Abstract
Background:
The purpose of this study was to investigate how 8-isoprostanes, used as a marker of airway oxidative stress, were related to sinus disease and asthma.
Methods:
We analyzed samples and data from two separate studies, one investigating sinonasal disease in asthma, the other investigating the effect of BMI on airway disease. We measured airway (nasal lavage) 8-isoprostanes and investigated the relationship with measures of sinus and asthma symptoms, asthma control and lung function.
Results:
The study of people with sinonasal disease and poorly controlled asthma included 48 obese, 31 overweight and 23 lean participants. In multivariate analysis, nasal lavage 8-isoprostane levels increased with increasing BMI (p<0.01), and were higher in Caucasian than African American participants (p=0.01). Sinus symptoms were inversely related to nasal 8-isoprostanes (p=0.02) independent of BMI and Race. In the study investigating the effect of BMI on airway disease, we enrolled 13 controls with obesity and 21 people with obesity and asthma: 8-isoprostane levels were higher in obese controls than in obese people with asthma (p<0.01), and levels were inversely related to sinus symptoms (p=0.02) and asthma control (p<0.01).
Interpretation:
8-isoprostanes in nasal lavage are increased in obesity, and increased in Caucasians compared with African Americans. However, levels are higher in obese controls than obese people with asthma, and appear inversely related to symptoms of airway disease.
Clinical Implication:
Airway 8-isoprostanes likely reflect complex oxidative signaling pathways, which are altered in obesity and those of different race, rather than being a simple marker of airway oxidative injury.
Capsule Summary:
Increased airway oxidative signaling (8-isoprostanes), may reflect normal physiology in the setting of obesity, as decreased levels are associated with disease activity in people with chronic sinonasal disease and asthma.
Keywords: reactive oxygen species, oxidative stress, obesity, race
INTRODUCTION
Oxidative stress refers to the presence of increased levels of reactive oxygen species (ROS) which may cause cell and tissue damage in a variety of conditions.(1) Many studies have investigated airway oxidative stress using a biomarker of lipid peroxidation, 8-isoprostanes,(2) to investigate how ROS might contribute to airway disease in people with asthma. Obese people with asthma have higher levels of 8-isoprostanes in exhaled breath condensate than lean people with asthma,(3) and it is hypothesized these higher levels could contribute to increased disease severity in obesity.(3–5) Other studies have found higher levels of 8-isoprostanes in people with severe asthma, though did not report BMI.(6) Combined, prior studies suggest that 8-isoprostanes are increased in those with severe asthma, and in those with higher BMI, though whether the association with severe asthma is related to higher BMI is not known, and the relationship to symptoms and physiology is unclear.
Oxidative stress levels may also differ by race, and might contribute to differences in disease patterns. For example, oxidative stress might contribute to more severe cardiovascular disease in the African American population,(7) and environmental factors may drive differences in oxidative stress that contribute to racial disparities in health.(8) African American people tend to have more severe asthma than Caucasians,(9) and increased oxidative stress could be one factors contributing to this, though we are not aware of prior studies examining the relationship between airway 8-isoprostanes and race.
A limitation to the routine measurement of 8-isoprostanes is access to airway samples. Bronchoalveolar lavage is not suitable for routine monitoring, and exhaled breath condensate and induced sputum are not readily available outside of a research setting. The nasal cavity, as the first part of the respiratory symptom, is often used as a marker of lower airway disease in people with asthma. (10, 11) We were interested in whether nasal lavage might be suitable to measure 8-isoprostanes, and how this might be related to airway disease activity.
The current study was designed to investigate the relationship of nasal lavage 8-isoprostanes with demographic factors, and then airway disease activity. We hypothesized that 8-isoprostanes would be elevated in the nasal lavage in proportion to BMI, and associated with worse lung function and increased nasal and asthma symptoms. A secondary hypothesis was that levels of ROS would be higher in African American than Caucasian participants, and so might contribute to more severe disease in this population.
METHODS
Study Design
We assessed samples and disease activity in two separate populations. Studies were approved by local institutional review boards, and informed consent obtained from all participants.
Data and samples from people with sinonasal disease and poorly controlled asthma were collected as part of the Study of Asthma and Nasal Steroids (STAN), a multi-center, randomized, placebo-controlled, double-blinded trial conducted by the American Lung Association – Airways Clinical Research Centers Network; comprehensive details of the STAN trial have been described elsewhere.(12) In short, this was a 24-week trial investigating whether treatment of chronic sinonasal disease with intranasal corticosteroids (mometasone 50mcg, 2 sprays per nostril per day) improved asthma control in adults and children with poorly controlled asthma. The current study included baseline data, and data obtained after 24 weeks of treatment.
In a separate single center study, the “Effect of BMI on Airway Disease” (EBAD study), people with and without asthma were enrolled in an observational study prior to bariatric surgery and asthma control, sinus symptoms and lung function were assessed.
