Abstract
Previous work has shown that lacZ fusions to the cysK, astD, tnaB, and gabT genes in Escherichia coli are activated by self-produced extracellular signals. Using a combination of ethyl acetate extraction, reversed-phase C18 chromatography, and thin-layer chromatography, we have purified an extracellular activating signal from E. coli supernatants. Mass spectrometry revealed a molecule with an m/z peak of 117, consistent with indole. Nuclear magnetic resonance analysis of the purified E. coli factor and synthetic indole revealed identical profiles. Using synthetic indole, a dose-dependent activation was observed with lacZ fusions to the gabT, astD, and tnaB genes. However, cysK::lacZ and several control fusions were not significantly activated by indole. Conditioned medium prepared from a tnaA (tryptophanase) mutant, deficient in indole production, supported 26 to 41% lower activation of the gabT and astD fusions. The residual level of activation may be due to a second activating signal. Activation of the tnaB::lacZ fusion was reduced by greater than 70% in conditioned medium from a tnaA mutant.
The use of chemical signals for bacterial communication is a widespread phenomenon (10, 11, 20, 23, 33). In gram-negative bacteria, these signals can be N-acyl derivatives of homoserine lactone, cyclic dipeptides, and quinolones (3, 8, 17, 28–30, 43). In gram-positive bacteria, small peptides appear to be the predominant signal (7, 15, 16, 18, 25, 36). In some cases, small proteins can mediate signaling (22, 40). These signals regulate a variety of functions, including bioluminescence, differentiation, virulence, DNA transfer, and biofilm maturation (1, 2, 4, 5, 9, 19, 24, 27, 31, 32).
Indole production is a common diagnostic marker for the identification of Escherichia coli (37). Among the Enterobacteriaceae, indole is produced by E. coli and certain members of the Proteeae, such as Proteus vulgaris, Providencia spp., and Morganella spp. (37). Indole is formed from tryptophan by the tryptophanase enzyme, encoded by the tnaA gene (35). At very high concentrations (5 mM), indole is toxic to E. coli, possibly by causing membrane changes that result in the generation of superoxide (12). However, the concentration at which indole is toxic is approximately 15-fold higher than the physiological concentration seen in stationary-phase supernatants of E. coli (see below). The efflux of indole from E. coli is mediated by the AcrEF pump, and acrEF mutants exhibit enhanced indole sensitivity (21). The primary pathway for indole transport into the cell is via the Mtr permease (42).
For E. coli, the role of cell-to-cell signaling in a variety of functions, including regulation of ftsQAZ, expression of type III secretion systems, inhibition of DNA replication, and activation of degradative pathways, has been described (1, 13, 34, 38, 39, 41). However, the extracellular signals involved in these processes are poorly understood. Previous studies from our lab have identified the E. coli genes cysK, astD, tnaB, and gabT, which are activated by extracellular signals (1). We have utilized a lacZ fusion to one of these genes (gabT) as a biosensor to purify an activating signal from E. coli supernatants. Our data indicate that this signal is indole. In addition to the activation of gabT, indole is also capable of activating lacZ fusions to the astD and tnaB genes, indicating that it may affect a specific signaling pathway.
MATERIALS AND METHODS
Strains and growth conditions.
A luxS mutant of E. coli strain DH5α, obtained from B. Bassler, Princeton University, was used for the preparation of conditioned medium for signal purification. Strains MT9 (cysK::lacZ), MT48 (astD::lacZ), MT113 (tnaB::lacZ), and MT114 (gabT::lacZ) have been described previously (1). Strain TM1061 is an MC1061 derivative that contains a tnaA::mini-Tn5 Cm null allele and is unable to produce indole. All strains were grown in 0.5× Luria broth (LB) at pH 7.5 for β-galactosidase assays by the method of Miller (26).
Bioassay conditions.
Strain MT114 gabT::lacZ was used to monitor purification of the activating signal. Assays were conducted in 3 ml of 0.5× LB at pH 7.5, and mixtures were shaken at 280 rpm in 13- by 100-mm test tubes. Cultures were inoculated at a 1:1,000 dilution with a dilute overnight culture of MT114, and cells were harvested at an optical density at 600 nm (OD600) of 0.35. This represented approximately 5 to 6 h of growth. The effects of indole were examined on MT9, MT48, MT113, and MT114 using the above conditions. Crude preparations of conditioned medium were prepared as described previously (1).
