This cohort study examines data for hospital length of stay to determine its association with early use of steroids among pediatric patients hospitalized for COVID-19 who did not have multisystem inflammatory syndrome in children.
Key Points
Question
What is the association between the early use of steroids and outcomes for pediatric patients hospitalized for COVID-19 without non–multisystem inflammatory syndrome in children (MIS-C)?
Findings
In this cohort study involving 1163 children, hospital length of stay for patients who received steroids within 2 days of admission did not differ significantly from those who did not receive early steroids. The margin of error does not rule out benefits in some patients.
Meaning
Early use of steroids may not affect the disease course in children with non–MIS-C COVID-19, but a definitive determination of benefit or harm from early steroid therapy in children cannot be made from this study.
Abstract
Importance
There is limited evidence for therapeutic options for pediatric COVID-19 outside of multisystem inflammatory syndrome in children (MIS-C).
Objective
To determine whether the use of steroids within 2 days of admission for non–MIS-C COVID-19 in children is associated with hospital length of stay (LOS). The secondary objective was to determine their association with intensive care unit (ICU) LOS, inflammation, and fever defervescence.
Design, Setting, and Participants
This cohort study analyzed data retrospectively for children (<18 years) who required hospitalization for non–MIS-C COVID-19. Data from March 2020 through September 2021 were provided by 58 hospitals in 7 countries who participate in the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS) COVID-19 registry.
Exposure
Administration of steroids within 2 days of admission.
Main Outcomes and Measures
Length of stay in the hospital and ICU. Adjustment for confounders was done by mixed linear regression and propensity score matching.
Results
A total of 1163 patients met inclusion criteria and had a median (IQR) age of 7 years (0.9-14.3). Almost half of all patients (601/1163, 51.7%) were male, 33.8% (392/1163) were non-Hispanic White, and 27.9% (324/1163) were Hispanic. Of the study population, 184 patients (15.8%) received steroids within 2 days of admission, and 979 (84.2%) did not receive steroids within the first 2 days. Among 1163 patients, 658 (56.5%) required respiratory support during hospitalization. Overall, patients in the steroids group were older and had greater severity of illness, and a larger proportion required respiratory and vasoactive support. On multivariable linear regression, after controlling for treatment with remdesivir within 2 days, country, race and ethnicity, obesity and comorbidity, number of abnormal inflammatory mediators, age, bacterial or viral coinfection, and disease severity according to ICU admission within first 2 days or World Health Organization ordinal scale of 4 or higher on admission, with a random intercept for the site, early steroid treatment was not significantly associated with hospital LOS (exponentiated coefficient, 0.94; 95% CI, 0.81-1.09; P = .42). Separate analyses for patients with an LOS of 2 days or longer (n = 729), those receiving respiratory support at admission (n = 286), and propensity score–matched patients also showed no significant association between steroids and LOS. Early steroid treatment was not associated with ICU LOS, fever defervescence by day 3, or normalization of inflammatory mediators.
Conclusions and Relevance
Steroid treatment within 2 days of hospital admission in a heterogeneous cohort of pediatric patients hospitalized for COVID-19 without MIS-C did not have a statistically significant association with hospital LOS.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, has caused an ongoing pandemic for 2 years.1 Most children affected by COVID-19 have a mild illness,2,3 but severe disease and mortality are well described.4,5 While many large trials have been conducted on therapeutic options for COVID-19 in adults, the data for pediatric COVID-19 outside of multisystem inflammatory syndrome in children (MIS-C) is sparse. MIS-C is a postinfectious inflammatory condition that is not directly caused by the virus,6 and as such, current evidence on the management of MIS-C, including intravenous immunoglobulin and steroids,7,8,9 may not be directly applicable to non–MIS-C COVID-19.
Severe COVID-19 illness is characterized by a systemic inflammatory response that leads to acute respiratory distress syndrome, and corticosteroids are proposed to be a beneficial treatment.10 In fact, many large randomized clinical trials have shown beneficial effects of steroids in adults hospitalized with COVID-19,11,12,13 and steroids for hospitalized adults who require supplemental oxygen have been accepted as a standard of care.14,15 However, children are generally not included in adult clinical trials, and there are no large studies on the effect of steroids on the disease course of non–MIS-C COVID-19 in children. Therefore, although the National Institutes of Health recommend systemic steroids for children with severe COVID-19 requiring high levels of respiratory support, they acknowledge that the extrapolation of this recommendation for children is uncertain.16
The primary objective of this study was to compare outcomes (hospital length of stay [LOS] as the primary outcome) for children admitted to the hospital with non–MIS-C COVID-19 who were treated with and without early steroids. We hypothesized that steroids modulate the clinical course of COVID-19 in children, which would result in a shorter LOS. The study’s secondary objective was to compare the clinical course (ICU LOS, fever defervescence, resolution of inflammation, etc) during hospitalization between the 2 groups.
Methods
Database
We performed a retrospective review of the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry. VIRUS is a prospective, cross-sectional, observational study including all eligible adult and pediatric patients admitted to the hospital.17,18 The VIRUS registry was approved by the Mayo Clinic institutional review board, and by all participating sites, with a waiver of informed consent. Each site collected deidentified data through manual medical record abstraction or automation and entered it into a centralized REDCap database19 hosted by the Mayo Clinic.