Eligibility criteria
Inclusion criteria for the STAN study were poor asthma control (as defined by a score of 19 or less on the asthma control test),(13) and chronic symptoms of rhinitis and sinusitis (as defined by a mean score of ≥ 1 on the Sinonasal Questionnaire).(14) Exclusion criteria for the STAN study were history of an upper respiratory infection within the last 8 weeks; fever ≥ 38.3·°C within the last 10 days; sinus surgery within the last 6 months; use of systemic or nasal corticosteroids within the last 4 weeks, or anti-leukotriene medication within the last 2 weeks; or, a greater than 10 pack year smoking history or smoking within the last 6 months. The current study was limited to adults aged 18 years and older of both sexes who had airway hyperresponsiveness to methacholine.
Inclusion criteria for the EBAD study for asthmatic participants were as follows: age 18 ≥ years, physician diagnosis of asthma, positive methacholine challenge, and an FEV1 of at least 60%. Inclusion criteria for non-asthmatic participants included age ≥ 18 years, no physician diagnosis of asthma, negative methacholine challenge test, undergoing bariatric surgery and an FEV1 ≥ 60%. Exclusion criteria for the EBAD study were chronic lung disease other than asthma, ≥ 20 pack year smoking history, smoking within the last 6 months and pregnancy.
Spirometry, methacholine challenge, and nasal lavage
Spirometry (Koko, Ferraris Respiratory Inc., Louisville, CO, USA) and methacholine challenge were performed as previously described; trial participants in the STAN study with an FEV1 ≤ 70% did not perform a methacholine challenge.(12) Closing index was calculated as percent decrease in FVC divided by the percent decrease in FEV1, and airway narrowing was calculated as percent decrease in FEV1 divided by FVC during maximal methacholine induced bronchoconstriction.(15) Nasal lavage was performed as previously described.(16) Exhaled nitric oxide was measured as previously described and according to ATS guidelines.(16, 17)
8-isprostane measurement
In the STAN study, 8-isoprostane levels were measured in the nasal lavage fluid at baseline and end of study (after treatment with nasal placebo or nasal corticosteroid). In the EBAD study, 8-isoprostanes were measured prior to bariatric study. Levels were measured by assay of 8-isoprostane levels using the OxiSelect 8-iso-Prostaglandin F2α ELISA Kit (Cellbio Labs, San Diego, CA, USA),(4) The ELISA was run according to manufacturer instructions—however, to avoid over dilution of the already dilute nasal lavage samples, specimens were pH balanced using 10N HCl and 10N NaOH prior to addition of any diluent.(20)
Statistical analysis
Data are shown as mean ± SD. All data were assessed for homogenous variability using the Brown and Forsythe’s Test for Homogeneity. All data were found to have homogenous variability with the exception of 8-isoprostane levels, which required log10 transformation. Differences between groups were analyzed using T-test, ANOVA or Chi-squared test where appropriate. Multivariate linear regression was used to investigate the relationship between 8-isoprostane levels with demographic factors (including age, race and sex), and then markers of disease activity in the STAN study. Univariate regression between 8-isoprostane levels and markers of disease activity was performed for the EBAD study. Data were analyzed with GraphPad Prism 8.0.0 (GraphPad Software Inc, La Jolla, CA, USA), SAS 9.4 (SAS, Cary, NC, USA) or STATA 16 (StataCorp, College Station, TX, USA).
RESULTS
Demographics
In participants in the study of sinonasal disease and asthma (STAN), one hundred and two adult participants completed methacholine challenge testing and had nasal lavage samples. Table I presents the baseline demographics of those included in the current study according to BMI group. There was a higher proportion of African Americans in the obese category. There was a numerically higher closing index in obese participants, as has been previously described.(18)
Table I.
