This cohort study evaluates the association between antidepressant use in pregnancy and neurodevelopmental outcomes in children.
Key Points
Question
Is antidepressant exposure during pregnancy associated with an increased risk of specific neurodevelopmental disorders in children?
Findings
In this cohort study including 145 702 antidepressant-exposed pregnancies, antidepressant exposure during pregnancy was not associated with autism spectrum disorder, attention-deficit/hyperactivity disorder, specific learning disorders, developmental speech/language disorders, developmental coordination disorders, intellectual disabilities, or behavioral disorders after accounting for confounding through various design and analytic approaches. Results were generally consistent across antidepressant medication classes, commonly used individual drugs, and gestational exposure windows.
Meaning
These findings suggest that antidepressant use in pregnancy does not increase the risk of neurodevelopmental disorders in children.
Abstract
Importance
Antidepressant use during pregnancy has been associated with neurodevelopmental disorders in children in some studies. However, results may be explained by uncontrolled confounding by parental mental health status, genetics, and environmental factors.
Objective
To evaluate the association between antidepressant use in pregnancy and neurodevelopmental outcomes in children.
Design, Setting, and Participants
This cohort study of health care utilization data was separated into cohorts of publicly and privately insured pregnant individuals and their children nested in the Medicaid Analytic eXtract (MAX; 2000-2014) and the IBM MarketScan Research Database (MarketScan; 2003-2015). A total of 1.93 million pregnancies in MAX and 1.25 million pregnancies in MarketScan were recorded. Children were followed from birth until outcome diagnosis, disenrollment, death, or end of study (maximum 14 years). Analyses were conducted between August 2020 and July 2021.
Exposures
Dispensing of antidepressant medication from gestational week 19 until delivery, the period of synaptogenesis.
Main Outcomes and Measures
Neurodevelopmental disorders in children defined using validated algorithms. Early pregnancy exposure was considered in sensitivity analyses, and approaches to confounding adjustment included propensity score fine stratification, discontinuers comparison, and sibling analyses.
Results
Among the individuals included in the analysis, there were 145 702 antidepressant-exposed and 3 032 745 unexposed pregnancies; the mean (SD) age among the antidepressant exposed and unexposed was 26.2 (5.7) and 24.3 (5.8) years in MAX and 32.7 (4.6) and 31.9 (4.6) years in MarketScan, respectively; and in MAX, which collected information on race and ethnicity, 72.4% of the antidepressant-exposed and 37.1% of the unexposed individuals were White. Crude results suggested up to a doubling in risk of neurodevelopmental outcomes associated with antidepressant exposure; however, no association was observed in the most fully adjusted analyses. When comparing antidepressant-exposed and unexposed siblings, hazard ratios were 0.97 (95% CI, 0.88-1.06) for any neurodevelopmental disorder, 0.86 (95% CI, 0.60-1.23) for autism spectrum disorder, 0.94 (95% CI, 0.81-1.08) for attention-deficit/hyperactivity disorder, 0.77 (95% CI, 0.42-1.39) for specific learning disorders, 1.01 (95% CI, 0.88-1.16) for developmental speech/language disorder, 0.79 (95% CI, 0.54-1.17) for developmental coordination disorder, 1.00 (95% CI, 0.45-2.22) for intellectual disability, and 0.95 (95% CI, 0.80-1.12) for behavioral disorders. Results were generally consistent for antidepressant classes and drugs and across exposure windows.
Conclusions and Relevance
The results of this cohort study suggest that antidepressant use in pregnancy itself does not increase the risk of neurodevelopmental disorders in children. However, given strong crude associations, antidepressant exposure in pregnancy may be an important marker for the need of early screening and intervention.
Introduction
Mental health disorders, and in particular depressive disorders, are common among women of reproductive age, including during pregnancy.1,2,3 Accordingly, antidepressants and other psychiatric medications are frequently prescribed, especially for women with severe illness. In the US, estimates for the prevalence of antidepressant use during pregnancy range from 6% to 8%.4,5,6,7 Several studies have attempted to clarify whether antidepressant exposure in utero increases the risk of neurodevelopmental disorders (NDDs) in children. Most of this research has focused on autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD).8 Results have largely been conflicting, and whether antidepressant exposure increases the risk of these childhood disorders remains controversial.8 It is possible that confounding by indication of antidepressants for severe depression and by genetic and environmental factors associated with NDDs in children can explain the observed associations.9 Moreover, few studies have considered other specific diagnosed NDDs outside of small, hospital- or clinic-based settings.10 Limited data for intellectual disabilities and motor development are largely inconclusive owing to lack of confounding control and inconsistent measurement of outcomes.11
In this cohort study, we used 2 large health care utilization databases to quantify the association between antidepressant use in pregnancy and various NDDs in children. We controlled for confounding by indication, environment, and genetics through various design and analytic approaches. The large sample size allowed for the assessment of antidepressant classes and individual drugs, and of various exposure windows.
Methods
Data Sources and Study Cohorts
We defined pregnancy cohorts nested in the Medicaid Analytic eXtract (MAX), which includes health care utilization data for Medicaid beneficiaries nationwide, from 2000 to 2014 and the MarketScan Commercial Claims Database (MarketScan), which includes private health insurance claims data, from 2003 to 2015. The development of the MAX pregnancy cohort has been previously described,12 and similar methods were used in the development of the MarketScan cohort. Both data sources include information on demographics, diagnoses, and procedures received during all health care encounters, including all inpatient, outpatient, or emergency department visits, and dispensed outpatient prescription medications. Individuals aged 12 to 55 years with live-birth deliveries linked to infants were required to have insurance coverage from 3 months before the date of the estimated last menstrual period (LMP) to 1 month after delivery.
This study was approved by the institutional review board of Brigham and Women’s Hospital, which waived the need for informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed for reporting.
Antidepressant Exposure
The primary exposed group included individuals with at least 1 dispensing of an antidepressant from 127 days after LMP (week 19 of gestation) to delivery, the approximate period of synaptogenesis. Antidepressant use was further categorized by class, including selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants. Exposure to individual antidepressant drugs was assessed for the 5 most commonly used drugs (sertraline, fluoxetine, bupropion, citalopram, and escitalopram). The unexposed group was defined as having no antidepressant dispensing from 90 days prior to pregnancy start through the day prior to delivery.
Neurodevelopmental Outcomes
We defined the following outcomes: ASD, ADHD, specific learning disorders, developmental speech/language disorder, developmental coordination disorder, intellectual disability, behavioral disorder, and any NDD (presence of any of the 7 specific disorders). Validated claims-based algorithms with high positive predictive values were used to define each outcome (eTable 1 in the Supplement).
Covariates
Potential confounders included demographics (age of pregnant individual, race and ethnicity [recorded in MAX only and determined based on information submitted to the Centers for Medicare & Medicaid Services by individual states], state, delivery year), indications for antidepressant use (depression, anxiety, bipolar disorder, other mental health disorders, NDDs in the pregnant individual), proxies for severity of mental health conditions (numbers of psychiatric visits, inpatient and emergency visits for mental health diagnoses, mental health diagnoses, dispensings of other psychotropic medications), lifestyle factors (smoking, alcohol, substance abuse), other medications, comorbidities, adequacy of prenatal care, and county-level socioeconomic level indicators (MAX only). There is a very small proportion of missing data for zip code (<1%), which is used to define county-level socioeconomic indicators; therefore, the adjusted analysis is a complete case analysis that excludes pregnancies without zip code data. The full list of covariates and their respective assessment periods is included in eTable 2 in the Supplement.
