Abstract
Objective.
To assess the impact of rural-urban residence on children with obstructive sleep-disordered breathing (SDB) who were candidates for tonsillectomy with or without adenoidectomy (TA).
Study Design.
Retrospective cohort study.
Setting.
Tertiary children’s hospital.
Methods.
A cohort of otherwise healthy children aged 2 to 18 years with a diagnosis of obstructive SDB between April 2016 and December 2018 who were recommended TA were included. Rural-urban designation was defined by ZIP code approximation of rural-urban commuting area codes. The main outcome was association of rurality with time to TA and loss to follow-up using Cox and logistic regression analyses.
Results.
In total, 213 patients were included (mean age 6 ± 2.9 years, 117 [55%] male, 69 [32%] rural dwelling). Rural-dwelling children were more often insured by Medicaid than private insurance (P < .001) and had a median driving distance of 74.8 vs 16.8 miles (P < .001) compared to urban-dwelling patients. The majority (94.9%) eventually underwent recommended TA once evaluated by an otolaryngologist. Multivariable logistic regression analysis did not reveal any significant predictors for loss to follow-up in receiving TA. Cox regression analysis that adjusted for age, sex, insurance, and race showed that rural-dwelling patients had a 30% reduction in receipt of TA over time as compared to urban-dwelling patients (hazard ratio, 0.7; 95% CI, 0.50-0.99).
Conclusion.
Rural-dwelling patients experienced longer wait times and driving distance to TA. This study suggests that rurality should be considered a potential barrier to surgical intervention and highlights the need to further investigate geographic access as an important determinant of care in pediatric SDB.
Keywords: tonsillectomy, rurality, health care disparity, travel, sleep-disordered breathing
Obstructive sleep-disordered breathing (SDB) is a prevalent condition among children and encompasses a spectrum of respiratory disorders, from primary snoring to severe obstructive sleep apnea (OSA).1 Negative sequelae of SDB in children may include excessive daytime sleepiness, cognitive deficits, behavioral problems, and poor academic performance.1-3 Generally, the first-line intervention is tonsillectomy with or without adenoidectomy (TA), constituting the most common major surgery performed in patients younger than 15 years in the United States.4-6 It is well established that children with limited resources and African American children are at higher risk for SDB.7-15 However, a growing body of literature suggests that these groups do not attain equitable rates of surgery.16-20 This observation is particularly concerning because resource-limited children are at baseline risk of poor developmental and psychosocial outcomes.21,22 Moreover, untreated SDB exacerbates other comorbid conditions that are more prevalent among vulnerable groups such as obesity, asthma, smoke exposure, poor school performance, and attention-deficit/hyperactivity disorder.23-27
Children who suffer most from SDB disparities may also encounter additional health disparities. Poverty has been positively associated with greater distances to pediatric subspecialty care.28 The lack of geographic access to health care among underserved rural populations has remained virtually unexplored in pediatric SDB. Rurality is most commonly defined by population density, proximity to urban health centers, and commuting patterns, and it often comprises communities with limited financial resources.29 Due to the relatively low supply of pediatric subspecialty providers in nonmetropolitan areas, families living in rural areas often face unique geographic barriers. Lengthy travel distances for surgical evaluation by an otolaryngologist impose transportation challenges and lost time from work for guardians, which can compound existing financial burdens. Additional barriers to care specific to rural residence may include decreased medial literacy, trust in health care providers, and lack of health care infrastructure, all of which may negatively affect timeliness to surgical treatment for pediatric SDB.
Inequities in attainment of TA have been documented separately in urban16,17 and rural19 settings, yet a comparison of differences in surgical attainment between rural and nonrural settings in the care of SDB has not been well delineated. The purpose of this study is to investigate geographic disparities for medically noncomplex children with SDB who were recommended for surgical intervention with TA. Specifically, we aim to investigate the impact of rural residence on timeliness of TA compared to children living in urban communities. Second, we aim to investigate whether guardians of children who travel greater distances for treatment experience delays in receiving surgery for SDB compared to patients living in closer proximity.