Signal purification.
For factor purification, 900 ml of LB (three preparations of 300 ml each) was inoculated with a dilute suspension of log-phase DH5α and allowed to shake overnight at 300 rpm. Cells were harvested at an A600 of 1.5 and were pelleted by centrifugation at 4,300 × g. The resulting supernatant was filter sterilized, and the pH was adjusted to 7.5. The supernatant was then sequentially extracted three times with 200 ml of ethyl acetate. The ethyl acetate phase was dried under a rotary evaporator at 40°C. The material was resuspended in 1 ml of ethyl acetate and loaded on a 5-g C18 column (Waters Corp.). The column was washed sequentially with 20-ml portions of H2O, 20% methanol, 50% methanol, 60% methanol, 80% methanol, and 100% methanol. The material in the 60% wash activated the gabT::lacZ fusion. The 60% methanol fraction was dried on a rotary evaporator and redissolved in 300 μl of ethyl acetate. The resulting material was applied to a silica gel thin-layer chromatography plate and eluted with hexane-ethyl acetate (3:2). Six individual bands were typically observed, and each band was cut out. The resulting material from each band was eluted in ethyl acetate and tested for activity in the bioassay described above.
Structural analysis.
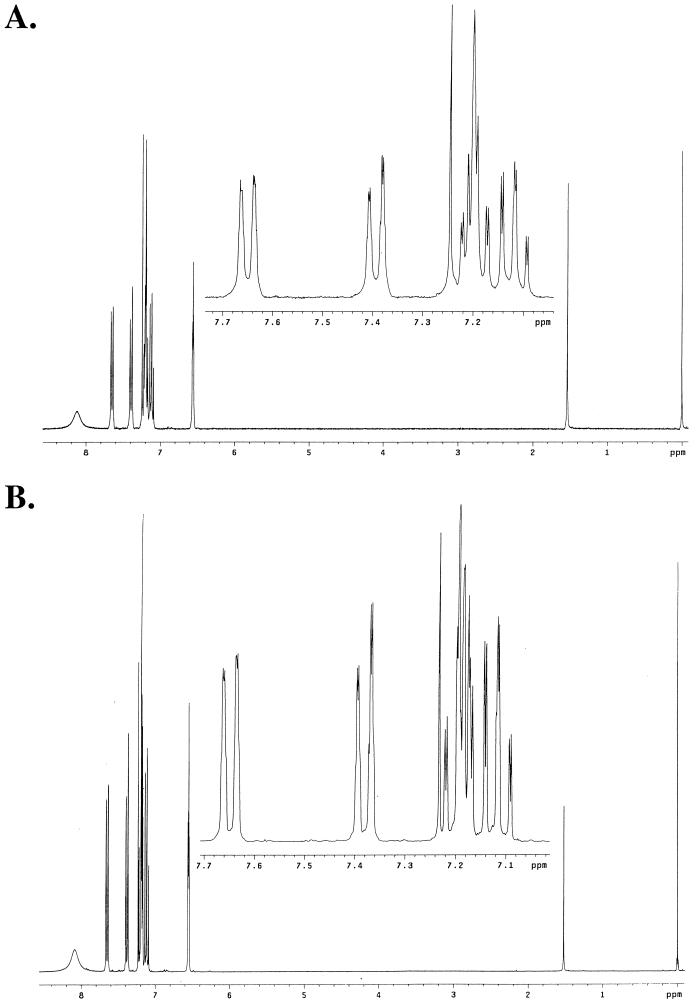
The high-resolution electron impact mass spectrum was recorded on a KRATOS MS25FA spectrometer. For the activating material, an m/z of 117.0584 was observed, corresponding to C8H7N with a calculated value of 117.0578. A database search of the spectrum for the activating factor identified a match with indole. 1H nuclear magnetic resonance (NMR) spectra in CDCl3 were recorded on a Varian 300-MHz spectrometer. The chemical shifts are reported in δ (parts per million). The 1H NMR spectra for the activating factor and synthetic indole (Aldrich Chemical Co. Inc.) were identical: 6.55 (m, 1H), 7.09 to 7.21 (m, 3H), 7.39 (d, J 8.1, 1H), 7.65 (d, J 7.8, 1H), 7.96 (br, s, 1H).
