Abstract
Objective
To compare the clinical results of the direct anterior approach (DAA) and posterolateral approach (PLA) in total hip arthroplasty (THA) patients.
Methods
From January 2017 to September 2019, 80 patients who received primary THA in our hospital were retrospectively selected based on the propensity score matching (PSM) method. Baseline characteristics of patients who underwent the DAA and PLA were collected. Moreover, the incision length, intraoperative blood loss, operative time, length of stay, and Harris hip score were compared between patients in the two groups. The CK level was used to assess muscle damage between patients in the DAA and PLA groups. The complications of these two approaches were also evaluated at patients' 12‐month follow‐up evaluation.
Results
There was no significant difference in baseline characteristics between patients in the two groups (p > 0.05). The patients in the DAA group had a shorter incision length (9.2 ± 0.2 vs 14.7 ± 0.5, respectively; p < 0.05) and shorter length of hospital stay (9.5 ± 0.7 vs 12.9 ± 0.8, respectively, p < 0.05) than patients in the PLA group. Moreover, the DAA was associated with a decrease in intraoperative blood loss compared with the PLA (109.1 ± 12.6 vs 305.1 ± 14.1 ml, respectively, p < 0.05). However, the operation time was longer in patients in the DAA group (130.7 ± 1.7) than in patients in the PLA group (112.6 ± 1.3 min, p < 0.05). The CK level of patients in the DAA group was lower than that of patients in the PLA group (p < 0.05). The CK level at 48 h post‐surgery was negatively correlated with the Harris hip scores at 6 months after THA (r = −0.538, p = 0.000). Compared with patients in the PLA group, the muscle strength of patients in the DAA group was significantly higher than that of patients in the DAA group at 4 days (p < 0.05) and 7 days (p < 0.05) after THA. The Harris hip scores of patients in the DAA group and PLA group were 81.0 ± 0.8 vs 70.8 ± 0.7 at 6 weeks, 93.4 ± 0.9 vs 86.4 ± 0.6 at 3 months, and 96.8 ± 1.1 vs 93.4 ± 0.8 at 6 months, respectively, both p < 0.05. There was no significant difference in the incidence of complications between patients in the DAA and PLA groups (p > 0.05).
Conclusion
DAA was superior to the PLA in improving hip function after THA. Compared with the PLA, the DAA could reduce muscle damage, which is negatively correlated with hip function. Further multi‐institution studies are required with longer follow‐up durations, and larger patient populations are needed to provide more definitive conclusions.
Keywords: direct anterior approach, functional recovery, muscle damage, posterolateral approach, total hip arthroplasty
Comparison of postoperative gross appearance and X‐ray images between patients who underwent the direct anterior approach (DAA) and posterolateral approach (PLA) for total hip arthroplasty (THA).

Introduction
Approximately 150,000 total hip arthroplasty (THA) surgeries are performed each year in the United States, and the number of THAs is increasing each year 1 , 2 . Hip dislocation is a major cause of complications after THA, with reported prevalence rates ranging from 0.3% to 3%. Thus, the ability to relieve postoperative dislocation, reduce the incidence of related complications, and improve patient satisfaction is urgently needed in clinical practice. Postoperative functional recovery represents an important factor affecting the satisfaction of the patient. The quality of the important muscles around the hip is an important determinant for functional recovery. A surgical approach has been identified that may affect perioperative complications and functional outcomes. There are many surgical approaches to THA, including the direct anterior approach (DAA), posterior approach (PA), and posterolateral approach (PLA) 3 .
Posterior approach or posterolateral approach could allow the surgical site to be fully exposed, but there are certain drawbacks to these methods. For example, PLA requires cutting off the short external rotator of the hip, and thus, the risk of posterior dislocation of the hip after surgery is higher. Currently, there is growing interest in the study of the DAA in patients undergoing THA. DAA uses the internervous plane and was first described by Hueter et al. in 1883 4 . The DAA incision enters from the muscle space and nerve space without damaging any muscles. DAA is performed on the anterior hip joint through the interval between the tensor fascia lata and sartorius muscle.
Compared with the outcomes of other approaches, the DAA has the advantages of less muscular damage, more rapid postoperative recovery, and less pain. Conversely, some other studies have suggested that THA patients treated with DAA had similar outcomes in the early period as those treated with PLA, although patients in the early surgery group achieved more rapid recovery and pain relief than patients in other groups 5 , 6 , 7 , 8 .
