Abstract
Macroscopic neuroimaging modalities in humans have revealed the organization of brain-wide activity into distributed functional networks that re-organize according to behavioral demands. However, the inherent coarse-graining of macroscopic measurements conceals the diversity and specificity in responses and connectivity of many individual neurons contained in each local region. New invasive approaches in animals enable recording and manipulating neural activity at meso- and microscale resolution, with cell-type specificity and temporal precision down to milliseconds. Determining how brain-wide activity patterns emerge from interactions across spatial and temporal scales will allow us to identify the key circuit mechanisms contributing to global brain states and how the dynamic activity of these states enables adaptive behavior.
Introduction
The brain is a complex network comprising dozens of highly specialized and tightly inter-connected regions that work in concert to control behavior. Even relatively basic behaviors, such as perceptual decisions or goal-directed movements, depend on the coordination of neural activity across long-range brain networks. The temporally coordinated activity of spatially distant regions is commonly referred to as functional connectivity [1]. Until recently, measurements of functional connectivity on the brain-wide scale were only possible with non-invasive neuroimaging methods, such as functional magnetic resonance imaging (fMRI), electroencephalography (EEG), or magnetoencephalography (MEG). These macroscale measurement techniques provide global coverage but with limited spatial resolution (Fig. 1A), as they measure the average activity and connectivity of local brain volumes at a resolution of ∼ 1 mm (fMRI) to ∼ 1 cm (EEG, MEG).
Figure 1. Brain-wide functional connectivity across spatial scales.
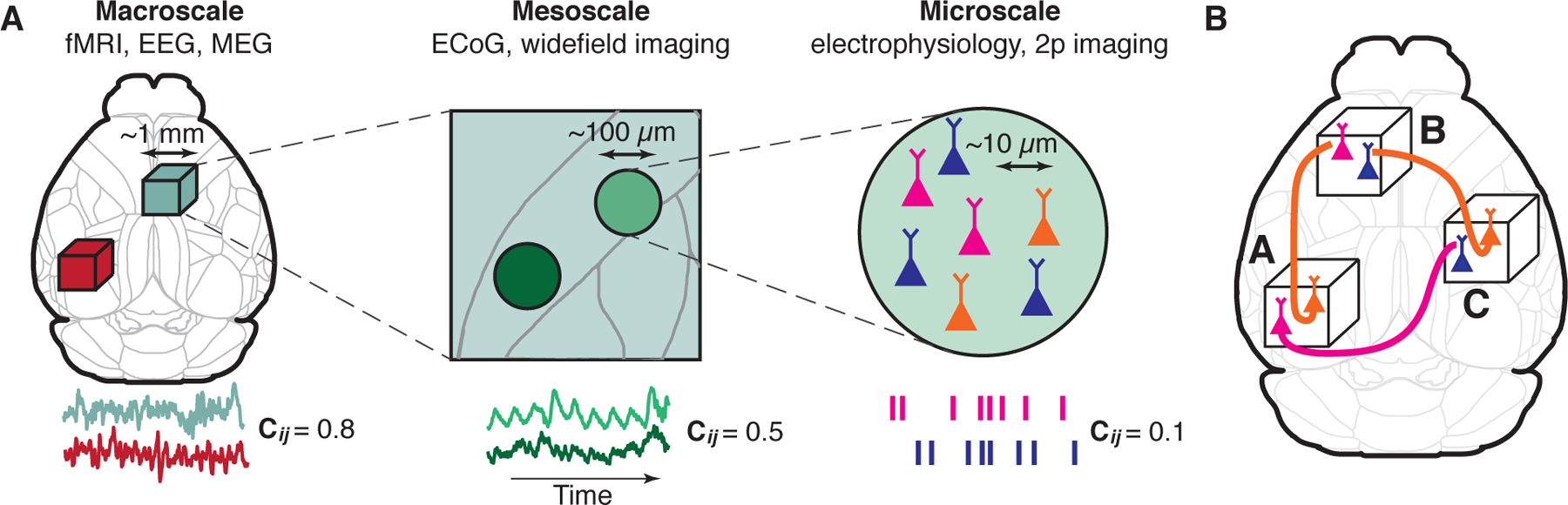
(A) Functional connectivity can be measured at the macro-, meso- and microscale resolution. Colored traces (lower row) illustrate time-series of brain activity recorded in two distant locations i and j. Functional connectivity cij is the correlation coefficient between these time-series. The correlation values typically increase with spatial scale. 2p imaging: two-photon optical imaging. See Box 1 for an explanation of the methods. (B) The diversity and specificity in responses and connectivity of different cell types can contribute to macroscale functional connectivity.
Macroscopic measurements reveal the global organization of distributed brain networks, but provide only a spatially coarse-grained estimation of the highly diverse and specific physiology and connectivity of the thousands of individual neurons contained in each local volume [2, 3, 4]. Indeed, consider an area A that has equally strong macroscale connectivity with two other areas B and C. It is possible that this connectivity is mediated by two distinct cell types within area A, each projecting to areas B and C exclusively without overlap (Fig. 1B). These cell types could have unique functional roles and communicate different information to their respective distal targets [5]. Conversely, similar inputs from areas A and C can target distinct cell types within area B, and the specific local connectivity of these cell types can process the inputs from A and C differently. Thus, identifying how the diversity and specificity of the brain’s microscale organization support the increasingly stable meso- and macroscale functional connectivity promises to reveal the specific brain computations which give rise to the coherent, distributed brain states supporting adaptive behavior.
The microscale cellular organization of brain networks can be probed with invasive recording methods, such as electrophysiology or optical imaging (Box 1). These microscopic measurements (Fig. 1A) reveal intricate mechanisms by which diverse cell types interact within local microcircuits to produce behaviorally relevant signals. Cellular-resolution methods have long been limited to small neural populations within a single or a few brain areas and thus could not reveal how neural signals are integrated across many areas to drive a unified behavioral output.
Box 1: Measuring and manipulating neural activity across scales.
Measurement
Electrophysiology:
Electrical activity in the brain can be measured using different techniques. While all electrical measurements have temporal resolution down to milliseconds, they vary greatly in their spatial resolution. Electrocorticography (ECoG) uses meso- or macroscale electrodes (~ 0.2–10 mm) to measure electrical activity at the brain’s surface. It is a complex mixture of the combined electrical potentials of approximately 1 cm3 around the ECoG electrode. Microelectrodes are small (~0.05–0.1 mm) and are inserted into the brain to measure the electrical potential in a small volume. Microelectrodes can detect both low frequency activity (local field potential) in a sphere with radius ~0.5–3 mm around the tip of the electrode, and high frequency activity (action potentials), within approximately ~0.05–0.35 mm. Compared to noninvasive EEG measurements, electrophysiology measurements have a similar temporal resolution but a more than 10-fold higher spatial resolution.
Optical imaging:
Optical imaging comprises a broad range of techniques which use intrinsic or fluorescent contrast to monitor brain activity. Two-photon imaging is a common technique which has subcellular spatial resolution (~1 µm) and an intermediate to slow temporal resolution (~0.01–1 s), depending on the scanning approach, volume imaged, and kinetics of the indicator. Widefield imaging sacrifices spatial resolution in favor of simultaneous imaging from large fields of view at the mesoscale (~0.2 mm) with an intermediate temporal resolution (~0.01 s). Compared with noninvasive fMRI measurements, invasive optical imaging techniques have a more than 10-fold higher spatial and temporal resolution.
Multimodal measurement:
Measurement modalities can be combined to overcome their shortcomings and benefit from their complementary features. For example, optical imaging can be combined with electrophysiological recordings or fMRI to enable simultaneous measurements of different signals across multiple scales.
Manipulation
Electrical stimulation:
Electrodes can be used not only for measuring electrical activity, but also for direct activation of cells and fibers in their vicinity. The spatial scale can be controlled based on the geometry of the stimulating electrode and the stimulation signal. However, with the exception of single cell stimulation, stimulation cannot be targeted to specific cell-types, or projections, and affects all membranes in the vicinity (including fibers of passage).
Optogenetics and pharmacogenetics:
Manipulating specific cell types is possible through the use of engineered receptors and channels that can be selectively expressed in cells of interest by means of genetic methods. Optogenetics refers to light-controlled proteins which can be expressed in specific cell types where they are integrated into the cell membrane and act as gates or pumps to hyperpolarize or depolarize neurons when activated by light (e.g., channelrhodopsin activates neurons when illuminated with blue light). In pharmacogenetics, selective activation is achieved through the use of small molecules. Extensive effort has gone into making opsins and DREADDs with a variety of actions (channels, pumps, and G-protein receptors), and which respond to different wavelengths or ligands.
Fortunately, recent technological advances enable measurements of cellular-resolution neural activity and connectivity on the global scale of the entire brain. In addition, new recording methods with global coverage and mesoscale spatial resolution (~100 µm, Fig. 1A), e.g., electrocorticography (ECoG) and widefield optical imaging, provide high-resolution spatial maps of neural activity bridging between the micro- and macro-scales. Mesoscale optical imaging can provide cell-type specificity through the use of calcium or voltage indicators expressed in specific genetically-defined cells and can also be combined with local cellular-resolution measurements, e.g., electrophysiology. Moreover, new technologies support perturbations of local and long-range functional connectivity with high temporal and spatial precision. These new approaches for mapping and perturbing large-scale brain dynamics with cellular-resolution open unique opportunities to reveal the mechanisms underlying macroscale functional connectivity, uncover functional specialization of diverse cell types, and test long-range connectivity through circuit perturbations.
Neural mechanisms contributing to functional connectivity across scales
In macroscale recordings, the coordination of whole-brain activity is traditionally characterized by pairwise correlations between brain regions, called functional connectivity. The correlations can arise in a variety of ways and do not imply a causal influence between functionally connected regions. For example, functional connectivity can reflect common inputs evoked by sensory events or driven by endogenous activity in other brain regions. One important distinction is therefore between correlations induced by task-evoked activity and correlations during spontaneous activity without task engagement, known as resting-state functional connectivity [1]. Concurrent task-related activation does not necessarily imply correlations during spontaneous activity. For example, a visuomotor task may cause concurrent activity in the visual and motor areas despite relatively low correlation between them at rest.
