Abstract
The dopamine-depleting agent tetrabenazine alters effort-based choice, suppressing food-reinforced behaviors with high response requirements, while increasing selection of low-cost options. In the present experiments, rats were tested on a concurrent fixed ratio 5/chow feeding choice task, in which high-carbohydrate Bio-serv pellets reinforced lever pressing and lab chow was concurrently available. Detailed timing of lever pressing was monitored with an event recording system, and the temporal characteristics of operant behavior seen after 1.0 mg/kg tetrabenazine or vehicle injections were analyzed. Tetrabenazine shifted choice, decreasing lever pressing but increasing chow intake. There was a small effect on the interresponse-time distribution within ratios, but marked increases in the total duration of pauses in responding. The postreinforcement-pause (PRP) distribution was bimodal, but tetrabenazine did not increase the duration of PRPs. Tetrabenazine increased time feeding and duration and number of feeding bouts, but did not affect feeding rate or total time spent lever pressing for pellets and consuming chow. Thus, TBZ appears to predominantly affect the relative allocation of lever pressing versus chow, with little alteration in consummatory motor acts involved in chow intake. Tetrabenazine is used to model motivational symptoms in psychopathology, and these effects in rats could have implications for psychiatric research.
Keywords: dopamine, effort, work, reinforcement, behavioral economics
Instrumental behavior tasks in rodents are increasingly being used to model features of psychopathology seen in humans. One example of this is the use of rodent tasks of effort-based choice behavior to model motivational symptoms seen in people with depression, schizophrenia and other disorders (Salamone et al. 2012, 2018; Yohn et al., 2016a,b,c,d). Instrumental behavior is regulated by a complex combination of factors, and a response-related factor that substantially affects instrumental behavior is work-related response costs (Foltin 1991; Hursh et al., 1988; Kaufman 1980; Kaufman et al. 1980; Salamone, 1986, 1987, 1992; Staddon 1979; Tustin, 1995). In complex environments, animals must make choices when there are multiple opportunities for accessing reinforcers, and different paths for obtaining them (Aparicio, 2001, 2007; Williams, 1988). A critical aspect of this process is that organisms make choices involving cost/benefit interactions based upon exertion of effort to overcome response costs versus reinforcement value (Hursh et al., 1988; Neill & Justice, 1981; Salamone 1987; Salamone & Correa 2002, 2012; Salamone et al. 2003, 2005, 2007, 2012; van den Bos et al. 2006; Walton et al. 2006). Considerable evidence indicates that brain dopamine (DA) transmission regulates the relative allocation of instrumental behavior in relation to response costs. DA is a nodal point in the cortical/basal ganglia/limbic circuitry regulating response vigor and exertion of physical effort (Salamone et al. 2018). Low systemic doses of DA antagonists, as well as local interference with nucleus accumbens DA transmission, affect the relative allocation of behavior in animals responding on tasks that assess effort-based choice behavior, biasing responding towards low-cost alternatives (Floresco, Onge et al. 2008, Floresco, Tse et al., 2008; Hauber & Sommer 2009; Salamone et al. 2003, 2005, 2007). One commonly used task for assessing effort-based choice is the concurrent fixed ratio (FR) 5/chow feeding choice task. This procedure offers rats the option of lever pressing reinforced by delivery of a relatively preferred food (e.g. Bio-Serv pellets) on a FR5 schedule versus approaching and consuming a less preferred food (lab chow) that is concurrently available in the chamber (Salamone et al., 1991). Trained rats under baseline or control conditions lever press at high rates, getting most of their food by lever pressing and consuming only small quantities of chow. Low-to-moderate doses of DA antagonists that block either D1 or D2 family receptor families substantially alter response allocation in rats performing on this task. These drugs decrease food-reinforced lever pressing but substantially increase intake of the concurrently available chow (Cousins et al., 1994; Koch et al., 2000; Salamone et al. 1991, 2002; Sink et al. 2008; Worden et al. 2009). The effects of DA antagonism on this task are not due to changes in food intake or preferences (see review by Salamone et al. 2012).
More recent studies have focused on the development of animal models employing the DA-depleting agent tetrabenazine (Nunes et al. 2013; Rotolo et al. 2019, 2020, 2021; Yohn, Collins et al. 2016; Yohn, Errante et al., 2016; Yohn, Lopez-Cruz et al., 2016). Tetrabenazine (TBZ) blocks vesicular storage of DA by inhibiting the vesicular monoamine transporter type-2 (Nunes et al. 2013), and was selected for these animal studies because human research has demonstrated that TBZ can induce symptoms of depression in humans, including effort-related symptoms such as fatigue (Chen et al., 2012; Frank, 2009, 2010; Guay, 2010). TBZ affects effort-based choice on a number of tasks, including the progressive ratio/chow feeding choice task (Randall et al. 2012, 2014), the T-maze barrier choice task (Yohn, Santerre et al. 2015; Yohn, Thompson et al., 2015), as well as several choice tasks in mice involving touchscreen panel pressing or wheel running as the high-effort choice (Yang et al. 2020; Carratala-Ros et al. 2021a,b). TBZ produces effects similar to DA antagonists in rats tested on the FR5/chow feeding choice task, decreasing lever pressing but increasing intake of the concurrently available chow (Nunes et al. 2013; Rotolo et al. 2019, 2020, 2021; Yohn, Collins et al. 2016; Yohn, Errante et al., 2016; Yohn, Lopez-Cruz et al., 2016). The doses of TBZ that shift effort-based choice do not affect intake of or preference for the two reinforcers in separate feeding preference tests (Nunes et al. 2013), and across multiple tasks in both rats and mice, the effects of TBZ do not resemble those of reinforcer devaluation by reduced food restriction or drugs that are known to suppress food intake, which decrease both instrumental responding and intake of the concurrently available food (Randall et al. 2012, 2014; Sink et al. 2008; Yang et al. 2020). Furthermore, TBZ has been shown to increase elasticity of demand for food (Salamone et al. 2017). The relevance of these actions of TBZ for psychopathology is validated by human research showing that people with depression and schizophrenia show reduced selection of high-effort choices when assessed for effort-based choice (Gold et al. 2013; Treadway et al. 2012). The ability of drugs to reverse the effects of TBZ on effort-based choice has become a useful translational tool for the development of potential treatments for motivational symptoms in humans (Nunes et al. 2013; Rotolo et al. 2019, 2020, 2021; Yohn, Collins et al. 2016; Yohn, Errante et al., 2016; Yohn, Lopez-Cruz et al., 2016).
Despite the large body of previous work on TBZ, these studies all involved analysis of global measures of behavior such as total number of responses and total food intake. One of the great contributions of the experimental analysis of behavior has been the focus on characterizing detailed features of behavior, including temporal parameters. In the present studies, rats were tested on the FR5/chow feeding choice task. The detailed temporal characteristics of lever pressing were monitored by a transistor–transistor level (TTL) recording system that was a component of an electrophysiological apparatus, and we then analyzed the temporal characteristics of streams of operant behavior seen after injection of TBZ or vehicle. Moreover, rats were observed during the drug and control sessions in order to obtain detailed parameters of feeding behavior that were altered by TBZ. These studies provide a rich and detailed characterization of the effects of TBZ on the underlying temporal characteristics of lever pressing (e.g. local rate of responding in ratios, postreinforcement pauses), and food intake, which has implications for understanding effort-related aspects of psychopathology. Also, they were conducted to develop a system that would allow for future studies that conduct parallel recordings of physiological and behavioral activity using the same system.