RESULTS
Purification of an extracellular signal that activates the gabT::lacZ fusion.
Strain MT114 gabT::lacZ was used as a biosensor to purify an activating signal. Previous work indicated that the LuxS-dependent signal of E. coli was not involved in the activation of gabT::lacZ (1). Preliminary extractions of conditioned medium indicated that both chloroform and ethyl acetate were capable of extracting an activating signal. However, ethyl acetate extracts gave higher activity and were used for further experiments. The stage in growth for optimal factor production was also examined. Although activation of gabT::lacZ was observed using conditioned medium from cells at mid-log phase (A600 of 0.5), conditioned medium prepared at an A600 of 1.5 gave the highest activity (data not shown).
To purify an activating signal, conditioned medium was prepared and extracted with ethyl acetate as described in Materials and Methods. The extract was applied to a reversed-phase C18 column and eluted with increasing concentrations of methanol. The material contained within the 60% elution displayed activity when tested with MT114 (gabT::lacZ) (data not shown). Thin-layer chromatography of this material resulted in six prominent bands under UV illumination. Individual bands were cut out, eluted with ethyl acetate and tested for activity. The material from one band was capable of activating the gabT::lacZ fusion approximately five-fold (data not shown).
Structural analysis of the activating signal.
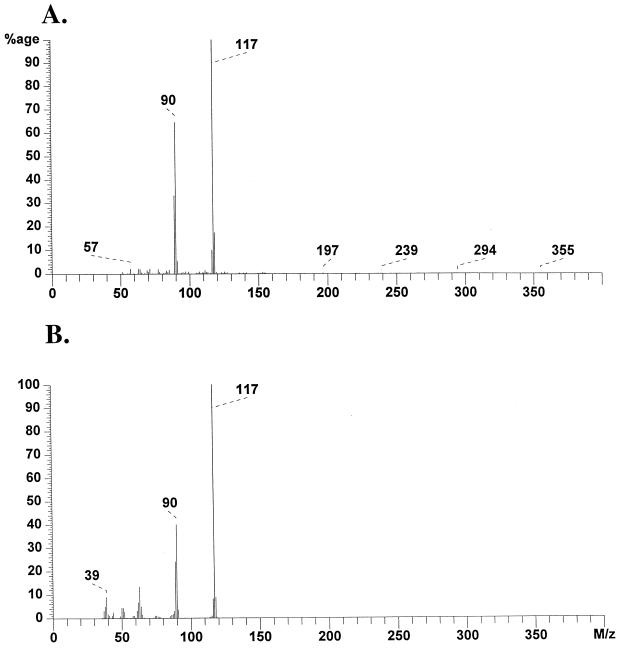
The high-resolution electron impact mass spectra of the activating material indicated a primary ion with an m/z of 117.0584 and a second peak at an m/z of 90.04767 (Fig. 1A). A database search indicated a match with indole (C8H7N), with an m/z of 117.0578. A comparison of the high-resolution spectrum for indole indicated that the profile was essentially identical to that for the activating signal (Fig. 1B). NMR analysis was then used to further confirm the chemical nature of the activating signal. The 1H NMR spectra of the activating signal (Fig. 2A) and synthetic indole (Fig. 2B) revealed identical profiles. In addition, the purified activating material gave an intense purple reaction with Kovács reagent. These data taken together indicate that the activating factor is indole.
FIG. 1.
Electron impact mass spectra of the activating fraction (A) and of synthetic indole (B). The m/z peaks were 117.0584 (C8H7N; calculated, 117.0578) and 90.04767 (C7H6-CHN+).
FIG. 2.
1H NMR spectra of the activating factor and synthetic indole. The chemical shifts are reported in δ (ppm) relative to residual trimethyl silane. The 1H NMR spectra for the activating factor (A) and synthetic indole (B) were identical (see Materials and Methods).
The concentration of extracellular indole produced by E. coli has previously been reported at 150 μM in minimal medium supplemented with tryptophan (12). However, we have observed that stationary-phase LB cultures of MG1655 have an indole concentration of 340 μM.
Synthetic indole activates the astD, tnaB, and gabT fusions in a dose-dependent manner.