The macroscale resting-state functional connectivity exposes the intrinsic organization of brain-wide interactions, which provide a backdrop for any task-related activity. Analyses of fMRI recordings during rest established that spontaneous activity fluctuations are bilaterally symmetric and organized in multiple distributed networks of regions that activate and deactivate together, referred to as resting-state networks [6]. The resting-state networks correspond well with the regional co-activation patterns evoked across multiple tasks, suggesting that regions intrinsically connected during rest become concurrently active during tasks [7, 8, 9]. The resting-state functional connectivity also corresponds with the structural (anatomical) connectivity measured with MRI. Structural connectivity is defined at the macroscale as the existence of white matter tracts physically interconnecting brain regions, but it is not equivalent to the synaptic connectivity between cells. Macroscale structural connectivity is symmetric, i.e. oblivious to the directionality (source versus target) of connections, and it is insensitive to microscale features, such as synaptic strength, i.e. it may discount connections between certain neurons or neuronal assemblies that are nonetheless impactful. The functional and structural connectivity are moderately correlated at the aggregate level, although their precise relationship is complex [10]. For example, strong functional connectivity commonly exists between regions with no direct anatomical connection. The anatomical structure nevertheless constrains the strengths and statistics of resting-state functional connectivity [11].
Correspondence between functional connectivity across scales.
The meso- and micro-scale recordings corroborate the large-scale organization of brain-wide networks found with macroscale neuroimaging. At mesoscale resolution, widefield optical imaging in mice allows for recording neural activity simultaneously across the dorsal cortex using voltage sensitive dyes (VSDs) or transgenic animals expressing fluorescent voltage or calcium sensors in specific neuronal cell types (Box 1). Widefield recordings reveal rich spatiotemporal activity which, like in the macroscale measurements, consists of bilaterally symmetric patterns that involve each part of the cortex and occur during behavioral tasks as well as spontaneously without engagement in a specific task [16, 17, 18]. The spatial organization of mesoscale functional networks corresponds with large-scale anatomical connectivity [19, 20], akin to the relationship between the macroscale functional and structural connectivity measured with MRI [11].
The correspondence between meso- and macroscale functional connectivity can be probed further by approaches that combine widefield calcium imaging of neural activity with widefield imaging of hemodynamic activity [21] or even whole-brain fMRI [12]. These studies found that mesoscopic calcium signals, which are a proxy of the neural activity, predicted slower blood-oxygen-level-dependent (BOLD) responses using models that optimize a transfer function between the calcium and hemodynamic signals. These results support the idea that resting-state hemodynamics are coupled to underlying patterns of excitatory neural activity. Moreover, data-driven parcellation of the cortex-wide calcium activity into functional regions yielded a strong similarity with the parcellation derived from the simultaneously recorded BOLD activity. Thus, functional parcellations of calcium and fMRI data identify shared brain organization.
At microscale resolution, brain-wide functional connectivity can be measured down to single neurons by employing large-field-of-view optical imaging [22, 23, 24] or large-scale multielectrode arrays [25, 26, 27], especially Neuropixels probes [28, 29, 14, 30, 31]. Microelectrode arrays open access to functional connectivity on fast timescales and can detect sharp millisecond-precision spike-time correlations indicating direct synaptic coupling between neurons [32]. Inter-area spike-time correlations, which can be detected in large datasets as with high-density Neuropixels probes, reveal that the organization of inter-area functional connectivity precisely mirrors the anatomical hierarchy of cortical areas [31], once again confirming the relationship between the macroscale functional and structural connectivity.
Heterogeneity of functional circuits at meso- and microscale.
Beyond merely confirming macroscale observations, new recording approaches begin to reveal the diversity and specificity of functional connectivity at meso- and microscales. In simultaneous widefield calcium imaging and fMRI, the relationship between the strength of functional connectivity measured with calcium or BOLD varied by region and within versus across hemispheres (Fig. 2A) [12]. Such differences may arise from distinct contributions of excitatory versus inhibitory neurons to the BOLD activity [33]. For example, increased interhemispheric inhibitory activity could be causing a simultaneous increase in BOLD and decrease in excitatory activity. Thus, a regional and functional dependence of the relationship between calcium and BOLD connectivity could be cell-type specific. Isolating functional connectivity of distinct cortical cell types could reveal the unique computations they perform within the circuit. Using neural activity indicators expressed in specific cell types, multimodal imaging will continue to disentangle how diverse cortical cell types contribute to BOLD in the future.
Figure 2. Diversity and specificity of functional connectivity at the meso- and microscale resolution.
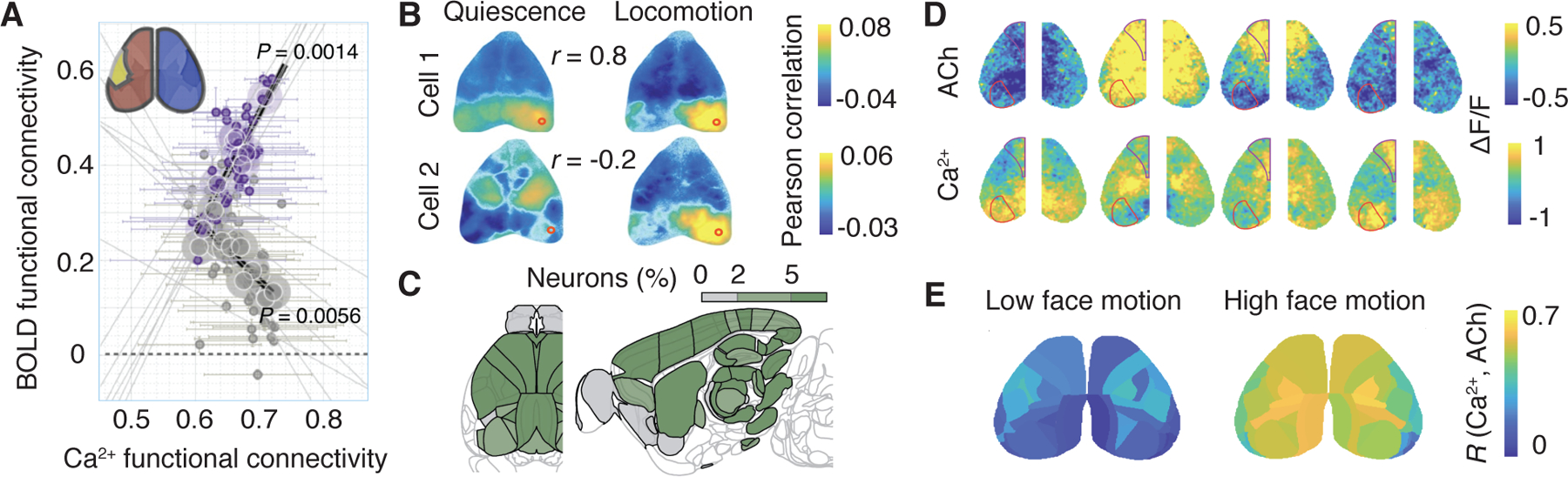
(A) Correlation between the calcium and BOLD functional connectivity in mice varies by region and within versus across hemispheres. For example, the calcium and BOLD functional connectivity strengths are correlated for the barrel field (yellow) and regions in the same hemisphere (red, purple dots), but anticorrelated for regions in the opposite hemisphere (blue, grey dots). Adapted from Ref. [12]. (B) Functional connectivity maps of nearby neurons are diverse and can change with behavioral state. Functional connectivity maps between spikes of two example neurons in V1 (rows, red circles indicate recording location) and the mesoscale calcium signal recorded simultaneously with widefield imaging during quiescence (left) and locomotion (right). r indicates 2D correlation of the quiescence versus locomotion functional connectivity maps. Adapted from Ref. [13] (C) Neural signals related to behavioral functions are widespread across many brain regions. Fraction of neurons in each brain region encoding action from Neuropixels recordings in mice during a decision-making task. Adapted from Ref. [14]. (D) The spatiotemporal variation of neuromodulation is differentially coupled to local neural activity. Example image frames showing fluctuations in simultaneously recorded cholinergic (upper row) and neural (lower row) activity across the dorsal cortical surface. Overlaid lines indicate primary visual and secondary motor areas. (E) The coupling between spatiotemporal cholinergic and neural activity depends on behavioral state. Average maps showing peak cross-correlation coefficients between cholinergic and neural activity for each cortical area during periods with low (left) and high (right) facial movement. D-E are adapted from Ref. [15].
The cell-type specificity of functional connectivity is most prominent at the level of single neurons. Microscale functional connectivity between single neurons can be characterized as correlations between firing rates (integrated over hundreds of milliseconds to seconds) known as spike-count (noise) correlations [34]. Spike-count correlations reflect coordinated changes in population firing rates within or across areas. Spike-count correlations depend on lateral distance, cortical layer, and cell type [23, 35, 36, 37, 38], pointing to a segregation of neural computations across anatomical dimensions of the cortex. At the microscale, inter-area correlations and communication rely on specific local neural ensembles [39, 40, 41, 42], suggesting a possible mechanism for selective routing of information across the brain. Brain-wide functional interactions of single neurons can be assessed efficiently with techniques that combine mesoscale widefield imaging with microelectrode recordings [43, 13, 44, 45] or two-photon imaging of single neuron populations [46, 45]. This multi-scale approach reveals that nearby cortical neurons can have distinct patterns of long-range functional connectivity, which depends on cell type and changes with behavioral state (Fig. 2B). These results suggest that large-scale brain organization may consist of multiple parallel networks that are supported by distinct cell types and carry out specific computations.
Changes in functional connectivity with behavioral state.
Functional connectivity is flexible, changing with behavioral state (e.g., resting versus alert) and task engagement, and meso- and microscale recordings are beginning to uncover underlying mechanisms. Neuropixels recordings show that neural signals related to cognitive and behavioral functions, such as sensory information, choice, action, or task engagement, are widespread across many brain regions [25, 26, 27, 14, 30]. These findings shift our perspective on information processing in the brain from local computations in specialized areas towards widely distributed processing across multi-area networks. These studies are enhanced by simultaneous monitoring of multidimensional behavioral information in mice, including pupil dilation, locomotion, facial movements, as well as ECoG [47, 48, 49, 50, 51]. In particular, movement-related signals, with or without any goal-directed task, dominate rodent neural activity across the brain (Fig. 2C) [50, 14, 51]. It is possible that the patterns of spontaneous movements characterize distinct behavioral states which can in part account for changes in functional connectivity.
