carbon sourcea |
PalkB promoter expression during:
|
|||
|---|---|---|---|---|
| Exponential phase
|
Stationary phase
|
|||
| Activityb | Repressionc | Activityb | Repressionc | |
| TetOH | 1,160 | 1 | 6,280 | 1 |
| LB | 74 | NA | 97 | NA |
| LB+TetOH | 100 | 12 | 99 | 63 |
| Spent LB | 74 | 15 | 115 | 54 |
| Spent LB+TetOH | 53 | 22 | 105 | 60 |
| TetOH+citrate | 82 | 14 | 102 | 61 |
| TetOH+fumarate | 64 | 18 | 80 | 78 |
| TetOH+lactate | 71 | 16 | 83 | 76 |
| TetOH+pyruvate | 101 | 11 | 97 | 65 |
| TetOH+succinate | 62 | 19 | 88 | 71 |
| TetOH+glutamate | 70 | 16 | 89 | 70 |
| TetOH+acetate | 129 | 9 | 100 | 62 |
| TetOH+glycerol | 140 | 8 | 80 | 78 |
| TetOH+glucose | 69 | 17 | 73 | 86 |
| TetOH+fructose | 79 | 15 | 123 | 51 |
| TetOH+lactose | 97 | 12 | 100 | 63 |
| TetOH+arabinose | 90 | 13 | 88 | 71 |
| TetOH+myristic acid | 292 | 4 | 379 | 16 |
Cells were grown in fresh or spent LB medium in the absence or presence of tetradecanol (1% [wt/vol]) or in minimal salts medium in the presence of either tetradecanol (1% [wt/vol]) or a combination of tetradecanol and the indicated compound (30 mM for organic acids or sugars, 1% for myristic acid). TetOH, tetradecanol.
Activity of the B. cepacia PalkB promoter (in Miller units) under each growth condition in exponentially growing or in stationary-phase cells. Values represent the averages of three independent assays; the standard deviation was less than 20%.
Repression (in folds) indicates the decrease in promoter activity observed in each case relative to the activity observed when tetradecanol was the sole carbon source. NA, nonapplicable.
The effects of several organic acids and sugars in cells cultured in minimal salts medium containing tetradecanol were also analyzed. As shown in Table 2, all the organic acids tested, as well as glucose, fructose, lactose, and arabinose, strongly repressed expression of the PalkB promoter in the presence of tetradecanol, both in the exponential and stationary phases of growth. The effect of myristic acid, the product of tetradecanol oxidation, was also analyzed. This fatty acid decreased the induction effect of tetradecanol by about 4-fold in exponential phase and by about 16-fold in stationary phase. This repression is interesting, since fatty acids are the product of alkane oxidation. It is tempting to speculate that accumulation of fatty acids may serve as a checkpoint to limit oxidation of alkanes when cells sense that the β-oxidation pathway is overloaded. In summary, the behavior of the PalkB::lacZ fusion is in full agreement with the mRNA analyses performed with S1 nuclease protection assays. Both assays indicate that expression of the PalkB promoter is induced by hydrocarbons and that the induction is strongly repressed by catabolite repression in the presence of other compounds that cells probably metabolize preferentially.
Expression of the alkB gene in cells grown at the expense of alkanes of different chain lengths.