Method
Subjects
Adult male Sprague Dawley rats (Envigo, Indianapolis, IN, USA) were housed in a vivarium that was maintained at 23 °C with a 12-hr light/dark cycle (lights on 07:00). Rats (n = 8) weighed 275–299 g at the beginning of the study, and were initially food restricted to 85% of their free-feeding body weight for operant training. Rats were fed supplemental lab chow to maintain body weight targets throughout the study, with water available ad libitum. Rats were allowed modest weight gain throughout the experiment based upon a growth curve approved by the animal care staff and attending veterinarian. This procedure made week to week adjustments in growth, allowing an average of 34.5% growth over the 22 weeks from the day of initial weighing until the day the animals were sacrificed. Animal protocols were approved by the University of Connecticut animal care and use committee, which followed NIH guidelines.
Apparatus
Behavioral sessions were conducted in operant chambers (28 × 23 × 23 cm; Med Associates, Fairfax, VT), with a single lever on the left side of the chamber, and a pellet dispenser in the middle of the wall containing the manipulandum. The sessions were controlled by Med PC software (version 5.0). Lever pressing and pellet dispensing data were connected with a Med Associates interface to a Digital Lynx SX Electrophysiology System (Neuralynx) via the TTL input system. This system can be used to acquire EEG activity from awake behaving rats in parallel with behavioral data, but for the present experiments, rats were not operated on and were not connected to the electrophysiology system, so that the utility of the TTL system for monitoring temporal characteristics of operant behavior could be assessed. Using a high sampling rate (1–2000 Hz, 4006 samples/s), behavioral events were recorded using the Neuralynx system (Cheetah Software version 6.4.1) and analyzed offline using MATLAB (MathWorks Inc, Natick, MA) and Python software.
Procedure
Pharmacological Agent and Drug Treatments: Experiments 1 And 2
TBZ (9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7, 11b hexahydrobenzo[a]quinolizin-2-one) was obtained from Tocris Bioscience (Ellisville, MO) and was dissolved in a solution of 20% dimethylsulfoxide (DMSO) and 80% physiological saline, and titrated with HCl to enhance solubility. The DMSO/saline solution also was administered as the vehicle control. The dose of 1.0 mg/kg TBZ was based on extensive published work in our laboratory (Rotolo et al. 2019, 2020, 2021). There were two experiments conducted on the same group of rats (n = 8). In both experiments, rats trained as described below received intraperitoneal (IP) injections of TBZ (1.0 mg/kg, 1.0 ml/kg volume) and vehicle 1.0 ml/kg 120 min before testing once per week in a randomly varied order. The first experiment did not involve direct observations of chow feeding behavior, while the second one did (see below). The order of TBZ or vehicle treatments was randomly assigned for both experiments, with half of the animals receiving vehicle first, and half receiving TBZ first. Both experiments used a within-groups design, with each rat receiving each treatment in a randomly varied order, once per week (no-drug baseline days were conducted 4 days per week). In most cases, rats were not tested the day after drug treatment, so that body weights could be adjusted with supplemental food. In the small number of cases in which rats were tested the day after treatment, behavior largely recovered to pretreatment baseline levels.
Behavioral Procedures
Concurrent FR5/Chow Feeding Choice Task.
Behavioral sessions were conducted in operant chambers (28 × 23 × 23 cm; Med Associates, Fairfax, VT). Thirty-minute sessions were conducted 5 days/week. After 2 days of magazine training rats were initially trained to lever press on a FR1 schedule to receive 45 mg high-carbohydrate pellets (Bio-Serv #F0021; Flemington, NJ, USA), which was followed by FR1 training for 3 additional days, and then followed by training on the FR5 schedule. After 5 weeks of FR5 training, lab chow was introduced into the chamber. Thus, during each FR5/chow feeding choice session, 15–20 g of lab chow (Laboratory Diet, 5P00 Prolab RMH 3000, Purina Mills, St. Louis, MO) was concurrently available on the floor of the chamber, and animals had a choice between the two sources of food. Rats were trained on this FR5/chow feeding choice procedure for 8 weeks, after which drug testing began. As an index of the stability of baseline lever pressing, we calculated the mean and standard deviation of last 3 baseline days prior to each drug treatment for each rat, and calculated the coefficient of variation for each rat, which ranged from 7.2% to 16.3%. Rats consumed all the operant pellets that were delivered during each baseline and drug test session. It is worth emphasizing that this procedure is not the same as contrafreeloading, because in those procedures the two foods are equivalent (e.g. Taylor 1975), while in studies of FR5/chow feeding choice the two foods are not the same, and Bio-serve high carbohydrate pellets are strongly preferred by rats over chow (Nunes et al. 2013; Salamone et al. 1991).
Observations of Feeding Behavior in Experiment 2
Rats were trained on the FR5/chow feeding choice task as described above, and 2 weeks after Experiment 1 was completed, the second experiment was conducted, during which a trained observer who was blind to the drug treatments was used to observe video recordings of the lab chow feeding during the 30-min sessions in the operant chamber. Feeding was defined as the presence of chewing behavior that occurred during direct mouth contact with the food pellet, or chewing that was initiated during mouth contact with the food pellet but was continued uninterrupted after mouth contact with the food had ceased. The observer also recorded feeding behavior in terms of when the rats used the two forepaws to hold the food pellet, and observed no instances of the animals failing to hold the food pellets. The total intake of lab chow (grams; weighed by someone who was blind to the treatments) and the total time spent consuming the lab chow (seconds) were recorded as the main variables. Rate of feeding (grams/min) was calculated by dividing the amount of food consumed by the total time spent feeding. The number of bouts for food pellets was also registered. A bout was recorded every time the animal initiated eating the food pellet. The number of bouts was used to calculate the average bout length (i.e. time spent/number of bouts). These procedures were based on Salamone et al. (1990, 1993). To determine interrater reliability, a second observer who was blind to the treatment was initially trained during baseline days, and made parallel observations of time spent eating in four treatment observation sessions. The correlation coefficient for these four data points was r = 1.0, and the total percent agreement was 97.2%. Also, a reliability check was done by calculating the correlation coefficient between time spent eating and the objective measure of food intake (gram intake). Across both vehicle and TBZ data in Experiment 2 (16 data points), the correlation was r = 0.98, and within the TBZ condition (8 data points) it was r = 0.95.
Analyses of Detailed Parameters of FR5 Lever Pressing
In order to study the effects of TBZ on lever pressing behavior, we performed analyses on three measures: number of completed ratios, interresponse interval, and postreinforcement pauses. The numbers of completed ratios were analyzed by paired-sample t-tests, comparing VEH and TBZ data. Interresponse time (IRT) was defined as the time interval between two lever presses within each ratio. Since the distributions of IRT were highly skewed (see Fig. 3), we used the median of IRT for each recording for further analysis (paired-sample t-tests) to compare the median IRT in VEH condition and TBZ conditions. Postreinforcement pause (PRP) was defined as the time interval between the last lever press in a ratio and the first lever press in the next ratio. We observed that the distributions of PRPs were bimodal, and in order to model these distributions, we found that the inverse Gaussian mixture model worked best. The PRP distribution often has a sharp onset and is highly skewed, which is consistent with the shape of an inverse Gaussian distribution. This may indicate that the rats have two behavioral modes after receiving a reward, a fast mode with a sharp distribution of shorter PRPs (likely ratio overruns), and a slow mode with a broader distribution of longer IRTs. To study whether TBZ affects these two behavioral modes, we fitted a mixture model to the PRP distribution for each recording in each individual animal. In particular, we fitted a mixture of a Wald (inverse Gaussian) distribution and a shifted Wald distribution (WW):
In this equation, t is the PRP duration, μ1 and λ1 are the mean and shape parameters of the Wald distribution (fast mode), μ2 and λ2 are the mean and shape parameters of the shifted Wald distribution (slow mode), in which s is the shift, and p is the proportion of PRPs in the first component (fast mode).