Cohort
Inclusion criteria for the VIRUS: COVID-19 registry encompass any patient admitted with COVID-19 in participating hospitals with laboratory-confirmed SARS-CoV-2 infection or high clinical suspicion of COVID-19. Patients with admissions not related to COVID-19 and repeated admissions are excluded. We extracted data for all pediatric patients (<18 years) for whom data were entered into the registry between March 2020 and September 2021. We excluded the following patients: those with missing age, those with readmissions, and those with COVID-19 not confirmed via laboratory test. To select patients where there is more clinical equipoise in receiving steroids, we further excluded patients who were diagnosed with MIS-C at participating institutions using Centers for Disease Control and Prevention criteria.20
Exposure
The primary exposure variable for this study was administration of steroids on or before day 2 of hospital admission. As an intention-to-treat analysis,21 patients who received steroids after day 2 (n = 22) were included in the control category. The dose and type of steroids were not available for most patients, and a discrete variable of administration of any steroid (yes/no) was used in the analysis.
Outcomes
The primary outcome variables for this analysis were hospital LOS and ICU LOS (for patients admitted to the ICU). In the VIRUS registry, LOS was entered in days with up to 2 decimal points of precision. The hospital LOS and ICU LOS for patients who died were replaced with the 99th percentile of hospital LOS (48.2 days) and ICU LOS (53.9 days).22 Multiple derived secondary outcome variables were created and included the following: nosocomial bacterial infection (positive bacterial cultures reported day ≥2), inotrope or ventilator requirement by day 2 or later (for patients for whom inotrope/ventilator was not needed at admission), number of days of inotropes, fever defervescence by day 3 (for patients who had fever on admission), and, when patients presented with abnormal inflammatory markers, the day of normalization of the following: white cell count, platelet count, and levels of C-reactive protein, procalcitonin, and ferritin.
Other Measured Variables and Confounders
Age was stratified into discrete categories: neonate (≤28 days), infant (>28 days to <2 years), child (≥2 years to <12 years), and adolescents (≥12 years).23 The patient’s weight, height, and body mass index were used to calculate the proportion of obese patients per standard guidelines.24,25 We used the previously published World Health Organization (WHO) ordinal scale to stratify patients by disease severity based on degree of respiratory and hemodynamic support at hospital admission.26 A total of 818 of 979 patients (84%) not treated with steroids had a WHO ordinal scale of 3, so the WHO ordinal scale was transformed into a variable with 2 levels indicating a score of 3 or 4 or more. Detailed description of other variables extracted from the VIRUS registry and included in the analysis, such as self-reported race and ethnicity, is provided in the eMethods in Supplement 1.
Missing Data Analysis
There were no missing data on age or mortality. Only 4 patients (0.3%) were missing race and ethnicity information. There was a high degree of missingness in the data on inflammatory mediators. The missing rate for these variables ranged from 57.4% to 97.6%. A total of 158 patients (37.5%) admitted to ICU were missing PRISM scores. The R package missforest was used to impute missing PRISM scores using random forest. Up to 271 (23.3%) patients were missing obesity classification based on weight and height. Whether or not the patient was missing BMI was significantly associated with obesity diagnosis as a comorbidity (more patients with obesity comorbidity were not missing BMI than patients who did not have obesity diagnosis as a comorbidity; 90.8% vs 75.8%, P = .005). The obesity by comorbidity variable served as a surrogate for BMI, and a composite category of obesity (yes/no) was created (with a preference for obesity by BMI). MIS-C categorization was not reported in 156 patients (13.4%). A separate sensitivity analysis for hospital length of stay was performed after excluding these patients. The remaining variables’ missingness is reported as absolute numbers and percentages wherever applicable.
Statistical Analysis
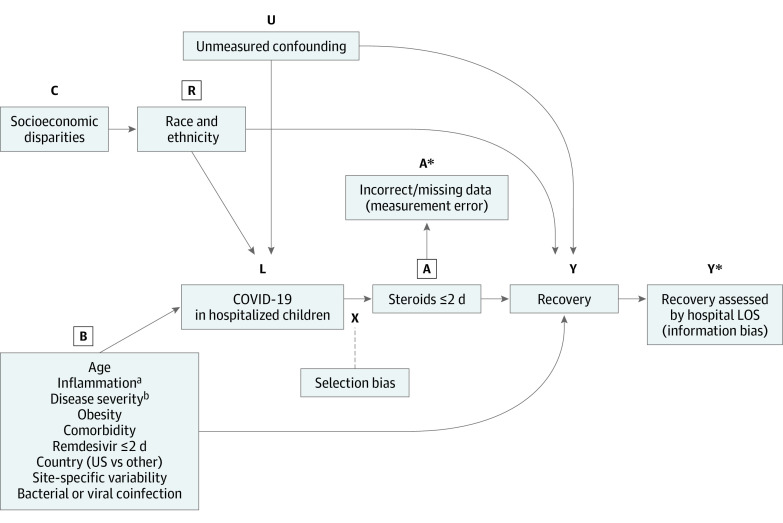
Standard descriptive and comparative analysis was performed using nonparametric Wilcoxon rank sum test or χ2/Fischer exact test. Steroid treatment within 2 days was included as an independent variable in a linear mixed regression model with a random intercept for the site. Treatment with remdesivir (≤2 days of admission), treatment country, presence of any comorbidity or obesity, number of abnormal inflammatory mediators on admission, bacterial or viral coinfection at admission, age, a composite variable of ICU admission (within ≤2 days) or WHO ordinal scale score 4 or higher on admission, and race and ethnicity (dichotomized as non-Hispanic White vs not), and the interaction effect of steroids with the number of abnormal inflammatory mediators on admission were also included as covariates. These factors were selected based on prior reports of their association with the severity of illness and the primary outcome of hospital length of stay.27,28 A causal directed acyclic graph29,30 was created to display these associations graphically and potentially identify potential biases in the analysis (Figure 1).