Several studies have shown that DAA was superior to PLA with regard to saving blood loss, reducing pain intensity, and shortening the length of hospital stay 9 , 10 . A retrospective diagnostic study using MRI found that the DAA has a better level of soft tissue protection than other traditional approaches. However, Meermans et al. 11 conducted a review and summarized that there is little evidence for improved kinematics or better long‐term outcomes following the use of the DAA for THA. There is a steep learning curve for this approach with similar rates of complications, length of stay, and outcomes. Moerenhout et al. 12 revealed that DAA appears to be a safe and effective option for THA. However, there was no significant difference in hospital length of stay or postoperative recovery between patients who underwent the DAA or PLA. Yoo et al. 13 performed gait analysis and found that gait speed and peak hip flexion within 3 months after surgery were significantly higher in patients in the DAA group than in patients in the anterolateral approach group. Sun et al. 14 revealed that patients in the DAA group had higher Harris hip scores within 6 months and shorter hospital stays than patients in the PLA group. They concluded that DAA could offer rapid early functional recovery after THA compared with PLA. However, patients in the DAA group often required a longer operative time and had more blood loss than those in the PLA group.
Previously, we conducted a systematic review and meta‐analysis and revealed that DAA could enhance functional recovery and reduce postoperative pain intensity compared with PA 15 . However, there is no consensus as to which approach offers fast recovery after THA.
This retrospective study compared clinical and functional outcomes in THA between patients in the DAA and PLA groups. We hypothesized that compared with PLA, DAA would lead to shorter incision length, less muscle damage, and better functional recovery after THA.
Therefore, the main purpose of this retrospective study was to (i) compare the surgery‐related results (incision length, total blood loss, hospital stay, and operation time) of total hip arthroplasty between patients in the DAA and PLA groups; (ii) explore muscle damage markers (CK level), Harris hip score, and hip joint function between patients in the DAA and PLA groups.
Materials and Methods
General Data
Patients (n = 80) with femoral neck fracture or end‐stage osteoarthritis who underwent total hip arthroplasty in Jingjiang People's Hospital from January 2017 to September 2019 were included in this study. Research protocol approval was provided by the Ethics Committee of Jingjiang People's Hospital (2016YLS007), and informed consent was obtained from all subjects and their families.
The included studies were required to meet the following criteria: (i) patients undergoing primary THA; (ii) patients underwent the DAA for THA; (iii) patients underwent the PLA for THA; (iv) the incision length, intraoperative blood loss, operative time, length of stay, Harris hip score, CK level, and complications were documented.
Exclusion criteria were as follows: Body mass index (BMI) ≥30 kg/m2; previous hardware, Crowe Type 3 or 4 dysplasia, nonelective (i.e. emergent) THA, and performance of other approaches for THA. All surgeons who performed THA with DAA or PLA had prior surgical experience and completed their learning curve during the study period. The patients were divided into a DAA group (n = 47) and PLA group (n = 33) according to the surgical approach. The general characteristics of the patients are shown in Table 1.
TABLE 1.
The general characteristics of the two groups
| Patients demographics | DAA | PLA | p Value |
|---|---|---|---|
| Age (years) | 65.2 ± 4.4 | 64.7 ± 5.2 | 0.096 |
| BMI (kg/m2) | 23.5 ± 1.1 | 23.2 ± 1.4 | 0.089 |
| Sex | |||
| Male | 25 | 19 | 0.698 |
| Female | 22 | 14 | |
| ASA score | 2.31 ± 0.45 | 2.41 ± 0.51 | 0.152 |
| Smoking status | |||
| Never | 20 | 16 | 0.852 |
| Previous | 15 | 10 | |
| Current | 12 | 7 | |
| Diabetes | 5 | 4 | 0.836 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; DAA, direct anterior approach; PLA, posterolateral approach.