Statistical Analysis
Main Analysis
Descriptive statistics were compared between exposure groups using standardized differences; covariates with standardized differences less than 10% were considered balanced. Cumulative incidence was calculated for each outcome stratified by data source and exposure status. Children were followed from birth until the time of the outcome or the time of censoring, marked by the end of enrollment, the end of the study period, or death. To control for measured confounding, weighting based on fine stratification of the propensity score (PS) was used with 50 strata after trimming the nonoverlapping regions of the PS distributions.13 Cox proportional hazards regression was used to calculate crude and weighted hazard ratios (HRs). The HRs were estimated in MAX and MarketScan separately and were pooled using fixed-effect meta-analysis (results for the publicly and privately insured cohorts separately are presented in eTables 3-12 in the Supplement).
Given the known potential for unmeasured confounding by treatment indication and by environmental and genetic factors, multiple design and analytic approaches to confounding adjustment were implemented. First, we compared pregnant individuals with antidepressant exposure to unexposed individuals and adjusted for nearly 70 measured covariates using fine stratification of the PS (adjusted). Second, we used a high-dimensional PS (HDPS adjusted) approach, including an additional 200 empirically selected covariates.14
Third, we compared individuals with exposure during late pregnancy to individuals who were unexposed during pregnancy but filled a prescription for an antidepressant prior to pregnancy (discontinuer referent). Discontinuers were defined as having a dispensing for any antidepressant in the window from 90 to 31 days prior to LMP but not during the window of 30 days prior to LMP through delivery. For class and drug-specific analyses, we additionally considered discontinuers to the specific exposure.
Fourth, we completed a sibling analysis to control for shared familial environmental and genetic confounders. Cox proportional hazards models were stratified on individual identifier. We adjusted for potential nonshared confounders, including all covariates included in the PS models except race and ethnicity, state, and county-level socioeconomic status variables; we additionally considered sibling birth order. The publicly and privately insured cohorts were pooled prior to analysis owing to sparse data challenges. We assessed potential carryover effects from the exposure of the first pregnancy to the outcome of the second pregnancy.15 Details on methods for the sibling analyses can be found in the eMethods in the Supplement. Because of limited study size, sibling analyses were not conducted for medication classes and individual drugs.
Sensitivity Analyses
The timing of the etiologically relevant window in pregnancy is not well established for these outcomes. Therefore, we defined a secondary exposure window during the first half of pregnancy (LMP through 126 days after LMP) and compared with antidepressant-unexposed pregnant individuals. To ensure that similarity in results between early and late exposure windows was not because of pregnancies with exposure in both windows, we additionally defined antidepressant exposure as only in late or only in early pregnancy. The discontinuer referent analysis and sibling analysis were also repeated using the early pregnancy exposure window.
Exposure misclassification is possible if individuals who fill a prescription for an antidepressant do not take it. This is expected to bias results toward the null. To address this, we required 2 dispensings during the exposure window to meet the exposure definition.
Interpretation
In interpretation of the results, we focused on estimating magnitude of associations in preference to dichotomizing the results as statistically significant or not.16 Specifically, we judged estimates to be similar or different from the reference group by 3 criteria: (1) strength of the adjusted HR (regardless of whether the 95% CI included the null), (2) degree to which the upper bound of the 95% CI indicated low compatibility between the data and strong adverse effects (ie, the upper bound of the 95% CI excludes a large increase in the risk of NDD), and (3) consistency of the estimates across multiple approaches to confounding adjustment.
All analyses were conducted using SAS, version 9.4 (SAS Institute Inc), and R, version 4.0.3 (R Foundation). No adjustments were made for multiple comparisons.
Results
There were 93 069 and 52 633 pregnancies with exposure to antidepressants in late pregnancy and 1 833 927 and 1 198 818 pregnancies unexposed to antidepressants in the MAX and MarketScan cohorts, respectively. Select characteristics of the pregnant individuals are presented in the Table17 (all characteristics are summarized in eTables 13 and 14 in the Supplement; early pregnancy exposure characteristics are summarized in eTables 15 and 16 in the Supplement). Across both populations, individuals exposed to antidepressants were older and had greater medication use during pregnancy than unexposed individuals. In the MAX population, antidepressant-exposed individuals were also more likely to be White. After PS weighting, all characteristics were balanced between groups (Table and eTables 13 and 14 in the Supplement).
Table. Select Characteristics of the Cohort for Individuals Exposed to Antidepressants During Late Pregnancy and Individuals Unexposed to Antidepressants Throughout Pregnancy by Data Source.
| Characteristic | MAX | MarketScan | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Standard difference, % | No. (%) | Standard difference, % | |||||
| Exposed (n = 93 069) | Unexposed (n = 1 833 927) | Crude | Weighted | Exposed (n = 52 633) | Unexposed (n = 1 198 818) | Crude | Weighted | |
| Age, mean (SD), y | 26.2 (5.7) | 24.3 (5.8) | 32.7 | 4.2 | 32.7 (4.6) | 31.9 (4.6) | 17.7 | 0.5 |
| Race and ethnicitya,b | ||||||||