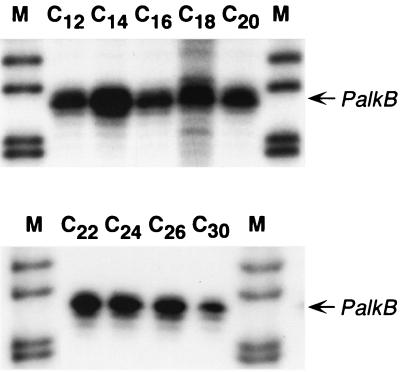
Some alkane-degrading bacterial strains are believed to contain only one alkane hydroxylase, while others have two different (albeit related) alkane hydroxylases. Examples of the former possibility are P. putida GPo1 (56, 58) and Acinetobacter sp. ADP1 (41). P. aeruginosa PAO1 contains two related alkane hydroxylases, although their individual contribution to alkane metabolism has not been elucidated yet. Acinetobacter sp. strain M-1 has two related alkane hydroxylases with different substrate specificities; one of them preferentially oxidizes alkanes of 12 to 16 carbon atoms, while the other shows higher activity with alkanes of more than 20 carbon atoms (32). It has been shown that each hydroxylase is differentially regulated by a specific transcription factor, each one responding to the presence of alkanes of the chain length recognized by the corresponding hydroxylase (53). To investigate the range of alkanes able to induce the B. cepacia RR10 alkB gene, cells were grown in minimal salts medium containing alkanes of either 12, 14, 16, 18, 20, 24, 26, or 30 carbon atoms as the sole carbon and energy source. The expression of the PalkB promoter was analyzed in each case by S1 nuclease protection assays. As shown in Fig. 5, all these alkanes induced the PalkB promoter to similar levels. Unless we assume a gratuitous induction by some of these alkanes, this suggests that the hydroxylase may oxidize all these substrates. Since the inherent difficulties in the genetic manipulation of B. cepacia RR10 has precluded us from obtaining a mutation in the alkB gene, it is at present unclear whether B. cepacia RR10 contains one or several alkane hydroxylases. However, several data are compatible with the idea that the isolated alkB gene is the only alkane hydroxylase gene present. First, the PCR amplification strategy to isolate the probe used for cloning, based on degenerated primers directed towards conserved regions of known alkane hydroxylases, identified a single gene in several independent assays. Second, the amplified DNA fragment afforded a single hybridization band in Southern blots performed with total B. cepacia RR10 chromosomal DNA (data not shown). Finally, the complementation analyses described above showed that transfer of the B. cepacia alkB gene to P. fluorescens KOB2Δ1 (lacking the alkane hydroxylase that allows it to grow in C12 to C18 alkanes) allowed it to grow at the expense of alkanes having from 12 to 22 carbon atoms, a range similar to that assimilated by the parental P. fluorescens CHAO strain in parallel control tests. In this case, factors other than the substrate range of the alkane hydroxylase possibly impede this strain to assimilate longer alkanes (J. B. van Beilen, unpublished observations).
FIG. 5.
Expression of the B. cepacia RR10 alkB gene in cells growing at the expense of alkanes of different chain lengths. B. cepacia RR10 was grown in minimal salts medium containing either dodecane (C12), tetradecane (C14), hexadecane (C16), octadecane (C18), eicosane (C20), docosane (C22), tetracosane (C24), hexacosane (C26), or triacontane (C30) as the sole carbon source. Samples were taken at the end of the exponential phase, and expression of the PalkB promoter was analyzed as described in the legend to Fig. 3. Lane M corresponds to the DNA size ladder. The two panels correspond to gels containing equivalent amounts of sample exposed for the same period of time.
Conclusions.
The work presented here shows that B. cepacia RR10 contains an alkane hydroxylase which is induced by alkanes of very diverse chain lengths, its expression being fivefold higher at the onset of the stationary phase than during exponential growth. Transcription of the alkB gene was strongly repressed by catabolite repression by many carbon sources that B. cepacia seems to prefer over alkanes. Many degradation pathways for diverse hydrocarbons are controlled by catabolite repression (reviewed in reference 10). However, the repression exerted on the B. cepacia alkB gene is significantly stronger than that observed in other alkane degradation pathways. For example, expression of the P. putida GPo1 alkane hydroxylase is also repressed by several organic acids, but the effect is much milder (about fourfold [50, 62]). Similarly, citrate generates a strong repression in B. cepacia but has no effect in strain GPo1. The repression observed in LB medium is also different in the two strains. In P. putida GPo1, expression of alkB is strongly repressed during exponential growth, but repression ceases abruptly at the onset of the stationary phase (62). Repression of the B. cepacia alkB gene in LB medium was tightly maintained during the stationary phase. Similarly, repression is not observed when P. putida GPo1 is grown in a spent LB medium (62), although it is strong in the case of B. cepacia. This may be due to metabolic and/or mechanistic differences in the catabolic repression strategies of these two bacterial species.
Hydrocarbons, and alkanes in particular, seem to be unfavorable growth substrates for bacteria. This could be due at least in part to the fact that many hydrocarbons show a certain level of toxicity for bacteria. For example, despite providing abundant growth, alkane degradation is known to impose a stress on cell physiology in P. putida GPo1 (11, 12). Solvents like toluene activate multiple responses in gram-negative bacteria (47), while methyl benzoates switch on a stress response in E. coli and probably in P. putida as well (34). Alternatively (or in addition), the low solubility of hydrocarbons and the high metabolic cost of biosurfactant production limit their usefulness as carbon sources. Catabolite repression, which is probably advantageous for bacterial fitness in their natural environments, can pose limitations on the use of hydrocarbon-degrading bacteria for diverse biotechnological applications. A detailed knowledge of the global and specific regulation mechanisms of bacterial pathways for hydrocarbons will surely help to optimize their applications.