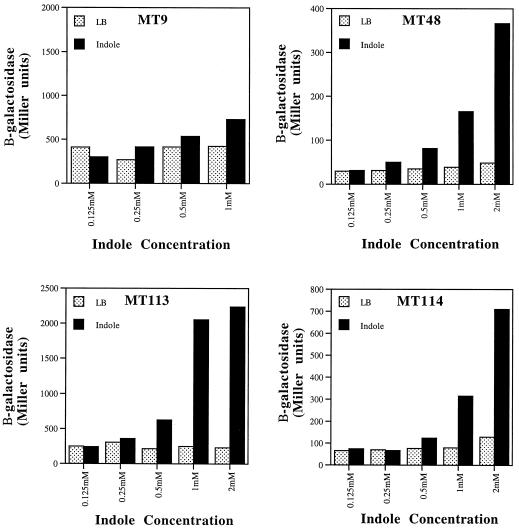
To confirm the identification of indole as an activating molecule, synthetic indole was tested for the ability to prematurely activate various quorum-sensing regulated lacZ fusions at early log phase. In Fig. 3, the effects of indole on β-galactosidase expression from each fusion are shown. The cysK::lacZ fusion (MT9) was not significantly activated by indole, with a 1.7-fold activation seen at 1 mM. In contrast, lacZ fusions to astD (MT48), tnaB (MT113), and gabT (MT114) were activated 4.3-, 8.3-, and 4.0-fold, respectively, at a concentration of 1 mM indole (Fig. 4). At 2 mM indole, the astD (MT48), tnaB (MT113), and gabT (MT114) fusions were activated 7.6-, 9.6-, and 6.6-fold, respectively. Indole did not stimulate growth, and at a concentration of 2 mM, it resulted in slower growth of MT48, MT113, and MT114. At 2 mM indole, MT9 was unable to reach an OD of 0.35. As a control, we examined the effects of indole at 1 mM on the expression of a random, uncharacterized lacZ fusion to a non-quorum-sensing activated gene. This fusion was not significantly activated and exhibited an induction value of 1.2-fold (data not shown). In addition, the expression of lacZ from its native chromosomal location in MG1655 was not altered by indole at 1 or 2 mM (data not shown).
FIG. 3.
Effects of indole on expression. The effects of synthetic indole on expression of lacZ fusions to cysK (MT9), atsD (MT48), tnaB (MT113), and gabT (MT114) were monitored by β-galactosidase expression (Miller units). Average results from duplicate experiments are shown. Standard deviations were less that 10% for each value. Duplicate experiments gave results similar to those shown.
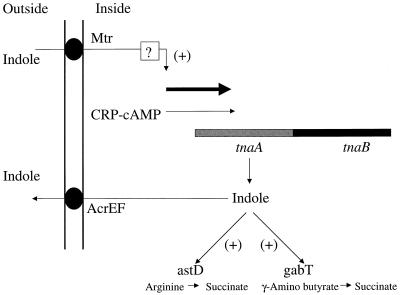
FIG. 4.
Possible model for indole signaling in E. coli. The tnaAB operon is depicted and is activated by CRP-cAMP as nutrients are depleted. This results in indole production via the TnaA (tryptophanase) enzyme. The indole is secreted and acts as an extracellular signal. The components of the signal response pathway are unknown and are indicated by a question mark.
Altered activation by conditioned medium from a tnaA (tryptophanase) mutant.
The production of indole from tryptophan depends on the tryptophanase enzyme (TnaA) (35). We investigated the role of secreted indole in conditioned medium on the activation of the fusions. Conditioned medium was prepared from MC1061 (wild type) and the isogenic derivative TM1061 (tnaA::mini-Tn5Cm) at an OD600 of 1.6 and was tested for the ability to activate lacZ fusions to the cysK, astD, tnaB, and gabT genes (Table 1). The TM1061 strain produced levels of indole that were below detection by standard methods (12). For the cysK (MT9), astD (MT48), and gabT(MT114) fusions, the activation values with conditioned medium from TM1061 were lower by 27, 41, and 26%, respectively, than the activation values with conditioned medium from MC1061 (wild type). For the tnaB fusion (MT113), the activation was lower by 70% with conditioned medium from TM1061, relative to MC1061 (Table 1).
TABLE 1.