| Asian or Other Pacific Islander | 883 (0.9) | 73 042 (4.0) | −19.7 | 2.5 | NA | NA | NA | NA |
| Black or African American | 13 642 (14.7) | 645 600 (35.2) | −48.9 | 3.6 | NA | NA | NA | NA |
| Hispanic or Latino | 5067 (5.4) | 279 317 (15.2) | −32.6 | 3.6 | NA | NA | NA | NA |
| White | 67 389 (72.4) | 680 748 (37.1) | 75.8 | −5.7 | NA | NA | NA | NA |
| Unknown or other | 6088 (6.5) | 155 220 (8.5) | −7.3 | 1.1 | NA | NA | NA | NA |
| Indications | ||||||||
| Depression | 43 721 (47.0) | 82 232 (4.5) | 111.2 | 5.6 | 18 305 (34.8) | 34 328 (2.7) | 90.4 | 0.6 |
| Anxiety | 24 222 (26.0) | 55 849 (3.0) | 69.0 | −0.3 | 12 349 (23.5) | 27 316 (2.3) | 66.7 | −3.0 |
| Bipolar disorder | 11 771 (12.6) | 25 178 (1.4) | 45.3 | −2.1 | 2119 (4.0) | 2756 (0.2) | 26.5 | −1.0 |
| Schizophrenia | 1218 (1.3) | 2726 (0.1) | 13.7 | −0.7 | 51 (0.1) | 93 (<0.1) | 3.9 | 0.3 |
| Psychosis | 1305 (1.4) | 4061 (0.2) | 13.2 | −0.1 | 145 (0.3) | 476 (<0.1) | 6.0 | −0.3 |
| Other mental health disordersc | 3585 (3.9) | 16 483 (0.9) | 19.5 | 0.5 | 1087 (2.1) | 6402 (0.5) | 13.6 | −1.0 |
| Epilepsy/convulsion | 2597 (2.8) | 17 701 (1.0) | 13.5 | −1.1 | 446 (0.8) | 4936 (0.4) | 5.5 | −0.3 |
| ADHD | 2934 (3.2) | 10 889 (0.6) | 19.0 | −1.1 | 1019 (1.9) | 4351 (0.4) | 14.8 | −0.6 |
| Adjustment disorder | 1697 (1.8) | 13 412 (0.7) | 9.7 | −0.7 | 581 (1.1) | 3449 (0.3) | 9.8 | −0.7 |
| Psychiatric visits | ||||||||
| 0 | 79 476 (85.4) | 1 789 696 (97.6) | −44.8 | −0.8 | 42 389 (80.5) | 1 167 770 (97.4) | −55.9 | 1.9 |
| 1 | 3579 (3.8) | 16 535 (0.9) | 19.4 | −0.2 | 1791 (3.4) | 5769 (0.5) | 21.3 | −1.0 |
| 2-3 | 3662 (3.9) | 11 254 (0.6) | 22.4 | 0.2 | 2492 (4.7) | 7052 (0.6) | 26.0 | −0.3 |
| ≥4 | 6352 (6.8) | 16 442 (0.9) | 31.1 | 1.1 | 5961 (11.3) | 18 227 (1.5) | 40.8 | −1.6 |
| Mental health diagnoses | ||||||||
| 0 | 41 625 (44.7) | 1 625 054 (88.6) | −105.2 | 2.4 | 29 921 (56.8) | 1 137 078 (94.8) | −99.1 | 2.7 |
| 1 | 18 301 (19.7) | 126 831 (6.9) | 38.2 | −4.5 | 9763 (18.5) | 37 534 (3.1) | 51.2 | −5.9 |
| 2-3 | 20 981 (22.5) | 65 301 (3.6) | 58.7 | −1.7 | 9940 (18.9) | 21 930 (1.8) | 58.3 | 0.3 |
| ≥4 | 12 162 (13.1) | 16 741 (0.9) | 49.1 | 4.0 | 3009 (5.7) | 2276 (0.2) | 33.1 | 4.0 |
| Mental health ER visits | ||||||||
| 0 | 85 412 (91.8) | 1 803 275 (98.3) | −30.6 | −1.0 | 51 070 (97.0) | 1 194 008 (99.6) | −20.1 | −0.6 |
| 1 | 5224 (5.6) | 25 482 (1.4) | 23.1 | −0.2 | 1099 (2.1) | 4134 (0.3) | 16.0 | −0.2 |
| 2-3 | 2084 (2.2) | 4819 (0.3) | 17.9 | 1.4 | 360 (0.7) | 592 (<0.1) | 10.5 | 1.2 |
| ≥4 | 349 (0.4) | 351 (<0.1) | 8.0 | 1.8 | 104 (0.2) | 84 (<0.1) | 6.0 | 0.9 |
| Mental health hospitalizations | ||||||||
| 0 | 87 502 (94.0) | 1 816 957 (99.1) | −28.0 | −1.8 | 51 786 (98.4) | 1 197 533 (99.9) | −16.3 | −1.4 |
| 1 | 2562 (2.8) | 11 636 (0.6) | 16.5 | 0.6 | 410 (0.8) | 787 (0.1) | 11.0 | 0.5 |
| 2-3 | 1976 (2.1) | 4248 (0.2) | 17.6 | 1.5 | 319 (0.6) | 418 (<0.1) | 10.1 | 1.3 |
| ≥4 | 1029 (1.1) | 1086 (0.1) | 13.8 | 1.2 | 118 (0.2) | 80 (<0.1) | 6.4 | 0.8 |
| Other psychiatric medications | ||||||||
| 0 | 71 611 (76.9) | 1 790 601 (97.6) | −65.4 | −2.0 | 45 778 (87.0) | 1 179 946 (98.4) | −45.1 | −1.6 |
| 1 | 7183 (7.7) | 21 778 (1.2) | 32.1 | −1.1 | 2773 (5.3) | 9403 (0.8) | 26.4 | −0.2 |
| 2-3 | 10 354 (11.1) | 17 799 (1.0) | 43.6 | 1.5 | 3383 (6.4) | 8688 (0.7) | 31.1 | 1.3 |
| ≥4 | 3921 (4.2) | 3749 (0.2) | 27.5 | 3.4 | 699 (1.3) | 781 (0.1) | 15.2 | 2.3 |
| Lifestyle behaviors | ||||||||
| Alcohol abuse | 3363 (3.6) | 13 921 (0.8) | 19.6 | 0.9 | 304 (0.6) | 806 (0.1) | 9.0 | 0.7 |
| Tobacco use | 17 583 (18.9) | 137 823 (7.5) | 34.1 | −2.4 | 1497 (2.8) | 12 022 (1.0) | 13.4 | −1.5 |
| Substance abuse | 10 385 (11.2) | 52 472 (2.9) | 32.9 | −0.3 | 524 (1.0) | 1728 (0.1) | 11.3 | 0.1 |
| Obstetric Comorbidity Index scored | ||||||||
| 0 | 38 972 (41.9) | 1 027 424 (56.0) | −28.6 | 0.7 | 17 755 (33.7) | 526 672 (43.9) | −21.0 | 1.9 |
| 1 | 21 941 (23.6) | 411 137 (22.4) | 2.8 | 0 | 14 585 (27.7) | 316 929 (26.4) | 2.9 | −0.5 |
| 2 | 15 169 (16.3) | 209 624 (11.4) | 14.1 | −0.5 | 9614 (18.3) | 186 353 (15.5) | 7.3 | −0.4 |
| ≥3 | 16 987 (18.3) | 185 742 (10.1) | 23.4 | −0.4 | 10 679 (20.3) | 168 864 (14.1) | 16.5 | −1.3 |
| Other prescription medications | ||||||||
| Anticonvulsants | 10 448 (11.2) | 26 298 (1.4) | 41.1 | 0.5 | 2878 (5.5) | 9325 (0.8) | 27.2 | 0.9 |
| Antipsychotics | 10 287 (11.1) | 10 544 (0.6) | 45.9 | 2.1 | 1471 (2.8) | 956 (0.1) | 23.0 | 3.5 |
| Anxiolytics | 3834 (4.1) | 4654 (0.3) | 26.7 | 1.0 | 1225 (2.3) | 1618 (0.1) | 20.0 | 0.6 |
| Benzodiazepines | 19 269 (20.7) | 33 710 (1.8) | 62.5 | 2.4 | 9220 (17.5) | 33 382 (2.8) | 50.3 | −0.1 |
| Psychostimulants | 3650 (3.9) | 9315 (0.5) | 23.4 | 0.1 | 1660 (3.2) | 6951 (0.6) | 19.1 | −0.8 |
| Opioids | 52 341 (56.2) | 532 059 (29.0) | 57.3 | −4.1 | 16 932 (32.2) | 199 561 (16.6) | 36.7 | −3.9 |
| Corticosteroids | 27 599 (29.7) | 323 243 (17.6) | 28.6 | −0.6 | 15 971 (30.3) | 229 744 (19.2) | 26.1 | −2.5 |
| Antidiabetics | 3262 (3.5) | 31 978 (1.7) | 11.0 | −0.3 | 3191 (6.1) | 45 342 (3.8) | 10.6 | −1.2 |
| Antihypertensives | 11 952 (12.8) | 94 612 (5.2) | 27.1 | −0.4 | 6302 (12.0) | 66 183 (5.5) | 23.0 | −2.0 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ER, emergency room; MarketScan, MarketScan Commercial Claims Database; MAX, Medicaid Analytic eXtract; NA, not applicable.
Race and ethnicity were determined on the basis of information submitted to the Centers for Medicare & Medicaid Services by individual states, which was based on information that had been collected and coded from Medicaid applications. The category Other or unknown included individuals who were American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Hispanic or Latino, more than 1 race, and unknown; the category Hispanic or Latino included those with missing race information, whereas data under Other or unknown included Hispanic or Latino with 1 or more races.
Information not available in MarketScan.
Other mental health disorders include delirium, dementia, tic disorder, somatoform spectrum disorder, eating disorder, psychotherapy, and self-inflicted injury.