Figure 1. Directed Acyclic Graph for Association of Steroids With Outcomes for Children Hospitalized With COVID-19 Without Multisystem Inflammatory Syndrome in Children.
Letters represent modified directed acyclic graph symbols. L represents the condition, A the treatment, and Y the effect. R represents a proxy confounder for C. B represents the variables listed below. A box around the letter represents blocking the backdoor path. A and Y represent reality and A* and Y* measurements in the study. The difference represents bias. Selection bias: factors that influence patients getting steroids also independently predict hospital length of stay (LOS). Information bias: hospital LOS may not be the best indicator of recovery.
aInflammation was indicated by the number of inflammatory mediators with abnormal values on admission.
bDisease severity was measured by a composite of World Health Organization ordinal scale score of 4 or higher at admission or intensive care unit admission within 2 days of hospital admission.
Because of the skewed distribution of hospital LOS, a log transformation was applied to the hospital and ICU LOS, resulting in geometric means being reported. Four separate sensitivity analyses were conducted: (1) an analysis limited to patients with a hospital LOS of 2 days or longer (to identify any immortal time bias31), (2) an analysis limited to patients with a WHO ordinal score at admission of 4 or higher (to determine whether steroids have a more significant association with the requirement of respiratory support11), (3) an analysis excluding patients who had missing MIS-C categorization, and (4) a propensity score–matched analysis to explore a potential effect of the choice of analytical method on the results. This analysis included propensity score matching and inverse probability treatment weighting (IPTW). Details about the propensity score methods are provided in the eAppendix in Supplement 1.
Semipartial correlations are reported as a measure of effect size for each independent variable in the regression model. Semipartial correlations, also referred to as part correlations, are the correlation between the dependent variables and an independent variable, controlling for all other independent variables in the model. As a type of correlation, these can be interpreted using benchmarks suggested by Cohen,32 with R = 0.10 considered a small effect, R = 0.30 a medium effect, and R = 0.50 a large effect.
Mixed logistic regression was performed for 2 secondary outcome variables (fever defervescence and normalization of inflammatory mediators by day 3) using the covariates listed previously. For secondary outcomes of interest that occurred in fewer than 2% of patients, analysis was restricted to simple bivariate comparisons. Statistical analysis was performed using JMP Pro version 16.0 (SAS Institute) and R version 4.0.1 against a 2-sided alternative hypothesis with a significance level of 5%. This study reporting conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.33
Results
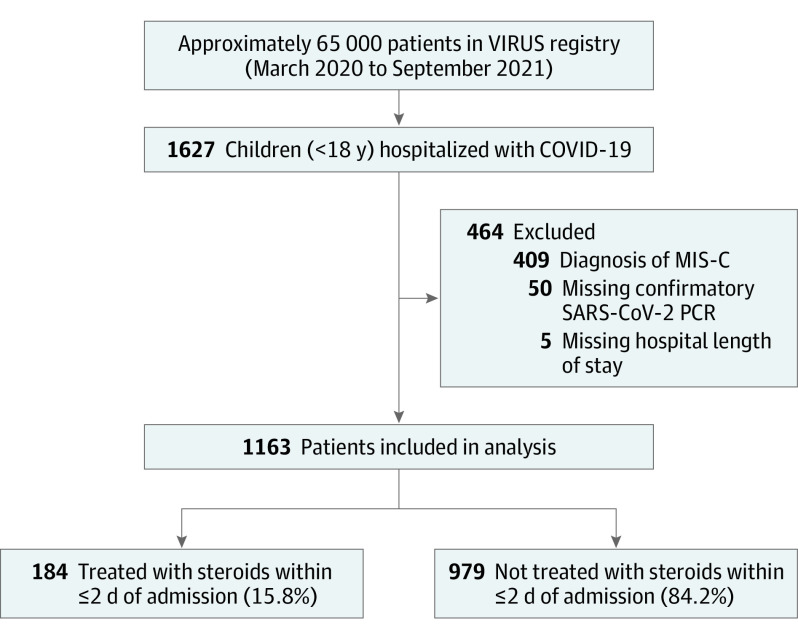
During the study period (March 2020 through September 2021), more than 65 000 patients were entered into the registry, of which 1627 patients were identified as children (aged <18 years), admitted with COVID-19–related illnesses. Data for 464 patients were excluded (patients with MIS-C [409], lack of confirmed SARS-CoV-2 polymerase chain reaction test results [50]). Five patients for whom hospital length of stay was missing were also excluded. The final analysis cohort included 1163 patients from 58 centers (1072 patients [92%] in the United States and 91 [7.8%] patients in 6 non-US countries). The median number of patients per hospital was 4 (range, 1-126). Of 1163 patients, 184 (15.8%) received steroids within 2 days of admission, and 979 (84.1%) did not (Figure 2).
Figure 2. Patient Selection CONSORT Flow Diagram.
MIS-C indicates multisystem inflammatory syndrome in children; PCR, polymerase chain reaction test result; VIRUS, Viral Infection and Respiratory Illness Universal Study.