Surgical Methods
All surgical procedures were performed by one experienced surgeon (Hong‐wei Bao, who exceeded the learning curve). For the DAA, the patient was placed in the supine position and the muscle interval was used. After disinfection, an incision was made at 2 cm rear of the anterior superior iliac spine to expose the hip joint. A soft tissue retractor was inserted to separate the tensor fascia latae muscle and sartorius. Then, the rectus femoris was inwardly pulled, and the gluteus minimus, gluteus maximus, and tensor fascia lata were moved to the outside. The articular capsule was incised, and then the lower limb was internally rotated to remove the femoral head with a saw. After exposing the superior dome of the acetabulum, we filed the acetabulum to a suitable size. Next, an acetabular prosthesis was implanted, and abduction up to 45° ± 10° and forward leaning to 15° ± 10° were identified. Then, the right femoral head was chosen and installed.
PLA is a modification of the Gibson–Moore approach 16 . The patient was placed in the lateral decubitus position. The curvilinear incision centered on the greater trochanter of the femur. Then, the skin, superficial fascia, fascia lata, and gluteus maximus were cut layer by layer. Then, the external rotator muscle was exposed and cut off. The hip joint capsule was cut open, and femoral head dislocation was performed. The femoral head was removed, and the acetabulum was exposed. The acetabulum was cut to an appropriate size to implant and fix the prosthesis. Then, the right femoral head was chosen and installed. Postoperative gross appearance and X‐rays of the DAA and PLA were obtained and are shown in Fig. 1.The schematic of the DAA and PLA is shown in Fig. 2.
Fig. 1.
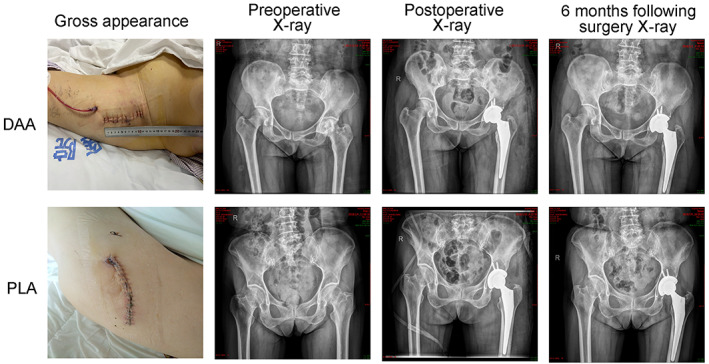
Comparison of postoperative gross appearance and X‐ray images between patients who underwent the direct anterior approach (DAA) and posterolateral approach (PLA) for total hip arthroplasty (THA)
Fig. 2.

The schematic of the direct anterior approach (DAA) and posterolateral approach (PLA)
Postoperative therapy in the DAA and PLA groups included antibiotic and anticoagulant therapy. Antibiotic prophylaxis was achieved by the administration of cefuroxime (1.5 g) at 0.5 to 1 h prior to skin incision and one more time immediately after THA.
For deep vein thrombosis (DVT) prophylaxis, low‐molecular‐weight heparin (0.4 ml 4250 IU; Kunming, China) was administered subcutaneously 12 h after THA, and this protocol continued for 10 days.
Data Collection
Patient general characteristics (age, BMI, sex [male or female], American Society of Anesthesiologists [ASA] score, smoking status, and diabetes) were recorded. In addition, the operation time, incision length, and total blood loss were recorded. Total blood loss was calculated according to the Nadler 17 and Gross formula 18 . Total blood loss = (total blood volume × [change in Hb level/preoperative Hb level]) x 1000 + volume transfused. The length of stay of all patients was recorded. The length of stay was measured from the admission day until discharge. The length of hospital stay was categorized as follows: less than or equal to 7 days of hospitalization (excellent) and greater than 7 days (poor), according to the calculated median 15 . Muscle strength was graded using the Modified Medical Research Council (MMRC) scale 19 at 1, 3, and 7 days after THA.
Harris hip scores 20 at 6 weeks, 3 months, and 6 months after THA were used to measure functional recovery. The HHS was used to evaluate postoperative recovery of hip function in an adult population. The HHS score system mainly includes four aspects: pain, function, absence of deformity, and range of motion. The score standard had a maximum of 100 points (best possible outcome). A total score <70 was considered poor, 70–80 was fair, 80–90 was good, and 90–100 was excellent.
Complications (loosening of the prosthesis, fracture of prosthesis, incidence of infection, and total complications) at 12‐month follow‐up between patients who underwent DAA and PLA were also recorded. The implant position, dislocation, and loosening of the prosthesis were evaluated by X‐ray examination. The muscle strength of hip flexion was quantified by using a handheld dynamometer (Anima Co.).