Changes in behavioral state are largely mediated by neuromodulation. Generally, increasing arousal leads to desynchronization in the cortex manifested in a reduction in correlated activity, e.g., drop in spike-count correlations [52], which is in part driven by dynamically changing neuromodulation [53]. The dual mesoscopic imaging approach opens a possibility to test how spatial and temporal changes in neuromodulation affect functional connectivity. Widefield imaging can record neural activity concurrently with neuromodulatory signals across the neocortex, using genetically-encoded reporters of neuromodulators such as acetylcholine (ACh) [15]. While the action of ascending neuromodulatory systems is usually assumed to be brain-wide and homogeneous, mesoscopic imaging clearly demonstrates that neuromodulator dynamics are spatially heterogeneous and differentially coupled to local neural activity across cortical areas (Fig. 2D). Moreover, behavioral states, defined by distinct patterns of motor activity, were associated with distinct spatiotemporal patterns of neuromodulation and their coupling to neural activity (Fig. 2E) [15], elucidating one mechanism for state-dependent changes in functional connectivity.
Together, these new approaches for mapping functional connectivity at the meso-, micro-,and multi-scale resolution open avenues not only for understanding the physiological sub-strates of macroscale connectivity, but also for revealing distributed mechanisms of information processing that depend on the diversity and specificity of single-neuron responses and connectivity.
Temporal dynamics of functional connectivity patterns
Timescales of neural correlations.
While static correlations are the basic building blocks of functional connectivity measurements, for the brain to flexibly adapt and act in a changing world, the correlation patterns must change dynamically. The timescale at which dynamic changes in connectivity can be observed is determined by the temporal window over which neural correlations are computed. There is overwhelming evidence that neural activity is correlated at all timescales. Correlations between the spike times of nearby neurons [54, 32] and even of neurons far apart in different areas [31] can happen on timescales as fast as several milliseconds (Fig. 3A). Spike-count (noise) correlations, on the other hand, reflect correlations between firing rates of neurons at the temporal resolution at which these firing rates are estimated (typically on the order of tens of milliseconds to seconds). Unlike spike-time correlations, which indicate direct synaptic interactions in a specific pair of neurons, spike-count correlations typically reflect changes of firing-rates coordinated across larger populations. Thus, macroscale measurements can reflect spike-count correlations but are oblivious to spike-time correlations. Mesoscopic population measures such as the LFP can show coupling between different areas for several seconds, visible as coherence in distinct frequency bands (Fig. 3A). Macroscopic measures of neural population activity such as EEG can exhibit coordination on even slower timescales. For example, fluctuations in electrophysiological signals in the 0.01–0.1 Hz range (slow cortical potentials or SCPs) [55] have a correlation structure similar to that observed with fMRI [56] (Fig. 3A). Similar widespread patterns of coordinated activity are also evident in the slow variation of single neuron spiking, which exhibit correlations at infraslow timescales that reflect the animal’s behavioral state [57, 58].
Figure 3. Temporal dynamics of functional connectivity measurements.
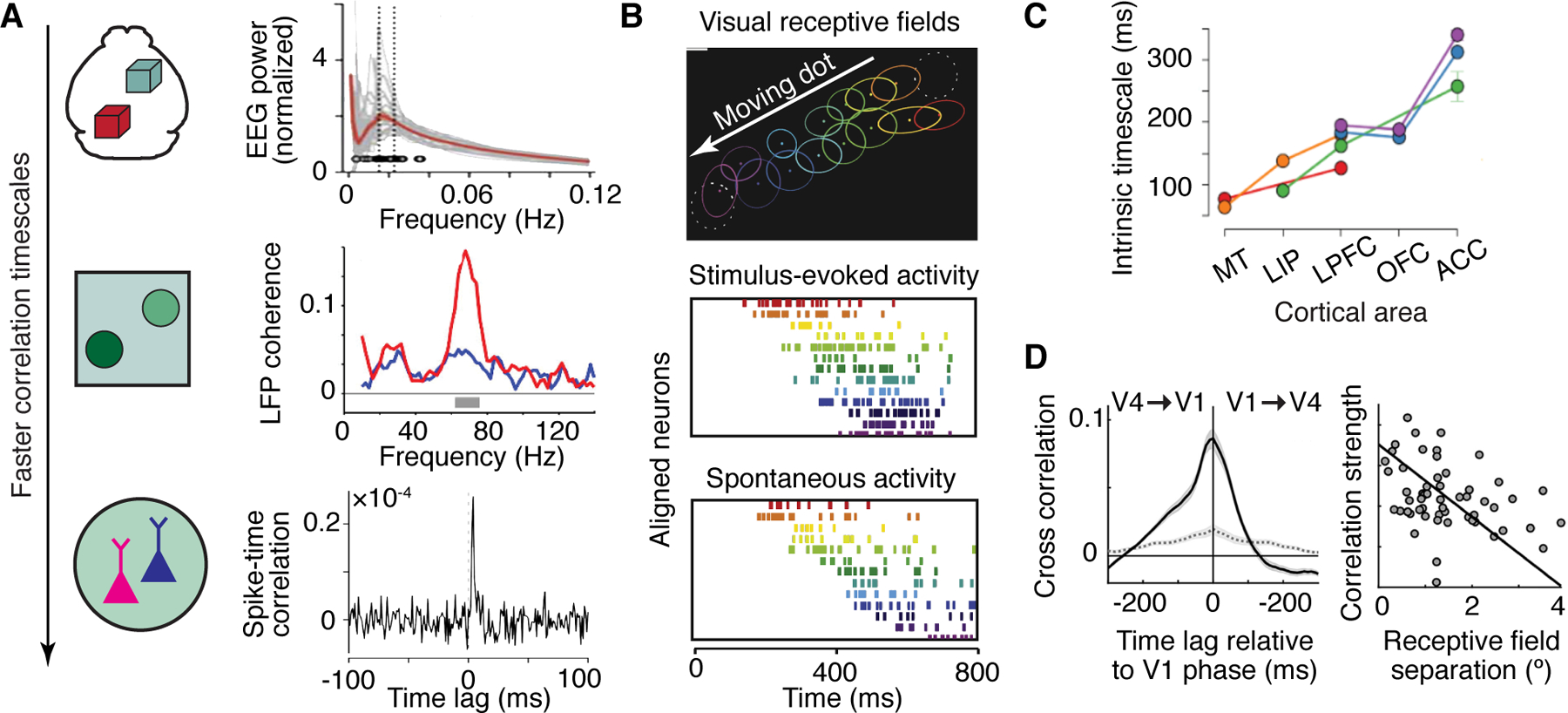
(A) The spatial scale at which functional connectivity is measured largely dictates its temporal resolution. Macroscale EEG measurements of resting-state networks show highest power in ultra-slow frequencies (top, adapted from Ref. [70]. Connectivity in the LFP signal of distant neural populations can appear as coherence in a particular frequency band, as shown here for visual cortical areas V1 and V4 in monkey that are coherent in the gamma band (middle, adapted from Ref. [64]). Pairs of single neurons can be synchronous in their spike times at the millisecond resolution, which manifests as a peak in their cross-correlogram shown here for two neurons in mouse areas V1 and LM (bottom, adapted from Ref. [31]). (B) Firing sequences of neurons can repeat precisely, shown here for rat visual cortex neurons with neighboring receptive fields (top), either as a result of visual experience (middle), or in spontaneous activity (bottom). Adapted from Ref. [67]. (C) Different brain areas display different intrinsic timescales, depending on their position in the cortical hierarchy, shown here for the macaque visual-frontal hierarchy. Different colors represent different data sets. Adapted from Ref. [71]. (D) Cross-correlation between time series of phases with high (On) and low (Off) spiking activity in V1 and V4 relative to V1 phase during a spatial attention task (left, dashed gray line - shuffle predictor). The correlation strengths (area under the curve) decreases with the receptive field separation (right). Adapted from Ref. [72].
Spatial coarse-graining is one possible reason for the prominent slow coordination of mesoscopic and macroscopic neural population activity, while faster timescales dominate in local spiking activity. In recurrent network models, local correlations on multiple timescales can arise from structured connectivity, and integrating activity over larger spatial scales eliminates faster timescales leaving only slower timescales in the coarse-grained activity [59]. Another possible explanation is the spatial scale at which neural activity is synchronized at different timescales [60]. While large-scale networks tend to synchronize at slower frequencies (1–30 Hz), local populations can synchronize at fast frequencies (30–200 Hz). This dissociation is likely the result of distinct generators. Slow synchronization might arise from cortical-subcortical interactions and be transmitted by long-range projections synchronously across large areas [61]. Fast synchronization, on the other hand, often relies on spatially localized feedback inhibition [62]. As a result, the summation of the highly distributed and synchronous currents underlying low frequency activity emerge as dominant in spatial averaging at the macroscale, while currents underlying faster synchronization are disparate and local, therefore canceling out. Indeed, in cases when neural populations are highly synchronized, such as during hippocampal ripples [63] or task-induced gamma oscillations [64], mesoscopic measurements reflect synchronous events at much faster timescales. The wide spread of temporal scales over which correlations can occur implies that many correlations stay under the radar for fMRI because of its low temporal resolution. Therefore, fMRI functional connectivity is an incomplete account of the correlations present in the brain at any one moment in time.
Dynamic correlations at the microscale.
One example of events that stay invisible to fMRI, are fast dynamic changes in correlations. Dynamic motifs such as sequences of active neurons that display precise repetitions of spike patterns can emerge with millisecond accuracy [65, 66]. The spontaneous firing sequences might be a reverberation of sensory experience and are often replayed with compressed timing [67] (Fig. 3B). A neuron’s participation in a particular firing sequence is strongly governed by stable factors such as its anatomical connections [68], as well as dynamic factors such as stimulus properties [69], highlighting the importance of specificity and diversity for functional dynamics at the microscale.
A well-known example of a dynamic motif is hippocampal replay activity. Firing sequences of hippocampal place cells during active exploration in the awake animal tend to be replayed during subsequent sleep [73, 74]. They can also be replayed immediately following the exploration in reverse order, which is consistent with reinforcement learning models posing that a sequence of events in reverse order is paired with a decaying dopamine signal, to associate the entire sequence with a reward at the end [75]. Hippocampal replay activity is largely dependent on the circuitry of the hippocampus [76], i.e. reflects anatomical connectivity. However, it plays a functional role, as it is thought to be essential for the consolidation of event memories in hippocampal-neocortical networks [77]. Direct evidence for this comes from fMRI recordings triggered on hippocampal ripples, which are high frequency waves in the LFP that coincide with replay events [63]. These fMRI recordings showed that most of the cortex is activated during the ripples, whereas most subcortical structures are silenced [78, 79]. Therefore, local replay events are part of a brain-wide phenomenon of interacting thalamocortical and hippocampal networks [80, 81] that orchestrate memory consolidation. Similar sequential patterns of activity are also found at a much coarser spatial and temporal scale in human fMRI data [82, 83].