Demographic Data
The median (IQR) age of the study population was 7 years (0.9-14.3), with a significantly higher median age in the steroids group (11 [2.9-16] years vs 5.6 [0.55-14] years; P < .001). Almost half (51.7%) of all patients were male, 33.8% (392/1163) were non-Hispanic White, and 27.9% (324/1163) were Hispanic. There was no difference in sex or race distribution between the 2 groups. Obesity and preexisting comorbidities were present in 22.1% (257/1163) and 42.0% (489/1163) of patients, with a significantly higher proportion of patients with obesity and comorbidities in the steroids group. A higher proportion of patients in the steroids group presented with multiple signs and symptoms (79.8% [147/184] vs 48.2% [472/979]; P < .001) and had abnormal inflammatory mediators at the time of admission (54.9% [101/184] vs 32.2% [316/979]; P < .001). Numbers of patients requiring ICU admission during hospitalization and those meeting criteria of critical illness were also significantly higher in the steroids group (Table 1). Details about individual laboratory parameters and their comparison between the 2 groups are provided in eTable 1 in Supplement 1.
Table 1. Demographic and Clinical Characteristics of the Study Cohort.
| Category | No. (%) | P value | ||
|---|---|---|---|---|
| Total cohort (N = 1163) | No early steroids (n = 979 [84.2%]) | Steroids group (n = 184 [15.8%]) | ||
| Age, median (IQR), y | 7 (0.9-14.3) | 5.6 (0.55-14) | 11 (2.9-16) | <.001 |
| Age categorya | <.001 | |||
| Neonate | 79 (6.7) | 78 (7.9) | 1 (0.5) | |
| Infant | 337 (28.9) | 299 (30.5) | 38 (20.6) | |
| Child | 317 (27.2) | 259 (26.5) | 58 (31.5) | |
| Adolescent | 430 (36.9) | 343 (35.0) | 87 (47.3) | |
| Male | 601 (51.7) | 496 (50.7) | 105 (57.1) | .11 |
| Female | 562 (48.3) | 483 (49.3) | 79 (42.9) | |
| Race and ethnicityb | .32 | |||
| Asian | 87 (7.5) | 72 (7.4) | 15 (8.2) | |
| Black | 244 (21.1) | 214 (21.9) | 30 (16.3) | |
| Hispanic | 324 (27.9) | 265 (27.2) | 59 (32.1) | |
| White | 392 (33.8) | 333 (34.2) | 59 (32.1) | |
| Other | 112 (9.6) | 91 (9.3) | 21 (11.4) | |
| Obesityc | 257 (22.1) | 185 (18.9) | 72 (39.1) | <.001 |
| Admitted to ICUd | 421 (36.1) | 309 (31.6) | 112 (60.8) | <.001 |
| Critical illnesse | 159 (13.7) | 101 (10.3) | 58 (31.5) | <.001 |
| WHO ordinal scalef | <.001 | |||
| 3 | 876 (75.3) | 818 (83.5) | 58 (31.5) | |
| 4 | 136 (11.7) | 91 (9.3) | 45 (24.5) | |
| 5 | 106 (9.1) | 40 (4.1) | 66 (35.8) | |
| 6 | 29 (2.5) | 21 (2.2) | 8 (4.3) | |
| 7 | 16 (1.4) | 9 (0.9) | 7 (3.8) | |
| Abnormal inflammatory mediatorsg | 417 (35.7) | 316 (32.2) | 101 (54.9) | <.001 |
| US centerh | 1072 (92.2) | 901 (92.0) | 171 (92.9) | .67 |
| ≥3 Signs or symptoms | 619 (53.2) | 472 (48.2) | 147 (79.8) | <.001 |
| Comorbidity | 489 (42.0) | 364 (37.2) | 125 (67.9) | <.001 |
| SARS-CoV-2 IgG positive | 58 (4.9) | 45 (4.6) | 13 (7.1) | .16 |
Abbreviations: ICU, intensive care unit; IgG, immunoglobulin G; WHO, World Health Organization.
Neonate was age ≤28 days; infant, >28 days to <2 years; child, ≥2 years to <12 years; adolescent, ≥12 years.
Race and ethnicity were self-reported separately and then later categorized as Hispanic, non-Hispanic Asian, non-Hispanic Black, non-Hispanic White, and non-Hispanic other. Other was used for any data that did not fit into the category of Asian, Black, Hispanic, or White. Race and ethnicity data were missing for 4 individuals.
According to body mass index or obesity listed as a comorbidity.
At any time during hospitalization.
Indicates invasive respiratory support (ventilator, nitric oxide) or invasive hemodynamic support (inotropes/vasopressors/extracorporeal membrane oxygenation) or invasive kidney support (dialysis) or hospital mortality.
A score of 3 indicates hospitalized, no oxygen therapy; 4, oxygen by mask or nasal prongs; 5, noninvasive ventilation or high-flow oxygen; 6, intubation and mechanical ventilation; 7, ventilation and additional organ support, including pressers, kidney replacement, or extracorporeal membrane oxygenation at admission.
High or low values for leukocyte count, platelets, and fibrinogen; high values for C-reactive protein, procalcitonin, ferritin, interleukin, or D-dimer; or low values for serum albumin on day 0 or 1 of admission.
Patients in non-US centers were located in Croatia (12, 1.0%), India (63, 5.3%), Japan (1, 0.08%), Pakistan (5, 0.4%), Saudi Arabia (1, 0.08%), or Nigeria (9, 0.8%).