Materials and Methods
This study was approved by the institutional review board at the Medical University of South Carolina (Pro#00088163). A retrospective chart review was performed at our tertiary children’s hospital to identify children with SDB from April 2016 to December 2018. Children were identified using the International Classification of Diseases, 10th Revision (ICD-10) codes for SDB (R0683, G4733, G4730, G478, J351, J353). Medical charts were reviewed and children ages 2 to 18 years with obstructive SDB who were recommended for surgical intervention with TA were included. Recommendation for surgery was determined from manual chart review of clinical notes in which the surgeon specifically cited TA as the next step in management for the child’s obstructive SDB. We extracted demographic information for each patient, including age, sex, race/ethnicity, insurance type, comorbidities, rural-urban status, and driving distance to the medical center. Race/ethnicity was categorized into non-Hispanic black, non-Hispanic white, and other group. Exclusion criteria were prior tonsillectomy and infectious indications of TA such as tonsillitis. To ensure a standardized study population of non–medically complex subjects, we excluded children who had other respiratory and cardiac comorbidities and patients who would otherwise warrant polysomnography (PSG) per the American Academy of Otolaryngology–Head and Neck Surgery guidelines (age less than 2 years, craniofacial anomalies, trisomy 21, neuromuscular disorders, mucopolysaccharidosis, sickle cell disease, and obesity).30 Given that most children who undergo TA for SDB do so without polysomnographic evidence31,32 and to keep the study population standardized to medically noncomplex patients who would otherwise be high utilizers of medical care, we did not include children who underwent preoperative PSG. Research Electronic Data Capture, a secure, web-based software platform, was used to collect and manage study data.33
Patients were followed for 12 months after the date of the surgical recommendation. Geographic distance was calculated using Google Maps to identify the travel distance from the patient’s residence to the treating hospital according to the fastest commuting route. Rural-urban status was defined using the University of Washington’s ZIP code approximation, designating a US Department of Agriculture’s rural-urban commuting area (RUCA) code to each ZIP code.34 RUCA is a 10-point scale of rural and urban status based on daily commuting patterns and population densities, which is then grouped into urban and rural categories using a strategy developed by the Rural Health Reference Center.34 Any areas that were not rural by this definition were stratified into the urban group. The main outcomes were (1) time to TA from date of initial recommendation by an otolaryngologist and (2) likelihood of loss to follow-up.
Statistical Analysis
Summary statistics are presented as means (SDs) for continuous independent variables and frequencies (percentages) for categorical independent variables. All continuous variables were assessed for normality using the Shapiro-Wilk test and were summarized by mean ± standard deviation or median (interquartile range [IQR]) when appropriate. Kaplan-Meier cumulative incidence curves were created to illustrate the associations between predictors (ie, rurality, distance to hospital) and time to surgical treatment. Multivariate Cox regression analysis was then performed to identify independent factors associated with time to surgical treatment. This model was adjusted for the following variables: age, race, sex, insurance status, and rurality. Results of the model were given as hazard ratios (HRs) and corresponding 95% CIs. Multivariate logistic regression analysis was also performed to identify factors associated with loss to follow-up and similarly included the aforementioned variables. P values <.05 were considered to indicate a significant difference for all statistical tests, all of which were performed using R software (version 3.6.0).
Results
A total of 399 patients with a diagnosis of SDB who were recommended TA were initially identified. From this, 184 children were excluded due to the presence of comorbidities or having received a preoperative PSG. Therefore, the analyses included a total of 213 children who met inclusion criteria. There were 117 (55%) male patients; the mean age was 6.0 years with an SD of 2.9 years. Table 1 highlights differences between patients who resided in rural vs urban areas. The cohort consisted of non-Hispanic white (57%), non-Hispanic black (27%), and other race (16%), which comprised Asian and Hispanic race/ethnicity. There was a greater proportion of African American children who lived in rural areas (35%) compared to urban (24%), but this difference did not meet statistical significance (P = .053).