Activation of gene expression by conditioned medium
| Strain | Growth conditiona | Mean β-galactosidase activity ± SD (Miller units)b |
|---|---|---|
| MT9 | LB | 262 ± 3 |
| CM (wild type) | 3,466 ± 66 (13.2) | |
| CM (tnaA::Cmr) | 2,526 ± 61 (9.6) | |
| MT48 | LB | 39 ± 3 |
| CM (wild type) | 341 ± 16 (9.0) | |
| CM (tnaA::Cmr) | 200 ± 6 (5.3) | |
| MT113 | LB | 177 ± 15 |
| CM (wild type) | 1,500 ± 46 (8.5) | |
| CM (tnaA::Cmr) | 464 ± 5 (2.6) | |
| MT114 | LB | 39 ± 3 |
| CM (wild type) | 405 ± 21 (10.4) | |
| CM (tnaA::Cmr) | 302 ± 8 (7.7) |
CM, conditioned medium.
Values in parentheses are fold induction relative to growth in LB only. Cells were harvested at an A600 of = 0.35.
The ability of indole at physiologically relevant concentrations to restore full activation of indole-deficient conditioned medium from the tnaA mutant was examined with the tnaB and gabT fusions (Table 2). Using tnaA mutant-conditioned medium supplemented with indole at 300 μM, the expression of β-galactosidase from the tnaB::lacZ and gabT::lacZ fusions was restored to a level that was 92 and 115%, respectively, of the levels with wild-type-conditioned medium (Table 2). With indole supplementation at 600 μM, the restored expression of β-galactosidase for the tnaB and gabT fusions corresponded to 116 and 127%, respectively, of the levels with wild-type-conditioned medium.
TABLE 2.
Effects of indole addition to conditioned medium from a tnaA mutant
| Strain | Growth conditiona | Mean β-galactosidase activity ± SD (Miller units)b |
|---|---|---|
| MT113 | LB | 299 ± 6 |
| CM (wild type) | 1,116 ± 57 | |
| CM (tnaA mutant) | 344 ± 17 | |
| CM (tnaA mutant) + 600 μM indole | 1,289 ± 39 | |
| CM (tnaA mutant) + 300 μM indole | 1,030 ± 79 | |
| CM (tnaA mutant) + 150 μM indole | 736 ± 11 | |
| MT114 | LB | 63 ± 2 |
| CM (wild type) | 252 ± 8 | |
| CM (tnaA mutant) | 210 ± 10 | |
| CM (tnaA mutant) + 600 μM indole | 320 ± 12 | |
| CM (tnaA mutant) + 300 μM indole | 289 ± 16 | |
| CM (tnaA mutant) + 150 μM indole | 244 ± 12 |
Conditioned medium (CM) was prepared at an A600 of = 1.4.
Cells were harvested at an A600 of 0.35.
DISCUSSION
Previous studies of gram-negative bacteria have shown that the primary signaling molecules involved in cell-cell communication are N-acyl derivatives of homoserine lactone, cyclic peptides, and quinolones (10, 11, 17, 29, 33). In this study, we have demonstrated that indole can act as an extracellular signaling molecule and activate the astD, tnaB, and gabT genes in a concentration-dependent manner. To date, there is no direct evidence that E. coli produces any of the N-acyl homoserine lactone signals commonly used in other gram-negative bacteria. Therefore, E. coli may have evolved to utilize alternative signals, such as the accumulation of certain metabolites. Signaling via metabolites may allow cells to fine-tune the regulation of target genes in response to changing environmental conditions. In addition, signaling by indole may not be limited to E. coli, as indole induces spore formation in the myxobacterium Stigmatella aurantiaca (14).
In E. coli, the addition of synthetic indole activated the astD, tnaB, and gabT fusions but did not activate cysK::lacZ or several control lacZ fusions. The use of a tnaA null mutant demonstrated that conditioned medium lacking indole exhibited a reduced ability to activate these fusions, relative to wild-type-conditioned medium (Table 1). In the case of cysK::lacZ, which is not activated by indole, it is unclear why conditioned medium from a tnaA mutant supported a lower level of activation. One possibility is that production of the signal for cysK activation is indirectly coupled to the activity of tryptophanase.