Hospital Management and Disease Course
A total of 658 patients (56.5%) required respiratory support with a significantly higher proportion in the steroids group (77.2% [142/184] vs 52.7% [516/979]; P < .001). Oxygen delivery by nasal cannula was the most frequent mode of respiratory support (268/1163, 23%), while invasive mechanical ventilation was needed in 7.1% of patients (83/1163), with a significantly higher proportion of patients requiring all individual modalities of respiratory support in the early steroids group. Only 4.5% of patients (52/1163) required vasopressors/inotropes, with a significantly higher proportion in the steroids group. Among the other common therapeutic agents for COVID-19, remdesivir was used most frequently, in 8.6% of patients (100/1163). All the measured therapeutic agents were used at a higher frequency among patients who were prescribed early steroids. There was no significant difference in the unadjusted duration of high-flow nasal cannula, noninvasive ventilator, or invasive ventilator between the 2 groups. The overall median (IQR) hospital and ICU LOS were 3 days (1.7-6) and 3.3 days (1.5-7.4), respectively, and were significantly longer in the early steroids group on unadjusted bivariate analysis (Table 2).
Table 2. Hospital Management and Outcomes.
| Category | Total cohort (N = 1163) | No early steroids (n = 979 [84.2%]) | Steroids group (n = 184 [15.8%]) | P value |
|---|---|---|---|---|
| Categorical variables, No. (%) | ||||
| Respiratory supporta | ||||
| Any respiratory support | 658 (56.5) | 516 (52.7) | 142 (77.2) | <.001 |
| Face mask oxygenation | 63 (5.4) | 40 (4.1) | 23 (12.5) | <.001 |
| Nasal cannula | 268 (23.0) | 177 (18.1) | 91 (49.5) | <.001 |
| High-flow nasal cannula | 135 (11.6) | 70 (7.2) | 65 (35.3) | <.001 |
| Noninvasive ventilation | 88 (7.5) | 49 (5.0) | 39 (21.2) | <.001 |
| Invasive ventilation | 83 (7.1) | 59 (6.0) | 24 (13.0) | .001 |
| Nitric oxideb | 7 (0.6) | 4 (0.4) | 3 (1.6) | .08 |
| Other organ supporta | ||||
| Vasopressors/inotropes | 52 (4.5) | 34 (3.5) | 18 (9.8) | <.001 |
| CKRT/HD | 3 (0.3) | 2 (0.2) | 1 (0.5) | .40 |
| ECLS | 6 (0.5) | 3 (0.3) | 3 (1.6) | .05 |
| Antiviral, immunomodulator, and other medicationsc | ||||
| Remdesivird | 100 (8.6) | 37 (3.8) | 63 (34.2) | <.001 |
| Azithromycin | 73 (6.3) | 48 (4.9) | 25 (13.6) | <.001 |
| Hydroxychloroquine | 22 (1.9) | 15 (1.5) | 7 (3.8) | .06 |
| Therapeutic anticoagulation | 59 (5.1) | 29 (2.9) | 30 (16.3) | <.001 |
| Aspirin | 23 (1.9) | 13 (1.3) | 10 (5.4) | .001 |
| Convalescent plasma | 6 (0.5) | 2 (0.2) | 4 (2.2) | .007 |
| IVIG | 13 (1.1) | 8 (0.8) | 5 (2.7) | .04 |
| Biologicals | 8 (0.6) | 4 (0.4) | 4 (2.2) | .02 |
| Vitamin C | 47 (4.0) | 32 (3.3) | 15 (8.2) | .006 |
| Vitamin D | 67 (5.7) | 46 (4.7) | 21 (11.4) | <.001 |
| Zinc | 43 (3.6) | 26 (2.6) | 17 (9.2) | <.001 |
| Continuous variables, median (IQR) [No.] | ||||
| Respiratory support duration, de | ||||
| High-flow nasal cannula | 2.5 (1.0-4.2) [120] | 2.7 (0.9-4.7) [62] | 2.2 (1.0-3.6) [58] | .49 |
| Noninvasive ventilator | 2.4 (1.0-7.0) [73] | 2.2 (0.7-6.9) [42] | 2.9 (1.2-7.2) [31] | .38 |
| Invasive ventilator | 3.8 (1.5-10.3) [78] | 3.5 (1.2-11.0) [55] | 4.9 (1.8-9.7) [23] | .54 |
| Length of stay, df | ||||
| Hospital | 3 (1.7-6) [1163] | 3 (1.6-5.5) [979] | 4.4 (2-7.9) [184] | <.001 |
| Intensive care unit | 3.3 (1.5-7.4) [416] | 3 (1.2-7.1) [305] | 3.8 (2-8.7) [111] | .04 |
Abbreviations: CKRT/HD, continuous kidney replacement therapy/hemodialysis; ECLS, extracorporeal life support; ICU, intensive care unit; IVIG, intravenous immunoglobulin.
Respiratory and other organ support represent support provided at any time during the hospitalization.
Or epoprostenol.
Medications given on any of day 0, 1, 2, 3, 7, 14, or 21 of hospitalization. Organ support and therapeutics not mutually exclusive.
Remdesivir is approved by the US Food and Drug Administration only for use in patients 12 years and older.
Missing durations of high-flow nasal cannula use for 15 of 135 patients (11%), noninvasive ventilator use for 15 of 88 patients (17%), and invasive ventilator use for 5 of 83 patients (6%).
Hospital and ICU length of stay of deceased patients represented as 99th percentile of total cohort (48.2 days and 53.9 days, respectively). Data are missing for ICU length of stay for 5 of 421 patients (1.2%).