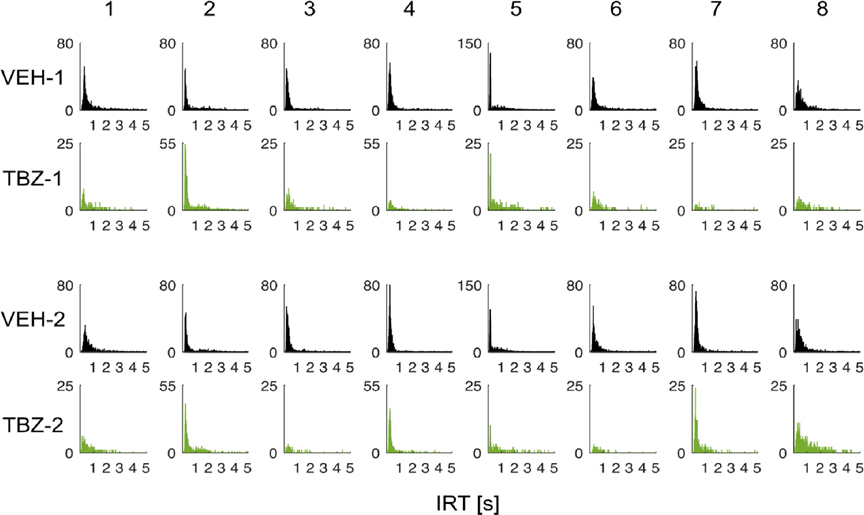
Figure 3. Distribution of IRTs in VEH and TBZ Conditions for Each Rat.
Note. This figure shows the IRT distribution for each rat in VEH (dark grey) and TBZ (green) conditions for the two experiments (experiment 1: VEH-1, TBZ-1; experiment 2: VEH-2, TBZ-2). Row: rat. Column: condition and experiment. Note that the y-axis scales for VEH and TBZ are different because of the low level of lever pressing in the TBZ condition.
For each recording, we found Bayes estimates of the model parameters with priors for each of the six parameters in the model. We used Gamma distributions as the priors of μ1 and μ2, where μ1 ~ Gamma(1, 10) and μ2 ~ Gamma(1, 10). We used half-Cauchy distributions (Cauchy distribution bounded at zero) as the priors of λ1 and λ2, where λ1 ~ | Cauchy(0, 10) | and λ2 ~ | Cauchy(0, 5) |. We used an exponential distribution as the prior of s, where s ~ Exponential(1), and a Dirichlet prior over p with . The priors here were chosen to regularize the parameter estimates and ensure stability. With many PRP observations the priors have little impact on the parameter estimates themselves. Parameters were estimated using Markov chain Monte Carlo (No-U-Turn Sampler) sampling in PyMC3with 4 Markov chains for 4000 iterations of burn-in/tuning followed by 4000 samples (Salvatier et al., 2016).
Using the posterior mean parameters, we then compare three statistics from the fits for the VEH condition and TBZ condition: the median of the Wald distribution (fast mode), the median of the shifted Wald distribution (slow mode), and the proportion of PRPs categorized as the fast mode p.
Total pause time was defined as the summation of the long periods of time during which there was no lever pressing. This measure was operationalized as the sum of the time before the first lever press, the time from the last lever press to the end of the session, and the PRPs and IRTs that were longer than 10 s. In experiment 2, total pause time and time spent eating were used to generate another measure, which was total time spent lever pressing for pellets and eating chow. This measure was calculated by subtracting the total pause time from the session time (30 min = 1800 s), and then adding the time spent eating chow to that value.
Results
Effects of TBZ on Parameters of Lever Pressing
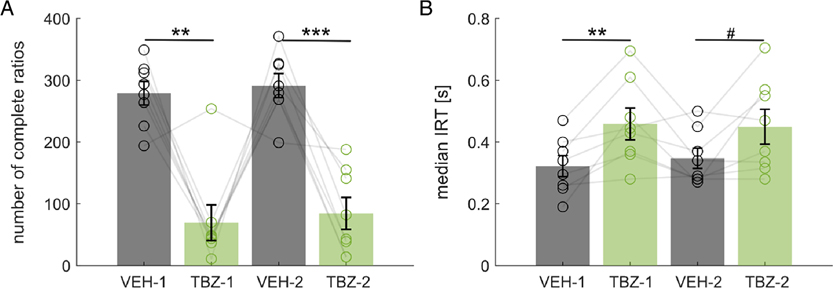
Here we recorded the detailed timing of lever presses during VEH and TBZ conditions, with repeated experiments in the same rats. Individual animals showed largely consistent patterns of behavior across the two experiments, with similar distributions of IRTs and PRPs (Fig. 1). For both experiments, we found that TBZ substantially reduced the number of completed ratios compared to VEH (Fig. 2A) with 279 ± 19 and 291 ± 19 ratios completed for VEH in Experiments 1 and 2, respectively, and 70 ± 29 and 85 ± 26 completed for TBZ on average (Mean ± SEM). Both of these differences were statistically significant (paired-sample t-test for experiment 1, t(7) = −5.04, p = .002, Cohen’ s d = −1.78, for Experiment 2, t(7) = −6.17, p < .001, d = −2.18). There were reliable individual differences across rats between Experiments 1 and 2 (Table 1), and it is worth noting that, out of eight rats, there was one rat (#2) that did not demonstrate a suppression of responding induced by TBZ. This lack of effect was consistent across both experiments, which suggests a reduced sensitivity to the drug in this animal.
Figure 1. Event Records of Lever Pressing in One Individual Rat.
Note. This figure shows the event record of lever presses from one rat (NIH14 #3) in VEH condition (dark grey) and TBZ condition (green) in two experiments. The top two panels show the times of lever presses. Each bar represents one lever press. The bottom panels show the distributions of IRTs and PRPs for these two experiments.
Figure 2. Effects of TBZ on Number of Completed Ratios and IRT.
Note. This figure presents the number of completed ratios and IRT in VEH condition (dark grey) and TBZ condition (green) for the two experiments (experiment 1: VEH-1, TBZ-1; experiment 2: VEH-2, TBZ-2). For each condition, each open circle represents the data from one rat, and the ladders connect the data points from the same rats in different recordings. The height of a bar represents the mean across all rats. Error bars represent 1 standard error of the mean. Panel A shows that the rats completed fewer ratios under TBZ in both experiments. Panel B shows that the rats show longer IRTs (median) under TBZ in both experiments. *: p<.01. ***: p<.001. #: p=.059.
Table 1.