Table 1.
Clinical and Demographic Overview of Rural vs Urban-Dwelling Patients.
| Variable | Total (N = 213a) | Rural (n = 69) | Nonrural (n = 144) | P valueb |
|---|---|---|---|---|
| Age, mean (SD), y | 6 (2.9) | 6.1 (2.9) | 6.0 (3.0) | .77 |
| Distance to hospital, miles (IQR range) | 23.3 (12.2-74.6) | 74.8 (68.3-88.8) | 16.8 (8.3-24.5) | <.001 |
| Sex, No. (%) | .98 | |||
| Female | 96 (45) | 3l (45) | 65 (45) | |
| Male | 117 (55) | 38 (55) | 79 (55) | |
| Race, No. (%) | .053 | |||
| White | 121 (57) | 3l (45) | 90 (62) | |
| African American | 58 (27) | 24 (35) | 34 (24) | |
| Other | 34 (16) | 14 (20) | 20 (14) | |
| Insurance, No. (%) | <.001 | |||
| Private | 85 (40) | 12 (17) | 73 (51) | |
| Medicaid | 107 (50) | 51 (74) | 56 (39) | |
| Other | 21 (10) | 6 (9) | 15 (10) | |
| LTFU, No. (%) | ||||
| Yes | 11 (5) | 5 (7) | 6 (4) | .34 |
| No | 202 (95) | 64 (93) | 138 (96) |
Abbreviations: IQR, interquartile range; LTFU, lost to follow-up.
Two patients were missing rurality data; therefore, N = 213.
P values were calculated using Wilcoxon rank-sum tests for continuous variables and χ2 tests for categorical variables.
Approximately half of the cohort was insured by Medicaid (50%), while 40% were privately insured, and 10% had another form of insurance coverage. Rural patients were also significantly less likely to have private health insurance and more likely to be insured by Medicaid (P < .001). When examining insurance type within each racial/ethnic group, 91% of African American children were insured by Medicaid, compared to 73% other and 26% white counterparts (P < .0001).
There was a significant difference in the median distance from the patient’s residence to the treating hospital, with patients from rural residences having to drive 74.8 (IQR, 68.3-88.8) miles one way to the hospital compared to nonrural patients, who only needed to drive 16.8 (IQR, 8.3-24.5) miles (P < .001). Overall, a high frequency of patients underwent surgery after recommendation by an otolaryngologist, with only a 5.1% rate of loss to follow-up.
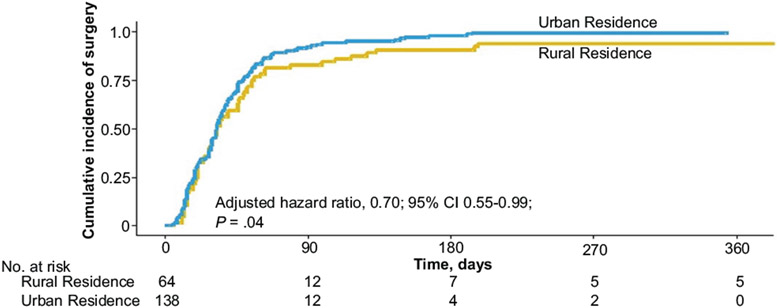
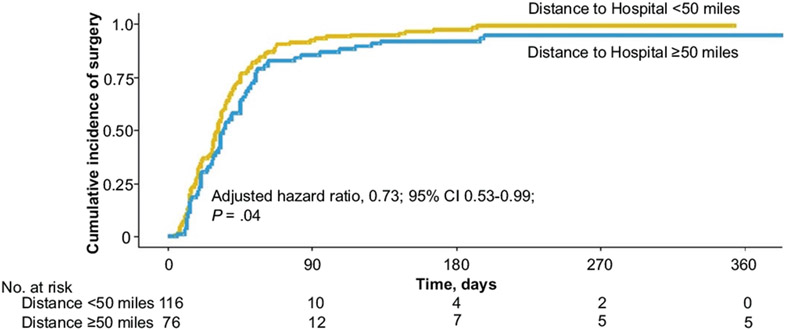
Cumulative incidence curves based on a Kaplan-Meier approach illustrate the fact that urban patients tend to receive surgery more quickly than rural patients (Figure 1). Figure 2 demonstrates that patients residing <50 miles from a hospital receive surgery more quickly than do patients living >50 miles from the hospital. For example, at 90 days postsurgical recommendation, 92% of the urban patients and 92% of the patients residing <50 miles from their treating hospital had had their surgery, compared to 83% of the rural patients and 85% of patients residing >50 miles from their treating hospital, respectively.