ACKNOWLEDGMENTS
We are grateful to L. Yuste for excellent technical assistance.
This work was supported by grants BIO97-0645-C02-01 and BIO2000-0939 from Comisión Interministerial de Ciencia y Tecnología to F.R.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas R M, Atlas M C. Biodegradation of oil and bioremediation of oil spills. Curr Opin Biotechnol. 1991;2:440–443. [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 4.Bauchop T, Eldsen S R. The growth of microorganisms in relation to their energy supply. J Gen Microbiol. 1960;23:457–569. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- 5.Becker G, Hengge-Aronis R. What makes an Escherichia coli promoter sigma S dependent? Role of the -13/-14 nucleotide promoter positions and region 2.5 of sigma S. Mol Microbiol. 2001;39:1153–1165. doi: 10.1111/j.1365-2958.2001.02313.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 7.Britton L N. Microbial degradation of aliphatic hydrocarbons. Vol. 13. New York, N.Y: Marcel Dekker; 1984. [Google Scholar]
- 8.Canosa I, Sanchez-Romero J M, Yuste L, Rojo F. A positive feedback mechanism controls expression of AlkS, the transcriptional regulator of the Pseudomonas oleovorans alkane degradation pathway. Mol Microbiol. 2000;35:791–799. doi: 10.1046/j.1365-2958.2000.01751.x. [DOI] [PubMed] [Google Scholar]
- 9.Canosa I, Yuste L, Rojo F. Role of the alternative sigma factor sigma S in expression of the AlkS regulator of the Pseudomonas oleovorans alkane degradation pathway. J Bacteriol. 1999;181:1748–1754. doi: 10.1128/jb.181.6.1748-1754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cases I, de Lorenzo V. Expression systems and physiological control of promoter activity in bacteria. Curr Opin Microbiol. 1998;1:303–310. doi: 10.1016/s1369-5274(98)80034-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Janssen D B, Witholt B. Growth on octane alters the membrane lipid fatty acids of Pseudomonas oleovorans due to the induction of alkB and synthesis of octanol. J Bacteriol. 1995;177:6894–6901. doi: 10.1128/jb.177.23.6894-6901.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Janssen D B, Witholt B. Physiological changes and alk gene instability in Pseudomonas oleovorans during induction and expression of alk genes. J Bacteriol. 1996;178:5508–5512. doi: 10.1128/jb.178.18.5508-5512.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 14.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin F C, Bagdasarian M, Bagdasarian M M, Timmis K N. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci USA. 1981;78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu H, Newcomb M, Wong C H. Pseudomonas oleovorans monooxygenase catalyzed asymmetric epoxidation of allyl alcohol derivatives and hydroxylation of a hypersensitive radical probe with the radical ring opening state exceeding the oxygen rebound site. J Am Chem Soc. 1991;113:5878–5880. [Google Scholar]
- 17.Geissdorfer W, Frosch S C, Haspel G, Ehrt S, Hillen W. Two genes encoding proteins with similarities to rubredoxin and rubredoxin reductase are required for conversion of dodecane to lauric acid in Acinetobacter calcoaceticus ADP1. Microbiology (UK) 1995;141:1425–1432. doi: 10.1099/13500872-141-6-1425. [DOI] [PubMed] [Google Scholar]
- 18.Geissdorfer W, Kok R G, Ratajczak A, Hellingwerf K J, Hillen W. The genes rubA and rubB for alkane degradation in Acinetobacter sp. strain ADP1 are in an operon with estB, encoding an esterase, and oxyR. J Bacteriol. 1999;181:4292–4298. doi: 10.1128/jb.181.14.4292-4298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 20.Grund A, Shapiro J, Fennewald M, Bacha P, Leahy J, Markbreiter K, Nieder M, Toepfer M. Regulation of alkane oxidation in Pseudomonas putida. J Bacteriol. 1975;123:546–556. doi: 10.1128/jb.123.2.546-556.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hengge-Aronis R. Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol Microbiol. 1996;21:887–893. doi: 10.1046/j.1365-2958.1996.511405.x. [DOI] [PubMed] [Google Scholar]