Demographics of STAN study participants
| BMI category | Lean | Overweight | Obese | p |
|---|---|---|---|---|
| n | 23 (22%) | 31 (31%) | 48 (47%) | |
| % Female | 56.5 | 62.5 | 74.5 | 0.28 |
| Age | 37.35 ± 15.95 | 36.78 ± 12.79 | 38.6 ± 13.48 | 0.84 |
| Race | <0.01 | |||
| Caucasian | 20 (86.9%) | 20 (62.5%) | 23 (48.9%) | |
| African American | 2 (8.7%) | 9 (28.1%) | 22 (46.8%) | |
| Other | 1 (4.4%) | 3 (9.4%) | 2 (4.3%) | |
| Age of asthma onset | 14.09 ± 16.32 | 13.47 ± 12.44 | 15.85 ± 14.17 | 0.74 |
| FEV1 (%predicted) | 89.97 ± 13.16 | 90.82 ± 14.10 | 88.13 ± 15.99 | 0.71 |
| FVC (%predicted) | 97.34 ± 13.24 | 103.10 ±14.58 | 96.88 ± 15.24 | 0.15 |
| FEV1/FVC | 0.92 ± 0.08 | 0.88 ± 0.09 | 0.90 ± 0.08 | 0.08 |
| Closing Index | 0.70 ± 0.26 | 0.68 ± 0.23 | 0.81 ± 0.18 | 0.05 |
| Airway Narrowing | 0.07 ± 0.08 | 0.11 ± 0.06 | 0.07 ± 0.08 | 0.11 |
| PC20 | 3.31 ± 3.78 | 2.23 ± 2.88 | 2.50 ± 3.64 | 0.54 |
| Peak Flow (%predicted) | 87.65 ± 16.22 | 89.13 ± 17.81 | 84.40 ± 18.07 | 0.48 |
| FeNO (ppb) | 39.42 ± 27.7 | 37.69 ± 33.3 | 32.10 ± 29.2 | 0.22 |
| ACT¥ | 15.65 ± 2.72 | 15.84 ± 3.30 | 15.72 ± 3.42 | 0.97 |
| ASUI¥ | 0.69 ± 0.20 | 0.72 ± 0.16 | 0.73 ± 0.19 | 0.70 |
| SNOT22 | 41.5 ± 23.9 | 39.9 ± 21.7 | 43.0 ± 22.7 | 0.83 |
Values are mean ± SD
p-values shown for one-way ANOVA, except for gender and race where a Fischer’s exact test was used.
Higher values indicative of better health
BMI, body mass index; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; ACT, Asthma Control Test; ASUI. Asthma Symptom Utility Index.
In a separate study of the effect of BMI on airway disease (EBAD), there were 34 participants: 13 obese controls and 21 obese people with asthma. Demographics of these participants can be found in Table II. There was a majority of females participating in this study, however the proportions of females did not differ between groups. All participants were enrolled prior to bariatric surgery, BMI’s were higher than the STAN study participants, but similar in controls and in participants with asthma.
Table II.
Demographics of EBAD study participants
| Controls | People with asthma | p | |
|---|---|---|---|
| n | 13 | 21 | |
| Female n (%) | 10 (77) | 17 (81) | 0.79 |
| BMI | 45.6 ± 7.9 | 46.9 ± 8.4 | 0.90 |
| Age | 41.4 ± 8.5 | 44.4 ± 12.3 | 0.45 |
| Age of asthma onset | - | 23.0 ± 13.7 | |
| FEV1 (%predicted)* | 97 (78–114) | 89 (76–108) | 0.07 |
| FVC (%predicted) | 93.5 ± 10.8 | 90.0 ± 7.5 | 0.28 |
| Sinonasal Questionnaire* | 0.2 (0–1.2) | 1.0 (0–2.4) | <0.01 |
| Asthma Control Questionnaire | - | 0.57 (0–3.3) |
Values shows are mean ±SD or median (range) for continuous variables
T-Test used to compare continuous variables, except where indicated, and
Chi-squared analysis to compared proportions
Wilcoxon rank-sum used to compare non-normally distributed variable
Airway 8-isoprostanes
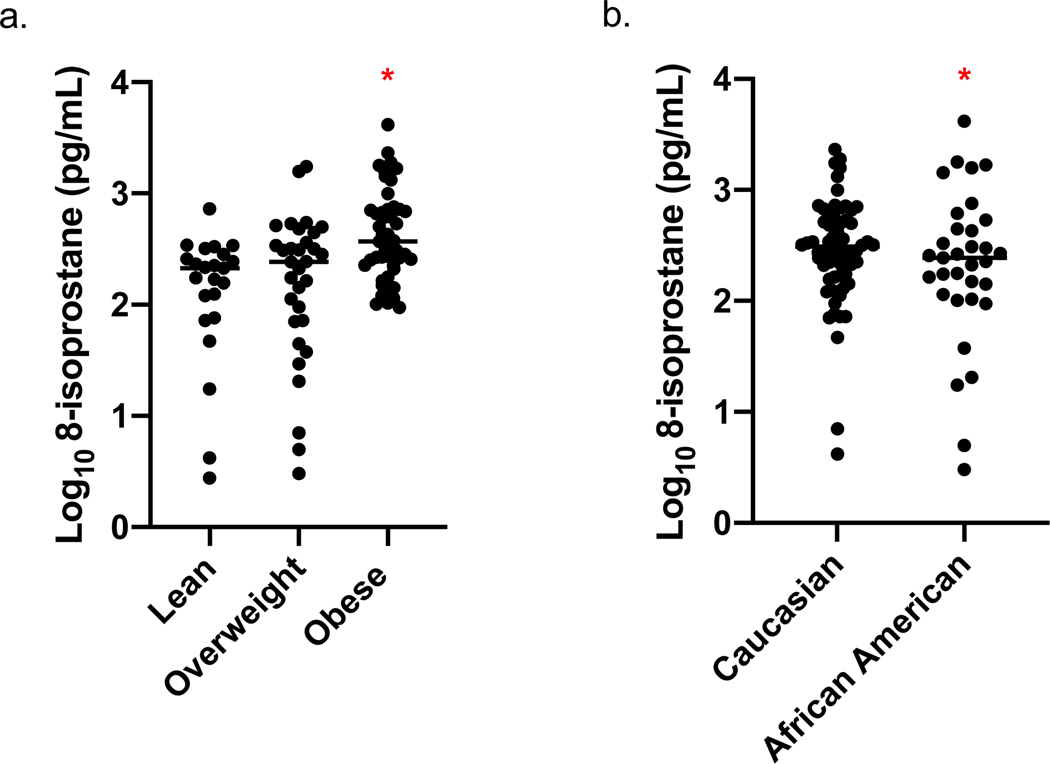
We performed a multivariate analysis to determine how demographic factors related to 8-isoprostanes including sex, age, BMI category and race in the model (Table III). BMI category and Race were significantly related to levels of 8-isoprostanes in nasal lavage, with increased BMI associated with higher levels of 8-isoprostanes (Figure 1a), and Caucasian versus African American Race (Figure 1b) associated with higher levels of 8-isoprostanes.