Functional specialization on different timescales.
The timescale at which neural correlation patterns change is governed by several factors, including the functional specialization of the respective neural populations or brain areas. Contrary to fast dynamic motifs, the resulting differences in correlations will be visible to fMRI. For example, in primates the frontal cortices, which exhibit persistent activity during mental tasks such as working memory [84], exhibit correlated fluctuations over longer timescales than the primary sensory areas, with shorter integration times of sensory input [71, 85] (Fig. 3C). Similarly, auditory cortex neurons in mice show functional coupling over much shorter timescales than posterior parietal cortex neurons, leading to a population code that represents stimulus and choice information much quicker [86]. Invasive ECoG recordings in humans confirm these findings, and associate the different timescales with a number of biological processes, such as gene activity responsible for excitatory and inhibitory connections between neurons [87]. In addition, the timescales can be flexible [59], e.g., lengthening when areas specialized in working memory are actively maintaining information, and shortening with age across many areas of the brain [87]. Temporal organization along the principal sensorimotor-to-association axis is also found at the macroscale in human fMRI data. Resting-state network activity is hierarchically organized as temporal gradients across the cerebral cortex [88] as well as subcortical structures [89] and divides into two distinct sets of networks. The transitions between networks occur at shorter timescales within, than between, these two sets, which represent the sensorimotor systems and higher order cognition, respectively [90].
The mesoscale functional connectivity can also be specific for timescales of interactions between brain regions. In particular, large-scale ECoG recordings in monkeys provide access to the broad spectrum of timescales in the local field potentials (LFP) [64, 91, 92]. Interactions on different timescales manifest in the inter-areal LFP coherence at different frequencies. This coherence is strongest in distinct frequency bands depending on the direction of interactions—feedforward versus feedback—through the visual hierarchy [93]. Feedforward influences occur in theta-band (~4 Hz) and gamma-band (~60–80 Hz), and feedback influences occur in beta-band (14–18 Hz). As gamma-band synchronization predominates in superficial and beta-band synchronization in deep cortical layers [94, 95], the asymmetries in directed influences are likely related to the laminar pattern of inter-area anatomical projections. Thus, the specificity of functional connectivity for timescales could result directly from its cell-type specificity.
Changes in dynamic correlations with cognitive states.
Dynamic changes in functional connectivity depend not only on stable factors, such as the functional properties of neurons, but also on fluctuating factors such as cognitive state and task context. Cognitive tasks affect local and long-range interactions between areas, and can thereby change functional connectivity with spatial specificity [96]. For example, the human brain traverses between functionally segregated states and states with a lot of integration between disparate brain regions, and during integrated states, performance on a cognitive task is faster and more accurate [97]. Moreover, integrated states induced by cognitive tasks amplify individual differences in the patterns of functional connectivity and thus better reveal existing brain-behavior relationships [98]. Importantly, changes in functional connectivity in this case result from fluctuations in brain state induced by the tasks and are not a (trivial) outcome of multiple brain areas being co-activated during the task. Another prominent example of brain state being altered locally and with temporal precision is spatially selective attention [99]. The coordination of inter-areal neural activity is also modulated during attention, in particular through increased coherence between neural populations with overlapping receptive fields [64, 91] and via top-down coordination of On- and Off-phases of spiking activity in a retino-topically precise manner [72] (Fig. 3D). Besides selective attention, other cognitive processes can also shape inter-areal functional connectivity. For example, flexible sensorimotor choices are the outcome of different integration processes of sensory cortex and frontoparietal cortex activity, depending on task context [25].
Thus, functional connectivity changes dynamically over a wide range of timescales defined by (besides anatomy) functional properties, behavioral state, as well as task context. Animal studies aid in elucidating the role of these factors by permitting a larger repertoire of behaviors than is possible in stationary humans (e.g., running mice) and by enlarging the palette of possible manipulations of cognitive state (e.g., manipulating acetylcholine levels to study its effect on attention [100]).
Perturbations to probe functional connectivity
While characterizing functional connectivity across spatial and temporal scales continues to reveal rich, intricate structure, it is ultimately desirable to test our understanding of these dynamics and their relation to behavior by perturbing brain activity. Perturbations have long provided valuable insight into brain function at multiple scales both through chronic interference and direct manipulation of brain activity [101]. Critically, the highly specific interventions necessary to probe brain dynamics across scales are currently only possible with invasive methods.
Determining how the brain’s microscale features give rise to meso- and macroscale organization requires precise control over perturbations. Innovations in the past decade have expanded the palette of interventional tools, introducing new engineered channels and receptors that increase the specificity of perturbations [102, 103, 104] (Box 1). These advances enable cells to be targeted using combinations of genetic, functional or anatomical features and facilitate temporally precise control with subcellular resolution in awake behaving animals (Fig. 4A). In the limit, perturbations can mimic physiological activity patterns observed in the same animals (Fig. 4A), and neuronal activity can influence experimental parameters in real-time by establishing a closed-loop [105]. Together with advances in recording technology, new perturbation methods have opened a world of possibilities for manipulating brain activity at a desired scale while tracking its effect across the intact system.
Figure 4. High specificity perturbations across spatial scales.
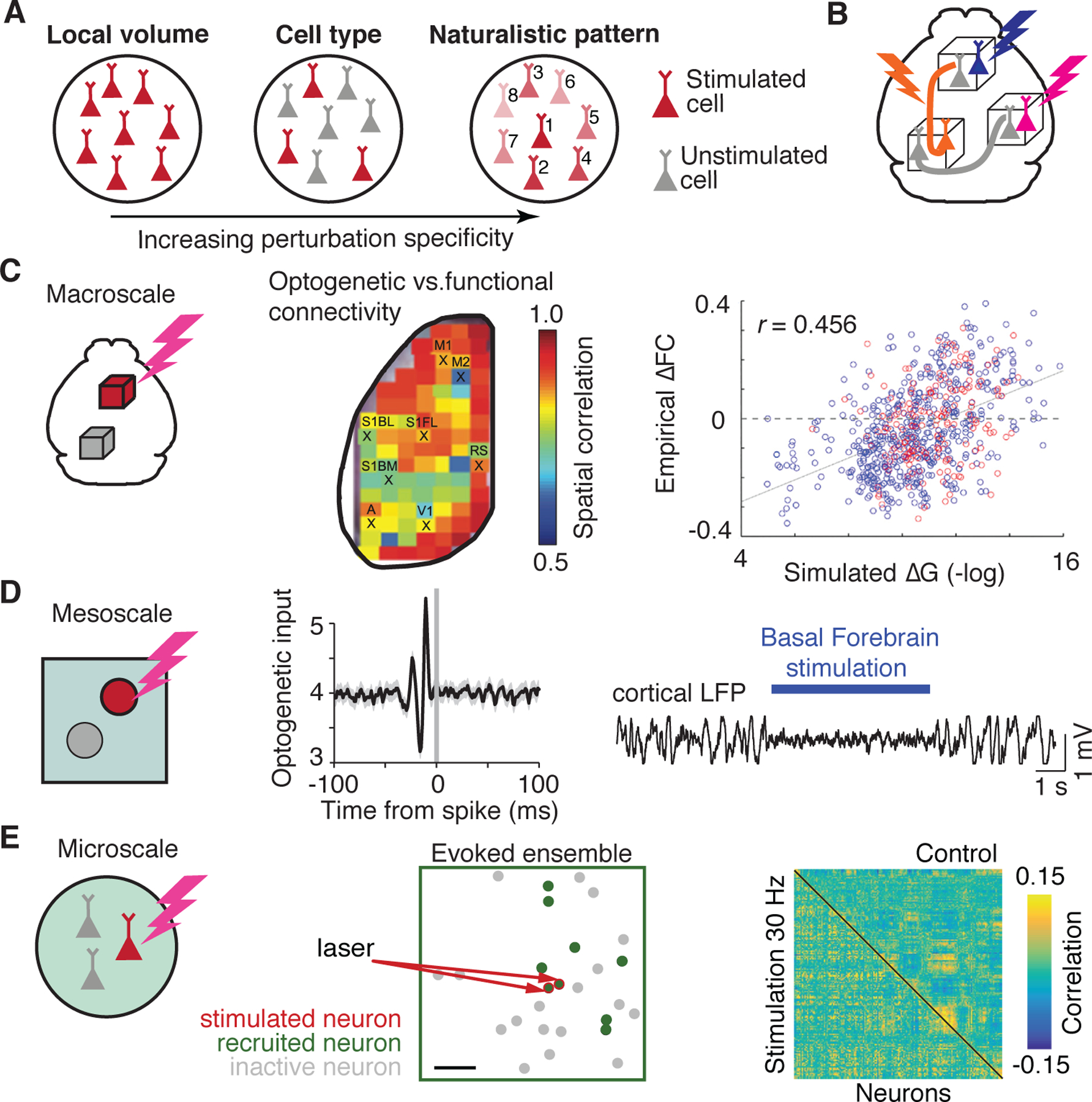
(A) Specificity and temporal precision of perturbations. Classical perturbations affect all membranes within a target volume (left). Genetic and viral approaches permit targeting of specific cell types (middle). Optical approaches permit cells to be targeted in naturalistic sequences (right). (B) Perturbations of high specificity and control. Individual neurons in distant brain areas targeted based on functional, genetic, or projection considerations and manipulated to assess their role in global brain states. (C) Macroscopic perturbations. Intrinsic optical imaging of hemodynamics was performed while local cortical areas were activated with an excitatory opsin. This enabled a comparison between optogenetic and functional connectivity (left, adapted from Ref. [112]). Whole brain fMRI was performed in rhesus monkeys while the amygdala was inhibited. Inhibition altered functional connectivity consistent with anatomical connectivity (right, adapted from Ref. [115]). (D) Mesoscopic perturbations. Electrophysiology and optogenetics were performed in visual cortex to assess the effect of temporally varying input. Input generated strong synchronization in the gamma band and led to preferential transfer of coherent inputs (left, adapted from Ref. [119]). Neuronal activity was monitored in the visual cortex while the basal forebrain was activated. Activation changed cortical state, increasing the discrimination performance of mice (right, adapted from Ref. [53]). (E) Microscopic perturbations with simultaneous 2-photon calcium imaging and single-cell optogenetics in mouse cortex. Neurons were functionally characterized according to visual selectivity. In the absence of visual stimuli, targeted stimulation of neurons recruited stimulus-related ensembles and induce behavior (left, adapted from Ref. [125]). Groups of neurons were artificially stimulated to alter their correlation structure. Targeted neurons increased and non-target neurons decreased their correlation relative to baseline (right, adapted from Ref. [126]).