- 22.Hengge-Aronis R. Interplay of global regulators and cell physiology in the general stress response of Escherichia coli. Curr Opin Microbiol. 1999;2:148–152. doi: 10.1016/S1369-5274(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 23.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hester K L, Madhusudhan K T, Sokatch J R. Catabolite repression control by crc in 2xYT medium is mediated by posttranscriptional regulation of bkdR expression in Pseudomonas putida. J Bacteriol. 2000;182:1150–1153. doi: 10.1128/jb.182.4.1150-1153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Højberg O, Schnider U, Winteler H V, Sorensen J, Haas D. Oxygen-sensing reporter strain of Pseudomonas fluorescens for monitoring the distribution of low-oxygen habitats in soil. Appl Environ Microbiol. 1999;65:4085–4093. doi: 10.1128/aem.65.9.4085-4093.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kok M, Shaw J P, Harayama S. Comparison of two hydrocarbon monooxygenases of Pseudomonas putida. In: Galli E, Silver S, Witholt B, editors. Pseudomonas: molecular biology and biotechnology. Washington, D.C.: American Society for Microbiology; 1992. pp. 214–222. [Google Scholar]
- 27.Leahy J G, Colwell R R. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990;54:305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee N R, Hwang M O, Jung G H, Kim Y S, Min K H. Physical structure and expression of alkBA encoding alkane hydroxylase and rubredoxin reductase from Pseudomonas maltophilia. Biochem Biophys Res Commun. 1996;218:17–21. doi: 10.1006/bbrc.1996.0004. [DOI] [PubMed] [Google Scholar]
- 29.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loewen P C, Hengge-Aronis R. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 31.Lutkenhaus J, Mukherjee A. Cell division. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: American Society for Microbiology; 1996. pp. 1615–1626. [Google Scholar]
- 32.Maeng J H, Sakai Y, Ishige T, Tani Y, Kato N. Diversity of dioxygenases that catalyze the first step of oxidation of long-chain n-alkanes in Acinetobacter sp. strain M-1. FEMS Microbiol Lett. 1996;141:177–182. [Google Scholar]
- 33.Marques S, Holtel A, Timmis K N, Ramos J L. Transcriptional induction kinetics from the promoters of the catabolic pathways of TOL plasmid pWW0 of Pseudomonas putida for metabolism of aromatics. J Bacteriol. 1994;176:2517–2524. doi: 10.1128/jb.176.9.2517-2524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marques S, Manzanera M, Gonzalez-Perez M M, Gallegos M T, Ramos J L. The XylS-dependent Pm promoter is transcribed in vivo by RNA polymerase with sigma 32 or sigma 38 depending on the growth phase. Mol Microbiol. 1999;31:1105–1113. doi: 10.1046/j.1365-2958.1999.01249.x. [DOI] [PubMed] [Google Scholar]
- 35.Maxam A M, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 36.Mermod N, Ramos J L, Lehrbach P R, Timmis K N. Vector for regulated expression of cloned genes in a wide range of gram-negative bacteria. J Bacteriol. 1986;167:447–454. doi: 10.1128/jb.167.2.447-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 38.Monsalve M, Mencía M, Rojo F, Salas M. Transcription regulation in Bacillus subtilis phage φ29: expression of the viral promoters throughout the infection cycle. Virology. 1995;207:23–31. doi: 10.1006/viro.1995.1048. [DOI] [PubMed] [Google Scholar]
- 39.Panke S, Meyer A, Huber C M, Witholt B, Wubbolts M G. An alkane-responsive expression system for the production of fine chemicals. Appl Environ Microbiol. 1999;65:2324–2332. doi: 10.1128/aem.65.6.2324-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson J A, Basu D, Coon M J. Enzymatic omega-oxidation. I. Electron carriers in fatty acid and hydrocarbon hydroxylation. J Biol Chem. 1966;241:5162–5164. [PubMed] [Google Scholar]
- 41.Ratajczak A, Geissdorfer W, Hillen W. Alkane hydroxylase from Acinetobacter sp. strain ADP1 is encoded by alkM and belongs to a new family of bacterial integral-membrane hydrocarbon hydroxylases. Appl Environ Microbiol. 1998;64:1175–1179. doi: 10.1128/aem.64.4.1175-1179.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratajczak A, Geissdorfer W, Hillen W. Expression of alkane hydroxylase from Acinetobacter sp. strain ADP1 is induced by a broad range of n-alkanes and requires the transcriptional activator AlkR. J Bacteriol. 1998;180:5822–5827. doi: 10.1128/jb.180.22.5822-5827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rehm H J, Reiff I. Mechanisms and occurrence of microbial oxidation of long-chain alkanes. Adv Biochem Eng. 1981;19:175–215. [Google Scholar]