A comorbidity index as defined by Bateman et al.17
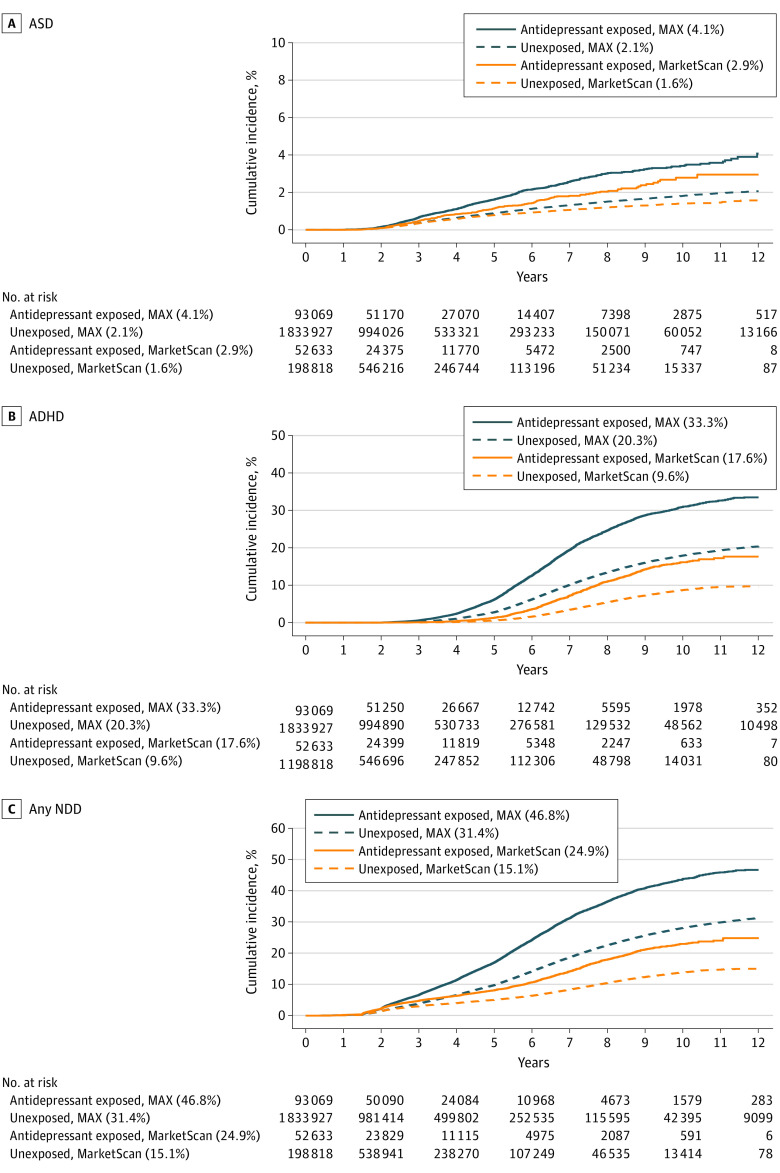
For all NDD outcomes, the cumulative incidence was higher in the MAX cohort than in the MarketScan cohort (Figure 1 and eFigure in the Supplement). Neurodevelopmental disorders were common among children of individuals with antidepressant exposure; by age 12 years, 46.8% of children (95% CI, 45.6%-48.1%) in the MAX cohort and 24.9% (95% CI, 23.0%-26.9%) in the MarketScan cohort with antidepressant exposure had any NDD, compared with 31.4% (95% CI, 31.1%-31.6%) and 15.1% (95% CI, 14.7%-15.4%), respectively, among unexposed individuals. The most common individual outcome assessed was ADHD; cumulative incidence at 12 years was 33.3% (95% CI, 32.2%-34.5%) and 20.3% (95% CI, 20.0%-20.5%) among children of antidepressant exposed and unexposed individuals, respectively, in MAX, and 17.6% (95% CI, 15.8%-19.5%) and 9.6% (95% CI, 9.3%-10.0%), respectively, in MarketScan. Incidence of ASD was similarly higher among antidepressant-exposed children and publicly insured children; cumulative incidence at 12 years was 4.1% (95% CI, 3.5%-4.7%) and 2.1% (95% CI, 2.0%-2.1%) among children of antidepressant-exposed and unexposed individuals, respectively, in MAX, and 2.9% (95% CI, 2.4%-3.6%) and 1.6% (95% CI, 1.4%-1.7%), respectively, in MarketScan.
Figure 1. Cumulative Incidence of ASD, ADHD, and Any NDD Stratified by Antidepressant Status and Data Source.
Crude cumulative incidence curves are presented for each outcome of interest stratified by antidepressant exposure status (defined as having an antidepressant dispensed between 127 days after last menstrual period to the day prior to delivery) and data source. The cumulative incidence at age 12 years for each outcome in each stratum is noted in the legend. ADHD indicates attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; MarketScan, MarketScan Commercial Claims Database; MAX, Medicaid Analytic eXtract; NDD, neurodevelopmental disorder.
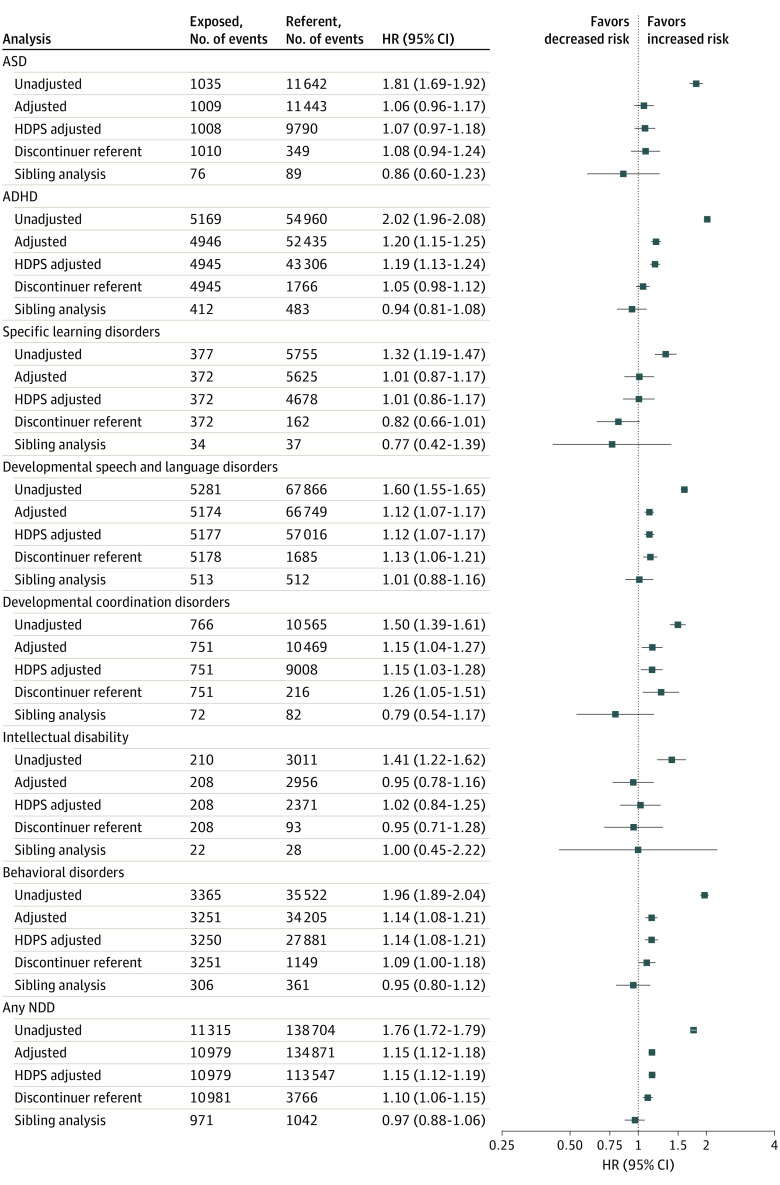
Crude HRs for all NDD outcomes suggested an increase in risk, with HRs ranging from 1.32 for specific learning disorders to 2.02 for ADHD, among children exposed to antidepressants in utero compared with unexposed children (Figure 2). Adjustment for measured covariates and proxies for unmeasured covariates via HDPS resulted in substantial attenuation of HRs for all NDDs; estimates from the adjusted analysis ranged from 1.01 (specific learning disorders) to 1.20 (ADHD). When comparing with children of individuals who discontinued use of antidepressants prior to pregnancy, HRs were consistent with the adjusted and HDPS results or further attenuated for all NDD outcomes. The sibling analyses indicated no increased risk for any of the outcomes. Sensitivity analyses for the sibling design did not suggest that carryover effects from the exposure in the first pregnancy to the outcome in the second pregnancy were present (eTable 17 in the Supplement). Additional results for the sibling analyses are available in eTables 21 and 22 in the Supplement.