For the tnaB fusion, indole appears to be the primary extracellular signal required for activation, as conditioned medium lacking indole exhibited a 70% reduction in tnaB activation. Previous studies by Yanofsky et al. reported that indole was not able to induce expression of the tna operon (42). We have obtained SVS1144 tnaA′-′lacZ, used by Yanofsky et al., and found that it is induced by indole during growth in LB. The use of different media and/or indole concentrations could account for the differences in our results.
With the astD and gabT fusions, there was significant residual activation with conditioned medium lacking indole (Table 1). In addition, the concentration of synthetic indole required for activation of the astD and gabT fusions in LB only was above 500 μM, a concentration higher than the 340 μM that we have observed in stationary-phase E. coli supernants. However, when indole was added back to conditioned medium from a tnaA mutant lacking indole, the level of gabT::lacZ activation could be restored to wild-type levels with physiologically relevant concentrations of indole (300 μM). In light of these results, we propose that a second extracellular signal is produced by E. coli and that the combination of both signals is required for full activation of gabT and possibly astD.
In Fig. 4, a model that represents a possible physiological role for signaling by indole is presented. The initial component of this model is the tnaAB operon, which is activated by cyclic AMP receptor protein-cyclic AMP complex (CRP-cAMP) (6). We hypothesize that nutrient depletion during the increase in cell density is the initial trigger that activates tnaAB via CRP-cAMP. This activation is predicted to result in an increase in indole production. In support of this model, our preliminary studies indicate that the concentration of extracellular indole increases when cells are starved at low cell density. The intracellular indole is then exported by the AcrEF efflux system (21) to the outside of the cell, where it accumulates in the growth medium. At this time, the components of the indole response pathway are unknown. The signaling pathway may involve the Mtr permease, which transports indole into the cell (42). The net result of this putative signaling pathway is predicted to be a positive amplifying loop for indole production via the tnaAB operon.
Two targets of indole-mediated signaling are the astD and gabT genes. These genes function in pathways that degrade amino acids to pyruvate or succinate (1). Furthermore, tryptophanase enzyme (TnaA) is able to catabolize tryptophan, cysteine, and serine to pyruvate (35). Studies by Zinser and Kolter have shown that the ability to catabolize amino acids is an important parameter in the ability to persist and compete in stationary phase (44). This raises the possibility that signaling by indole may play a role in a pathway which prepares the cells for a nutrient-poor environment when the catabolism of amino acids becomes important for energy production.
ACKNOWLEDGMENT
This work was funded by National Science Foundation award MCB9904766.
REFERENCES
- 1.Baca-DeLancey R R, South M M T, Ding X, Rather P N. Escherichia coli genes regulated by cell-to-cell signaling. Proc Natl Acad Sci USA. 1999;96:4610–4614. doi: 10.1073/pnas.96.8.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 3.Cao J G, Meighen E A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- 4.Cheng Q, Campbell E A, Naughton A M, Johnson S, Masure H R. The com locus controls genetic transformation in Streptococcus pneumoniae. Mol Microbiol. 1997;23:683–692. doi: 10.1046/j.1365-2958.1997.2481617.x. [DOI] [PubMed] [Google Scholar]
- 5.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 6.Deeley M C, Yanofsky C. Transcription initiation at the tryptophanase promoter of Escherichia coli K-12. J Bacteriol. 1982;151:942–951. doi: 10.1128/jb.151.2.942-951.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunny G M, Brown B L, Clewell D B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci USA. 1978;75:3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhard A, Burglingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 9.Engebrecht J, Nealson K, Silverman M. Bacterial luminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuqua W C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 12.Garbe T R, Kobayashi M, Yukawa H. Indole-inducible proteins in bacteria suggest membrane and oxidant toxicity. Arch Microbiol. 2000;173:78–82. doi: 10.1007/s002030050012. [DOI] [PubMed] [Google Scholar]
- 13.García-Lara J, Shang L H, Rothfield L I. An extracellular factor regulates expression of sdiA, a transcriptional activator of cell division genes in Escherichia coli. J Bacteriol. 1996;178:2742–2748. doi: 10.1128/jb.178.10.2742-2748.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerth K, Metzger R, Reichenbach H. Induction of myxospores in Stigmatella aurantiaca (myxobacteria): inducers and inhibitors of myxospore formation and mutants with a changed sporulation behavior. J Gen Microbiol. 1993;139:865–871. [Google Scholar]