For Each Rat and Each of the Two Experiments: the Number of Lever Presses, Total Pause Time, Completed Ratios, and Median Interresponse Time (IRT)
| Lever presses |
Total pause time |
Ratios VEH | IRT [s] |
PRP(fast) [s] |
PRP(slow) [s] |
Proportion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VEH | TBZ | VEH | TBZ | TBZ | VEH | TBZ | VEH | TBZ | VEH | TBZ | VEH | TBZ | ||
|
| ||||||||||||||
| rat | Experiment 1 | |||||||||||||
| 1 | 1562 | 245 | 187 | 1491 | 312 | 49 | 0.4 | 0.45 | 0.29 | 0.21 | 2.01 | 1.69 | 0.60 | 0.34 |
| 2 | 970 | 1270 | 1134 | 468 | 194 | 254 | 0.26 | 0.28 | 0.23 | 0.24 | 2.24 | 2.4 | 0.52 | 0.30 |
| 3 | 1130 | 220 | 925 | 1585 | 226 | 44 | 0.25 | 0.37 | 0.25 | 0.24 | 2.5 | 2.55 | 0.09 | 0.11 |
| 4 | 1320 | 236 | 676 | 1575 | 264 | 47 | 0.29 | 0.36 | 0.24 | 0.15 | 3.36 | 3.12 | 0.14 | 0.07 |
| 5 | 1590 | 354 | 585 | 1384 | 318 | 70 | 0.19 | 0.48 | 0.16 | 0.19 | 2.37 | 1.97 | 0.62 | 0.44 |
| 6 | 1386 | 220 | 691 | 1642 | 277 | 44 | 0.34 | 0.43 | 0.25 | 0.21 | 2.09 | 1.63 | 0.32 | 0.14 |
| 7 | 1745 | 56 | 447 | 1436 | 349 | 11 | 0.37 | 0.61 | 0.27 | 0.10 | 1.94 | 1.35 | 0.07 | 0.05 |
| 8 | 1470 | 186 | 677 | 1654 | 294 | 37 | 0.47 | 0.7 | 0.72 | 0.26 | 1.65 | 1.22 | 0.41 | 0.13 |
| Experiment 2 | ||||||||||||||
| 1 | 1347 | 215 | 860 | 655 | 269 | 43 | 0.5 | 0.47 | 0.27 | 0.23 | 1.69 | 1.56 | 0.69 | 0.43 |
| 2 | 997 | 944 | 1216 | 951 | 199 | 188 | 0.29 | 0.31 | 0.27 | 0.23 | 2.28 | 2.89 | 0.35 | 0.14 |
| 3 | 1400 | 70 | 529 | 1292 | 280 | 14 | 0.28 | 0.37 | 0.29 | 0.22 | 2.99 | 2.11 | 0.08 | 0.14 |
| 4 | 1455 | 775 | 415 | 971 | 291 | 155 | 0.28 | 0.28 | 0.36 | 0.22 | 3.44 | 4.04 | 0.08 | 0.11 |
| 5 | 1627 | 199 | 457 | 1068 | 325 | 39 | 0.27 | 0.57 | 0.15 | 0.18 | 1.23 | 1.82 | 0.54 | 0.22 |
| 6 | 1345 | 72 | 978 | 1769 | 269 | 14 | 0.37 | 0.55 | 0.39 | 0 | 1.98 | 1.69 | 0.21 | 0.03 |
| 7 | 1855 | 410 | 387 | 1425 | 371 | 82 | 0.34 | 0.33 | 0.25 | 0.44 | 1.73 | 1.63 | 0.18 | 0.12 |
| 8 | 1637 | 706 | 407 | 999 | 327 | 141 | 0.45 | 0.7 | 0.45 | 0.33 | 2.05 | 1.63 | 0.1 | 0.05 |
Note. Also shown are results from the two-component mixture model fitted to the distribution of postreinforcement pauses (PRPs): the median of the fast mode, median of the slow mode, and the proportion of PRPs in the fast mode.
When comparing the timing of lever presses, we found that there were also substantial differences in the median interresponse times (Fig. 2B). The overall tendency of TBZ injection was to shift the IRT distribution by lengthening the IRT with median times of 0.32 ± 0.03 s and 0.35 ± 0.03 s for VEH in Experiments 1 and 2, and 0.46 ± 0.05 s and 0.45 ± 0.06 s for TBZ (paired-sample t-test Experiment 1: t(7) = 3.83, p = .007, d = 1.35; Experiment 2 t(7) = 2.25, p = .059, d = 0.80). The distributions of IRTs for each rat were similar for Experiments 1 and 2 (Fig. 3), and these results suggest that TBZ induces a slight reduction in the local rate of lever pressing.
Since distributions of postreinforcement pauses were typically bimodal, we characterized them by fitting a mixture model (Fig. 4), in which the PRPs are described by two modes: a fast mode and a slow mode. Comparing the parameters of the model distributions (Fig. 5) we found that, for both experiments, there was not a statistically significant difference between the median fast mode under TBZ and Vehicle (Fig. 5A). Under VEH the average of the medians was 0.30 ± 0.06 s and 0.30 ± 0.04 s for Experiments 1 and 2, and under TBZ the average median was 0.20 ± 0.02 s and 0.26 ± 0.04 s (Experiment 1 t(7) = −1.81, p = .11, d = −0.64, and Experiment 2 t(6) = −0.65, p = .54, d = −0.24). Note that rat #6 (in Experiment 2) was excluded from the t-tests because it did not have fast mode PRPs in the TBZ condition (see Table 1 and Fig. 5). Rats treated with TBZ showed similar or slightly shorter median slow mode PRP durations under slow mode compared to VEH (Fig. 5B, Experiment 1: VEH 2.27 ± 0.2 s; TBZ 1.99 ± 0.25 s, t(7) = −3.04, p = .019, = −1.07; Experiment 2: VEH 2.18 ± 0.27 s; TBZ 2.17 ± 0.33 s, t(7) = −0.01, p = .99, d = −0.005). Although TBZ treatment led to the rats completing fewer lever presses and ratios, it did not substantially increase the median duration of fast or slow PRPs.
Figure 4. Distributions of PRPs in VEH and TBZ Conditions for Each Rat.
Note. This figure presents the distribution of PRP for each rat in VEH condition (dark grey) and TBZ condition (green) for the two experiments (experiment 1: VEH-1, TBZ-1; experiment 2: VEH-2, TBZ-2), and the model fittings of the fast mode (blue line) and the slow mode (red line). Row: condition and experiment. Column: rat. Note that the y-axis scales for VEH and TBZ are different because of the low level of lever pressing in the TBZ condition.
Figure 5. Effects of TBZ on PRP (Fast Mode PRP, Short Mode PRP, and Proportion of Short Mode PRP) and Total Pause Time.
Note. This figure presents the parameters of PRP distribution in VEH condition (dark grey) and TBZ condition (green) for the two experiments (experiment 1: VEH-1, TBZ-1; experiment 2: VEH-2, TBZ-2). Symbols are the same as described in Figure 2. Panel A shows that TBZ does not have a significant effect on the fast mode PRP durations. Panel B shows that TBZ has a significant effect on the slow mode PRP durations in experiment 1, but not in experiment 2. Panel C shows that the rats take fewer fast mode PRPs under TBZ in both experiments. Panel D shows that the rats spend longer time not pressing under TBZ in both experiments. *: p<.05. **: p<.01. #: p=.069.