Peripheral blood samples were collected preoperatively and immediately after surgery and on postoperative days (PODs) 1 and 2. The CK level was used to assess muscle damage. The total serum CK levels in the DAA and PLA groups were measured by a Cobas® 6000 analyzer (Hitachi High‐Technologies Corporation).
The blood transfusion rate was also recorded. The use of blood transfusions was standardized, which meant that the hemoglobin concentration was <70 g/L or a patient developed any anemia‐related organ dysfunction.
Statistical Analysis
Propensity score matching (PSM) was used to match patients in the two groups. SPSS 20.0 statistical software (SPSS, Inc.) was used for statistical analysis. The χ 2 test was applied for comparing discontinuous data (complications), x̅ ± s was applied for describing continuous data (incision length, total blood loss, hospital stay, operation time, CK level, muscle strength, Harris hip score, and hip joint function), and the t test was adopted for analysis. Correlations were calculated using the Pearson correlation. p < 0.05 was considered statistically significant.
Result
Comparison of Surgery‐Related Outcomes
There was no significant difference in any indicator of the general data (age, BMI, sex, ASA score, smoking status, and diabetes) of the patients in the two groups (p > 0.05, Table 1). The DAA significantly reduced the incision length (9.2 ± 0.2 vs 14.7 ± 0.5, respectively, t = 68.18, p < 0.05, Table 2), total blood loss (109.1 ± 12.6 vs 305.1 ± 14.1, respectively; t = 51.91, p = 0.00, Table 2), and hospital stay (9.5 ± 0.7 vs 12.9 ± 0.8, respectively, t = 14.24, p = 0.00, Table 2) compared with the outcome of patients who underwent the PLA. However, the operation time with the DAA was longer than that with the PLA (130.7 ± 1.7 vs 112.6 ± 1.3, respectively, t = 45.78, p = 0.00, Table 2). Transfusion rate in the DAA (10.64%) group was less than PLA (30.3%) group, the difference was statistically significant (χ 2 = 4.921, p = 0.027).
TABLE 2.
Comparison of clinical indexes between two kinds of patients during operation
| Groups | n | Incision length (cm) | Operation time (min) | Total blood loss (ml) | Hospital stay (d) |
|---|---|---|---|---|---|
| DAA group | 47 | 9.2 ± 0.2 | 130.7 ± 1.7 | 109.1 ± 12.6 | 9.5 ± 0.7 |
| PLA group | 33 | 14.7 ± 0.5 | 112.6 ± 1.3 | 305.1 ± 14.1 | 12.9 ± 0.8 |
| t | 68.18 | 45.78 | 51.91 | 14.24 | |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Abbreviations: DAA, direct anterior approach; PLA, posterolateral approach.
Comparison of Muscle Damage Markers
The preoperative CK levels of patients in the DAA group and PLA group were 109.38 ± 8.03 vs 118.52 ± 9.23, respectively (Fig. 3A, p > 0.05).
Fig. 3.

(A) Pre‐surgery CK levels in patients who underwent the direct anterior approach (DAA) and posterolateral approach (PLA) for total hip arthroplasty (THA); (B) Post‐surgery CK levels in patients who underwent the DAA and PLA for THA; (C) CK levels in patients who underwent the DAA and PLA at 24 h post‐THA; (D) CK levels in patients who underwent the DAA and PLA at 48 h post‐THA. *p < 0.05
In addition, the CK level of patients in the DAA group was lower than that of patients in the PLA group (226.29 ± 13.66 vs 246.00 ± 17.16 post‐surgery, 336.76 ± 11.06 vs 372.76 ± 13.09 and 351.57 ± 7.96 vs 398.19 ± 12.98, respectively, both p < 0.05, Fig. 3B–D).
Moreover, the CK level at 48 h post‐surgery was negatively correlated with the Harris hip scores at 6 months after THA (r = −0.538, p = 0.000, Fig. 4).
Fig. 4.