Macroscale perturbations reveal regional diversity in structure-function relationships.
Macroscale interventions can be used to probe signal flow across brain networks and the distributed effects of diverse projection systems. The activation of local inhibitory interneurons can be used to identify key nodes in distributed networks by producing temporary lesions [106, 107, 108]. Control over the location and timing of lesions has demonstrated that while task-related information may be broadly distributed across cortical sites, specific locations are critical to unique periods of task execution or learning [109, 106, 107, 108, 110]. Although the interpretation of such manipulations can be complicated by compensatory mechanisms and off-target effects [111], these experiments confirm that neural activity relevant for behavior is spatially and temporally specific.
While macroscopic perturbations generally affect global brain activity in a manner consistent with anatomical connectivity, altering activity in cortical sites has downstream effects that are region and projection specific [112, 113, 114]. Such regional variation was demonstrated by combining intrinsic hemodynamic imaging of the dorsal cortex of the awake mouse with optogenetic activation of local cortical populations [112]. This approach permitted a direct comparison of optogenetically-evoked cortical activation with mesoscale functional connectivity, revealing considerable regional heterogeneity (Fig. 4C). Optogenetic activation matched functional connectivity most for somatomotor and midline structures, but diverged for sensory and association areas. Interestingly, optogenetically-evoked activity and functional connectivity were more similar to each-other than either was to anatomical connectivity estimated by viral tracing. Some of these differences may result from the spontaneous behaviors of the animal which directly engage the somatomotor system. Further studies are necessary to probe how the degree of correspondence for individual cortical sites varies with the behavioral state of the animal and the scale at which connectivity is estimated.
Heterogeneity is similarly found when perturbing distinct subcortical sites, which may have widespread effects on brain dynamics depending on their projection pattern and synaptic features. Pharmacogenetic inhibition of the amygdala during fMRI in anaesthetized rhesus monkeys revealed wide-spread, network-specific alterations of functional connectivity that could be partially predicted using an anatomically-constrained computational model [115] (Fig. 4C). The limbic and default mode networks, which have a high degree of anatomical and functional connectivity to the amygdala, decreased their connectivity. Importantly, changes in functional connectivity occurred also for edges that did not include the amygdala, both as decreases in amygdala-related networks and as increases in amygdala-unrelated networks, such as the somatomotor network. Alternate subcortical structures have distinct effects on global brain activity and functional connectivity. For example, pharmacogenetic activation of the locus coeruleus results in network-specific increases in connectivity, notably in the salience network [116], whereas pharmacological inactivation of the basal forebrain reduces shared variability in a topographic manner, but it does so irrespective of network [117].
Overall, macroscale perturbations can be used to track signal flow across intact brain structures and evaluate the influence of specific projections on large-scale, distributed patterns of brain activity. The sites identified as key nodes relevant to particular behaviors, or projections that give rise to deviations from anatomical expectations indicate appealing targets for further investigation at finer scales.
Mesoscale perturbations probe the brain’s intrinisic dynamics.
Mesoscale perturbations permit increased spatial and temporal control enabling manipulations of higher fidelity. They have been targeted to long-range cortico-cortical [118, 110, 119, 120], thalamocortical [121, 122, 123, 124] and modulatory [53] projections. This refined spatial control permits investigation of specific cortico-cortical projections, such as those arising in frontal regions and targeting primary sensory areas, demonstrating highly specific alterations of population activity that are topographic, feature-dependent, and sensitive to the animal’s history of reward [118, 110].
Improved resolution also reveals fine-grained temporal features that are obscured by macroscopic investigations, either technically due to reduced resolution, or because the activation of large populations of diverse elements blurs the temporal precision of the constituents. Precise temporal control facilitates time-varying manipulations that can probe the dynamics of populations or isolated projections. For example, driving excitation in a population of cortical pyramidal cells with broadband temporal white-noise demonstrated preferential transmission of fast, gamma-band input components (Fig. 4D) [119], a feature that would be missed with slow or tonic stimulation. Another study used closed-loop coupling to link reward with focal brain activity, demonstrating that mice could rapidly establish volitional control over brain activity and alter brain-wide functional connectivity [120]. These results indicate the high degree of selectivity of cortico-cortical connectivity both in terms of spatial and temporal structure, but also its sensitivity to behavior and experience.
Mesoscale perturbations targeted to subcortical sites also demonstrate a high degree of specificity and sensitivity to behavior. For example, perturbation to primary thalamic nuclei can alter both cortical and subcortical activity in a behavior dependent manner [124]. Intriguingly, activation of higher order thalamic nuclei gives rise to sustained, reverberatory activity in the primary sensory cortex that is not observed for primary thalamic or cortical input [123], suggesting a role in amplifying or sustaining signals arriving from other sources. Activation of the basal forebrain projection to the primary visual cortex of mice markedly reduced synchronization and shortened the timescale of cortical activity (Fig. 4D) [53], leading to an increase in responsiveness and improved visual discrimination. Similar changes were observed when activating thalamic projections to the mouse somatosensory cortex [122], suggesting that multiple subcortical-cortical pathways can have partially overlapping mesoscopic profiles. Overall, mesoscopic studies have highlighted how long-range projections can shift the temporal dynamics of local circuits and underscored the importance of timing for interactions between populations.
Microscale perturbations reveal the exquisite specificity and plasticity of brain organization.
Microscale interventions have greater resolution still, revealing cellular and subcellular specificity in local populations. Studies have focused on monitoring activity densely within a population, often using two-photon calcium imaging, while perturbing individual neurons or small groups of neurons. Perturbations have focused on the effect of nearby neurons on the local population [127, 128, 125, 129, 130, 126, 131, 121], or long-range projections to the local circuit [132, 121, 110]. These studies highlight the heterogeneity of the brain’s microscale architecture, as the effects of a perturbation are highly dependent on the targeted population and on the characteristics of the local circuit. In general, the effects of microscale perturbations are similar to expectations from anatomical connectivity [133], and the timing of signals is of great importance for their effect on downstream elements and plasticity [134, 135]. Within a local circuit, local and long-range excitatory projections target specific cell classes with subcellular precision, determining their effect on the cell’s activity and the dynamics of the local circuit [121, 132]. Likewise, distinct classes of inhibitory neurons deferentially regulate lateral, as compared to, laminar flow of information within the local population [127]. Such studies continue to elaborate the exquisite specificity of the brain’s microcircuits, revealing the diverse circuit motifs that populate a given brain region, defining it’s computational capacity and determining signal flow [136].
Recently, experiments have attempted to probe [129, 130], or alter the correlation structure between excitatory cells in a local population [125, 126, 131]. Selective stimulation of functionally characterized neurons in the mouse visual cortex can recruit ensembles of similarly tuned cells and boost the animal’s visual discrimination (Fig. 4E) [125]. In some cases, the correlation structure between neurons can be rapidly altered based on imposed activation patterns (Fig. 4E) [126], while in others, the intrinsic correlation structure is robust to perturbation [131]. Likewise, local and long-range projections can have disparate effects on a circuit depending on their targets [132, 118], the similarity of the cells’ tuning [129, 125], and the behavioral state of the animal [110]. The heterogeneity of these results, combined with the closer relationship of anatomical and functional connectivity at the microscale provide an exciting platform on which to investigate structure-function relationships that are currently intractable at the macroscale. The fact that some circuits are more prone to modification than others, and the diversity of effects already observed at the microscale reflect the intricate and highly-specified wiring of neural circuits and point to their powerful computational capacity.
Bringing the levels together.
To date, most studies have targeted perturbations and assessed their effects at the same scale of organization. We therefore still have little understanding of how the high degree of diversity and specificity observed at fine spatial and temporal scales gives rise to stable patterns of activity at the meso- and macroscale. Understanding how microscale diversity gives rise to stability depends on studies that bridge scales, ideally perturbing sequences of cells in distributed brain areas while monitoring whole brain activity with cellular resolution. New perturbation methods provide unprecedented control over brain circuits and will continue to reveal how microscale signals are routed and integrated into distributed, whole-brain states.
Conclusions
The methods commonly used in human neuroscience (fMRI, EEG and MEG) measure functional connectivity at the macroscale, offering brain-wide spatial coverage but missing the diversity and specificity of the constituent neurons. Invasive approaches in animals overcome this limitation by measuring connectivity at the meso- and microscale, providing details about the intricacies and exquisite precision of the brain’s dynamics on these finer scales. The brain’s functional networks are coordinated across all levels of spatial and temporal organization to support flexible behavior. However, we still lack a cohesive perspective on how the detailed connectivity and dynamics at the micro- and mesoscales give rise to the global brain states observed with macroscale measures.
Promisingly, mesoscale measurements have confirmed that the large-scale networks observed at the macroscale are also present at the mesoscale. However, mesoscale measurements have revealed a large degree of variability in the temporal and spatial dynamics of these large-scale networks, and implicate a variety of underlying sources, obscured at the macroscale. It seems likely that the coarse-grained estimates at the macroscale reflect consistent biases that are present in finer scales of organization, while micro- and mesoscale measurements reveal a greater degree of specificity and variability. It is an important open question how individual neuronal elements contribute to large-scale patterns and how their variation around the coarse-grained patterns reflect important features of computation in the brain. Increasing evidence suggests that such higher-order variation reflects behaviorally relevant activity, rather than physiological noise [137, 138, 139, 140]. The degree to which integrated brain states depend on the organization at fine resolutions requires additional studies.
New technologies for measurement and perturbation continue to rapidly develop and, together with advances in new behavioral approaches, will increase the scope of questions experimentally addressable. New activity indicators will reveal the unique spatial and temporal dynamics of the diverse neurotransmitters and signalling molecules active in the brain. More precise measurement and greater control over interventions will enable a better understanding of how the activity of identified cells and pathways is integrated and distributed into global brain states in the service of behavior. Additionally, the further refinement of analysis tools and the close integration of modelling and experiment will continue to improve our understanding of brain function and how the brain integrates arriving signals from the periphery into ongoing patterns of distributed brain activity. Ultimately, this will aid in determining the organizational principles underlying global brain states and how brain activity is dynamically routed and integrated across spatial and temporal scales.