Figure 1.
Cumulative incidence curves reflecting the proportion of patients who underwent tonsillectomy, stratified by rural vs urban residency. The hazard ratio reported adjusted for age, sex, insurance status, and race.
Figure 2.
Cumulative incidence curves reflecting the proportion of patients who underwent tonsillectomy, stratified by distance from the patient’s residence to the hospital. The hazard ratio reported adjusted for age, sex, insurance status, and race.
The results of the multivariate Cox regression modeling that analyzed time to surgery and adjusted for age, sex, insurance, and race are listed in Table 2. Even after adjusting for racial and insurance differences, rural patients were found to have a 30% lower rate of surgery over time compared to urban-dwelling patients (adjusted HR [aHR], 0.70; 95% CI, 0.55-0.99; P = .04). For each additional increment of 1 SD (2.9 years) from the mean age (6 years), the rate of surgery decreased by 15% (aHR, 0.85; 95% CI, 0.74-0.98; P = .02). No other variables were found to be significantly associated with time to surgery. The multivariate logistic regression model, analyzing likelihood of receiving surgery at our institution vs being lost to follow-up after recommendation for surgery, indicated that none of the predictors (age, sex, race, rurality, and insurance) were significantly associated with loss to follow-up in this model.
Table 2.
Cox Regression Analysis to Assess Factors Associated With Time to Surgery.
| Variable | Adjusted hazard ratio | 95% CI | P value |
|---|---|---|---|
| Agea | 0.85 | 0.74-0.98 | .02 |
| Rurality | |||
| Nonrural | 1 [Reference] | ||
| Rural | 0.70 | 0.55-0.99 | .04 |
| Sex | |||
| Female | 1 [Reference] | ||
| Male | 1.16 | 0.87-1.55 | .31 |
| Insurance | |||
| Medicaid | 1 [Reference] | ||
| Private insurance | 0.85 | 0.57-1.25 | .40 |
| Other insurance | 1.30 | 0.78-2.16 | .31 |
| Race | |||
| White | 1 [Reference] | ||
| Nonwhite | 1.01 | 0.72-1.43 | .94 |
Each unit increase in age corresponded to a 1 SD (2.9 years) increase above the mean age.
Discussion
This study of a racially and socioeconomically diverse sample of patients with obstructive SDB raises concern for equitable and timely surgical access for rural children compared to their urban peers. The results also indicate that demographic differences exist between rural and urban dwellers, with children of families living in rural areas more likely to be insured by Medicaid compared to private health insurance. In addition, there was a 58-mile difference in median driving distance to the treating hospital between urban and rural groups. Our findings show that even after accounting for important covariates, rural status significantly affected the timeliness of receiving TA for children who were recommended surgical intervention, suggesting that geographic barriers may play an important role in access to surgical treatment for SDB independent of race or insurance type. Consequently, rurality warrants further investigation as a social determinant that may contribute to disparities for this common childhood condition.