Correlation between Harris hip scores and CK levels 48 h after total hip arthroplasty (THA)
Comparison of Muscle Strength Changes Between Patients in the DAA and PLA Groups
There was no significant difference in muscle strength between patients in the two groups at day 1 after THA (1.91 ± 0.22 vs 1.89 ± 0.24, respectively, p = 0.546). Compared with patients in the PLA group, the muscle strength of patients in the DAA group was significantly higher than that of patients in the DAA group at 4 days (2.81 ± 0.36 vs 2.51 ± 0.29, respectively, t = 6.392, p < 0.05;Table 3) and 7 days (3.95 ± 0.41 vs 2.89 ± 0.34, respectively, t = 9.653, p < 0.05; Table 3) after THA.
TABLE 3.
Comparison of muscle strength changes between the two groups on the 1st, 4th, and 7th day after operation [ ± s/level]
| Groups | 1st day | 4th day | 7th day |
|---|---|---|---|
| DAA group | 1.91 ± 0.22 | 2.81 ± 0.36 | 3.95 ± 0.41 |
| PLA group | 1.89 ± 0.24 | 2.51 ± 0.29 | 2.89 ± 0.34 |
| t‐value | 0.605 | 6.392 | 9.653 |
| p‐value | 0.546 | 0.000 | 0.000 |
Abbreviations: DAA, direct anterior approach; PLA, posterolateral approach.
Comparison of Harris Hip Scores
The Harris hip scores of patients in the DAA group and PLA group were 81.0 ± 0.8 vs 70.8 ± 0.7 at 6 weeks, 93.4 ± 0.9 vs 86.4 ± 0.6 at 3 months, and 96.8 ± 1.1 versus 93.4 ± 0.8 at 6 months, respectively (both p < 0.05, Table 4 ).
TABLE 4.
Comparison of Harris hip scores between the two groups
| Groups | n | 6 weeks | 3 months | 6 months |
|---|---|---|---|---|
| DAA group | 47 | 81.0 ± 0.8 | 93.4 ± 0.9 | 96.8 ± 1.1 |
| PLA group | 33 | 70.8 ± 0.7 | 86.4 ± 0.6 | 93.4 ± 0.8 |
| t‐value | 64.840 | 38.975 | 45.517 | |
| p‐value | 0.000 | 0.000 | 0.000 |
Abbreviations: DAA, direct anterior approach; PLA, posterolateral approach.
Comparison of Hip Joint Function
The hip joint function, including pain degree (35.12 ± 1.47 vs 29.55 ± 2.76, respectively), activity ability (30.65 ± 1.39 vs 24.90 ± 2.28, respectively), life ability (12.71 ± 0.54 vs 10.65 ± 0.47, respectively), and activity degree (4.15 ± 0.26 vs 3.72 ± 0.19, respectively), in patients in the DAA group at 7 days after THA were better than those in patients in the PLA group (p < 0.05,Table 5 ).
TABLE 5.
Comparison of hip joint function between the two groups at 7 days after operation [( ± s),score]
| Groups | Pain degree | Activity ability | Life ability | Activity degree |
|---|---|---|---|---|
| DAA group | 35.12 ± 1.47 | 30.65 ± 1.39 | 12.71 ± 0.54 | 4.15 ± 0.26 |
| PLA group | 29.55 ± 2.76 | 24.90 ± 2.28 | 10.65 ± 0.47 | 3.72 ± 0.19 |
| t | 6.253 | 15.226 | 20.347 | 9.442 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Abbreviations: DAA, direct anterior approach; PLA, posterolateral approach.
Comparison of the Occurrence of Complications
There was no significant difference in the incidence of loosening of the prosthesis, fracture of the prosthesis, infection, stiffness, or total complications between patients in the DAA and PLA groups (p > 0.05, Table 6).
TABLE 6.
Comparison of the incidence of complications between the two groups
| Groups | Loosening of the prosthesis | Fracture of the prosthesis | Incidence of infection | Stiffness | Incidence of complications |
|---|---|---|---|---|---|
| DAA group (47) | 0 (0.0%) | 1 (2.13%) | 0 (0.00%) | 0 (0.00%) | 1 (2.13%) |
| PLA group (33) | 0 (0.0%) | 1 (3.03%) | 0 (0.00%) | 1 (3.03%) | 2 (6.06%) |
| χ 2 | — | 0.012 | — | 1.177 | 0.523 |
| p | — | 0.914 | — | 0.278 | 0.470 |
Abbreviations: DAA, direct anterior approach; PLA, posterolateral approach.