However, TBZ did appear to alter the proportion PRPs that fell within each of these two modes (Fig. 5C). The median proportion of fast PRPs was 0.37 and 0.19 under VEH for Experiments 1 and 2 and 0.13 and 0.13 under TBZ (Wilcoxon signed-rank test for Eexperiment 1 z = −2.38, p = .017, and Experiment 2, z = −1.82, p = .069). These results suggest that instead of affecting the PRP duration itself, TBZ mainly affected the pattern of PRPs, altering the relative number of fast and slow ones. Although there was a tendency for some TBZ-treated rats to stop lever pressing earlier in the session, there was not a significant difference from VEH (time to emit last response: Experiment 1: VEH 1690.82 + 62.75 s; TBZ 1393.19 + 110.99 s, t(7) = −2.05, p = .0795, d = 0.72; Experiment 2: VEH 1676.81 + 77.63 s; TBZ 1438.82 + 141.13 s, t(7) = −1.28, p = .243, d = −0.45).
We also noted that TBZ appears to induce more long pauses that are rare relative to the main distribution of PRPs (>10s). In TBZ-treated rats, the total pause time showed a very robust increase compared to VEH conditions, which was evident across both experiments (Fig. 5D). Under VEH the average total pause time was 665 ± 108 s and 621 ± 110 s for Experiments 1 and 2, and under TBZ the average total pause time was 1404 ± 148 s and 1141 ± 130 s (Experiment 1, t(7) = 3.50, p = .009, d = 1.24, Experiment 2, t(7) = 2.91, p = .023, d = 1.03). Note that, as with the other behavioral measures, the total pause times of rat #2 showed a different pattern compared to other rats, and this was consistent across both experiments.
Effect of TBZ on Intake of the Concurrently Available Chow During the Operant Sessions
As stated above, the FR5/chow feeding choice task is conducted under conditions in which chow is concurrently available in the operant chamber. In Experiment 1, there were no direct observations of feeding behavior, only measurements of gram quantity of chow intake during the operant session. Injection of TBZ induced a shift in choice behavior; while it significantly decreased lever pressing as described above, it significantly and substantially increased intake of the concurrently available chow (paired t-test t(7) = −6.11, p < .01; Experiment 1 mean ± SEM: VEH 0.95 ± 0.32 grams, TBZ 5.51 ± 0.89 grams).
For Experiment 2 (see Table 2; data expressed as mean ± SEM), in which feeding behavior was observed, TBZ significantly increased multiple measures of chow intake during the operant sessions, including total grams of chow consumed (t(7) = −5.98, p < .01), the total time spent eating the lab chow (t(7) = −5.17, p < .01), number of bouts of feeding upon lab chow (t(7) = −4.47, p < .01), the average duration of each bout (t(7) = −2.09, p < .05), and the total amount of food intake (pellets + chow; t(7) = −6.05, p < .01). However, t-tests for dependent samples failed to demonstrate significant differences between VEH and TBZ conditions on feeding rate in grams/min spent feeding (t(7) = 0.508, p = .627), and total time spent lever pressing for pellets and feeding on chow (t(7) = 0.83, p = .432).
Table 2.
Effect of TBZ on Feeding Behavior and Total Time Spent Lever Pressing and Chow Feeding During 30- Min Sessions in Experiment 2
| Parameter | VEH | TBZ |
|---|---|---|
|
| ||
| Chow intake (g) | 1.0 ± 0.3 | 5.2 ± 0.8** |
| Chow Feeding time (s) | 99 ± 30 | 730 ± 123** |
| Chow Feeding rate (g/min) | 0.50 ± 0.14 | 0.43 ± 0.03 |
| Bouts of chow feeding | 5.1 ± 1.8 | 20.1 ± 3.9** |
| Average bout duration (s) | 16 ± 5 | 42 ± 11* |
| Total intake (g) pellets + chow | 14.07 ± 0.04 | 8.98 ± 0.49** |
| Time lever pressing + chow feeding | 1243.2 ± 104.5 | 1353.0 ± 113.7 |
Note. Mean (± SEM) grams, seconds, or counts in 30 min.
p < .05.
p < .01 significantly different from VEH.
Correlations Between Lever Presses and Feeding Behavior
Pearson’s correlations for the Experiment 2 data were calculated to provide a measure of linear association between lever press variables and those related to chow intake. Under TBZ conditions in Experiment 2 (n = 8), we found statistically significant, robust negative correlations between number of lever presses and chow intake (r = −0.92, p < .001; r2 = 0.85), number of lever presses and time spent eating the chow (r = −0.97, p < .001; r2 = 0.94), and time spent eating and chow intake (r = −0.95, p < .001; r2 = 0.91). As lever pressing increased, chow intake and time spent eating decreased. None of the other measures of lever pressing (IRT, PRP, total pause time) were significantly related to the chow intake measures or the number of lever presses (all r2 <0.5).
Discussion
The present experiments focused on obtaining a detailed behavioral characterization of the effects of the VMAT-2 inhibitor and DA depleting agent TBZ on performance of rats tested on the FR5/chow feeding choice task. Previous work employing a variety of effort-based choice tasks (Salamone et al., 2018) has shown that TBZ shifts choice behavior away from components of the task with a higher workload (e.g. lever pressing, barrier climbing, wheel running), and increases selection of the low effort option (e.g. approach and consumption of concurrently available food, such as lab chow). In the present experiments, TBZ reliably induced a shift in choice behavior, substantially decreasing lever pressing and increasing chow intake, confirming what has been previously reported (Nunes et al. 2013; Rotolo et al. 2019, 2020, 2021; Yohn et al. 2016a,b,c,d). Thus, TBZ altered response allocation between these alternative sources of food, shifting animals away from lever pressing but increasing the tendency to approach and consume substantial amounts of chow. Although the FR5/chow feeding choice task is not technically a contrafreeloading procedure, because the Bio-serv pellets and lab chow are not equivalent foods, it is reasonable to suggest that both areas of research are tapping into similar behavioral processes. For example, Taylor (1975) and others have discussed contrafreeloading in terms of the idea that rats have a preference for working for reinforcement. Published studies from our lab and others indicate that the main effect of TBZ on effort-based choice is to reduce preference for the choice with the higher work requirements, but not the preference for the food alternatives per se (e.g. Nunes et al. 2013; Salamone et al. 2018; Yohn et al. 2015a,b).
Although TBZ at higher doses can affect all monoamines, previous research has shown that at low doses in rats, TBZ has its greatest effects on DA. Pettibone et al. (1984) showed that a dose of 1.0 mg/kg tetrabenazine in rats reduced striatal DA by 70%−75%, while this same dose reduced 5-HT and NE by about 15%−30%. Moreover, that paper reported that 10.0 mg/kg tetrabenazine would be needed to suppress 5-HT about as much as 1.0 mg/kg reduces striatal DA, and that hypothalamic NE was very resistant to the effects of TBZ. A microdialysis study from our laboratory showed that 0.75 mg/kg TBZ decreased extracellular DA in nucleus accumbens by about 75% (Nunes et al. 2013). Moreover, the effects of TBZ on FR5/chow feeding choice performance are reversed by drugs that enhance DA transmission (i.e., DA transport blockers) but not by drugs that selectively enhance norepinephrine or serotonin transmission (e.g. Yohn et al. 2016a,b). Taken together, this pattern of results suggests that the effects of TBZ on FR5/chow feeding choice performance are largely due to actions on DA, which is consistent with the well-characterized effects of nucleus accumbens DA depletions and selective DA receptor antagonists on effort-based choice (Cousins et al. 1994; Salamone et al. 1991, 2002, 2018; Sink et al. 2008).