Figure 2. Hazard Ratios (HRs) and 95% CIs for Neurodevelopmental Disorders (NDDs) Among Children Exposed to Antidepressants During Late Pregnancy.
The exposed cohort is measured by antidepressant dispensing in late pregnancy. For all analyses except the discontinuer referent, the referent is defined as no antidepressant dispensing from 90 days prior to last menstrual period (LMP) through 1 day prior to delivery. For the discontinuer referent analysis, the referent is defined as antidepressant dispensing in the 31 to 90 days prior to LMP but no dispensing from 30 days prior to LMP through the day prior to delivery. The high-dimensional propensity score (HDPS) analysis used an 80% random sample of the unexposed cohort in the Medicaid Analytic eXtract data owing to sample size constraints in the SAS macro (SAS Institute) used for HDPS analysis. Results from the crude and adjusted analysis using the 80% sample of the referent cohort were very similar to the main results. Event counts for all adjusted analyses are based on the cohort after trimming of nonoverlapping regions of the propensity scores. Counts for the sibling analysis are based on families that had discordance on both antidepressant exposure status and outcome timing. ADHD indicates attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder.
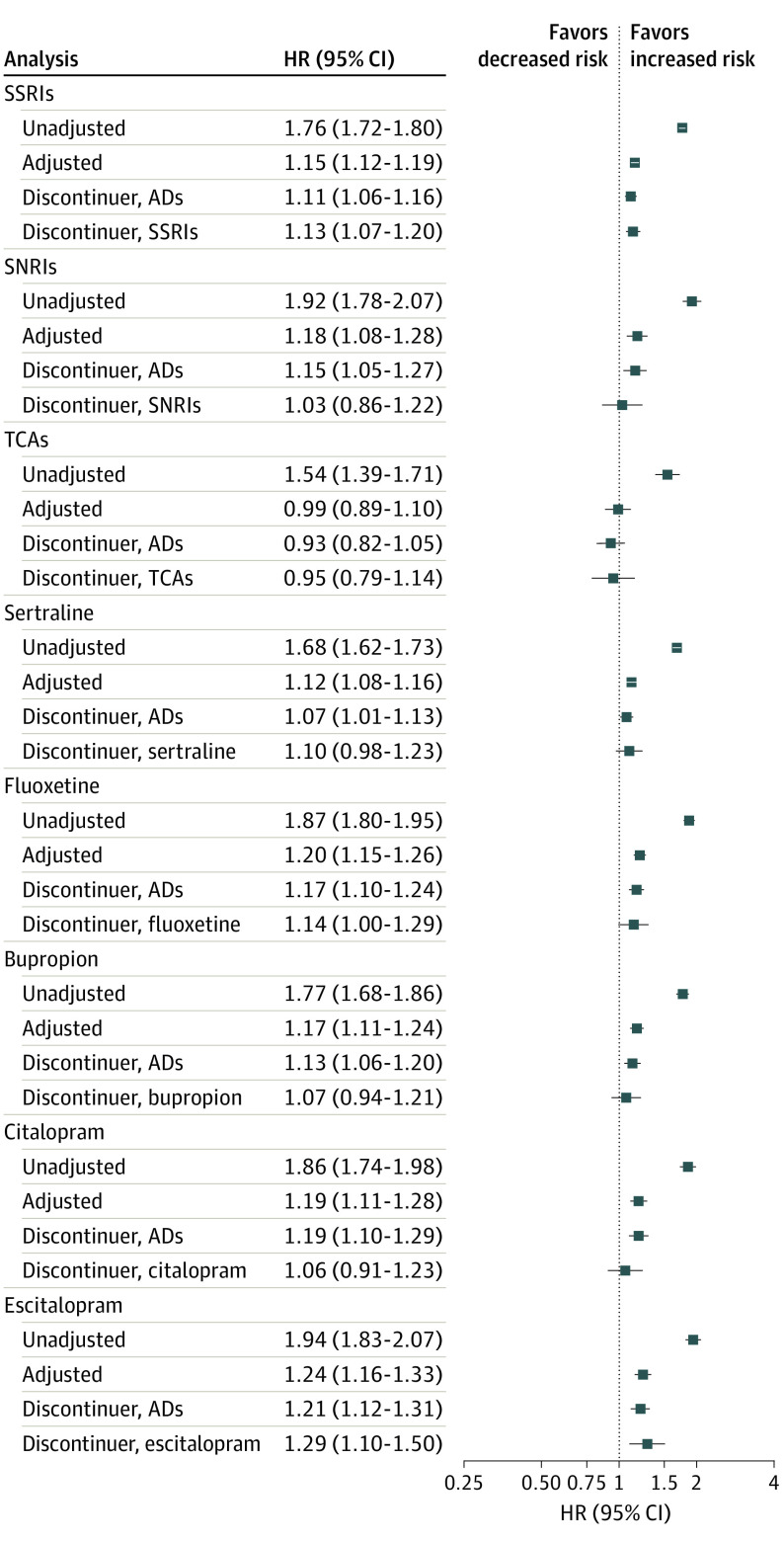
Results by antidepressant class and individual drugs are presented in Figure 3 and eTables 5 through 12 in the Supplement (characteristics are summarized in eTables 18 and 19 in the Supplement). Results for the composite outcome of any NDD indicate no meaningful increase in risk for any of the classes or drugs, with the possible exception of escitalopram. Comparison of escitalopram results across all outcomes evaluated revealed adjusted HRs that tended to be slightly higher than estimates for other drugs and classes. The evidence for ASD, ADHD, specific learning disorders, intellectual disability, developmental speech/language disorder, and behavioral disorders suggests no increased risk that is consistent across all analyses for any class or drug (eTables 5-12 in the Supplement). For developmental coordination disorder, point estimates were elevated in the adjusted analysis and in both discontinuer referent analyses for some individual antidepressants considered, but HRs were imprecisely estimated (eTables 9-12 in the Supplement).
Figure 3. Hazard Ratios (HRs) and 95% CIs for Any Neurodevelopmental Disorders (NDDs) by Specific Antidepressant (AD) Classes and Drugs During Late Pregnancy.
The exposed cohort is measured by antidepressant dispensing in late pregnancy. For all analyses except the discontinuer referents, the referent is defined as no antidepressant dispensing from 90 days prior to last menstrual period (LMP) through 1 day prior to delivery. For the AD discontinuer referent analysis, the referent is defined as dispensing of any antidepressant from 90 to 31 days prior to LMP but not during the window of 30 days prior to LMP through delivery. For the class/drug specific discontinuer referent analyses, the referent is defined as dispensing of the specific class or drug from 90 to 31 days prior to LMP but no dispensing of any antidepressant during the window of 30 days prior to LMP through delivery. Event counts for all analyses can be found in eTables 5 through 12 in the Supplement. SNRIs indicates serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants.