Discussion
There has been controversy about the optimal surgical approach for THA. Some studies suggested that the DAA is better than the PLA in improving postoperative function 21 , whereas another study reported that the DAA has comparable functional effects but a longer learning curve than the PLA 22 . Therefore, we conducted this study to assess whether the DAA was superior to the PLA for functional recovery after THA.
The DAA Enhanced the Functional Recovery of Patients Who Underwent THA
The DAA could enhance functional recovery compared with the PLA in THA patients. We measured the Harris hip scores at 6 months after THA. Based on our results, the DAA may be preferred for better functional recovery at mid‐term follow‐up (6 months). Early functional outcomes following the DAA for THA compared with the PA and PLA have been reported 3 , 23 . Retrespo et al. 24 analyzed a total of 122 patients and found statistically significant differences in functional recovery between patients in the DAA and LA groups after 1 year of follow‐up.
The operation time in patients in the DAA group was longer than that in patients in the PLA group. As with most complex hip procedures, the learning curve is steep, and long operating times are needed. A previous study identified that the complication rate of the DAA decreases as the surgeon goes through a learning curve 25 . Additionally, it should be noted that obese THA patients who underwent DAA were associated with an increase in complications compared to other patients who underwent the DAA for THA 26 . Another study also suggested that wound complications should be taken into consideration for obese patients when choosing the DAA for THA 27 .
Operation time with the DAA was longer than that with the PLA (130.7 ± 1.7 vs 112.6 ± 1.3). It is mainly due to the learning curve of DAA being longer than that of PLA. In most studies, the DAA learning curve mainly focused on operating time and complications 28 , 29 . The learning curve for DAA is long, with a reported range from 20 to 100 THAs 7 , 30 , 31 , 32 . Nairn et al. 32 revealed that the operative time of THA plateaued after approximately 100 cases. From our experience, the operative time and complications were stabilized after nearly 80 cases. Learning time is required before generalization about THA outcomes.
Strengths and Limitations
A major strength of this study was that the incision length of patients who underwent the DAA was shorter and hip function was better at 6 months than those who underwent the PLA. Moreover, we calculated the kappa value to check for consistency between evaluators. A shorter incision length signified less damage to the muscle. Zhao et al. 33 assessed muscle damage markers after THA between patients in the DAA and PLA groups. The results revealed that the DAA could decrease muscle damage markers compared with that of the PLA. The reason could be that the DAA uses an intermuscular plane. Kwak et al. 34 also identified that CK, IL‐6,IL‐10, and IL‐1α levels were significantly lower in patients in the DAA group than in patients in the PLA group. Another important finding was that the DAA could decrease the length of hospital stay compared with that when the PLA was used. The results of this study are in line with those results in previous studies 10 , 35 , 36 , 37 . Moreover, we performed a meta‐analysis previously and found that the DAA was associated with a reduction in the VAS at 6 weeks and total blood loss for THA patients 38 .
However, some limitations exist in our research. First, the present study was retrospective with a small sample size. Second, another limitation of our study was the relatively short follow‐up period (6 months). The last limitation was that we only included patients with BMI <30 kg/m2. Future studies should focus on the DAA for the clinical outcomes of THA patients with BMI >40 kg/m2.
Conclusion
In conclusion, the DAA was associated with shorter incision length and better hip function than the PLA. However, the DAA was associated with a longer operating time than the PLA in THA patients. In light of study limitations, further multi‐institution studies are required with longer follow‐up durations, and larger patient populations are needed to provide more definitive conclusions.
Author Contributions
Zhao Wang and Hong‐Wei Bao conceived the idea for this manuscript. Jing‐Zhao Hou and Bin Ju performed statistical analysis. Can‐Hua Wu and Yao‐Jiang Zhou wrote the primary manuscript. Xiao‐Ming Gu and Hai‐Hong Wang edited the manuscript. All authors read the final version of this paper and approved the final version.
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Statement
Research protocol approval was provided by the Ethics Committee of Jingjiang People's Hospital (2016YLS007).
Acknowledgments
We thank the authors of the included studies for their help.
Data Availability Statement
All data generated or analyzed during this study are included in published articles.