Table III.
Factors affecting 8-isoprostane levels in multivariate regression in STAN study participants
| coefficient | CI | p | |
|---|---|---|---|
| Overweight | 0.19 | −1.00–0.48 | 0.17 |
| Obese | 0.66 | 0.38–0.94 | <0.001 |
| Race* | −0.31 | −0.55–−0.08 | 0.01 |
| Sex† | −0.07 | −0.90–0.15 | 0.85 |
| Age | 0 | −0.01–0.0 | 0.45 |
8-isoprostanes log10 transformed for the analysis
Race refers to African American versus Caucasian
Sex refers to female versus male in this analysis
Adj R2 for model=0.22, p< 0.0001
Figure 1: Nasal lavage 8-isoprostane levels in relation to BMI and Race:
Multivariate regression adjusted for sex, age and race in the model; N=102, Adj R2=0.22 (a) levels are significantly increased in obese compared with lean and overweight people (p< 0.0001), and (b) levels are higher in Caucasian than African American participants (p=0.01).
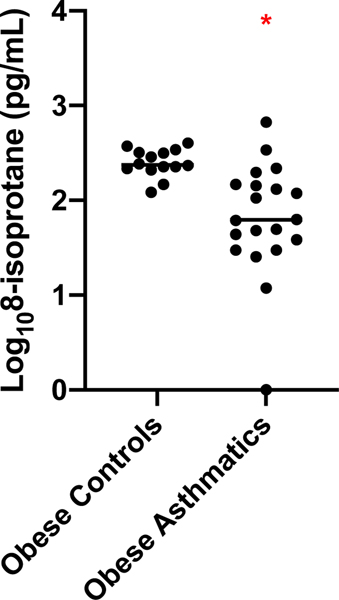
In the EBAD study—in which all participants were morbidly obese—8-isoprostanes were not related to BMI (r2=0.01, p=0.66) (Figure E1). As most participants were Caucasian, we could not determine effects of race. Overall, levels of 8-isoprostanes were significantly higher in obese controls compared to participants with asthma (T-test, 2.40±0.15 vs 1.81±0.60 log10 8-isoprostane pg/mL, p<0.01) (Figure 2).
Figure 2. Nasal Lavage 8-isoprostane levels in obese healthy and obese people with asthma.
Overall levels were significantly higher in people without asthma (N=13, 2.40 ± 0.15 log10 8-isoprostane pg/mL) as compared to those with asthma (N=21, 1.81 ± 0.60 log10 8-isoprostane pg/mL), p<0.001, T-test).
Effect of corticosteroid treatment on 8-isoprostane levels
In the STAN study, 8-isoprostanes were unaffected by the study intervention: there was no change in 8-isoprostanes in participants receiving 6 months of nasal mometasone (2.15±0.57 log10 pg/mL vs 2.33±0.66 log10 pg/mL; p=0.71), or placebo (2.41±0.53 log10 pg/mL vs 2.32±0.63 log10 pg/mL; p=0.89) (Figure E2).
Association of 8-isoprostane with lung function
There was no relationship between serum 8-isoprostanes and any lung function parameter in either study (data not shown).
