Abstract
Objectives
Epilepsy is the most prevalent chronic neurologic disorder in children. One-third of patients with epilepsy do not respond to antiepileptic drugs. This condition is known as intractable epilepsy. Previous studies have shown the beneficial effects of curcumin in the treatment of epilepsy. There are no randomized controlled clinical trials assessing the use of curcumin in epilepsy. This study aimed to evaluate the effects of nanomicelle curcumin on intractable pediatric epilepsy.
Materials & Methods
This double-blinded randomized crossover clinical trial was performed by a consecutive sampling to select 22 patients with intractable epilepsy divided into two groups. Patients received a daily dose of 4 mg/kg of curcumin or placebo as add-on therapy for 4 weeks. After a 2-week washout period, the treatment was replaced, and the new treatment was given for another 4 weeks. The SPSS software version 16 was used for statistical analysis. The study was approved by the Ethics Committee of Mashhad University of Medical Sciences, Iran.
Results
A total of 22 children were enrolled in this study, 11 of which were boys. The mean age of the patients was 4.28±5 years. A female patient taking a placebo was excluded in the first week of the trial due to parental dissatisfaction. The most common type of seizure among our patients was a generalized myoclonic seizure (42.9%). The mean number of seizure attacks among the subjects was 68.76±69.26 pre-intervention and 39.85±39.41at the end of the intervention, which represents a statistically significant difference (P=0.01).
Conclusion
Nanomicelle curcumin reduced the number of seizures significantly. Our results imply that curcumin treatment can help treat patients with intractable pediatric epilepsy.
Key Words: Intractable epilepsy, Nanomicelle curcumin, Pediatric, Seizures
Introduction
Approximately 70 million people are affected by epilepsy worldwide (1). Epilepsy is commonly diagnosed in younger ages and above 60 years (2). Various antiepileptic drugs (AEDs) are available nowadays, and these medications control nearly 70% of seizures. However, the other one-third of patients are resistant to AEDs and are known as intractable epilepsy (3). Although some patients are candidates for resective surgery, there is no appropriate treatment option available for most patients, and those who undergo surgery experience significant side effects (3). Therefore, novel therapeutic strategies are required. Herbal treatment can be a good alternative due to its easy accessibility, low cost, and fewer side effects.
Curcumin is the main active ingredient of Turmeric (Curcuma Longa), which is an Indian dietary spice (4). It has been shown to positively affect various conditions, including epilepsy (5-7). However, the exact antiepileptic mechanism of curcumin is not well understood. It is known that curcumin has anti-inflammatory and antioxidant properties. On the other hand, oxidative stress and neuroinflammation play dominant roles in the pathophysiology of epilepsy. As a result, curcumin properties may be beneficial for the treatment of epilepsy (8, 9). The epigenetic mechanism of regulating miRNA expression is another pathway that explains the long-term antiepileptic effects of this remedy (10).
Curcumin has shown anti-seizure potential in various animal models. However, the low bioavailability of curcumin in the brain limits its clinical use (11). Curcumin has low bioavailability, and it seems that increasing its bioavailability can lead to better therapeutic results (12, 13). In a recent study by Bertoncello et al., micronized-curcumin, which has a significantly higher bioavailability than curcumin, reduced the number of seizure attacks and slowed seizure progression, similar to valproate, which is a common AED (14). This study used nano-curcumin (SinaCurcumin®), a nanomicelle containing curcumin. Each soft gel of SinaCurcumin® contains 80 mg of curcumin as a nanomicelle and has five times higher bioavailability than curcumin powder (15).
Curcumin has not shown any interactions with other medications in previous studies. Therefore, it is considered safe as add-on therapy. Nevertheless, no randomized controlled clinical trial has assessed the effect of curcumin in patients with epileptic seizures (11). In the present pilot randomized double-blinded controlled clinical trial, we aimed to evaluate the effects of add-on nano-curcumin therapy on intractable epilepsy symptoms in pediatric patients and the serum levels of inflammatory cytokines.
Materials & Methods
Patients
This double-blinded randomized crossover clinical trial was conducted through convenience sampling. Considering the low prevalence of intractable epilepsy, this study being a pilot, and the results of a previous study conducted by these researchers regarding the effects of curcumin on pediatric myoclonic epilepsy, a minimum sample size of 11 patients for each group was selected. Based on the clinical history, electrodiagnostic, and paraclinical findings, patients with intractable epilepsy, including generalized myoclonic, generalized tonic, infantile spasms, generalized tonic-clonic, and complex partial seizures, were selected. The subjects were included in the study after assessment for eligibility based on the inclusion and exclusion criteria. If the physician doubted the diagnosis, the parents were asked to record their child`s seizures.