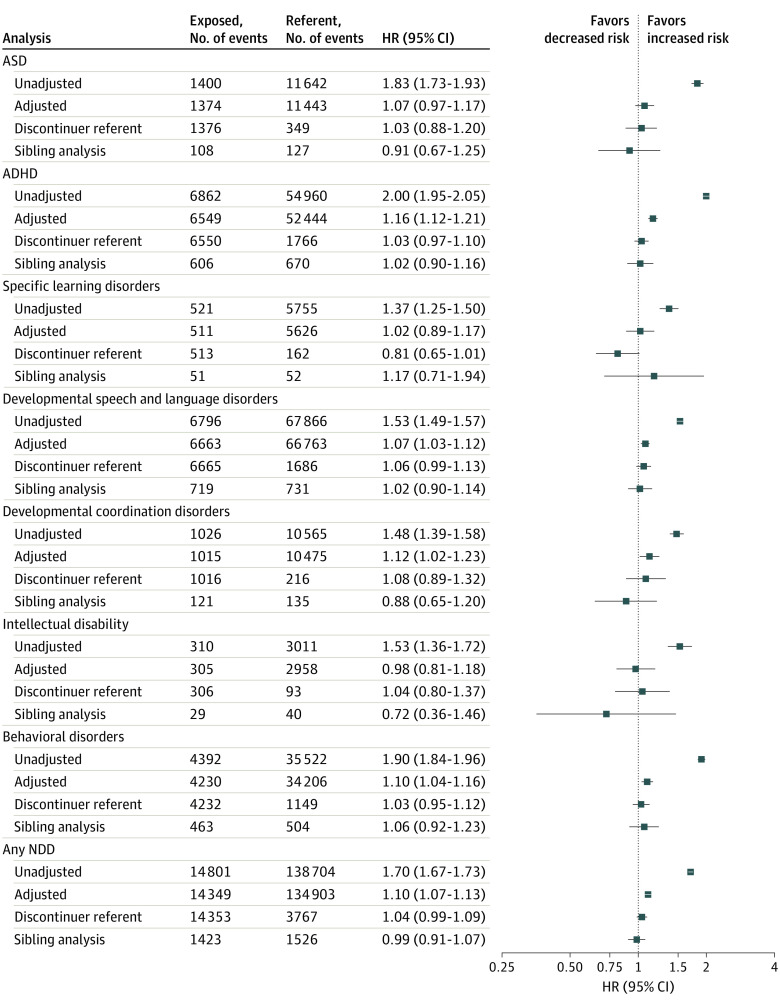
When exposure to antidepressants was defined by dispensings in the first half of pregnancy, 129 358 exposed pregnancies in MAX and 69 138 exposed pregnancies in MarketScan were available for analysis. Results were similar to the main analysis (Figure 4). The discontinuer referent and sibling analyses similarly resulted in null estimates for all outcomes. When antidepressant exposure was defined as only in late pregnancy or only in early pregnancy, results were similar to the main results for each exposure window (eTable 20 in the Supplement). Results from analyses requiring 2 fills of an antidepressant to meet the exposure definition were also consistent with those of the main analysis (eTable 3 in the Supplement).
Figure 4. Hazard Ratios (HRs) and 95% CIs for Neurodevelopmental Disorders (NDDs) Among Children Exposed to Antidepressants During Early Pregnancy.
The exposed cohort is measured by antidepressant dispensing in early pregnancy, and the referent is measured by no antidepressant dispensing from 90 days prior to last menstrual period (LMP) through 1 day prior to delivery. For the discontinuer referent analysis, the referent is defined as antidepressant dispensing in the 31 to 90 days prior to LMP but no dispensing from 30 days prior to LMP through the day prior to delivery. Event counts for all adjusted analyses are based on the cohort after trimming of nonoverlapping regions of the propensity scores. Counts for the sibling analysis are based on families that had discordance on both antidepressant exposure status and outcome timing. ADHD indicates attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder.
Discussion
In this study of 2 large cohorts of publicly and privately insured parent-child pairs, individuals using antidepressants during pregnancy had a higher risk of having a child with an NDD than individuals not using antidepressants. After adjustment for an extensive list of measured potential confounders, elevated crude HRs shifted substantially toward the null. Comparing with antidepressant discontinuers further shifted estimates to the null, and comparison with unexposed siblings resulted in null estimates for all outcomes. These results point to an increased risk of NDDs owing to factors associated with antidepressant use during pregnancy, but not the medication itself.
Autism spectrum disorder is the most widely studied NDD in relation to antidepressant exposure during pregnancy. Studies using population-based registries in Nordic countries and Canadian provinces have reported estimates of a 20% to 80% increase in ASD risk with antidepressant exposure.18,19,20,21,22,23,24,25 Sensitivity analyses suggested that uncontrolled confounding by indication and environment may be a likely explanation in some21,22,23,25 but not all of these studies.18,20 Using a broad range of confounding variables, we did not observe an association after adjustment.
Prior studies of the risk of ADHD have also been conflicting, suggesting either no association with antidepressant exposure,26,27 an increase in risk,28,29,30 or an increase in risk when comparing with unexposed children that was not sustained in sensitivity analyses.22,23,31,32 Consistent with this latter group of studies,22,23,31,32 the association we observed after adjustment for measured confounders was no longer present in analyses that targeted residual confounding by using discontinuer referents or sibling analyses.
Previous studies evaluating the risk of other NDDs have tended to be small and hospital, clinic, or classroom based, and often did not adjust for confounders.10,11 Prenatal exposure to antidepressants has been associated with various neurodevelopmental characteristics at different time points in childhood: in the first year of life as a risk factor for delayed motor development and motor control33,34,35 and longer-term risks in the domains of intelligence, social problems, anxiety/depression, and internalizing problems.36,37 However, the outcomes are too diverse and the studies are too heterogeneous to draw clear conclusions.10,11 The present results suggest no association between antidepressant use and specific learning disorders, developmental speech/language disorders, intellectual disabilities, and behavioral disorders.
While the class and drug-specific results showed some residual associations in the adjusted analyses and discontinuer analyses for developmental coordination disorder, it is unlikely that these associations are causal. The adjusted and discontinuers analysis for antidepressants overall similarly showed an association, but this was no longer present when accounting for shared environmental and genetic factors in the sibling analysis. While sample size was not adequate to repeat sibling analyses for individual drugs, it is likely that drug-specific sibling comparisons would have resulted in a similar shift to the null.
Compared with exposure to other specific antidepressant classes and drugs, exposure to escitalopram resulted in slightly elevated HRs for all outcomes except for ASD. We did not observe clear differences in patient characteristics (eTables 18 and 19 in the Supplement) that might explain these associations, and all measured covariates were balanced after PS weighting. In a Danish study, no association was found between escitalopram users and antidepressant discontinuers for the outcome of any psychiatric disorders in children.19 In contrast with the present null results for ASD, an analysis of Swedish mother-child pairs reported an association between citalopram and escitalopram use and ASD, but not with other selective serotonin reuptake inhibitors.24 Further investigation of the association with escitalopram may be warranted.
Limitations
Some limitations of the data and design must be noted. Records of antidepressant dispensing are used as a proxy for actual medication use. However, sensitivity analyses that required 2 fills during the exposure window were consistent with the main results. Most children ended follow-up much sooner than the maximum of 14 years because of administrative censoring (Figure 1). Previous analyses of this cohort found no evidence of informative censoring because of loss of insurance enrollment,38 and follow-up did not differ by antidepressant exposure status. Survival analyses properly account for noninformative, right censoring. Outcomes were defined using algorithms with high positive predictive values, which minimizes the potential bias owing to outcome misclassification. However, in the absence of universal screening for these outcomes, it is possible that parental depression status effects the likelihood of diagnosis and results in differential outcome misclassification. We see no evidence in the present data, however, that individuals with depression are less likely to bring their children in for well-child visits where screening would occur (eTables 13-16 in the Supplement). Despite the extensive list of confounders considered, residual confounding by imperfectly measured variables (eg, lifestyle factors, mental health) and unmeasured factors (eg, childhood environment) may be present. Results from the sibling analysis suggested that there is no association between antidepressant use and any neurodevelopmental outcome. Sibling analyses are useful to control residual confounding that is not easily measured in conventional analysis, such as genetic and environmental factors. However, other potential explanations for this result must be acknowledged. Siblings that differ in exposure status are expected to differ strongly on other nonshared confounders, which can lead to more bias than in the full population analysis.39 However, adjustment for all measured covariates in the sibling analysis resulted in little difference in HR estimates from the crude results (eTable 21 in the Supplement). Sibling comparisons are also more susceptible to exposure misclassification owing to the comparison of siblings with nonshared causes of exposure,39,40 which may bias estimates toward the null. Sibling comparisons control for shared mediators by design and therefore provide an estimate of the direct causal effect, which could result in the attenuation we observed.41 Finally, while sibling comparisons are vulnerable to carryover effects,15 such effects are implausible for in utero antidepressant exposure, and the present analyses ruled out the likelihood that exposure in the first pregnancy had consequences on the outcome in the second pregnancy. In summary, the sibling analyses increased our confidence in the conclusion that there is no association between in utero exposure to antidepressants and NDDs in the offspring.