Primary Outcome Variable (Length of Stay)
On multivariable linear mised-effects regression using the variables described previously, steroid treatment within the first 2 days of hospital admission was not significantly associated with hospital LOS (exponentiated coefficient, 0.94; 95% CI, 0.81-1.09; P = .42). Holding all other variables constant at their mean or reference level, patients who received early steroids had an adjusted geometric mean LOS of 2.09 days (95% CI, 1.74-2.67) compared with patients not treated with early steroids (2.17 days; 95% CI, 1.74-2.67). The semipartial correlation of the treatment with steroids and LOS was small at 0.023. There was no significant interaction effect of steroid treatment and number of abnormal inflammatory mediators (Table 3). Results of 3 sensitivity analyses (only including patients with LOS ≥2 days [n = 729], only including patients who required respiratory support at admission [n = 286], and excluding patients with missing MIS-C categorization [n = 1003]) are presented in eTable 2 in Supplement 1. Overall results were similar, with no association between early steroid administration and LOS in either model. For patients with an LOS of 2 days or longer, the interaction effect of early steroid treatment and number of abnormal inflammatory mediators was significant (exponentiated coefficient, 1.07; 95% CI, 1.00-1.15; P = .04). The propensity score–matched and IPTW analyses results are presented in the eAppendix in Supplement 1. Both analyses found no significant association between steroid treatment and hospital LOS.
Table 3. Regression Analysis of Hospital and ICU Length of Stay.
| Variable | Exponentiated coefficient (95% CI) | P value | R a |
|---|---|---|---|
| Hospital length of stayb | |||
| Steroid treatment (≤2 d) | 0.94 (0.81-1.09) | .42 | 0.02 |
| Remdesivir treatment (≤2 d) | 1.30 (1.08-1.56) | .006 | 0.08 |
| Non-US center | 2.20 (1.60-3.02) | <.001 | 0.25 |
| No. of abnormal inflammatory mediators | 1.10 (1.06-1.14) | <.001 | 0.14 |
| Bacterial or viral coinfection at admission | 1.19 (1.04-1.38) | .01 | 0.07 |
| Obesity or comorbidity | 1.18 (1.07-1.30) | .001 | 0.10 |
| WHO ordinal scale ≥4 or ICU admission within ≤2 d of admission | 1.48 (1.34-1.64) | <.001 | 0.22 |
| Not non-Hispanic White | 0.98 (0.90-1.08) | .72 | 0.01 |
| Age | 1.01 (1.00-1.02) | .01 | 0.07 |
| Steroid treatment (≤2 d) × No. of abnormal inflammatory mediators | 1.04 (0.98-1.11) | .20 | 0.04 |
| ICU length of stayc | |||
| Steroid treatment (≤2 d) | 1.03 (0.79-1.35) | .83 | 0.01 |
| No. of abnormal inflammatory mediators | 1.07 (0.99-1.15) | .09 | 0.09 |
| Non-US center | 2.05 (1.27-3.29) | .004 | 0.23 |
| Remdesivir treatment (≤2 d) | 1.10 (0.83-1.47) | .50 | 0.03 |
| Bacterial or viral coinfection at admission | 1.30 (1.00-1.69) | .05 | 0.10 |
| WHO ordinal scale (≥4) at admission | 1.35 (1.10-1.66) | .004 | 0.15 |
| Obesity or comorbidity | 1.08 (0.88-1.32) | .45 | 0.04 |
| Not non-Hispanic White | 0.97 (0.81-1.17) | .77 | 0.02 |
| Age | 1.00 (0.99-1.02) | .93 | 0.004 |
| Steroid treatment (≤2 d) × No. of abnormal inflammatory mediators | 1.07 (0.97-1.19) | .17 | 0.07 |
Abbreviations: ICU, intensive care unit; WHO, World Health Organization.
R is a semipartial correlation between the predictor and the outcome, or the correlation between the predictor and the outcome controlling for the other predictors in the model, and can be interpreted as simple correlations are, with R = 0.10 being considered a small effect, R = 0.30 a medium effect, and R = 0.50 a large effect.
R2 of the full model 23.9%; n = 1159 because of missing race and ethnicity data for 4 patients.
R2 of the full model 17.5%.
Multivariable linear regression of ICU LOS also showed no significant association between steroid treatment within 2 days of admission and ICU LOS (exponentiated coefficient, 1.03; 95% CI, 0.79-1.35; P = .83). There was also no significant interaction between early steroid treatment and number of abnormal inflammatory mediators (Table 3). The model’s R2 was 17.5%. The addition of PRISM scores improved R2 to 23%, and a likelihood ratio test found that the addition of mean PRISM scores significantly improved the model (P < .001). However, the association of early steroids and ICU LOS was not significant in the model including PRISM scores (data not shown).
Secondary Outcomes
On multivariable logistic regression including all the variables described previously, early steroid treatment was not significantly associated with a higher fever defervescence rate (odds ratio [OR], 1.02; 95% CI, 0.86-1.21; P = .83). No significant difference was observed on separate multivariable logistic regression for normalization of inflammatory mediators by day 3 with steroids within 2 days (OR, 1.03; 95% CI, 0.84-1.26; P = .75) (eTable 3 in Supplement 1). Simple bivariate comparisons for secondary outcomes that occurred rarely are presented in eTable 4 in Supplement 1. Overall hospital mortality was 1% and was significantly higher on an unadjusted analysis in the early steroid group (2.7% [5/184] vs 0.61% [6/979]; P = .02).
Discussion
In this international multicenter study from 58 hospitals, including children who required hospitalization with laboratory-confirmed COVID-19 without MIS-C, we did not detect a statistically significant association between early use of steroids and hospital LOS. To the best of our knowledge, this is the largest and most comprehensive evaluation of the early use of steroids in the treatment of COVID-19 in this age group.