Previous institutional studies have demonstrated barriers to care in either exclusively urban or rural settings. For example, white children were more likely to receive TA than African American children in rural Mississippi.19 In a study set in a metropolitan location, Boss et al16 found delays in PSG and tonsillectomy for children with public insurance compared to private insurance among a cohort of urban children. Our findings are novel in that we identified differences in timing of TA between rural and urban-dwelling children with SDB. From a demographic standpoint, rural children in this study were significantly more likely to be insured by Medicaid, which among this otherwise healthy group of patients is likely a proxy for low-income status given the state’s Medicaid eligibility criteria of 2.1 times the federal poverty level.35 Our study was conducted in South Carolina, a state that exemplifies a diverse, underserved rural population. In 2018, 21.8% of residents living in rural areas in South Carolina were considered low income compared to 14.0% in urban areas of the state.36 Nationally, all extreme poverty counties were located in rural communities, with the southeastern states representing the greatest rural poverty rates of at least 20%.37 Childhood poverty rates in nonmetropolitan areas have historically been higher, partly due to higher unemployment rates and a greater share of low-wage jobs.38 Low-income levels may preclude one’s ability to access health care and can also create delays in treatment due to concerns regarding affordability of care.39
In addition to limited financial resources, rural residency is associated with an educational achievement gap. Children living in rural areas are more likely to have younger and less educated parents compared to metropolitan-dwelling children.38 For example, 17.5% of South Carolina residents in rural areas have a college degree compared to 29.2% of urban residents.36 It has been demonstrated that lower education levels correlate with decreased health literacy and may lead to poorer awareness of health conditions, management, and engagement in the decision-making process with health care professionals.40,41 Financial strains coupled with decreased health literacy may cause barriers in interactions with health care teams and contribute to delays in treatment. In fact, individuals from rural communities have been shown to be less willing to seek out health care based on their health literacy level and experience more difficulties navigating the health care system.42,43 This decreased willingness to seek out health care may be explained by decreased trust in health care providers, leaving rural patients less apt to establish strong physician-patient relationships with resultant poorer patient outcomes.44 Thus, an emphasis on establishing rapport with rural patients (eg, by adhering to a patient-centered model of health care delivery) may be paramount to increasing trust and overall communication between physician and families.45
Rural settings have a sparser distribution of medical practices and health care services that can lead to differential utilization, spending, and geographic distribution of health care centers and providers.29 Studies have shown that pediatricians in rural settings have difficulty coordinating timely referral to surgical subspecialty consultations compared to their urban counterparts.46,47 This may be in part due to the fact that approximately 66% of all counties in the United States lack a practicing otolaryngologist, and only 4 total practicing otolaryngologists served counties with less than 10,000 people.48 The decreased supply of local subspecialists requires that rural residents travel prolonged distances, as exemplified by our findings of significant differences between median one-way travel distances for urban-dwelling (17 miles) compared to rural-dwelling (75 miles) families. Our results of less timely surgical intervention for children living in rural areas may be explained by these lengthy distances to treatment facilities, time required for the commute, lack of transportation, and incurring financial strain from cost of travel and missed work.49,50
Geographic inaccessibility, demographic composition, health literacy with resulting health behaviors, and lack of health care infrastructure are all implicated in access to care for rural populations.51 Possible solutions include electronic consultation platforms and telemedicine that may serve to address health care disparities in rural communities by allowing for more accessible consultations with otolaryngologists.52 However, efforts to diminish the digital divide between rural and urban communities, as evidenced by decreased access to broadband services in remote areas, will be necessary. In addition to the development and expansion of broadband infrastructure, it will be critical to increase affordability of electronic telehealth-compatible devices for underserved rural populations.53
This study has limitations that are inherent to a retrospective chart review, including coding and billing methods. Given the inclusion criteria of otherwise healthy children who were recommended TA without a formal PSG, the findings do not apply to medically complex children. One prior study showed high rates of loss to follow-up after referrals from urban primary care providers for PSG and TA,54 but we were not able to investigate this question as referrals to our clinics are from outside of our institution and were not reliably captured in the electronic medical record. Therefore, information regarding loss to follow-up from referrals could not be evaluated, and rural barriers to SDB care are likely underestimated in this study since we did not capture families that were referred but ultimately did not seek evaluation. Currently, there is no clinical consensus as to what period of time defines a delay in tonsillectomy for pediatric SDB. In addition, the patients in this study constituted one geographic region of the country, and therefore results may not be generalizable to other rural regions in the United States. Last, we could not determine from this study whether delays in care arose from parental decision making, trust in health care providers, challenges within the health care infrastructure, public policy, or a combination of these factors.