Patients aged 3 months to 16 years experiencing at least four seizures during the last month and taking anticonvulsant medications for at least one month were included. Patients with curable seizures, epilepsy due to progressive or neurodegenerative brain disorders, a history of status epilepsy within the past 3 months, a history of pseudoseizures, multiple and uncountable seizures, a history of exacerbating seizures, a cardiovascular, liver, or kidney disorder, and patients who were not cooperative in taking the medications were excluded.
Procedure
The study was conducted at the Pediatric Neurology Clinic of Ghaem Hospital, affiliated with Mashhad University of Medical Sciences, Mashhad, Iran. The patients were randomly allocated to two groups by simple randomization based on computer-generated random numbers. The medicine was a curcumin solution, which is a registered curcumin product (SinaCurcumin®) for oral use and has been developed in the Nanotechnology Research Center of the Mashhad University of Medical Sciences and marketed by "Exir Nano Sina" Company in Tehran, Iran (IRC:1228225765). Each drop contained 4 mg nanomicelle curcumin.
Sealed drug containers were used to conceal the treatments from the clinician, patients, and their parents. Drug or placebo containers were distinguished with codes and were not disclosed until the end of the study. The patients received a daily dose of 4 mg/kg of medication or placebo for four weeks. After a two-week washout interval, the treatments were crossed over, and the new treatment was given for another four weeks.
The primary outcome was the number of seizures. The number of seizures was recorded at the beginning and end of each treatment course (baseline and the end of weeks 4, 6, and 10). In addition, 5 cc of blood was obtained at the baseline and one week after each treatment course (baseline and the end of weeks 5 and 11) to evaluate the changes in inflammatory cytokines, including interleukin-1α (IL-1α), IL-1β, IL-2, IL-4, L-6, IL-8, IL-10, monocyte chemoattractant protein (MCP-1), tumor necrosis factor-α (TNF-α), epidermal growth factor (EGF), and interferon-γ (IFN-γ). Parents were asked to call the doctor immediately if the child showed any concerning symptoms, such as nausea, vomiting, exanthema, impaired consciousness, increased duration, or the number of seizures. If the duration or number of seizures augmented significantly, the medication was immediately discontinued. During the study, clinical signs were also monitored.
Statistical Analysis
The SPSS software version 16 was used for statistical analysis. Paired t-test and independent t-test were used to analyze the hypotheses. P-value<0.05 was considered statistically significant.
Ethical Issues
The parents of all patients signed informed consent. The study procedures were conducted following the Declaration of Helsinki and were approved by the Ethics Committee of Mashhad University of Medical Sciences. The study was registered in the Iranian Registry of Clinical Trials with the registration number IRCT20130811014330N7 (https://irct.ir/trial/35406).
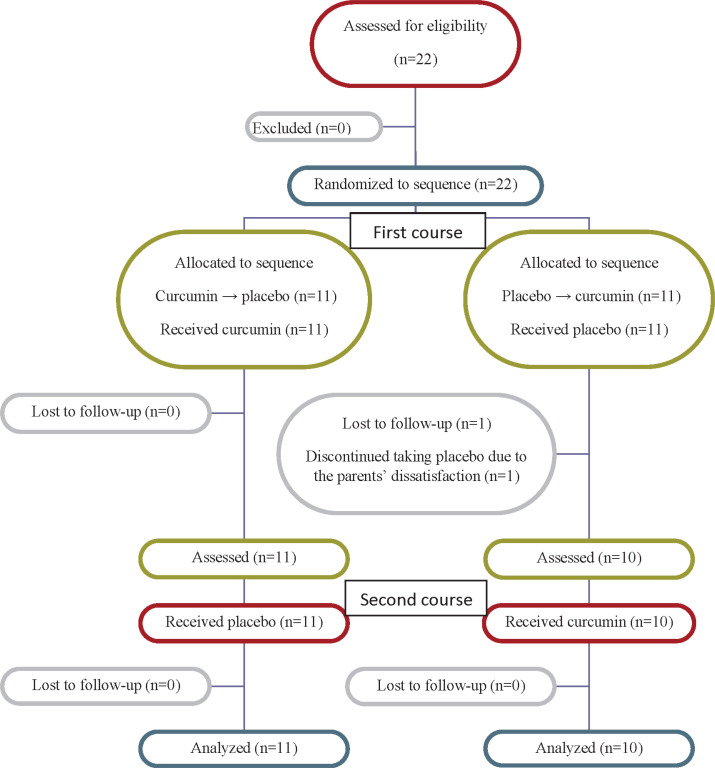
Figure 1.