Association of 8-isoprostane with nasal and asthma symptoms
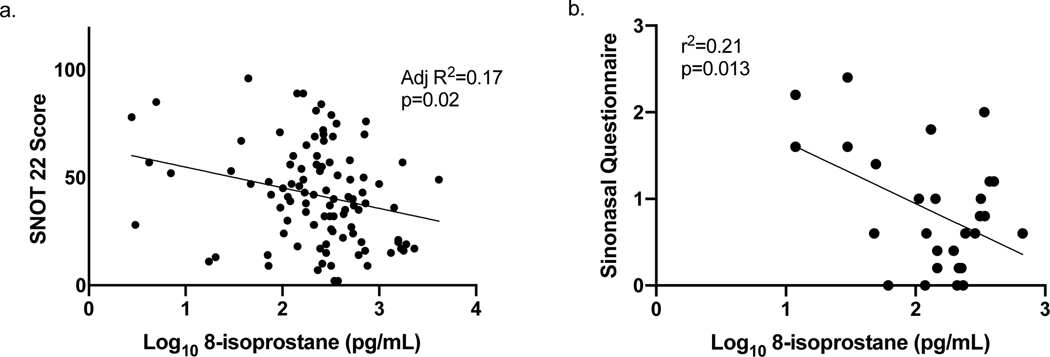
In the STAN study, sinus symptoms were quantified using the SNOT22 questionnaire.(19) 8-isoprostane levels were inversely associated with sinus symptoms, independent of BMI and Race category (p=0.02) (Figure 3). In the EBAD study, sinus symptoms were measured by the SNQ questionnaire and were inversely correlated with sinus symptoms (r2=0.17; p=0.02) (Figure 3).
Figure 3: Relationship between sinus symptoms and nasal lavage 8-isoprostane levels.
a) In the STAN study, higher levels of nasal lavage were significantly associated with fewer sinus symptoms (SNOT 22 questionnaire), (Multiple regression adjusted for BMI and race, N=101, Adj R2=0.17, p=0.02), and b) in the EBAD study, higher levels of nasal lavage 8-isoprostane were associated with fewer sinus symptoms (Simple linear regression, N=29, r2=0.21, p= 0.02).
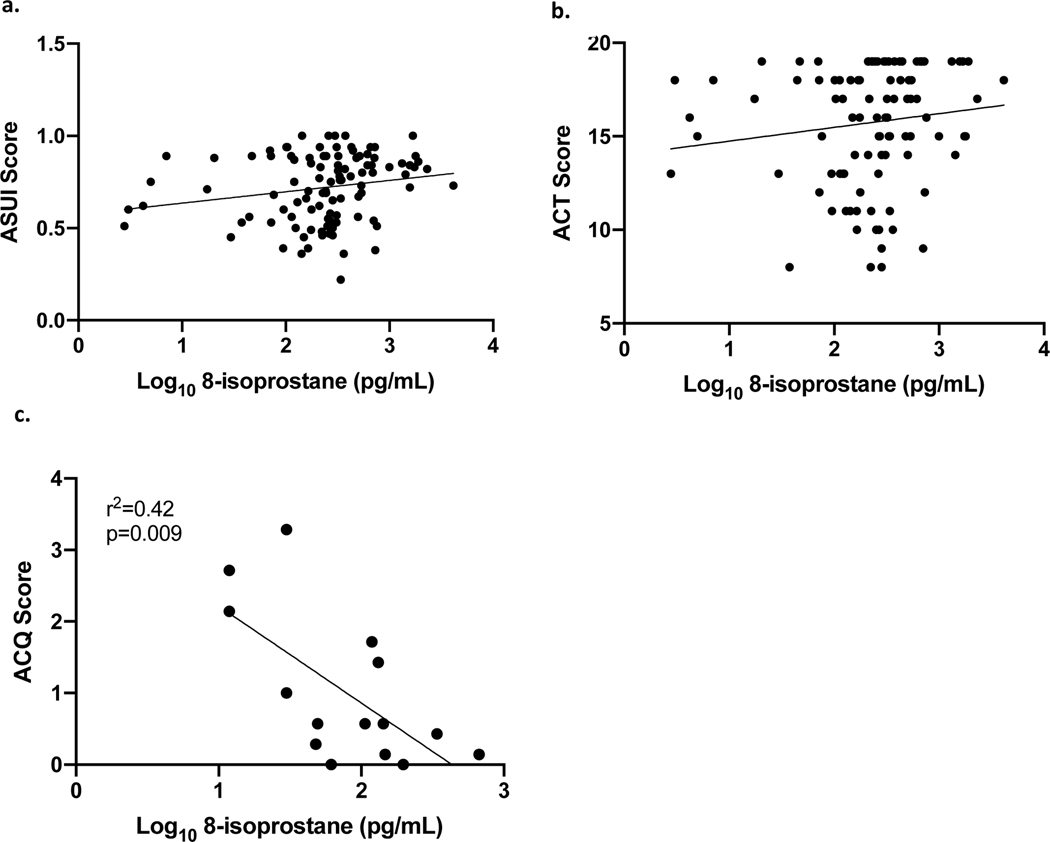
In the STAN study, asthma symptoms were quantified using the Asthma Symptom Utility Index (20) and the Asthma Control Test.(13) In multivariate regression, including BMI and Race, 8-isoprostanes appeared to be associated with fewer asthma symptoms, though this did not reach statistical significance. In the EBAD study, higher 8-isoprostane correlated with fewer asthma symptoms (r2=0.37; p=0.006) (Figure 4).