Highlights.
Nearby neurons exhibit diverse patterns of local and long-range functional connectivity, that depend on cell type and change with behavioral state.
Neural activity is coordinated within and across areas on timescales from milliseconds to minutes and hours.
Perturbing neuronal activity with high specificity and precision permits control over the diverse pathways facilitating global brain states.
Acknowledgements
The authors acknowledge financial support by NIH grants R01 EB026949 and RF1DA055666 (T.A.E.), Alfred P. Sloan Foundation Research Fellowship (T.A.E.), the Max Planck Society (M.L.S.), Forschungskredit from the University of Zürich (project K-41220-04, C.M.L.) and the European Research Council (ERC Advanced Grant BRAINCOMPATH, project 670757, C.M.L.).
Footnotes
Conflict of interest
None.
Contributor Information
Tatiana A. Engel, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724.
Marieke L. Schölvinck, Ernst Strüngmann Institute for Neuroscience in Cooperation with Max Planck Society, Frankfurt am Main, Germany.
Christopher M. Lewis, Laboratory of Neural Circuit Dynamics, Brain Research Institute, University of Zürich, Zürich 8057, Switzerland.
References
- 1.Friston KJ. Functional and Effective Connectivity: A Review. Brain Connectivity 2011;1(1):13–36. [DOI] [PubMed] [Google Scholar]
- 2.Kebschull JM, Garcia da Silva P, Reid AP, Peikon ID, Albeanu DF, Zador AM. High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA. Neuron 2016;91(5):975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris JA, Mihalas S, Hirokawa KE, Whitesell JD, Choi H, Bernard A, Bohn P, Caldejon S, Casal L, Cho A, Feiner A, Feng D, Gaudreault N, Gerfen CR, Graddis N, Groblewski PA, Henry AM, Ho A, Howard R, Knox JE, Kuan L, Kuang X, Lecoq J, Lesnar P, Li Y, Luviano J, McConoughey S, Mortrud MT, Naeemi M, Ng L, Oh SW, Ouellette B, Shen E, Sorensen SA, Wakeman W, Wang Q, Wang Y, Williford A, Phillips JW, Jones AR, Koch C, Zeng H. Hierarchical organization of cortical and thalamic connectivity. Nature 2019;508:207–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitesell JD, Liska A, Coletta L, Hirokawa KE, Bohn P, Williford A, Groblewski PA, Graddis N, Kuan L, Knox JE, Ho A, Wakeman W, Nicovich PR, Nguyen TN, van Velthoven CTJ, Garren E, Fong O, Naeemi M, Henry AM, Dee N, Smith KA, Levi B, Feng D, Ng L, Tasic B, Zeng H, Mihalas S, Gozzi A, Harris JA. Regional, Layer, and Cell-Type-Specific Connectivity of the Mouse Default Mode Network. Neuron 2021;109(3):545–559.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang ZJ. Toward a Genetic Dissection of Cortical Circuits in the Mouse. Neuron 2014;83(6):1284–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8(9):700–11. [DOI] [PubMed] [Google Scholar]
- 7.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America 2009;106(31):13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di X, Gohel S, Kim EH, Biswal BB. Task vs. rest-different network configurations between the coactivation and the resting-state brain networks. Front Hum Neurosci 2013;7(SEP):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron 2014;83(1):238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uddin LQ. Complex relationships between structural and functional brain connectivity. Trends Cogn Sci 2013;17(12):600–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America 2009;106(6):2035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lake EMR, Ge X, Shen X, Herman P, Hyder F, Cardin JA, Higley MJ, Scheinost D, Papademetris X, Crair MC, Constable RT. Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI. Nat Methods 2020;13:157–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clancy KB, Orsolic I, Mrsic-Flogel TD. Locomotion-dependent remapping of distributed cortical networks. Nature neuroscience 2019;503:51–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinmetz NA, Zatka-Haas P, Carandini M, Harris KD. Distributed coding of choice, action and engagement across the mouse brain. Nature 2019;551:232–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohani S, Moberly AH, Benisty H, Landa B, Jing M, Li Y, Higley MJ, Cardin JA. Dual color mesoscopic imaging reveals spatiotemporally heterogeneous coordination of cholinergic and neocortical activity. bioRxiv preprint available at 10.1101/2020.12.09.418632v1 2020;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohajerani MH, McVea DA, Fingas M, Murphy TH. Mirrored Bilateral Slow-Wave Cortical Activity within Local Circuits Revealed by Fast Bihemispheric Voltage-Sensitive Dye Imaging in Anesthetized and Awake Mice. J Neurosci 2010;30(10):3745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanni MP, Chan AW, Balbi M, Silasi G, Murphy TH. Mesoscale Mapping of Mouse Cortex Reveals Frequency-Dependent Cycling between Distinct Macroscale Functional Modules. The Journal of Neuroscience 2017;37(31):7513–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDowell CJ, Buschman TJ. Low-Dimensional Spatiotemporal Dynamics Underlie Cortex-wide Neural Activity. Current Biology 2020;30(14):2665–2680.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohajerani MH, Chan AW, Mohsenvand M, LeDue J, Liu R, McVea DA, Boyd JD, Wang YT, Reimers M, Murphy TH. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nature neuroscience 2013;16(10):1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Kebschull JM, Furth D, Musall S, Kaufman MT, Churchland AK, Zador AM. BRICseq Bridges Brain-wide Interregional Connectivity to Neural Activity and Gene Expression in Single Animals. Cell 2020;182(1):177–188.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y, Shaik MA, Kozberg MG, Kim SH, Portes JP, Timerman D, Hillman EMC. Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proceedings of the National Academy of Sciences 2016;113(52):E8463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sofroniew NJ, Flickinger D, King J, Svoboda K. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. Elife 2016;5(JUN2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Stirman JN, Dorsett CR, Smith SL. Mesoscale correlation structure with single cell resolution during visual coding. bioRxiv 2019;:469114. [Google Scholar]
- 24.Kauvar IV, Machado TA, Yuen E, Kochalka J, Choi M, Allen WE, Wetzstein G, Deisseroth K. Cortical Observation by Synchronous Multifocal Optical Sampling Reveals Widespread Population Encoding of Actions. Neuron 2020;:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel M, Buschman TJ, Miller EK. Cortical information flow during flexible sensorimotor decisions. Science 2015;348(6241):1352–5. URL: https://science.sciencemag.org/content/348/6241/1352. doi: 10.1126/science.aab0551. arXiv:https://science.sciencemag.org/content/348/6241/1352.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dotson NM, Hoffman SJ, Goodell B, Gray CM. Feature-Based Visual Short-Term Memory Is Widely Distributed and Hierarchically Organized. Neuron 2018;99(1):215–226.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang H, Bartolo R, Averbeck BB. Reward-related choices determine information timing and flow across macaque lateral prefrontal cortex. Nat Comms 2021;12(1):894–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, Lee AK, Anastassiou CA, Andrei A, Aydin C, Barbic M, Blanche TJ, Bonin V, Couto J, Dutta B, Gratiy SL, Gutnisky DA, Häusser M, Karsh B, Ledochowitsch P, Lopez CM, Mitelut C, Musa S, Okun M, Pachitariu M, Putzeys J, Rich PD, Rossant C, Sun Wl, Svoboda K, Carandini M, Harris KD, Koch C, O’Keefe J, Harris TD. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017;551(7679):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinmetz NA, Koch C, Harris KD, Carandini M. Challenges and opportunities for large-scale electrophysiology with Neuropixels probes. Current Opinion in Neurobiology 2018;50:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen WE, Chen MZ, Pichamoorthy N, Tien RH, Pachitariu M, Luo L, Deisseroth K. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 2019;364(6437):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegle JH, Jia X, Durand S, Gale S, Bennett C, Graddis N, Heller G, Ramirez TK, Choi H, Luviano JA, Groblewski PA, Ahmed R, Arkhipov A, Bernard A, Billeh YN, Brown D, Buice MA, Cain N, Caldejon S, Casal L, Cho A, Chvilicek M, Cox TC, Dai K, Denman DJ, de Vries SEJ, Dietzman R, Esposito L, Farrell C, Feng D, Galbraith J, Garrett M, Gelfand EC, Hancock N, Harris JA, Howard R, Hu B, Hytnen R, Iyer R, Jessett E, Johnson K, Kato I, Kiggins J, Lambert S, Lecoq J, Ledochowitsch P, Lee JH, Leon A, Li Y, Liang E, Long F, Mace K, Melchior J, Millman D, Mollenkopf T, Nayan C, Ng L, Ngo K, Nguyen T, Nicovich PR, North K, Ocker GK, Ollerenshaw D, Oliver M, Pachitariu M, Perkins J, Reding M, Reid D, Robertson M, Ronellenfitch K, Seid S, Slaughterbeck C, Stoecklin M, Sullivan D, Sutton B, Swapp J, Thompson C, Turner K, Wakeman W, Whitesell JD, Williams D, Williford A, Young R, Zeng H, Naylor S, Phillips JW, Reid RC, Mihalas S, Olsen SR, Koch C. Survey of spiking in the mouse visual system reveals functional hierarchy. Nature 2021;592(7852):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi D, Hirabayashi T, Tamura K, Miyashita Y. Reversal of interlaminar signal between sensory and memory processing in monkey temporal cortex. Science 2011;331(6023):1443–7. [DOI] [PubMed] [Google Scholar]
- 33.Buzsáki G, Kaila K, Raichle ME. Inhibition and brain work. Neuron 2007;56(5):771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nature neuroscience 2011;14(7):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandy AS, Nassi JJ, Reynolds JH. Laminar Organization of Attentional Modulation in Macaque Visual Area V4. Neuron 2017;93(1):235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi YL, Steinmetz NA, Moore T, Boahen K, Engel TA. Influence of On-Off dynamics and selective attention on the spatial pattern of correlated variability in neocortex. bioRxiv 2020;:2020.09.02.279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni AM, Ruff DA, Alberts JJ, Symmonds J, Cohen MR. Learning and attention reveal a general relationship between population activity and behavior. Science (New York, NY) 2018;359(6374):463–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najafi F, Elsayed GF, Cao R, Pnevmatikakis E, Latham PE, Cunningham JP, Churchland AK. Excitatory and Inhibitory Subnetworks Are Equally Selective during Decision-Making and Emerge Simultaneously during Learning. Neuron 2020;105(1):165–179.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noudoost B, Clark KL, Moore T. Working Memory Gates Visual Input to Primate Prefrontal Neurons. bioRxiv 2020;:2020.11.09.375287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregoriou GG, Gotts SJ, Desimone R. Cell-type-specific synchronization of neural activity in FEF with V4 during attention. Neuron 2012;73(3):581–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semedo JD, Zandvakili A, Machens CK, Yu BM, Kohn A. Cortical Areas Interact through a Communication Subspace. Neuron 2019;102(1):249–259.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semedo JD, Jasper AI, Zandvakili A, Aschner A, Machens CK, Kohn A, Yu BM. Feedforward and feedback interactions between visual cortical areas use different population activity patterns. bioRxiv 2021;:2021.02.08.430346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao D, Vanni MP, Mitelut CC, Chan AW, LeDue JM, Xie Y, Chen AC, Swindale NV, Murphy TH. Mapping cortical mesoscopic networks of single spiking cortical or sub-cortical neurons. Elife 2017;6:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters AJ, Fabre JMJ, Steinmetz NA, Harris KD, Carandini M. Striatal activity topographically reflects cortical activity. Nature 2021;19:1100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renz AF, Lee J, Tybrandt K, Brzezinski M, Lorenzo DA, Cerra Cheraka M, Lee J, Helmchen F, Vörös J, Lewis C. Opto-E-Dura: A Soft, Stretchable ECoG Array for Multimodal, Multiscale Neuroscience. Advanced Healthcare Materials 2020;9(17):e2000814. [DOI] [PubMed] [Google Scholar]