Conclusions
Results of this cohort study suggest that antidepressant use in pregnancy does not increase the risk of NDDs in children. However, elevated crude cumulative incidence estimates for NDDs suggest that antidepressant use by pregnant individuals is a robust marker of NDD risk in children. Therefore, antidepressant exposure during pregnancy could be an important marker for early screening and intervention in children, with the goal of improving outcomes for NDDs.
eMethods. Additional methods and results for the sibling analyses
eTable 1. Outcome definitions for neurodevelopmental disorders
eTable 2. Full list of covariates included in propensity score models and assessment periods
eTable 3. Hazard ratios and 95% confidence intervals for antidepressant use in late pregnancy and neurodevelopmental disorders for the main analysis and sensitivity analyses, by data source
eTable 4. Hazard ratios and 95% confidence intervals for antidepressant use in early pregnancy and neurodevelopmental disorders for the main analysis and sensitivity analyses, by data source
eTable 5. Hazard ratios and 95% confidence intervals for SSRI use in late pregnancy and neurodevelopmental disorders, by data source
eTable 6. Hazard ratios and 95% confidence intervals for SNRI use in late pregnancy and neurodevelopmental disorders, by data source
eTable 7. Hazard ratios and 95% confidence intervals for TCA use in late pregnancy and neurodevelopmental disorders, by data source
eTable 8. Hazard ratios and 95% confidence intervals for sertraline use in late pregnancy and neurodevelopmental disorders, by data source
eTable 9. Hazard ratios and 95% confidence intervals for fluoxetine use in late pregnancy and neurodevelopmental disorders, by data source
eTable 10. Hazard ratios and 95% confidence intervals for bupropion use in late pregnancy and neurodevelopmental disorders, by data source
eTable 11. Hazard ratios and 95% confidence intervals for citalopram use in late pregnancy and neurodevelopmental disorders, by data source
eTable 12. Hazard ratios and 95% confidence intervals for escitalopram use in late pregnancy and neurodevelopmental disorders, by data source
eTable 13. Characteristics of the cohort for individuals exposed to antidepressants during late pregnancy and individuals unexposed to antidepressants throughout pregnancy, in MAX, crude and propensity score weighted results
eTable 14. Characteristics of the cohort for individuals exposed to antidepressants during late pregnancy and individuals unexposed to antidepressants throughout pregnancy, in MarketScan, crude and propensity score weighted results
eTable 15. Characteristics of the cohort for individuals exposed to antidepressants during early pregnancy and individuals unexposed to antidepressants throughout pregnancy, in MAX, crude and propensity score weighted results
eTable 16. Characteristics of the cohort for individuals exposed to antidepressants during early pregnancy and individuals unexposed to antidepressants throughout pregnancy, in MarketScan, crude and propensity score weighted results
eTable 17. Sibling analysis for antidepressant use during late pregnancy and NDDs stratified by exposure status of first sibling, to investigate carry-over effects
eTable 18. Characteristics of the cohort for individuals exposed to specific antidepressant classes or drugs during late pregnancy, in MAX
eTable 19. Characteristics of the cohort for individuals exposed to specific antidepressant classes or drugs during late pregnancy, in MarketScan
eTable 20. Hazard ratios and 95% confidence intervals for antidepressant exposed exclusive to early or late gestational windows and neurodevelopmental disorders, results pooled across data sources
eTable 21. Counts and crude and adjusted HR (95% CI) for sibling analysis for antidepressant use during late and early pregnancy compared to unexposed, pooled MAX and MarketScan data
eTable 22. Crude and adjusted HR (95% CI) within the full sibling population for antidepressant use during late pregnancy compared to unexposed, pooled MAX and MarketScan data
eFigure. Unadjusted cumulative incidence of select neurodevelopment disorders stratified by antidepressant exposure status during pregnancy and data source
References
- 1.Ashley JM, Harper BD, Arms-Chavez CJ, LoBello SG. Estimated prevalence of antenatal depression in the US population. Arch Womens Ment Health. 2016;19(2):395-400. doi: 10.1007/s00737-015-0593-1 [DOI] [PubMed] [Google Scholar]
- 2.Ko JY, Farr SL, Dietz PM, Robbins CL. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005-2009. J Womens Health (Larchmt). 2012;21(8):830-836. doi: 10.1089/jwh.2011.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farr SL, Bitsko RH, Hayes DK, Dietz PM. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-542.e9. doi: 10.1016/j.ajog.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 4.Huybrechts KF, Palmsten K, Mogun H, et al. National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry. 2013;35(3):265-271. doi: 10.1016/j.genhosppsych.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ailes EC, Simeone RM, Dawson AL, Petersen EE, Gilboa SM. Using insurance claims data to identify and estimate critical periods in pregnancy: an application to antidepressants. Birth Defects Res A Clin Mol Teratol. 2016;106(11):927-934. doi: 10.1002/bdra.23573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1-194.e5. doi: 10.1016/j.ajog.2007.07.036 [DOI] [PubMed] [Google Scholar]
- 7.Molenaar NM, Bais B, Lambregtse-van den Berg MP, et al. The international prevalence of antidepressant use before, during, and after pregnancy: a systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J Affect Disord. 2020;264:82-89. doi: 10.1016/j.jad.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 8.Uguz F. Neonatal and childhood outcomes in offspring of pregnant women using antidepressant medications: a critical review of current meta-analyses. J Clin Pharmacol. 2021;61(2):146-158. doi: 10.1002/jcph.1724 [DOI] [PubMed] [Google Scholar]
- 9.Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med. 2018;16(1):6. doi: 10.1186/s12916-017-0993-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Marroun H, White T, Verhulst FC, Tiemeier H. Maternal use of antidepressant or anxiolytic medication during pregnancy and childhood neurodevelopmental outcomes: a systematic review. Eur Child Adolesc Psychiatry. 2014;23(10):973-992. doi: 10.1007/s00787-014-0558-3 [DOI] [PubMed] [Google Scholar]
- 11.Al-Fadel N, Alrwisan A. Antidepressant use during pregnancy and the potential risks of motor outcomes and intellectual disabilities in offspring: a systematic review. Drugs Real World Outcomes. 2021;8(2):105-123. doi: 10.1007/s40801-021-00232-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One. 2013;8(6):e67405-e67405. doi: 10.1371/journal.pone.0067405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28(2):249-257. doi: 10.1097/EDE.0000000000000595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512-522. doi: 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjölander A, Frisell T, Kuja-Halkola R, Öberg S, Zetterqvist J. Carryover effects in sibling comparison designs. Epidemiology. 2016;27(6):852-858. doi: 10.1097/EDE.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 16.Wasserstein RL, Lazar NA. The ASA statement on P values: context, process, and purpose. Am Stat. 2016;70(2):129-133. doi: 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 17.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122(5):957-965. doi: 10.1097/AOG.0b013e3182a603bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2016;170(2):117-124. doi: 10.1001/jamapediatrics.2015.3356 [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Agerbo E, Ingstrup KG, et al. Antidepressant use during pregnancy and psychiatric disorders in offspring: Danish nationwide register based cohort study. BMJ. 2017;358:j3668. doi: 10.1136/bmj.j3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, Vigod SN. Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA. 2017;317(15):1544-1552. doi: 10.1001/jama.2017.3415 [DOI] [PubMed] [Google Scholar]