References
- 1. Hall MJ, Owings MF. 2000 National Hospital Discharge Survey. Advance Data. 2002;329:1–18. [PubMed] [Google Scholar]
- 2. Bougherara H, Zdero R, Shah S, Miric M, Papini M, Zalzal P, et al. A biomechanical assessment of modular and monoblock revision hip implants using FE analysis and strain gage measurements. J Orthop Surg Res. 2010;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Putananon C, Tuchinda H, Arirachakaran A, Wongsak S, Narinsorasak T, Kongtharvonskul J. Comparison of direct anterior, lateral, posterior and posterior‐2 approaches in total hip arthroplasty: network meta‐analysis. Eur J Orthop Surg Traumatol. 2018;28(2):255–67. [DOI] [PubMed] [Google Scholar]
- 4. Light TR, Keggi KJ. Anterior approach to hip arthroplasty. Clin Orthop Relat Res. 1980;152:255–60. [PubMed] [Google Scholar]
- 5. Graves SC, Dropkin BM, Keeney BJ, Lurie JD, Tomek IM. Does surgical approach affect patient‐reported function after primary THA? Clin Orthop Relat Res. 2016;474(4):971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Geest T, Fennema P, Lenaerts G, De Loore G. Adverse effects associated with the direct anterior approach for total hip arthroplasty: a Bayesian meta‐analysis. Arch Orthop Trauma Surg. 2015;135(8):1183–92. [DOI] [PubMed] [Google Scholar]
- 7. Bhandari M, Matta JM, Dodgin D, Clark C, Kregor P, Bradley G, et al. Outcomes following the single‐incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am. 2009;40(3):329–42. [DOI] [PubMed] [Google Scholar]
- 8. Ogonda L, Wilson R, Archbold P, Lawlor M, Humphreys P, O'Brien S, et al. A minimal‐incision technique in total hip arthroplasty does not improve early postoperative outcomes. A prospective, randomized, controlled trial. J Bone Joint Surg Am. 2005;87(4):701–10. [DOI] [PubMed] [Google Scholar]
- 9. Cichos KH, Mabry SE, Spitler CA, McGwin G Jr, Quade JH, Ghanem ES. A comparison between the direct anterior and posterior approaches for Total hip arthroplasty performed for femoral neck fracture. J Orthop Trauma. 2020;35(1):41–8. [DOI] [PubMed] [Google Scholar]
- 10. Chen W, Sun JN, Zhang Y, Zhang Y, Chen XY. Direct anterior versus posterolateral approaches for clinical outcomes after total hip arthroplasty: a systematic review and meta‐analysis. J Orthop Surg Res. 2020;15(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meermans G, Konan S, Das R, Volpin A, Haddad FS. The direct anterior approach in total hip arthroplasty: a systematic review of the literature. Bone Joint J. 2017;99‐b(6):732–40. [DOI] [PubMed] [Google Scholar]
- 12. Moerenhout K, Derome P, Laflamme GY, Leduc S, Gaspard HS, Benoit B. Direct anterior versus posterior approach for total hip arthroplasty: a multicentre, prospective, randomized clinical trial. Can J Surg. 2020;63(5):E412–e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoo JI, Cha YH. Gait analysis after total hip arthroplasty using direct anterior approach versus anterolateral approach: a systematic review and meta‐analysis. BMC Musculoskelet Disord. 2019;20(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun X, Zhao X, Zhou L, Su Z. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a meta‐analysis of results on early post‐operative period. J Orthop Surg Res. 2021;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Z, Hou JZ, Wu CH, Zhou YJ, Gu XM, Wang HH, et al. A systematic review and meta‐analysis of direct anterior approach versus posterior approach in total hip arthroplasty. J Orthop Surg Res. 2018;13(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibson A. Posterior exposure of the hip joint. J Bone Joint Surg Br. 1950;32‐b(2):183–6. [DOI] [PubMed] [Google Scholar]
- 17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32. [PubMed] [Google Scholar]
- 18. Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58(3):277–80. [DOI] [PubMed] [Google Scholar]
- 19. Melià MJ, Kubota A, Ortolano S, Vílchez JJ, Gámez J, Tanji K, et al. Limb‐girdle muscular dystrophy 1F is caused by a microdeletion in the transportin 3 gene. Brain. 2013;136(Pt 5):1508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end‐result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737–55. [PubMed] [Google Scholar]