- 46.Barson D, Hamodi AS, Shen X, Lur G, Constable RT, Cardin JA, Crair MC, Higley MJ. Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits. Nat Methods 2020;17(1):107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS. Pupil Fluctuations Track Fast Switching of Cortical States during Quiet Wakefulness. Neuron 2014;84(2):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinck M, Batista-Brito R, Knoblich U, Cardin JA. Arousal and Locomotion Make Distinct Contributions to Cortical Activity Patterns and Visual Encoding. Neuron 2015;86(3):740–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGinley MJ, Vinck M, Reimer J, Batista-Brito R, Zagha E, Cadwell CR, Tolias AS, Cardin JA, McCormick Da. Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 2015;87(6):1143–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musall S, Kaufman MT, Gluf S, Churchland AK. Movement-related activity dominates cortex during sensory-guided decision making. Nature Neuroscience 2019;22:1677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stringer C, Pachitariu M, Steinmetz NA, Reddy CB, Carandini M, Harris KD. Spontaneous behaviors drive multidimensional, brainwide activity. Science 2019;364(6437):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci 2011;12(9):509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature Neuroscience 2013;16(12):1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maffei L, Galli-Resta L. Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life. Proceedings of the National Academy of Sciences 1990;87(7):2861–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiological Reviews 1990;70(1):1–41. URL: 10.1152/physrev.1990.70.1.1. doi: 10.1152/physrev.1990.70.1.1. arXiv: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- 56.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proceedings of the National Academy of Sciences of the United States of America 2008;105(41):16039–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okun M, Steinmetz NA, Lak A, Dervinis M, Harris KD. Distinct Structure of Cortical Population Activity on Fast and Infraslow Timescales. Cerebral cortex (New York, NY: 1991) 2019;240:141–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cowley BR, Snyder AC, Acar K, Williamson RC, Yu BM, Smith MA. Slow Drift of Neural Activity as a Signature of Impulsivity in Macaque Visual and Prefrontal Cortex. Neuron 2020;108(3):551–567.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeraati R, Shi YL, Steinmetz NA, Gieselmann MA, Thiele A, Moore T, Levina A, Engel TA. Attentional modulation of intrinsic timescales in visual cortex and spatial networks. bioRxiv preprint available at 10.1101/2021.05.17.444537v1 2021;:2021.05.17.444537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nature reviews Neuroscience 2012;. [DOI] [PubMed] [Google Scholar]
- 61.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993;262(5134):679–85. [DOI] [PubMed] [Google Scholar]
- 62.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science 2004;304(5679):1926–9. [DOI] [PubMed] [Google Scholar]
- 63.Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nature neuroscience 2007;10(10):1241–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, Weerd PD, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron 2012;75(5):875–88. URL: https://www.sciencedirect.com/science/article/pii/S089662731200623X. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: Temporal modules of cortical activity. Science 2004;304(5670):559–64. URL: https://science.sciencemag.org/content/304/5670/559. doi: 10.1126/science.1093173. arXiv:https://science.sciencemag.org/content/304/5670/559.full.pdf. [DOI] [PubMed] [Google Scholar]
- 66.Hemberger M, Shein-Idelson M, Pammer L, Laurent G. Reliable Sequential Activation of Neural Assemblies by Single Pyramidal Cells in a Three-Layered Cortex. Neuron 2019;104(2):353–369.e5. [DOI] [PubMed] [Google Scholar]
- 67.Xu S, Jiang W, Poo Mm, Dan Y. Activity recall in a visual cortical ensemble. Nature neuroscience 2012;15(3):449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadovsky AJ, MacLean JN. Mouse visual neocortex supports multiple stereotyped patterns of microcircuit activity. Journal of Neuroscience 2014;34(23):7769–77. URL: https://www.jneurosci.org/content/34/23/7769. doi: 10.1523/JNEUROSCI.0169-14.2014. arXiv:https://www.jneurosci.org/content/34/23/7769.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Havenith MN, Yu S, Biederlack J, Chen NH, Singer W, Nikolić D. Synchrony makes neurons fire in sequence, and stimulus properties determine who is ahead. Journal of Neuroscience 2011;31(23):8570–84. URL: www.jneurosci.org/content/31/23/8570. doi: 10.1523/JNEUROSCI.2817-10.2011. arXiv:https://www.jneurosci.org/content/31/23/8570.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson BO. Cognitive and Physiologic Impacts of the Infraslow Oscillation. Frontiers in systems neuroscience 2018;12:213–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murray JD, Bernacchia A, Freedman DJ, Romo R, Wallis JD, Cai X, Padoa-Schioppa C, Pasternak T, Seo H, Lee D, Wang XJ. A hierarchy of intrinsic timescales across primate cortex. Nature neuroscience 2014;17(12):1661–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Kempen J, Gieselmann MA, Boyd M, Steinmetz NA, Moore T, Engel TA, Thiele A. Top-down coordination of local cortical state during selective attention. Neuron 2021;109(5):894–904.e8. URL: https://www.sciencedirect.com/science/article/pii/S0896627320309958. doi: 10.1016/j.neuron.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson M, McNaughton B. Reactivation of hippocampal ensemble memories during sleep. Science 1994;265(5172):676–9. URL: https://science.sciencemag.org/content/265/5172/676. doi: 10.1126/science.8036517. arXiv:https://science.sciencemag.org/content/265/5172/676.full.pdf. [DOI] [PubMed] [Google Scholar]
- 74.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 2001;29(1):145–56. URL: https://www.sciencedirect.com/science/article/pii/S0896627301001866. doi: 10.1016/S0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 75.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 2006;440(7084):680–3. [DOI] [PubMed] [Google Scholar]
- 76.Buzsáki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015;25(10):1073–188. URL: 10.1002/hipo.22488. doi: 10.1002/hipo.22488. arXiv: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nature neuroscience 2009;12(7):913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. Hippocampal–cortical interaction during periods of subcortical silence. Nature 2012;491(7425):547–53. [DOI] [PubMed] [Google Scholar]
- 79.Ramirez-Villegas JF, Besserve M, Murayama Y, Evrard HC, Oeltermann A, Logothetis NK. Coupling of hippocampal theta and ripples with pontogeniculooccipital waves. Nature 2021;589(7840):96–102. [DOI] [PubMed] [Google Scholar]
- 80.Karimi Abadchi J, Nazari-Ahangarkolaee M, Gattas S, Bermudez-Contreras E, Luczak A, McNaughton BL, Mohajerani MH. Spatiotemporal patterns of neocortical activity around hippocampal sharp-wave ripples. eLife 2020;9:e51972. URL: 10.7554/eLife.51972. doi: 10.7554/eLife.51972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu X, Ren C, Lu Y, Liu Y, Kim JH, Leutgeb S, Komiyama T, Kuzum D. Multimodal neural recordings with neuro-fitm uncover diverse patterns of cortical–hippocampal interactions. Nature Neuroscience 2021;24(6):886–96. URL: 10.1038/s41593-021-00841-5. doi: 10.1038/s41593-021-00841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitra A, Snyder AZ, Hacker CD, Raichle ME. Lag structure in resting-state fmri. Journal of Neurophysiology 2014;111(11):2374–91. URL: 10.1152/jn.00804.2013. doi: 10.1152/jn.00804.2013. arXiv: 10.1152/jn.00804.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitra A, Snyder AZ, Tagliazucchi E, Laufs H, Raichle ME. Propagated infraslow intrinsic brain activity reorganizes across wake and slow wave sleep. Elife 2015;4(NOVEMBER2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldman-Rakic P Cellular basis of working memory. Neuron 1995;14(3):477–85. URL: https://www.sciencedirect.com/science/article/pii/0896627395903046. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 85.Rossi-Pool R, Zainos A, Alvarez M, Parra S, Zizumbo J, Romo R. Invariant timescale hierarchy across the cortical somatosensory network. Proceedings of the National Academy of Sciences of the United States of America 2021;118(3):e2021843118–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Runyan CA, Piasini E, Panzeri S, Harvey CD. Distinct timescales of population coding across cortex. Nature 2017;548(7665):92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao R, van den Brink RL, Pfeffer T, Voytek B. Neuronal timescales are functionally dynamic and shaped by cortical microarchitecture. eLife 2020;9:e61277. URL: 10.7554/eLife.61277. doi: 10.7554/eLife.61277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, Bezgin G, Eickhoff SB, Castellanos FX, Petrides M, Jefferies E, Smallwood J. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences 2016;113(44):12574–9. URL: https://www.pnas.org/content/113/44/12574. doi: 10.1073/pnas.1608282113. arXiv:https://www.pnas.org/content/113/44/12574.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raut RV, Snyder AZ, Raichle ME. Hierarchical dynamics as a macroscopic organizing principle of the human brain. Proceedings of the National Academy of Sciences 2020;117(34):20890–7. URL: https://www.pnas.org/content/117/34/20890. doi: 10.1073/pnas.2003383117. arXiv:https://www.pnas.org/content/117/34/20890.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vidaurre D, Smith SM, Woolrich MW. Brain network dynamics are hierarchically organized in time. Proceedings of the National Academy of Sciences 2017;114(48):12827–32. URL: https://www.pnas.org/content/114/48/12827. doi: 10.1073/pnas.1705120114. arXiv:https://www.pnas.org/content/114/48/12827.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grothe I, Neitzel SD, Mandon S, Kreiter AK. Switching neuronal inputs by differential modulations of gamma-band phase-coherence. Journal of Neuroscience 2012;32(46):16172–80. URL: https://www.jneurosci.org/content/32/46/16172. doi: 10.1523/JNEUROSCI.0890-12.2012. arXiv:https://www.jneurosci.org/content/32/46/16172.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lewis CM, Bosman CA, Womelsdorf T, Fries P. Stimulus-induced visual cortical networks are recapitulated by spontaneous local and interareal synchronization. Proceedings of the National Academy of Sciences of the United States of America 2016;113(5):E606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P. Visual Areas Exert Feedforward and Feedback Influences through Distinct Frequency Channels. Neuron 2015;85(2):390–401. [DOI] [PubMed] [Google Scholar]