Figure 4. Relationship between asthma symptoms and nasal lavage 8-isoprostane levels.
a) STAN study: relationship between ASUI scores (higher levels indicate fewer symptoms) and 8-isoprostane levels, (Multivariate regression including BMI and race, N=102, Adj R2=0.12, p=0.09). b) STAN study: relationship between ACT scores (higher levels indicate better control) and 8-isoprostane, (Multivariate regression including BMI and race, N=102, Adj R2=0.12, p=0.08). c) EBAD study: relationship between 8-isoprostanes and ACQ scores (lower scores indicate better control) (Simple linear regression, N=15, r2=0.37, p=0.006).
DISCUSSION
The lipid peroxidation marker, 8-isoprostane is reported to be increased in people with severe asthma (21, 22) and also in obese people with asthma.(3) The functional significance of increased levels of 8-isoprostanes in the airway are not known, but are assumed to reflect oxidative stress and damage. The goal of the current study was to determine if 8-isoprostanes were associated with pathophysiology of airway disease, particularly in people with obesity and asthma. We found that nasal lavage 8-isoprostanes are significantly elevated in proportion to BMI in people with asthma and sinonasal disease, though lower in African Americans compared with Caucasians (when controlled for BMI). We also found that levels were higher in obese people without asthma than they were in obese people with asthma. Levels were not related to lung function abnormalities; higher levels were associated with fewer sinus symptoms, and also appeared related to fewer asthma symptoms. Our study suggests that 8-isoprostane levels in the airway do not simply reflect damage and disease severity, but are likely to be a marker of redox signaling pathways involved in airway homeostasis that are altered in obesity.
A number of prior publications have measured 8-isoprostanes in the airways of people with asthma, in exhaled breath condensate, (23) induced sputum(24) and bronchoalveolar lavage.(21) These studies have reported that 8-isoprostane levels are associated with severe asthma, but have not determined if this is independent of BMI (obese people tend to have more severe asthma).(6) Komakula et al found exhaled breath condensate 8-isoprostanes levels increased in relation to BMI, but did not determine if levels were related to asthma severity.(3) Our study shows that while obese people with asthma have higher levels of airway 8-isoprostanes than lean people with asthma, obese controls have higher levels than obese people with asthma. These data suggest that the relationship between airway disease and 8-isoprostanes is driven by BMI rather than asthma.
We found lower levels of nasal lavage 8-isoprostanes in African Americans compared with Caucasians when controlling for BMI. We are not aware of any previous reports investigating racial differences in airway 8-isoprostanes. However, others have found lower systemic levels of 8-isoprostanes in African Americans compared with Caucasians, and that these lower levels are associated with an increased risk of developing diabetes(25) and weight gain.(26) It has been suggested that lower levels of 8-isoprostanes might reflect slower mitochondrial oxidative metabolism, which has been linked to African ancestry.(27) African Americans have more severe asthma than Caucasian counterparts.(9) Given that previous work has found that lower 8-isoprostane levels are associated with a predilection for the development of disease, and our study found that lower 8-isoprostane levels were associated with worse nasal and asthma symptoms it could be that lower levels of 8-isoprostane for a given BMI might reflect a maladaptive response rather than be an indicator of disease activity.
Some prior studies have investigated the relationship between 8-isoprostanes with asthma symptoms and lung function. Levels in induced sputum increase during exacerbations (28), this differs from our study which did not assess participants during exacerbations. A few small studies have assessed the relation between 8-isoprostanes and asthma control, and found inconsistent results, though none controlled for BMI.(29–31) Our study is the first, of which we are aware, to investigate the relationship between markers of lung function and asthma symptoms when controlling for BMI, and suggest that while levels go up in relation to BMI, there is no relationship to lung function, and perhaps an inverse relationship with asthma symptoms. Together, our current study and prior publications suggest that 8-isoprostanes are not useful to monitor chronic disease activity in people with asthma.
We found that higher levels of 8-isoprostanes correlated with fewer sinus symptoms (all participants in the STAN study had chronic sinonasal disease and asthma), and were unaffected by topical corticosteroids. We are not aware of any prior studies investigating the relationship between chronic sinus symptoms and levels of nasal lavage 8-isoprostanes. Prior studies suggest that nasal lavage 8-isoprostanes are increased during pollen season,(32) and nasal lavage and exhaled breath 8-isoprostanes are increased after nasal allergen challenge.(11) This may reflect acute inflammatory changes, and perhaps mirrors the finding of increased 8-isoprostanes during asthma exacerbations.(28) Our data suggest that chronically, higher levels of 8-isoprostanes levels are associated with lower levels of sinonasal symptoms.