Many of the management options for non–MIS-C COVID-19 in children are extrapolated from adult data. Our results of a lack of favorable association between the outcomes and the early use of steroids in children admitted to the hospital with non–MIS-C COVID-19 are in contrast to adult data11 and meta-analysis.15 The RECOVERY trial11 showed the most significant benefit in patients needing mechanical ventilation at randomization and no benefit among patients who did not require oxygen. Patients included in our study were overall less sick, with only 25% requiring respiratory support on admission, which may have affected the effect size. Nonetheless, a separate analysis including only the patients requiring respiratory support did not support benefits in our population. In our data, early steroids were used more in sicker patients. Because of the limitations of data granularity, sickness cannot be adjusted entirely. It is more likely than not that early steroid treatment has a stronger beneficial effect than what is observed in our data. A similar bias is also observed in other observational studies, such as those for steroids in pediatric septic shock, where some retrospective studies have shown steroid use to be associated with either no benefit or worse outcomes.34 Despite such studies, there remains uncertainty as to whether such data are due to bias by selective treatment of the sickest patients with septic shock.35 While methylprednisolone36 and hydrocortisone12 use for COVID-19 in adults has been studied, the best data on survival benefits with steroids are with dexamethasone. In our study, we could not differentiate between steroid types. Although most pediatric sites likely used dexamethasone based on adult studies, it is still possible that a sizable proportion were using other steroids at variable dosing, which may have affected our results.
The disease course of our patients may also have been affected by the use of other medications. Remdesivir was used in 90 patients (7.7%) within 2 days. Its association with LOS can be variable.37 Our analysis was adjusted for the influence of remdesivir. It is still possible that other therapeutic agents may have affected the disease course, but their effect could not be adjusted because of low absolute numbers. The interaction effects of early steroids and number of abnormal inflammatory mediators in 1 of the sensitivity analyses (LOS ≥2 days) suggest that patients treated with early steroids who had more abnormal mediator values would be expected to have a longer stay. Because this interaction was specific to, and seems to be, driven by patients with abnormal results for 3 inflammatory mediators only, it is possible that it is a false-positive association, or it is due to presence of another variable that is not controlled for in our analysis.
Assessing the correct outcome variable in pediatrics is challenging. In our study, evaluating the mortality difference was not possible because of the very low mortality rate (approximately 1%). Thus, hospital LOS was used as the primary outcome variable in this study with a hypothesis that resolution of illness due to early steroids may allow a physician to discharge the patient early. However, hospital LOS is influenced by multiple factors unrelated to the disease itself38 and may not be the most sensitive marker to detect the difference. We chose multiple secondary outcome variables because of the limitation of the primary outcome variables; however, because of low numbers, sophisticated analyses on many of the secondary outcome variables could not be performed.
Strengths and Limitations
The study’s strength is its large multicenter cohort, the inclusion of only patients with non–MIS-C COVID-19, selection of confounders based on causal inference models, and use of more than 1 sensitivity analysis to account for design choices on the outcome. The study has limitations inherent to a registry-based analysis. The expectation for research such as ours is that both groups were similar in all regards and were randomly prescribed early steroids or not. Although we attempted to adjust for the observed differences using linear regression, there will always be unmeasured confounders in retrospective analysis, and interpretation is subject to bias. Missingness of inflammatory mediator measures may affect the severity of illness adjustment in our models. This study specifically excluded patients with MIS-C to include patients only with COVID-19; however, occasionally MIS-C diagnosis is ambiguous. Furthermore, heterogeneity is also expected in non–MIS-C COVID-19 (respiratory illnesses or abdominal presentations, etc). Patients with an incidental diagnosis of COVID-19 were excluded from the registry; however, occasionally this distinction is inexplicit. It is possible that site investigators may have entered borderline cases in the registry (and vice versa). Lastly, even with vigilance about data quality, incorrect data are always possible in voluntary registries such as VIRUS.
Conclusions
Although our analysis did not find an association between the early use of steroids in children hospitalized with non–MIS-C COVID-19 and improved outcomes, the 95% CIs did not rule out benefit (or possibly, but less likely, harm) from early treatment with steroids. The definitive determination of the potential benefit or harm from early steroid therapy for children with COVID-19 cannot be made from retrospective data such as ours. There is a need for a prospective randomized clinical trial regarding the use of steroids to treat children with non–MIS-C COVID-19.