Conclusion
Rural children and their families likely face multiple barriers to care in the treatment of SDB, including limited resources and longer travel distances. We found lower rates of surgery over time among rural-dwelling patients compared to urban-dwelling patients after accounting for age, sex, race, and insurance coverage. The need to provide equitable specialty care to underserved rural children is a high priority. Future studies are warranted to further investigate the barriers to care for rural children with SDB and potential interventions to mitigate these disparities.
Funding source:
This project was funded in part by grants from the National Institutes of Health (NCATS grant UL1-TR001450, NIAMS grant P30-AR072582, NIGMS grant U54-GM104941). Phayvanh P. Pecha is supported through the BSM PRIDE grant R25HL105444 and the AAO-HNS Health Services Research Grant.
Footnotes
Competing interests: None.
These data were presented in a virtual poster presentation at the 2020 annual meeting of the American Society of Pediatric Otolaryngology, May 15, 2020.
References
- 1.Katz ES, D’Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suratt PM, Peruggia M, D’Andrea L, et al. Cognitive function and behavior of children with adenotonsillar hypertrophy suspected of having obstructive sleep-disordered breathing. Pediatrics. 2006;118(3):e771–e781. [DOI] [PubMed] [Google Scholar]
- 3.Bourke RS, Anderson V, Yang JS, et al. Neurobehavioral function is impaired in children with all severities of sleep disordered breathing. Sleep Med. 2011;12(3):222–229. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell RB, Archer SM, Ishman SL, et al. Clinical practice guideline: tonsillectomy in children (update). Otolaryngol Head Neck Surg. 2019;160(1)(suppl):S1–S42. [DOI] [PubMed] [Google Scholar]
- 5.Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017;(102):1–15. [PubMed] [Google Scholar]
- 6.Redline S, Amin R, Beebe D, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5, pt 1):1527–1532. [DOI] [PubMed] [Google Scholar]
- 8.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997; 155(1):186–192. [DOI] [PubMed] [Google Scholar]
- 9.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003; 142(4):383–389. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein NA, Abramowitz T, Weedon J, Koliskor B, Turner S, Taioli E. Racial/ethnic differences in the prevalence of snoring and sleep disordered breathing in young children. J Clin Sleep Med. 2011;7(2):163–171. [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin JL, Babar SI, Kaemingk KL, et al. Symptoms related to sleep-disordered breathing in white and Hispanic children: the Tucson Children’s Assessment of Sleep Apnea Study. Chest. 2003;124(1):196–203. [DOI] [PubMed] [Google Scholar]
- 12.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149(3):342–347. [DOI] [PubMed] [Google Scholar]
- 13.Pagel JF, Forister N, Kwiatkowki C. Adolescent sleep disturbance and school performance: the confounding variable of socioeconomics. J Clin Sleep Med. 2007;3(1):19–23. [PubMed] [Google Scholar]
- 14.Quan SF, Goodwin JL, Babar SI, et al. Sleep architecture in normal Caucasian and Hispanic children aged 6-11 years recorded during unattended home polysomnography: experience from the Tucson Children’s Assessment of Sleep Apnea Study (TuCASA). Sleep Med. 2003;4(1):13–19. [DOI] [PubMed] [Google Scholar]
- 15.Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. J Pediatr. 2011;158(5):789–795.e781. [DOI] [PubMed] [Google Scholar]
- 16.Boss EF, Benke JR, Tunkel DE, Ishman SL, Bridges JFP, Kim JM. Public insurance and timing of polysomnography and surgical care for children with sleep-disordered breathing. JAMA Otolaryngol Head Neck Surg. 2015;141(2):106–111. [DOI] [PubMed] [Google Scholar]