It is perhaps unexpected that a marker usually utilized to measure oxidative stress should have an inverse relationship to symptoms. However, a study describing 8-isoprostane in exhaled breath condensate levels in adults participating in the Epidemiological Study of the Genetics and Environment of Asthma found that higher levels tended to be associated with a lower risk of poor asthma control (odds ratio 0.86 [CI 0.61–1.22] adjusted for age, sex and smoking status), whereas fluorescent oxidation products were associated with in an increased risk of poor asthma control (adjusted odds ratio 1.30 [CI 1.02–1.66]).(33) This suggests that measuring markers of oxidative signaling may yield different insights depending on the end product being measured. It is possible that 8-isoprostanes chronically reflect homeostatic pathways. 8-isoprostanes are formed by peroxidization of arachidonic acid by free radicals,(34) while in acute inflammatory states these may be produced by inflammatory cells, chronically one of the major sources of reactive oxygen species contributing to peroxidation of lipids is ROS production during oxidative phosphorylation. Higher levels of peroxidation may reflect increased mitochondrial function rather than inflammation. Oxidative signaling is involved in many critical pathways, and interpreting increased levels of a single marker as an indicative of damage is clearly an oversimplification of complicated biologic pathways.
There are some important limitations to the current study. We included only STAN participants who had a baseline FEV1≥70% (and were eligible for methacholine), and the EBAD study only included those with a baseline FEV1≥60%, and so how these data might relate to those with lower lung function is unclear. Additionally, as all participants had both severe asthma and chronic sinonasal disease and we do not have controlled groups of asthma without sinonasal disease and sinonasal disease without asthma, we are unable to determine whether the changes seen in 8-isoprostane levels are primarily due to asthma, sinonasal disease or both; however our results are generalizable to those with concomitant sinonasal disease and asthma. The STAN measurements were on stored samples since the end of the study: levels of 8-isoprostane might increase with significant storage time and the stability of 8-isoprostane levels have only been assessed in urine and serum (35, 36); however, we would anticipate this would affect all samples in a similar manner in this study. The EBAD study was small single center study and predominately consisted of females. However, the fact that these two very different populations showed similar results supports the validity of the findings.
In summary, we have shown that 8-isoprostanes in nasal lavage are increased in proportion to BMI, and lower in African Americans than in Caucasians. Levels are higher in obese people without asthma than in obese people with asthma. 8-isoprostane levels are inversely associated with sinus symptoms, and perhaps asthma symptoms. Our study suggests that 8-isoprostanes are not simply a marker of oxidative damage; high levels in obese subjects may reflect normal airway homeostasis in obesity rather than active airway disease.
Supplementary Material
Acknowledgements:
We would like to thank Dr. Jeff Priest for consulting on statistical analyses.
Funding:
Supported by grants from the American Lung Association, and NIH grants UO1HL089464 and R01 HL133920.
Abbreviation list
- ACT
asthma control test
- ACQ
Juniper asthma control questionnaire
- ASUI
asthma symptoms utility index
- BMI
body mass index
- EBAD
Effect of BMI on Airway Disease
- FeNO
fractional excretion of nitric oxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- ICS
inhaled corticosteroid
- ROS
reactive oxygen species
- STAN
Study of Asthma and Nasal Steroids
- SNOT 22
sino-nasal outcome test
- SNQ
sinonasal questionnaire
REFERENCES
- 1.Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrow JD. The isoprostanes - unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr Pharm Des. 2006;12(8):895–902. [DOI] [PubMed] [Google Scholar]
- 3.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winnica D, Corey C, Mullett S, Reynolds M, Hill G, Wendell S, et al. Bioenergetic Differences in the Airway Epithelium of Lean Versus Obese Asthmatics Are Driven by Nitric Oxide and Reflected in Circulating Platelets. Antioxid Redox Signal. 2019;31(10):673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh VP, Aggarwal R, Singh S, Banik A, Ahmad T, Patnaik BR, et al. Metabolic Syndrome Is Associated with Increased Oxo-Nitrative Stress and Asthma-Like Changes in Lungs. PLoS One. 2015;10(6):e0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract. 2018;6(2):545–54 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feairheller DL, Park JY, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci. 2011;4(1):32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emeny RT, Carpenter DO, Lawrence DA. Health disparities: Intracellular consequences of social determinants of health. Toxicol Appl Pharmacol. 2021:115444. [DOI] [PubMed]
- 9.Shanawani H. Health disparities and differences in asthma: concepts and controversies. Clin Chest Med. 2006;27(1):17–28, v. [DOI] [PubMed] [Google Scholar]