The present paper differs from previous work by providing the first detailed characterization of the effects of TBZ on both lever pressing and feeding behavior. A drug could reduce the total number of lever presses in many ways, either by dramatically increasing the IRTs, or selectively increasing PRPs, or by leaving those measures intact but increasing long pauses in operant behavior. The results showed that TBZ produced a variable alteration in the IRT distribution, lengthening the median IRT in most rats (Figs. 2 and 3, Table 1), with no change in some animals and increases ranging from 0.02 s to 0.3 s in others. TBZ appears to induce a slowing of the local rate of responding within ratios in most rats. However, it does not appear that individual differences in the effect of TBZ on the IRT distributions explain the overall reduction in responding produced by the drug. There were no significant correlations between the median IRT and total lever presses or ratios completed in TBZ-treated rats in either experiment. Furthermore, some rats showed very substantial suppression of lever pressing induced by TBZ (rat #1: VEH 1347, TBZ 215; rat #4: VEH 1455, TBZ 775), but no increase in the median IRT.
Analysis of the PRP distributions of each individual rat (Fig. 4) revealed that they were bimodal, characterized by a fast mode with a sharp distribution of shorter PRPs, and a slow mode with a broader distribution of longer IRTs. It is likely that the fast mode represents situations in which the rats continue lever pressing despite the fact that reinforcement has been delivered (i.e., ratio overruns), while the slow mode occurs in situations in which the rats take a longer pause in their lever pressing, collect the reinforcer, and cease responding for a time. There were no effects of TBZ on fast mode IRTs in either experiment, and there was a small but significant reduction in the median length of the slow PRPs in Experiment 1, but not Experiment 2. As shown in Figure 5C, there was an alteration of the proportion of fast (short) PRPs relative to slow PRPs. This relative decrease in the fast PRPs could have meant that the TBZ-treated rats are more directed towards food, which is consistent with the increase in chow intake. Nevertheless, it appears that like the IRT measure, the PRP measures are not major determinants of the overall reduction in lever press output induced by TBZ. There was a decrease in the median length of the slow PRPs resulting from TBZ treatment, but this is indicative of a faster return to lever pressing after reinforcement delivery, and thus does not appear to be a marker of reduced lever press output. Several individual animals showed very substantial reductions in lever pressing induced by TBZ, but had reduced, rather than increased lengths of slow PRPs (Table 1).
One measure of lever pressing that showed robust effects across both experiments was total pause time, which was defined as the sum of the long periods of time during which there was no lever pressing (see Method). TBZ significantly increased the total pause time in both experiments (Fig. 5D). While one animal failed to show a substantial reduction of lever pressing or total pause time after administration of TBZ, of the 14 injections of TBZ in the seven other rats that resulted in decreased lever pressing across both experiments, 13 of these were marked by large increases in total pause time as well (Table 1). This pause time measure is important in the context of the choice component of the task, because animals only eat chow during periods in which they are not lever pressing.
Another feature of Experiment 2 was that it was the first formal study of the effects of TBZ on the detailed characteristics of feeding behavior. As in previous studies, TBZ shifted choice behavior, decreasing lever pressing but increasing chow intake. TBZ also increased the number of feeding bouts, the average length of feeding bouts, and total time spent eating. Interestingly, local rate of feeding (in grams/min spent feeding) was not substantially altered by TBZ. The 0.43 grams/min average feeding rate after TBZ administration is in the normal range for control subjects that were previously observed using similar methods (Salamone et al. 1990, 1993). Also, it was reported by the observers that all rats showed normal motor patterns of eating behavior after TBZ injection, in that they held the chow pellets with both forepaws during all the bouts of feeding. Previous studies reported that substantial neurotoxic depletions of DA in the ventrolateral neostriatum produced motor impairments marked by significantly reduced rates of feeding and disrupted forepaw use during food handling (Salamone et al. 1993). However, the 1.0 mg/kg dose of TBZ used in the present research, which is in the low end of the overall dose range, did not produce these effects. Thus, there were no indications that use of the forepaws and mouth for the motor act of feeding behavior was substantially impaired by TBZ.
TBZ reduced the total amount of food consumed (i.e., combining pellet reinforcers and chow intake, Table 2). However, a difference in total food intake between vehicle and TBZ in this context is an artifact of the different rates of baseline consumption of the two different foods. A rat pressing 1200–1700 times on an FR5 schedule consumes 10.8–15.3 grams of Bio-serv pellets. With no other food present, previous studies from our laboratory showed that food-restricted Sprague Dawley rats with no water present only ate a maximum of 7–8 grams of laboratory chow in a 30-min session (e.g. Randall et al. 2010; Salamone et al. 1993). Thus, anything that shifts behavior from FR5 lever pressing reinforced by Bio-serv pellets to intake of laboratory chow would by definition reduce total intake because intake is constrained by the ceiling levels of chow intake. The most direct way to study the effect of a drug on food intake is to directly conduct feeding studies, and previous research has demonstrated that TBZ (0.25–1.0 mg/kg) did not alter preference between Bio-serv pellets and lab chow, and did not reduce intake of either food, in free-feeding preference tests (Nunes et al., 2013). Moreover, Experiment 2 demonstrated that TBZ did not reduce the total combined time of lever pressing for pellets plus feeding on chow, indicating that the overall allocation of time towards both food sources was not altered by TBZ. Rather, it was the allocation of behavior between these sources of food that was affected.
Taken together, it appears that the detailed characteristics of lever pressing are affected by TBZ in different ways in different rats. Most rats (7 of 8) injected by TBZ showed decreased lever pressing and substantial increases in total pause time. There also was an alteration of the IRT distribution, with median IRTs being slightly longer in most rats after TBZ injection. Nevertheless, TBZ-treated rats remained directed towards the acquisition and consumption of food, and thus select an alternative food source (the concurrently available chow). TBZ appears to predominantly affect the initiation and maintenance of food-reinforced FR5 lever pressing, but it leaves fundamental aspects of food-motivated behavior intact. Interestingly, the behavioral measures that were most closely related to each other in TBZ-treated rats were the global measures of total number of lever presses, total chow intake, and total time spent feeding. TBZ and other VMAT-2 inhibitors produce depressive symptoms in humans, including self-reported motivational dysfunctions (Chen et al., 2012; Frank, 2009, 2010; Guay, 2010). Studies of the effects of TBZ on effort-based choice are important because these methods are used to model deficits that are seen in psychiatric and neurological patients (Salamone et al. 2016a,b, 2018), and are useful for the development of novel drugs for treating these symptoms (Rotolo et al. 2019, 2020, 2021; Salamone et al. 2018). The present studies yielded a rich and detailed characterization of the behavioral effects of TBZ that is useful for further studies involving comparisons between the electrophysiological and behavioral effects of this drug, and may offer clues to clinical researchers studying the effects of TBZ and other VMAT-2 inhibitors (valbenazine, deutetrabenazine) in humans.
Acknowledgments
This research was supported by a grant to JDS, JC and IS from the US NIH/NIMH (MH121350).