eMethods. Other measured variables and confounders
eReferences
eAppendix. Propensity score matched analysis
eTable 1. Laboratory values
eTable 2. Hospital length of the supplementary analysis
eTable 3. Logistic regression of secondary outcomes
eTable 4. Bivariate comparison of rare secondary outcomes
Nonauthor collaborators
References
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhalala US, Gist KM, Tripathi S, et al. Characterization and outcomes of hospitalized children with coronavirus disease 2019: a report from a multicenter, viral infection and respiratory illness universal study (coronavirus disease 2019) registry. Crit Care Med. 2022;50(1):e40-e51. doi: 10.1097/CCM.0000000000005232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative . Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868-873. doi: 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachdeva R, Rice TB, Reisner B, et al. The impact of coronavirus disease 2019 pandemic on US and Canadian PICUs. Pediatr Crit Care Med. 2020;21(9):e643-e650. doi: 10.1097/PCC.0000000000002510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripathi S, Gist KM, Bjornstad EC, et al. Coronavirus disease 2019-associated PICU admissions: a report from the Society of Critical Care Medicine Discovery Network Viral Infection and Respiratory Illness Universal Study Registry. Pediatr Crit Care Med. 2021;22(7):603-615. doi: 10.1097/PCC.0000000000002760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383(4):334-346. doi: 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z, Auriau J, Méot M, et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation. 2020;142(23):2282-2284. doi: 10.1161/CIRCULATIONAHA.120.050147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouldali N, Toubiana J, Antona D, et al. ; French Covid-19 Paediatric Inflammation Consortium . Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325(9):855-864. doi: 10.1001/jama.2021.0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son MBF, Murray N, Friedman K, et al. ; Overcoming COVID-19 Investigators . Multisystem inflammatory syndrome in children: initial therapy and outcomes. N Engl J Med. 2021;385(1):23-34. doi: 10.1056/NEJMoa2102605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awasthi S, Wagner T, Venkatakrishnan AJ, et al. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discov. 2021;7(1):55. doi: 10.1038/s41420-021-00429-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angus DC, Derde L, Al-Beidh F, et al. ; Writing Committee for the REMAP-CAP Investigators . Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317-1329. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dequin P-F, Heming N, Meziani F, et al. ; CAPE COVID Trial Group and the CRICS-TriGGERSep Network . Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298-1306. doi: 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health . COVID-19 treatment guidelines: clinical management of adults summary. Accessed January 28, 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/clinical-management-summary/
- 15.Sterne JAC, Murthy S, Diaz JV, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330-1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health . COVID-19 treatment guidelines: special considerations in children. Accessed January 28, 2022. https://www.covid19treatmentguidelines.nih.gov/special-populations/children/
- 17.Walkey AJ, Kumar VK, Harhay MO, et al. The Viral Infection and Respiratory Illness Universal Study (VIRUS): an international registry of coronavirus 2019-related critical illness. Crit Care Explor. 2020;2(4):e0113. doi: 10.1097/CCE.0000000000000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walkey AJ, Sheldrick RC, Kashyap R, et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med. 2020;48(11):e1038-e1044. doi: 10.1097/CCM.0000000000004572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention . Multisystem inflammatory syndrome (MIS). Accessed September 13, 2020. https://www.cdc.gov/mis-c/index.html
- 21.Gagne JJ, Polinski JM, Avorn J, Glynn RJ, Seeger JD. Standards for causal inference methods in analyses of data from observational and experimental studies in patient-centered outcomes research. Published March 15, 2012. https://www.pcori.org/assets/Standards-for-Causal-Inference-Methods-in-Analyses-of-Data-from-Observational-and-Experimental-Studies-in-Patient-Centered-Outcomes-Research.pdf
- 22.Lin W, Halpern SD, Prasad Kerlin M, Small DSA. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res. 2017;26(1):292-311. doi: 10.1177/0962280214545121 [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration . Pediatric exclusivity study age group. Accessed October 25, 2020. https://www.fda.gov/drugs/data-standards-manual-monographs/pediatric-exclusivity-study-age-group
- 24.Centers for Disease Control and Prevention . Use and interpretation of the WHO and CDC growth charts for children from birth to 20 years in the United States. Updated May 2013. https://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/growthchart.pdf
- 25.Centers for Disease Control and Prevention . A SAS program for the 2000 CDC growth charts (ages 0 to <20 years). Page last reviewed February 18, 2022. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 26.World Health Organization R&D Blue Print . COVID-19 therapeutic trial synopsis. Accessed December 24, 2021. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis
- 27.Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi: 10.1016/j.ijid.2020.11.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripathi S, Christison AL, Levy E, et al. ; Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group . The impact of obesity on disease severity and outcomes among hospitalized children with COVID-19. Hosp Pediatr. 2021;11(11):e297-e316. doi: 10.1542/hpeds.2021-006087 [DOI] [PubMed] [Google Scholar]
- 29.edX, Hernan M. Causal diagrams: draw your assumptions before your conclusions. June 4, 2021. https://learning.edx.org/course/course-v1:HarvardX+PH559x+2T2020/home
- 30.Shpitser I, Kudchadkar SR, Fackler J. Causal inference from observational data: it is complicated. Pediatr Crit Care Med. 2021;22(12):1093-1096. doi: 10.1097/PCC.0000000000002847 [DOI] [PubMed] [Google Scholar]
- 31.Vail EA, Gershengorn HB, Wunsch H, Walkey AJ. Attention to immortal time bias in critical care research. Am J Respir Crit Care Med. 2021;203(10):1222-1229. doi: 10.1164/rccm.202008-3238CP [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. Erlbaum; 1988. [Google Scholar]
- 33.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerman JJ. Corticosteroids in pediatric septic shock are not helpful. Crit Care Med. 2018;46(4):637-639. doi: 10.1097/CCM.0000000000002980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agus MSD, Parker MM. Glucocorticoid equipoise. Crit Care Med. 2018;46(4):493. doi: 10.1097/CCM.0000000000002981 [DOI] [PubMed] [Google Scholar]
- 36.Tang X, Feng Y-M, Ni J-X, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100(2):116-126. doi: 10.1159/000512063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman DL, Aldrich ML, Hagmann SHF, et al. Compassionate use of remdesivir in children with severe COVID-19. Pediatrics. 2021;147(5):e2020047803. doi: 10.1542/peds.2020-047803 [DOI] [PubMed] [Google Scholar]
- 38.Buttigieg SC, Abela L, Pace A. Variables affecting hospital length of stay: a scoping review. J Health Organ Manag. 2018;32(3):463-493. doi: 10.1108/JHOM-10-2017-0275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Other measured variables and confounders
eReferences
eAppendix. Propensity score matched analysis
eTable 1. Laboratory values
eTable 2. Hospital length of the supplementary analysis
eTable 3. Logistic regression of secondary outcomes
eTable 4. Bivariate comparison of rare secondary outcomes
Nonauthor collaborators