- 15.Grossman A D, Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci USA. 1988;85:4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Håvarstein L S, Coomaraswamy G, Morrison D A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holden M T G, Ram Chhabra S, de Nys R, et al. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol Microbiol. 1999;33:1254–1266. doi: 10.1046/j.1365-2958.1999.01577.x. [DOI] [PubMed] [Google Scholar]
- 18.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones S, Yu B, Bainton N J, Birdsall M, Bycroft B W, Chhabra S R, Cox A J R, Golby P, Reeves P J, Stephens S, Winson M K, Salmond G P C, Stewart G S A B, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser D, Losick R. How and why bacteria talk to each other. Cell. 1993;73:873–885. doi: 10.1016/0092-8674(93)90268-u. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura-Sato K, Shibayama K, Horii T, Iimuma Y, Arkawa Y, Ohta M. Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol Lett. 1999;179:345–352. doi: 10.1111/j.1574-6968.1999.tb08748.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim S K, Kaiser D. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of Myxococcus xanthus. Cell. 1990;61:19–26. doi: 10.1016/0092-8674(90)90211-v. [DOI] [PubMed] [Google Scholar]
- 23.Kleerebezem M, Quadri L E N, Kuipers O P, deVos W M. Quorum sensing by peptide pheromones and two-component signal transduction systems in gram positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 24.Latifi A, Winson M K, Foglino M, Bycroft B W, Stewart G S A B, Lazdunski A, Williams P. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson R, Solomon J, Grossman A D. Biochemical and genetic characterization of a competence pheromone from Bacillus subtilis. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 26.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 27.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell to cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 28.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pesci E C, Milbank J B J, Pearson J P, McKnight S, Kende A S, Greenberg E P, Iglewski B H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pesci E C. New signal molecules on the quorum sensing block: response. Trends Microbiol. 2000;8:103–104. doi: 10.1016/s0966-842x(00)01717-0. [DOI] [PubMed] [Google Scholar]
- 31.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature (London) 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 32.Pirhonen M, Flego D, Heikinheimo R, Palva E T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993;12:2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmond G P C, Bycroft B W, Stewart G S A B, Williams P. The bacterial enigma: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 34.Sitnikov D M, Schineller J B, Baldwin T O. Control of cell division in Escherichia coli: regulation of transcription of ftsQA involves both rpoS and SdiA mediated autoinduction. Proc Natl Acad Sci USA. 1996;93:336–341. doi: 10.1073/pnas.93.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snell E E. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol. 1975;42:287–333. doi: 10.1002/9780470122877.ch6. [DOI] [PubMed] [Google Scholar]
- 36.Solomon J M, Lazazzera B A, Grossman A D. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 1996;10:2014–2024. doi: 10.1101/gad.10.16.2014. [DOI] [PubMed] [Google Scholar]
- 37.Sonnenwirth A C. The enteric bacteria and bacteroides. In: Davis B D, Dulbecco R, Eisen H N, Ginsberg H S, editors. Microbiology. 3rd ed. Philadelphia, Pa: Harper & Row, Publishers, Inc.; 1980. pp. 645–672. [Google Scholar]
- 38.Sperandio V, Mellies J L, Nguyen W, Shin S, Kaper J B. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 1999;96:15196–15201. doi: 10.1073/pnas.96.26.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surette M G, Bassler B L. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willey J, Santamaria R, Guijarro J, Geistlich M, Losick R. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by S. coelicolor. Cell. 1991;65:641–650. doi: 10.1016/0092-8674(91)90096-h. [DOI] [PubMed] [Google Scholar]
- 41.Withers H L, Nordstrom K. Quorum-sensing acts at initiation of chromosome replication in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:15694–15699. doi: 10.1073/pnas.95.26.15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanofsky C, Horn V, Gollnick P. Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J Bacteriol. 1991;173:6009–6017. doi: 10.1128/jb.173.19.6009-6017.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-homoserine lactones. Nature (London) 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- 44.Zinser E R, Kolter R. Mutations enhancing amino acid catabolism confer a growth advantage in stationary phase. J Bacteriol. 1999;181:5800–5807. doi: 10.1128/jb.181.18.5800-5807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]