References
- Aparicio CF (2001). Overmatching in rats: The barrier choice paradigm. Journal of the Experimental Analysis of Behavior, 75, 93–106. 10.1901/jeab.2001.75-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio CF (2007). Haloperidol, dynamics of choice, and the parameters of the matching law. Behavioral Processes, 75, 206–212. 10.1016/j.beproc.2007.02.024 [DOI] [PubMed] [Google Scholar]
- Carratalá-Ros C, López-Cruz L, Martínez-Verdú A, Olivares-García R, Salamone JD, & Correa M (2021a). Impact of fluoxetine on behavioral invigoration of appetitive and aversively motivated responses: Interaction with dopamine depletion. Frontiers in Behavioral Neuroscience, 15, 700182. 10.3389/fnbeh.2021.700182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carratala-Ros C, Olivares-García R, Martínez-Verdú A, Arias-Sandolva E, Salamone JD, & Correa M (2021b). Energizing effects of bupropion on effortful behaviors in mice under positive and negative test conditions: Modulation of DARPP-32 phosphorylation patterns. Psychopharmacology, 238(12), 3357–3373. 10.1007/s00213-021-05950-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Ondo WG, Dashtipour K, & Swope DM (2012). Tetrabenazine for the treatment of hyperkinetic movement disorders: A review of the literature. Clinical Therapeutics, 34, 1487–1504. 10.1016/j.clinthera.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Cousins MS, Wei W, & Salamone JD (1994). Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: Effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology, 116, 529–537. 10.1007/bf02247489 [DOI] [PubMed] [Google Scholar]
- Floresco SB, St. Onge JR, Ghods-Sharifi S, & Winstanley CA (2008a). Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive Affective Behavioral Neuroscience, 8, 375–389. 10.3758/CABN.8.4.375 [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, & Ghods-Sharifi S (2008b). Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology, 33, 1966–1979. 10.1038/sj.npp.1301565 [DOI] [PubMed] [Google Scholar]
- Foltin RW (1991). An economic analysis of “demand” for food in baboons. Journal of the Experimental Analysis of Behavior, 56, 445–454. 10.1901/jeab.1991.56-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S (2009). Tetrabenazine as anti-chorea therapy in Huntington Disease: An open-label continuation study. Huntington Study Group/TETRA-HD Investigators. BMC Neurology, 9, 62. 10.1186/1471-2377-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S (2010). Tetrabenazine: The first approved drug for the treatment of chorea in US patients with Huntington’s disease. Neuropsychiatric Disease and Treatment, 5, 657–665. 10.2147/NDT.S6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, & Frank MJ (2013). Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biological Psychiatry, 74, 130–136. 10.1016/j.biopsych.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay DR (2010). Tetrabenazine, a monoamine-depleting drug used in the treatment of hyperkinectic movement disorders. American Journal of Geriatric Pharmacotherapy, 8, 331–373. 10.1016/j.amjopharm.2010.08.006 [DOI] [PubMed] [Google Scholar]
- Hauber W, & Sommer S (2009). Prefrontostriatal circuitry regulates effort-related decision making. Cerebral Cortex, 10, 2240–2247. 10.1093/cercor/bhn241 [DOI] [PubMed] [Google Scholar]
- Hursh SR, Raslear TG, Shurtleff D, Bauman R, & Simmons L (1988). A cost-benefit analysis of demand for food. Journal of the Experimental Analysis of Behavior, 50, 419–440. 10.1901/jeab.1988.50-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman LW (1980). Foraging costs and meal patterns in ferrets. Physiology & Behavior, 25, 139–141. https://psycnet.apa.org/doi/10.1016/0031-9384(80)90194-8 [DOI] [PubMed] [Google Scholar]
- Kaufman LW, Collier G, Hill WL, & Collins K (1980). Meal cost and meal patterns in an uncaged domestic cat. Physiological Behavior, 25, 135–137. https://psycnet.apa.org/doi/10.1016/0031-9384(80)90193-6 [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, & Schnitzler HU (2000). Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology, 152, 67–73. 10.1007/s002130000505 [DOI] [PubMed] [Google Scholar]
- Neill DB, & Justice JB (1981). An hypothesis for a behavioral function of dopaminergic transmission in nucleus accumbens. In Chronister RB& Defrance JF (Eds.), The neurobiology of nucleus accumbens. Huer Institute. [Google Scholar]
- Nunes EJ, Randall PA, Hart EE, Freeland C, Yohn S, Baqi Y, Müller CE, López-Cruz L, Correa M, & Salamone JD (2013). Effort-related motivational effects of the VMAT-2 inhibitor tetrabenazine: Implications for animal models of the motivational symptoms of depression. Journal of Neuroscience, 33, 19120–19130. 10.1523/JNEUROSCI.2730-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettibone DJ, Totaro JA, & Pflueger AB (1984). Tetrabenazine-induced depletion of brain monoamines: Characterization and interaction with selected antidepressants. European Journal of Pharmacology, 102(3–4), 425–430. 10.1016/0014-2999(84)90562-4 [DOI] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Nunes EJ, Yohn SE, Nowak V, Khan B, Shah P, Pandit S, Vemuri VK, Makriyannis A, Baqi Y, Müller CE, Correa M, & Salamone JD (2014). The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: Reversal with antidepressant drugs. PLoS One, 9. 10.1371/journal.pone.0099320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, López Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Müller CE, Correa M, & Salamone JD (2012). Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: Pharmacological studies and the role of individual differences. PLoS One, 7. 10.1371/journal.pone.0047934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ, Santerre JL, Makriyannis A, & Salamone JD (2010). The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacology, Biochemistry, and Behavior, 97, 179–184. 10.1016/j.pbb.2010.07.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotolo RA, Dragacevic V, Kalaba P, Urban K, Zehl M, Roller A, Wackerlig J, Langer T, Pistis M, De Luca MA, Caria F, Schwartz R, Presby RE, Yang JH, Samels S, Correa M, Lubec G, & Salamone JD (2019). The novel atypical dopamine uptake inhibitor (S)-CE-123 partially reverses the effort-related effects of the dopamine depleting agent tetrabenazine and increases progressive ratio responding. Frontiers in Pharmacology, 10. 10.3389/fphar.2019.00682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotolo RA, Kalaba P, Dragacevic V, Presby RE, Neri J, Robertson E, Yang JH, Correa M, Bakulev V, Volkova NN, Pifl C, Lubec G, & Salamone JD (2020). Behavioral and dopamine transporter binding properties of the modafinil analog (S, S)-CE-158: Reversal of the motivational effects of tetrabenazine and enhancement of progressive ratio responding. Psychopharmacology, 237, 3459–3470. 10.1007/s00213-020-05625-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotolo RA, Presby RE, Tracy O, Asar S, Yang JH, Correa M, Murray F, & Salamone JD (2021). The novel atypical dopamine transport inhibitor CT-005404 has pro-motivational effects in neurochemical and inflammatory models of effort-based dysfunctions related to psychopathology. Neuropharmacology, 183. 10.1016/j.neuropharm.2020.108325 [DOI] [PubMed] [Google Scholar]
- Salamone JD (1986). Different effects of haloperidol and extinction on instrumental behaviours. Psychopharmacology, 88, 18–23. 10.1007/bf00310507 [DOI] [PubMed] [Google Scholar]
- Salamone JD (1987). The actions of neuroleptic drugs on appetitive instrumental behaviors. In Iversen LL, Iversen SD, & Snyder SH (Eds.), Handbook of psychopharmacology (pp. 575–608). Plenum Press. [Google Scholar]
- Salamone JD (1992). Complex motor and sensorimotor functions of striatal and accumbens dopamine: Involvement in instrumental behavior processes. Psychopharmacology, 107, 160–174. 10.1007/bf02245133 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, & Aberman JE (2002). Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology, 160, 371–380. 10.1007/s00213-001-0994-x [DOI] [PubMed] [Google Scholar]