- 44.Rosenberg E, Ron E Z. Bioremediation of petroleum contamination. In: Crawford R L, Crawford D L, editors. Bioremediation: principles and applications. London, United Kingdom: Cambridge University Press; 1996. pp. 100–124. [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 46.Schellhorn H E, Hassan H M. Transcriptional regulation of katE in Escherichia coli K-12. J Bacteriol. 1988;170:4286–4292. doi: 10.1128/jb.170.9.4286-4292.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segura A, Duque E, Mosqueda G, Ramos J L, Junker F. Multiple responses of gram-negative bacteria to organic solvents. Environ Microbiol. 1999;1:191–198. doi: 10.1046/j.1462-2920.1999.00033.x. [DOI] [PubMed] [Google Scholar]
- 48.Shanklin J, Whittle E, Fox B G. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry. 1994;33:12787–12794. doi: 10.1021/bi00209a009. [DOI] [PubMed] [Google Scholar]
- 49.Smits T H M, Röthlisberger M, Witholt B, van Beilen J B. Molecular screening for alkane hydroxylase genes in Gram-negative and Gram-positive strains. Environ Microbiol. 1999;1:307–317. doi: 10.1046/j.1462-2920.1999.00037.x. [DOI] [PubMed] [Google Scholar]
- 50.Staijen I E, Marcionelli R, Witholt B. The PalkBFGHJKL promoter is under carbon catabolite repression control in Pseudomonas oleovorans but not in Escherichia coli alk+ recombinants. J Bacteriol. 1999;181:1610–1616. doi: 10.1128/jb.181.5.1610-1616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sze C C, Moore T, Shingler V. Growth phase-dependent transcription of the sigma 54-dependent Po promoter controlling the Pseudomonas-derived (methyl)phenol dmp operon of pVI150. J Bacteriol. 1996;178:3727–3735. doi: 10.1128/jb.178.13.3727-3735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tani A, Ishige T, Sakai Y, Kato N. Gene structures and regulation of the alkane hydroxylase complex in Acinetobacter sp. strain M-1. J Bacteriol. 2001;183:1819–1823. doi: 10.1128/JB.183.5.1819-1823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor S E, Calvin M. Hydrocarbons from plants: biosynthesis and utilization. Agric Food Chem. 1987;1:1–26. [Google Scholar]
- 55.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Beilen J B, Panke S, Lucchini S, Franchini A G, Röthlisberger M, Witholt B. Analysis of Pseudomonas putida alkane degradation gene clusters and flanking insertion sequences: evolution and regulation of the alk-genes. Microbiology. 2001;147:1621–1630. doi: 10.1099/00221287-147-6-1621. [DOI] [PubMed] [Google Scholar]
- 57.van Beilen J B, Wubbolts M G, Chen Q, Nieboer M, Witholt B. Effects of two-liquid-phase systems and expression of alk genes on the physiology of alkane-oxidizing strains. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of Pseudomonas. Washington, D.C.: ASM Press; 1996. pp. 35–47. [Google Scholar]
- 58.van Beilen J B, Wubbolts M G, Witholt B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation. 1994;5:161–174. doi: 10.1007/BF00696457. [DOI] [PubMed] [Google Scholar]
- 59.Witholt B, de Smet M J, Kingma J, van Beilen J B, Kok M, Lageveen R G, Eggink G. Bioconversions of aliphatic compounds by Pseudomonas oleovorans in multiphase bioreactors: background and economic potential. Trends Biotechnol. 1990;8:46–52. doi: 10.1016/0167-7799(90)90133-i. [DOI] [PubMed] [Google Scholar]
- 60.Wosten M M. Eubacterial sigma-factors. FEMS Microbiol Rev. 1998;22:127–150. doi: 10.1111/j.1574-6976.1998.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 61.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 62.Yuste L, Canosa I, Rojo F. Carbon-source-dependent expression of the PalkB promoter from the Pseudomonas oleovorans alkane degradation pathway. J Bacteriol. 1998;180:5218–5226. doi: 10.1128/jb.180.19.5218-5226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuste L, Corbella M E, Turiegano M J, Karlson U, Puyet A, Rojo F. Characterization of bacterial strains able to grow on high molecular mass residues from crude oil processing. FEMS Microbiol Ecol. 2000;32:69–75. doi: 10.1111/j.1574-6941.2000.tb00700.x. [DOI] [PubMed] [Google Scholar]