- 21.Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med. 2013;369(25):2406-2415. doi: 10.1056/NEJMoa1301449 [DOI] [PubMed] [Google Scholar]
- 22.Malm H, Brown AS, Gissler M, et al. Gestational Exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry. 2016;55(5):359-366. doi: 10.1016/j.jaac.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sujan AC, Rickert ME, Öberg AS, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA. 2017;317(15):1553-1562. doi: 10.1001/jama.2017.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viktorin A, Uher R, Reichenberg A, Levine SZ, Sandin S. Autism risk following antidepressant medication during pregnancy. Psychol Med. 2017;47(16):2787-2796. doi: 10.1017/S0033291717001301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449-459. doi: 10.2147/CLEP.S53009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castro VM, Kong SW, Clements CC, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry. 2016;6(1):e708. doi: 10.1038/tp.2015.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupattelli A, Mahic M, Handal M, Ystrom E, Reichborn-Kjennerud T, Nordeng H. Attention-deficit/hyperactivity disorder in children following prenatal exposure to antidepressants: results from the Norwegian Mother, Father and Child Cohort Study. BJOG. 2021;128(12):1917-1927. doi: 10.1111/1471-0528.16743 [DOI] [PubMed] [Google Scholar]
- 28.Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry. 2015;20(6):727-734. doi: 10.1038/mp.2014.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Figueroa R. Use of antidepressants during pregnancy and risk of attention-deficit/hyperactivity disorder in the offspring. J Dev Behav Pediatr. 2010;31(8):641-648. doi: 10.1097/DBP.0b013e3181e5ac93 [DOI] [PubMed] [Google Scholar]
- 30.Boukhris T, Sheehy O, Bérard A. Antidepressant use in pregnancy and the risk of attention deficit with or without hyperactivity disorder in children. Paediatr Perinat Epidemiol. 2017;31(4):363-373. doi: 10.1111/ppe.12378 [DOI] [PubMed] [Google Scholar]
- 31.Laugesen K, Olsen MS, Telén Andersen AB, Frøslev T, Sørensen HT. In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ Open. 2013;3(9):e003507. doi: 10.1136/bmjopen-2013-003507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man KKC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357(8108):j2350. doi: 10.1136/bmj.j2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142(4):402-408. doi: 10.1067/mpd.2003.139 [DOI] [PubMed] [Google Scholar]
- 34.Hanley GE, Brain U, Oberlander TF. Infant developmental outcomes following prenatal exposure to antidepressants, and maternal depressed mood and positive affect. Early Hum Dev. 2013;89(8):519-524. doi: 10.1016/j.earlhumdev.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 35.Mortensen JT, Olsen J, Larsen H, Bendsen J, Obel C, Sørensen HT. Psychomotor development in children exposed in utero to benzodiazepines, antidepressants, neuroleptics, and anti-epileptics. Eur J Epidemiol. 2003;18(8):769-771. doi: 10.1023/A:1025306304635 [DOI] [PubMed] [Google Scholar]
- 36.Lupattelli A, Wood M, Ystrom E, Skurtveit S, Handal M, Nordeng H. Effect of time-dependent selective serotonin reuptake inhibitor antidepressants during pregnancy on behavioral, emotional, and social development in preschool-aged children. J Am Acad Child Adolesc Psychiatry. 2018;57(3):200-208. doi: 10.1016/j.jaac.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singal D, Chateau D, Struck S, et al. In utero antidepressants and neurodevelopmental outcomes in kindergarteners. Pediatrics. 2020;145(5):e20191157. doi: 10.1542/peds.2019-1157 [DOI] [PubMed] [Google Scholar]
- 38.Straub L, Bateman BT, Hernandez-Diaz S, et al. Neurodevelopmental disorders among publicly or privately insured children in the United States. JAMA Psychiatry. 2022;79(3):232-242. doi: 10.1001/jamapsychiatry.2021.3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713-720. doi: 10.1097/EDE.0b013e31825fa230 [DOI] [PubMed] [Google Scholar]
- 40.Frisell T. Invited commentary: sibling-comparison designs, are they worth the effort? Am J Epidemiol. 2021;190(5):738-741. doi: 10.1093/aje/kwaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjölander A, Zetterqvist J. Confounders, mediators, or colliders: what types of shared covariates does a sibling comparison design control for? Epidemiology. 2017;28(4):540-547. doi: 10.1097/EDE.0000000000000649 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Additional methods and results for the sibling analyses
eTable 1. Outcome definitions for neurodevelopmental disorders
eTable 2. Full list of covariates included in propensity score models and assessment periods
eTable 3. Hazard ratios and 95% confidence intervals for antidepressant use in late pregnancy and neurodevelopmental disorders for the main analysis and sensitivity analyses, by data source
eTable 4. Hazard ratios and 95% confidence intervals for antidepressant use in early pregnancy and neurodevelopmental disorders for the main analysis and sensitivity analyses, by data source
eTable 5. Hazard ratios and 95% confidence intervals for SSRI use in late pregnancy and neurodevelopmental disorders, by data source
eTable 6. Hazard ratios and 95% confidence intervals for SNRI use in late pregnancy and neurodevelopmental disorders, by data source
eTable 7. Hazard ratios and 95% confidence intervals for TCA use in late pregnancy and neurodevelopmental disorders, by data source
eTable 8. Hazard ratios and 95% confidence intervals for sertraline use in late pregnancy and neurodevelopmental disorders, by data source
eTable 9. Hazard ratios and 95% confidence intervals for fluoxetine use in late pregnancy and neurodevelopmental disorders, by data source
eTable 10. Hazard ratios and 95% confidence intervals for bupropion use in late pregnancy and neurodevelopmental disorders, by data source
eTable 11. Hazard ratios and 95% confidence intervals for citalopram use in late pregnancy and neurodevelopmental disorders, by data source
eTable 12. Hazard ratios and 95% confidence intervals for escitalopram use in late pregnancy and neurodevelopmental disorders, by data source
eTable 13. Characteristics of the cohort for individuals exposed to antidepressants during late pregnancy and individuals unexposed to antidepressants throughout pregnancy, in MAX, crude and propensity score weighted results
eTable 14. Characteristics of the cohort for individuals exposed to antidepressants during late pregnancy and individuals unexposed to antidepressants throughout pregnancy, in MarketScan, crude and propensity score weighted results
eTable 15. Characteristics of the cohort for individuals exposed to antidepressants during early pregnancy and individuals unexposed to antidepressants throughout pregnancy, in MAX, crude and propensity score weighted results
eTable 16. Characteristics of the cohort for individuals exposed to antidepressants during early pregnancy and individuals unexposed to antidepressants throughout pregnancy, in MarketScan, crude and propensity score weighted results
eTable 17. Sibling analysis for antidepressant use during late pregnancy and NDDs stratified by exposure status of first sibling, to investigate carry-over effects
eTable 18. Characteristics of the cohort for individuals exposed to specific antidepressant classes or drugs during late pregnancy, in MAX
eTable 19. Characteristics of the cohort for individuals exposed to specific antidepressant classes or drugs during late pregnancy, in MarketScan
eTable 20. Hazard ratios and 95% confidence intervals for antidepressant exposed exclusive to early or late gestational windows and neurodevelopmental disorders, results pooled across data sources
eTable 21. Counts and crude and adjusted HR (95% CI) for sibling analysis for antidepressant use during late and early pregnancy compared to unexposed, pooled MAX and MarketScan data
eTable 22. Crude and adjusted HR (95% CI) within the full sibling population for antidepressant use during late pregnancy compared to unexposed, pooled MAX and MarketScan data
eFigure. Unadjusted cumulative incidence of select neurodevelopment disorders stratified by antidepressant exposure status during pregnancy and data source