- 94.Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proceedings of the National Academy of Sciences 2011;108(27):11262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xing D, Yeh CI, Burns S, Shapley RM. Laminar analysis of visually evoked activity in the primary visual cortex. Proceedings of the National Academy of Sciences 2012;109(34):13871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Medaglia JD, Lynall ME, Bassett DS. Cognitive Network Neuroscience. Journal of Cognitive Neuroscience 2015;27(8):1471–91. URL: 10.1162/jocn_a_00810.doi: 10.1162/jocn_a_00810. arXiv:https://direct.mit.edu/jocn/article-pdf/27/8/1471/1783500/jocn_a_00810.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, Moodie CA, Poldrack RA. The dynamics of functional brain networks: Integrated network states during cognitive task performance. Neuron 2016;92(2):544–54. URL: https://www.sciencedirect.com/science/article/pii/S0896627316305773. doi: 10.1016/j.neuron.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greene AS, Gao S, Scheinost D, Constable RT. Task-induced brain state manipulation improves prediction of individual traits. Nature Communications 2018;9(1):2807. URL: 10.1038/s41467-018-04920-3. doi: 10.1038/s41467-018-04920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Engel TA, Steinmetz NA, Gieselmann MA, Thiele A, Moore T, Boahen K. Selective modulation of cortical state during spatial attention. Science 2016;354(6316):1140–4. [DOI] [PubMed] [Google Scholar]
- 100.Dasilva M, Brandt C, Gotthardt S, Gieselmann MA, Distler C, Thiele A. Cell class-specific modulation of attentional signals by acetylcholine in macaque frontal eye field. Proceedings of the National Academy of Sciences 2019;116(40):20180–9. URL: https://www.pnas.org/content/116/40/20180. doi: 10.1073/pnas.1905413116. arXiv:https://www.pnas.org/content/116/40/20180.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bassett DS, Sporns O. Network neuroscience. Nature Neuroscience 2017;20(3):353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nature reviews Neuroscience 2017;18(4):222–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Packer AM, Roska B, Häusser M. Targeting neurons and photons for optogenetics. Nature Neuroscience 2013;:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron 2008;57(5):634–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grosenick L, Marshel JH, Deisseroth K. Closed-Loop and Activity-Guided Optogenetic Control. Neuron 2015;86(1):106–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT, Feng G, Svoboda K. Flow of Cortical Activity Underlying a Tactile Decision in Mice. Neuron 2014;81(1):179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo ZV, Inagaki HK, Daie K, Druckmann S, Gerfen CR, Svoboda K. Maintenance of persistent activity in a frontal thalamocortical loop. Nature 2017;:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pinto L, Rajan K, DePasquale B, Thiberge SY, Tank DW, Brody CD. Task-Dependent Changes in the Large-Scale Dynamics and Necessity of Cortical Regions. Neuron 2019;:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allen WE, Kauvar IV, Chen MZ, Richman EB, Yang SJ, Chan K, Gradinaru V, Deverman BE, Luo L, Deisseroth K. Global representations of goal-directed behavior in distinct cell types of mouse neocortex. Neuron 2017;94(4):891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Banerjee A, Parente G, Teutsch J, Lewis C, Voigt FF, Helmchen F. Value-guided remapping of sensory cortex by lateral orbitofrontal cortex. Nature 2020;585(7824):245–50. [DOI] [PubMed] [Google Scholar]
- 111.Otchy TM, Wolff SBE, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SMH, Ölveczky BP. Acute off-target effects of neural circuit manipulations. Nature 2015;528(7582):358–63. [DOI] [PubMed] [Google Scholar]
- 112.Bauer AQ, Kraft AW, Baxter GA, Wright PW, Reisman MD, Bice AR, Park JJ, Bruchas MR, Snyder AZ, Lee JM, Culver JP. Effective connectivity measured using optogenetically evoked hemodynamic signals exhibits topography distinct from resting state functional connectivity in the mouse. Cerebral Cortex 2018;28(1):370–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Markicevic M, Fulcher BD, Lewis C, Helmchen F, Rudin M, Zerbi V, Wenderoth N. Cortical excitation:inhibition imbalance causes abnormal brain network dynamics as observed in neurodevelopmental disorders. Cerebral Cortex 2020;30(9):4922–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosenthal ZP, Raut RV, Yan P, Koko D, Kraft AW, Czerniewski L, Acland B, Mitra A, Snyder LH, Bauer AQ, Snyder AZ, Culver JP, Raichle ME, Lee JM. Local Perturbations of Cortical Excitability Propagate Differentially Through Large-Scale Functional Networks. Cerebral Cortex 2020;30(5):3352–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, Fair DA, Amaral DG. The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic Inactivation of the Amygdala. Neuron 2016;91(2):453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zerbi V, Floriou-Servou A, Markicevic M, Vermeiren Y, Sturman O, Privitera M, von Ziegler L, Ferrari KD, Weber B, De Deyn PP, Wenderoth N, Bohacek J. Rapid Reconfiguration of the Functional Connectome after Chemogenetic Locus Coeruleus Activation. Neuron 2019;103(4):702–718.e5. [DOI] [PubMed] [Google Scholar]
- 117.Turchi J, Chang C, Ye FQ, Russ BE, Yu DK, Cortes CR, Monosov IE, Duyn JH, Leopold Da. The Basal Forebrain Regulates Global Resting-State fMRI Fluctuations. Neuron 2018;97(4):940–952.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y. Long-range and local circuits for top-down modulation of visual cortex processing. Science 2014;345(6197):660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lewis CM, Ni J, Wunderle T, Jendritza P, Lazar A, Diester I, Fries P. Cortical gamma-band resonance preferentially transmits coherent input. Cell reports 2021;35(5):109083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clancy KB, Mrsic-Flogel TD. The sensory representation of causally controlled objects. Neuron 2021;109(4):677–689.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature 2009;457(7233):1142–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poulet JFA, Fernandez LMJ, Crochet S, Petersen CCH. Thalamic control of cortical states. Nature Neuroscience 2012;15(3):370–2. [DOI] [PubMed] [Google Scholar]
- 123.Zhang W, Bruno RM. High-order thalamic inputs to primary somatosensory cortex are stronger and longer lasting than cortical inputs. eLife 2019;8:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sych Y, Chernysheva M, Sumanovski LT, Helmchen F. High-density multifiber photometry for studying large-scale brain circuit dynamics. Nature Methods 2019;16(6):553–60. [DOI] [PubMed] [Google Scholar]
- 125.Carrillo-Reid L, Han S, Yang W, Akrouh A, Yuste R. Controlling Visually Guided Behavior by Holographic Recalling of Cortical Ensembles. Cell 2019;178(2):447–457.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mardinly AR, Oldenburg IA, Pégard NC, Sridharan S, Lyall EH, Chesnov K, Brohawn SG, Waller L, Adesnik H. Precise multimodal optical control of neural ensemble activity. Nature Neuroscience 2018;:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature 2012;490(7419):226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beltramo R, D’Urso G, Dal Maschio M, Farisello P, Bovetti S, Clovis Y, Lassi G, Tucci V, De Pietri Tonelli D, Fellin T. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nature Neuroscience 2013;16(2):227–34. [DOI] [PubMed] [Google Scholar]
- 129.Chettih SN, Harvey CD. Single-neuron perturbations reveal feature-specific competition in V1. Nature 2019;:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marshel JH, Kim YS, Machado TA, Quirin S, Benson B, Kadmon J, Raja C, Chibukhchyan A, Ramakrishnan C, Inoue M, Shane JC, McKnight DJ, Yoshizawa S, Kato HE, Ganguli S, Deisseroth K. Cortical layer-specific critical dynamics triggering perception. Science (New York, NY) 2019;365(6453). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McKenzie S, Huszár R, English DF, Kim K, Christensen F, Yoon E, Buzsáki G. Pre-existing hippocampal network dynamics constrain optogenetically induced place fields. Neuron 2021;:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anastasiades PG, Collins DP, Carter AG. Mediodorsal and Ventromedial Thalamus Engage Distinct L1 Circuits in the Prefrontal Cortex. Neuron 2020;:1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ko H, Hofer SB, Pichler B, Buchanan KA, Sjöström PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature 2011;473(7345):87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Branco T, Clark BA, Häusser M. Dendritic discrimination of temporal input sequences in cortical neurons. Science 2010;329(5999):1671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Caporale N, Dan Y. Spike timing–dependent plasticity: a hebbian learning rule. Annu Rev Neurosci 2008;31:25–46. [DOI] [PubMed] [Google Scholar]
- 136.Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, Patel S, Tolias AS. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 2015;350(6264). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Valente M, Pica G, Bondanelli G, Moroni M, Runyan CA, Morcos AS, Harvey CD, Panzeri S. Correlations enhance the behavioral readout of neural population activity in association cortex. Nature Neuroscience 2021;:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ni AM, Ruff DA, Alberts JJ, Symmonds J, Cohen MR. Learning and attention reveal a general relationship between population activity and behavior. Science 2018;359(6374):463–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bányai M, Lazar A, Klein L, Klon-Lipok J, Stippinger M, Singer W, Orban G. Stimulus complexity shapes response correlations in primary visual cortex. Proceedings of the National Academy of Sciences 2019;116(7):2723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stringer C, Pachitariu M, Steinmetz N, Carandini M, Harris KD. High-dimensional geometry of population responses in visual cortex. Nature 2019;571(7765):361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