- 10.Giavina-Bianchi P, Aun MV, Takejima P, Kalil J, Agondi RC. United airway disease: current perspectives. J Asthma Allergy. 2016;9:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serrano CD, Valero A, Bartra J, Roca-Ferrer J, Munoz-Cano R, Sanchez-Lopez J, et al. Nasal and bronchial inflammation after nasal allergen challenge: assessment using noninvasive methods. J Investig Allergol Clin Immunol. 2012;22(5):351–6. [PubMed] [Google Scholar]
- 12.American Lung Association-Asthma Clinical Research Centers’ Writing C, Dixon AE, Castro M, Cohen RI, Gerald LB, Holbrook JT, et al. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. J Allergy Clin Immunol. 2015;135(3):701–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–56. [DOI] [PubMed] [Google Scholar]
- 14.Dixon AE, Sugar EA, Zinreich SJ, Slavin RG, Corren J, Naclerio RM, et al. Criteria to screen for chronic sinonasal disease. Chest. 2009;136(5):1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J. 2008;32(6):1563–9. [DOI] [PubMed] [Google Scholar]
- 16.Kanagalingam S, Shehab SS, Kaminsky DA, Wise RA, Lang JE, Dixon AE. Effect of obesity on sinonasal disease in asthma. The Journal of asthma : official journal of the Association for the Care of Asthma. 2018;55(5):525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaminsky DA, Chapman DG, Holbrook JT, Henderson RJ, Sugar EA, Mastronarde J, et al. Older age and obesity are associated with increased airway closure in response to methacholine in patients with asthma. Respirology. 2019;24(7):638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–54. [DOI] [PubMed] [Google Scholar]
- 20.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998;114(4):998–1007. [DOI] [PubMed] [Google Scholar]
- 21.Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-beta1 and oxidant stress in children with severe asthma: association with airflow limitation. J Allergy Clin Immunol. 2012;129(2):388–96, 96 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. American journal of respiratory and critical care medicine. 1999;160(1):216–20. [DOI] [PubMed] [Google Scholar]
- 23.Peel AM, Crossman-Barnes CJ, Tang J, Fowler SJ, Davies GA, Wilson AM, et al. Biomarkers in adult asthma: a systematic review of 8-isoprostane in exhaled breath condensate. J Breath Res. 2017;11(1):016011. [DOI] [PubMed] [Google Scholar]
- 24.Ono E, Dutile S, Kazani S, Wechsler ME, Yang J, Hammock BD, et al. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. American journal of respiratory and critical care medicine. 2014;190(8):886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Il’yasova D, Spasojevic I, Base K, Zhang H, Wang F, Young SP, et al. Urinary F2-isoprostanes as a biomarker of reduced risk of type 2 diabetes. Diabetes Care. 2012;35(1):173–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB Jr., Wagenknecht LE. Urinary F2-isoprostanes, obesity, and weight gain in the IRAS cohort. Obesity (Silver Spring). 2012;20(9):1915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annor F, Goodman M, Thyagarajan B, Okosun I, Doumatey A, Gower BA, et al. African Ancestry Gradient Is Associated with Lower Systemic F2- Isoprostane Levels. Oxid Med Cell Longev. 2017;2017:8319176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood LG, Garg ML, Simpson JL, Mori TA, Croft KD, Wark PA, et al. Induced sputum 8-isoprostane concentrations in inflammatory airway diseases. American journal of respiratory and critical care medicine. 2005;171(5):426–30. [DOI] [PubMed] [Google Scholar]
- 29.Piotrowski WJ, Majewski S, Marczak J, Kurmanowska Z, Gorski P, Antczak A. Exhaled breath 8-isoprostane as a marker of asthma severity. Arch Med Sci. 2012;8(3):515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keskin O, Balaban S, Keskin M, Kucukosmanoglu E, Gogebakan B, Ozkars MY, et al. Relationship between exhaled leukotriene and 8-isoprostane levels and asthma severity, asthma control level, and asthma control test score. Allergol Immunopathol (Madr). 2014;42(3):191–7. [DOI] [PubMed] [Google Scholar]
- 31.Robroeks CM, van de Kant KD, Jobsis Q, Hendriks HJ, van Gent R, Wouters EF, et al. Exhaled nitric oxide and biomarkers in exhaled breath condensate indicate the presence, severity and control of childhood asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2007;37(9):1303–11. [DOI] [PubMed] [Google Scholar]
- 32.Ciebiada M, Gorski P, Antczak A. Evaluation of eicosanoids in nasal lavage as biomarkers of inflammation in patients with allergic rhinitis. Arch Med Sci. 2014;10(6):1123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrianjafimasy M, Zerimech F, Akiki Z, Huyvaert H, Le Moual N, Siroux V, et al. Oxidative stress biomarkers and asthma characteristics in adults of the EGEA study. The European respiratory journal. 2017;50(6). [DOI] [PubMed] [Google Scholar]
- 34.Janssen LJ. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1067–82. [DOI] [PubMed] [Google Scholar]
- 35.Kitano S, Hisatomi H, Hibi N, Kawano K, Harada S. Improved method of plasma 8-Isoprostane measurement and association analyses with habitual drinking and smoking. World J Gastroenterol. 2006;12(36):5846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chehne F, Oguogho A, Lupattelli G, Budinsky AC, Palumbo B, Sinzinger H. Increase of isoprostane 8-epi-PGF(2alpha)after restarting smoking. Prostaglandins Leukot Essent Fatty Acids. 2001;64(6):307–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.