- Salamone JD, & Correa M (2002). Motivational views of reinforcement: Implications for understanding the behavioral functions of nucleus accumbens dopamine. Behavioural Brain Research, 137, 3–25. 10.1016/s0166-4328(02)00282-6 [DOI] [PubMed] [Google Scholar]
- Salamone JD, & Correa M (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron, 76, 470–485 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, & Mingote SM (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology, 191, 461–482. 10.1007/s00213-006-0668-9 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Ferrigno S, Yang JH, Rotolo RA, & Presby RE (2018). The psychopharmacology of effort-related decision making: Dopamine, adenosine, and insights into the neurochemistry of motivation. Pharmacology Review, 70, 747–762. 10.1124/pr.117.015107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, & Weber SM (2003). Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: Implications for studies of natural motivation, psychiatry, and drug abuse. Journal of Pharmacology and Experimental Therapeutics, 305, 1–8. 10.1124/jpet.102.035063 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, & Weber SM (2005). Beyond the reward hypothesis: Alternative functions of nucleus accumbens dopamine. Current Opinion in Pharmacology, 5, 34–41. 10.1016/j.coph.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Nunes EJ, Randall PA, & Pardo M (2012). The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine and beyond. Journal of the Experimental Analysis of Behavior, 97(1), 125–146. 10.1901/jeab.2012.97-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yohn S, Lopez-Cruz L, San Miguel N, & Alatorre L (2016b). The pharmacology of effort-related choice behavior: Dopamine, depression, and individual differences. Behavioural Processes, 127, 3–17. 10.1016/j.beproc.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yohn SE, Yang JH, Somerville M, Rotolo RA, & Presby RE (2017). Behavioral activation, effort-based choice, and elasticity of demand for motivational stimuli: Basic and translational neuroscience approaches. Motivation Science, 3, 208–229. 10.1037/mot0000070 [DOI] [Google Scholar]
- Salamone JD, Mahan K, & Rogers S (1993). Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacology Biochemistry and Behavior, 44, 605–610. 10.1016/0091-3057(93)90174-r [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, & Mahan K (1991). Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology, 104, 515–521. 10.1007/bf02245659 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Yohn S, Lopez-Cruz L, San Miguel N, & Correa M (2016a). Activational and effort-related aspects of motivation: Neural mechanisms and implications for psychopathology. Brain, 139, 1325–1347 10.1093/brain/aww050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Zigmond MJ, & Stricker EM (1990). Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions. Neuroscience, 39, 17–24. 10.1016/0306-4522(90)90218-S [DOI] [PubMed] [Google Scholar]
- Salvatier J, Wiecki TV, & Fonnesbeck C (2016). Probabilistic programming in Python using PyMC3. PeerJ Computer Science, 2, e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, & Salamone JD (2008). Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology, 196, 565–574. 10.1007/s00213-007-0988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon JER (1979). Operant behavior as adaptation to constraint. Journal of Experimental Psychology: General, 108, 48–67. https://psycnet.apa.org/doi/10.1037/0096-3445.108.1.48 [Google Scholar]
- Taylor GT (1975). Discriminability and the contrafreeloading phenomenon. Journal of Comparative and Physiological Psychology, 88(1), 104–109. 10.1037/h0076222 [DOI] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, & Zald DH (2012). Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. Journal of Abnormal Psychology, 121, 553–558. 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustin RD (1995). Assessing preference for reinforcers using demand curves, work-rate functions, and expansion paths. Journal of the Experimental Analysis of Behavior, 64, 313–329. 10.1901/jeab.1995.64-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos R, van der Harst J, Jonkman S, Schilders M, & Spruijt B (2006). Rats assess costs and benefits according to an internal standard. Behavioural Brain Research, 171, 350–354. https://psycnet.apa.org/doi/10.1016/j.bbr.2006.03.035 [DOI] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PE, & Rushworth MF (2006). Weighing up the benefits of work: Behavioral and neural analyses of effort-related decision making. Neural Network, 19, 1302–1314. 10.1016/j.neunet.2006.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA (1988). Reinforcement, choice, and response strength. In Atkinson RC, Herrnstein RJ, Lindsey G, & Luce RD (Eds.), Stevens’ handbook of experimental psychology. vol. 2 (pp. 167–174). John Wiley and Sons. [Google Scholar]
- Worden LT, Shahriari M, Farrar AM, Sink KS, Hockemeyer. J, Müller C, & Salamone JD (2009). The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: Differential interaction with D1 and D2 family antagonists. Psychopharmacology, 203, 489–499. 10.1007/s00213-008-1396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Presby RE, Jarvie AA, Rotolo RA, Fitch RH, Correa M, & Salamone JD (2020). Pharmacological studies of effort-related decision making using mouse touchscreen procedures: Effects of dopamine antagonism do not resemble reinforcer devaluation by removal of food restriction. Psychopharmacology, 237, 33–43. 10.1007/s00213-019-05343-8 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Collins SL, Contreras-Mora HM, Errante EL, Rowland MA, Correa M, & Salamone JD (2016a). Not all antidepressants are created equal: Differential effects of monoamine uptake inhibitors on effort-related choice behavior. Neuropsychopharmacology, 41, 686–694. 10.1038/npp.2015.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn SE, Errante EL, Rosenbloom-Snow A, Sommerville M, Rowland MA, Tokarski K, Zafar N, Correa M, & Salamone JD (2016c). Blockade of uptake for dopamine, but not norepinephrine or 5-HT, increases selection of high effort instrumental activity: Implications for treatment of effort-related motivational symptoms in psychopathology. Neuropharmacology, 109, 270–280. 10.1016/j.neuropharm.2016.06.018 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Gogoj A, Haque A, Lopez-Cruz L, Haley A, Huxley P, Baskin P, Correa M, & Salamone JD (2016d). Evaluation of the effort-related motivational effects of the novel dopamine uptake inhibitor PRX-14040. Pharmacology Biochemistry and Behavior, 148, 84–91. 10.1016/j.pbb.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Lopez-Cruz L, Hutson PH, Correa M, & Salamone JD (2016b). Effects of lisdexamfetamine and s-citalopram, alone and in combination, on effort-related choice behavior in the rat. Psychopharmacology, 233, 949–960. 10.1007/s00213-015-4176-7 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Santerre JL, Nunes EJ, Kozak R, Podurgiel SJ, Correa M, & Salamone JD (2015b). The role of dopamine D1 receptor transmission in effort-related choice behavior: Effects of D1 agonists. Pharmacology Biochemistry and Behavior, 135, 217–226. 10.1016/j.pbb.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Thompson C, Randall PA, Lee CA, Müller CE, Baqi Y, Correa M, & Salamone JD (2015a). The VMAT-2 inhibitor tetrabenazine alters effort-related decision making as measured by the T-maze barrier choice task: Reversal with the adenosine A2A antagonist MSX-3 and the catecholamine uptake blocker bupropion. Psychopharmacology, 232, 1313–1323. 10.1007/s00213-014-3766-0 [DOI] [PubMed] [Google Scholar]