- 21. Higgins BT, Barlow DR, Heagerty NE, Lin TJ. Anterior vs posterior approach for total hip arthroplasty, a systematic review and meta‐analysis. J Arthroplasty. 2015;30(3):419–34. [DOI] [PubMed] [Google Scholar]
- 22. Connolly KP, Kamath AF. Direct anterior total hip arthroplasty: comparative outcomes and contemporary results. World J Orthop. 2016;7(2):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rüdiger HA, Dittrich M, Robinson J, Mansour T, Schwab T, Stadelmann VA, et al. The impact of heterotopic ossification on self‐reported outcomes after total hip arthroplasty using the direct anterior approach. J Bone Joint Surg Am. 2020;102(Suppl 2):91–8. [DOI] [PubMed] [Google Scholar]
- 24. Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty. 2010;25(5):671–9. [DOI] [PubMed] [Google Scholar]
- 25. Kong X, Grau L, Ong A, Yang C, Chai W. Adopting the direct anterior approach: experience and learning curve in a Chinese patient population. J Orthop Surg Res. 2019;14(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russo MW, Macdonell JR, Paulus MC, Keller JM, Zawadsky MW. Increased complications in obese patients undergoing direct anterior total hip arthroplasty. J Arthroplasty. 2015;30(8):1384–7. [DOI] [PubMed] [Google Scholar]
- 27. Watts CD, Houdek MT, Wagner ER, Sculco PK, Chalmers BP, Taunton MJ. High risk of wound complications following direct anterior total hip arthroplasty in obese patients. J Arthroplasty. 2015;30(12):2296–8. [DOI] [PubMed] [Google Scholar]
- 28. Shen K, Feng E, Lin F, Weng Y, Chen J. Learning curve of total hip arthroplasty in direct anterior approach without requiring corrective osteotomy for hip dysplasia. Orthop Surg. 2022;14(5):840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burnham RR Jr, Kiernan H, Ortega LF, Wesolowski M, Tauchen A, Russo M, et al. Defining the learning curve of anterior total hip arthroplasty after fellowship‐specific training. J Am Acad Orthop Surg. 2022;30(1):e131–8. [DOI] [PubMed] [Google Scholar]
- 30. Woolson ST, Pouliot MA, Huddleston JI. Primary total hip arthroplasty using an anterior approach and a fracture table: short‐term results from a community hospital. J Arthroplasty. 2009;24(7):999–1005. [DOI] [PubMed] [Google Scholar]
- 31. D'Arrigo C, Speranza A, Monaco E, Carcangiu A, Ferretti A. Learning curve in tissue sparing total hip replacement: comparison between different approaches. J Orthop Traumatol. 2009;10(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nairn L, Gyemi L, Gouveia K, Ekhtiari S, Khanna V. The learning curve for the direct anterior total hip arthroplasty: a systematic review. Int Orthop. 2021;45:1971–82. [DOI] [PubMed] [Google Scholar]
- 33. Zhao HY, Kang PD, Xia YY, Shi XJ, Nie Y, Pei FX. Comparison of early functional recovery after total hip arthroplasty using a direct anterior or posterolateral approach: a randomized controlled trial. J Arthroplasty. 2017;32(11):3421–8. [DOI] [PubMed] [Google Scholar]
- 34. Kwak S, Chun Y, Rhyu K, Cha J, Cho Y. Quantitative analysis of tissue injury after minimally invasive total hip arthroplasty. Clin Orthop Surg. 2014;6(3):279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mjaaland KE, Kivle K, Svenningsen S, Pripp AH, Nordsletten L. Comparison of markers for muscle damage, inflammation, and pain using minimally invasive direct anterior versus direct lateral approach in total hip arthroplasty: a prospective, randomized, controlled trial. J Orthop Res. 2015;33(9):1305–10. [DOI] [PubMed] [Google Scholar]
- 36. Bergin PF, Doppelt JD, Kephart CJ, Benke MT, Graeter JH, Holmes AS, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang XT, Huang HF, Sun L, Yang Z, Deng CY, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta‐analysis of randomized controlled studies. Orthop Surg. 2020;12(4):1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Z, Bao HW, Hou JZ. Direct anterior versus lateral approaches for clinical outcomes after total hip arthroplasty: a meta‐analysis. J Orthop Surg Res. 2019;14(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in published articles.