- 17.Boss EF, Smith DF, Ishman SL. Racial/ethnic and socioeconomic disparities in the diagnosis and treatment of sleep-disordered breathing in children. Int J Pediatr Otorhinolaryngol. 2011;75(3):299–307. [DOI] [PubMed] [Google Scholar]
- 18.Heller MA, Lind MN, Boss EF, Cooper JN. Differences in tonsillectomy use by race/ethnicity and type of health insurance before and after the 2011 tonsillectomy clinical practice guidelines. J Pediatr. 2020;220:116–124.e113. [DOI] [PubMed] [Google Scholar]
- 19.Kum-Nji P, Mangrem CL, Wells PJ, Klesges LM, Herrod HG. Black/white differential use of health services by young children in a rural Mississippi community. South Med J. 2006;99(9):957–962. [DOI] [PubMed] [Google Scholar]
- 20.Morton S, Rosen C, Larkin E, Tishler P, Aylor J, Redline S. Predictors of sleep-disordered breathing in children with a history of tonsillectomy and/or adenoidectomy. Sleep. 2001;24(7):823–829. [DOI] [PubMed] [Google Scholar]
- 21.Poverty and child health in the United States. Pediatrics. 2016; 137(4):e20160339. [DOI] [PubMed] [Google Scholar]
- 22.Gupta RP-S, de Wit ML, McKeown D. The impact of poverty on the current and future health status of children. Paediatr Child Health. 2007;12(8):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery-Downs HE, Gozal D. Sleep habits and risk factors for sleep-disordered breathing in infants and young toddlers in Louisville, Kentucky. Sleep Med. 2006;7(3):211–219. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Andrew M, Gebremariam A, Lumeng JC, Lee JM. Longitudinal associations between poverty and obesity from birth through adolescence. Am J Public Health. 2014;104(5):e70–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell AE, Ford T, Williams R, Russell G. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440–458. [DOI] [PubMed] [Google Scholar]
- 26.Singh GK, Siahpush M, Kogan MD. Disparities in children’s exposure to environmental tobacco smoke in the United States, 2007. Pediatrics. 2010;126(1):4–13. [DOI] [PubMed] [Google Scholar]
- 27.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28(7):885–890. [DOI] [PubMed] [Google Scholar]
- 28.Mayer ML. Disparities in geographic access to pediatric subspecialty care. Matern Child Health J. 2008;12(5):624–632. [DOI] [PubMed] [Google Scholar]
- 29.Hartley D. Rural health disparities, population health, and rural culture. Am J Public Health. 2004;94(10):1675–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roland PS, Rosenfeld RM, Brooks LJ, et al. Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1)(suppl):S1–S15. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: survey of current practice. Laryngoscope. 2006;116(6):956–958. [DOI] [PubMed] [Google Scholar]
- 32.Friedman NR, Perkins JN, McNair B, Mitchell RB. Current practice patterns for sleep-disordered breathing in children. Laryngoscope. 2013;123(4):1055–1058. [DOI] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WWAMI Rural Health Research Center. ZIP code RUCA approximation. Accessed July 15, 2020. https://depts.washington.edu/uwruca/ruca-approx.php
- 35.Kaiser Family Foundation. Medicaid and CHIP eligibility, enrollment, renewal, and cost sharing policies as of January 2017: findings from a 50-state survey. Published 2017. Accessed October 10, 2020. https://www.kff.org/medicaid/report/medicaid-and-chip-eligibility-enrollment-renewal-and-cost-sharing-policies-as-of-january-2017-findings-from-a-50-state-survey/
- 36.US Department of Agriculture Economic Research Service. State fact sheets: South Carolina. Published 2020. Accessed July 14, 2020. https://data.ers.usda.gov/reports.aspx?StateFIPS=45&StateName=South%20Carolina&ID=17854#Pca004512a01741768697bd5f29a4f0ce_2_oHit0
- 37.Housing Assistance Council. Poverty in rural America. Published 2012. Accessed July 15, 2020. http://www.ruralhome.org/storage/research_notes/rrn_poverty.pdf
- 38.US Department of Agriculture Economic Research Service. Rural children at a glance. Published 2005. Accessed July 15, 2020. https://www.ers.usda.gov/webdocs/publications/43664/18708_eib1_2_.pdf?v=9618.8
- 39.Samuelsen H, Tersbøl BP, Mbuyita SS. Do health systems delay the treatment of poor children? A qualitative study of child deaths in rural Tanzania. BMC Health Serv Res. 2013;13(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gazmararian JA, Williams MV, Peel J, Baker DW. Health literacy and knowledge of chronic disease. Patient Educ Counsel. 2003;51(3):267–275. [DOI] [PubMed] [Google Scholar]
- 41.Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med. 2009;69(12):1805–1812. [DOI] [PubMed] [Google Scholar]
- 42.Spleen AM, Lengerich EJ, Camacho FT, Vanderpool RC. Health care avoidance among rural populations: results from a nationally representative survey. J Rural Health. 2014;30(1): 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood FG. Health literacy in a rural clinic. Online J Rural Nurs Health Care. 2012;5(1):9–18. [Google Scholar]
- 44.Birkhäuer J, Gaab J, Kossowsky J, et al. Trust in the health care professional and health outcome: a meta-analysis. PLoS One. 2017;12(2):e0170988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espinel AG, Shah RK, Beach MC, Boss EF. What parents say about their child’s surgeon: parent-reported experiences with pediatric surgical physicians. JAMA Otolaryngol Head Neck Surg. 2014;140(5):397–402. [DOI] [PubMed] [Google Scholar]
- 46.Pletcher BA, Rimsza ME, Cull WL, Shipman SA, Shugerman RP, O’Connor KG. Primary care pediatricians’ satisfaction with subspecialty care, perceived supply, and barriers to care. J Pediatr. 2010;156(6):1011–1015.e1011. [DOI] [PubMed] [Google Scholar]
- 47.Brems C, Johnson ME, Warner TD, Roberts LW. Barriers to healthcare as reported by rural and urban interprofessional providers. J Interprofessional Care. 2006;20(2):105–118. [DOI] [PubMed] [Google Scholar]
- 48.Vickery TW, Weterings R, Cabrera-Muffly C. Geographic distribution of otolaryngologists in the United States. Ear Nose Throat J. 2016;95(6):218–223. [PubMed] [Google Scholar]
- 49.Arcury TA, Gesler WM, Preisser JS, Sherman J, Spencer J, Perin J. The effects of geography and spatial behavior on health care utilization among the residents of a rural region. Health Serv Res. 2005;40(1):135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocque GB, Williams CP, Miller HD, et al. Impact of travel time on health care costs and resource use by phase of care for older patients with cancer. J Clin Oncol. 2019;37(22): 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McEvoy CS, Ross-Li D, Held JM, et al. Geographic distance to pediatric surgical care within the continental United States. J Pediatr Surg. 2019;54(6):1112–1117. [DOI] [PubMed] [Google Scholar]
- 52.Marcin JP, Shaikh U, Steinhorn RH. Addressing health disparities in rural communities using telehealth. Pediatr Res. 2016; 79(1):169–176. [DOI] [PubMed] [Google Scholar]
- 53.Lori AD, Lamie RD, Brian EW. The struggle for broadband in rural America. Choices. 2010;25(4):1–8. [Google Scholar]
- 54.Harris VC, Links AR, Kim JM, Walsh J, Tunkel DE, Boss EF. Follow-up and time to treatment in an urban cohort of children with sleep-disordered breathing. Otolaryngol Head Neck Surg. 2018;159(2):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]