CONSORT flow diagram for crossover trial (16)
Results
In this study, 22 children were enrolled, 11 of whom were boys. A 1.9-year-old girl receiving placebo was excluded from the study because she was diagnosed with pneumonia in the first week of the trial. Her family decided to stop the treatment. She had developmental delay and myoclonic seizures induced by hypoglycemia since she was 3 days old. The most common type of seizure among our patients was a generalized myoclonic seizure (Table 1). The mean age of patients was 4.28±5 years, and the mean duration of epilepsy was 4.07±4.01 years. Five (23.8%) of our patients had a family history of seizures in their first-degree relatives, and 19 (90.5%) had developmental delay. The number of seizure attacks at the baseline and the end of intervention (after 10 weeks) in the study population had a range of 2-210 per week (mean: 68.76±69.26) and 0-130 (mean: 39.85±39.41), respectively. The latter difference was statistically significant (P=0.01).
Table 1.
Characteristics of patients
| Age (years) | Patients (%) |
|---|---|
| < 3 | 7 (33.3) |
| 3-6 | 8 (38.1) |
| >6 | 6 (28.6) |
| Gender | |
| Male | 11 |
| Female | 10 |
| Type of seizures | |
| Generalized myoclonic | 9 (42.9) |
| Generalized tonic | 8 (38.1) |
| Infantile spasms | 2 (9.5) |
| Generalized tonic-clonic | 1 (4.8) |
| Complex partial seizures | 1 (4.8) |
| Possible cause of seizures | |
| Asphyxia | 3 (14.3) |
| Febrile seizures | 3 (14.3) |
| Chromosomal anomalies | 1 (4.8) |
| Hypoglycemia | 1 (4.8) |
| Kernicterus | 1 (4.8) |
| TORCH syndrome | 1 (4.8) |
| Trauma | 1 (4.8) |
| Congenital hernia surgery | 1 (4.8) |
| Unknown | 9 (42.9) |
In the first group, which initially received curcumin, the mean number of seizure attacks after 4 weeks of treatment with curcumin dropped from 69.4±73.89 to 27.73±32.77, with a significant difference (P=0.016). In this group, the mean number of seizure attacks at the beginning and the end of the placebo period was not significantly different (P=0.41). Furthermore, the difference between the beginning and end of the whole 10-week study period was significant (P=0.02). In this group, after receiving curcumin, changes in the levels of IL-6, IL-8, MCP, TNF-α, and EGF were significant (P=0.01, 0.03, <0.001, <0.001, and <0.001, respectively). In the second course, after receiving the placebo, changes in the levels of TNF-α were significant (P<0.001). At the end of 10 weeks, changes in IL-6 MCP, TNF-α, and EGF were significant compared to the beginning of the research (P=0.02, <0.001, <0.001, and 0.01, respectively).
In the second group, which initially received the placebo, neither the placebo nor the curcumin treatment caused a significant difference (Table 2). In this group, alterations in the level of TNF-α were significant after receiving a placebo (P<0.001). In the second course, after receiving curcumin, changes in the concentrations of MCP and TNF-α were significant (P<0.001). This significance remained compared to the beginning and the end of the study (p-values: 0.03 and <0.001, respectively).
Table 2.
Changes in the number of seizure attacks
| First Course | Second Course | P -value 10 week | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | P -value | Week 6 | Week 10 | P -value | |||
|
Group 1,
(Curcumin → placebo) |
69.40±73.89 | 27.73±32.77 | 0.01 | Two-week washout period | 42.54±41.03 | 45.95±46.08 | 0.41 | 0.02 |
|
Group 2,
(Placebo → curcumin) |
40.83±31.73 | 28.00±21.54 | 0.08 | 30.16±22.45 | 28.66±22.18 | 0.55 | 0.42 | |
Table 3.
Changes in the concentration of measured cytokines and growth factors in each study group
| Cytokines | Baseline | After the first course | After the second course | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| Curcumin→placebo group | ||||||
| IL1-α | 0.55 (0.33) | 0.55 (0.18) | 0.49 (0.14) | 0.377 | 0.583 | 0.333 |
| IL1-β | 0.59 (1.61) | 0.58 (0.2) | 0.60 (0.39) | 0.075 | 0.378 | 0.113 |
| IL-2 | 3.19 (2.14) | 2.75 (0.8) | 2.75 (0.75) | 0.128 | 0.618 | 0.148 |
| IL-4 | 1.99 (0.72) | 1.72 (0.33) | 1.82 (0.45) | 0.264 | 0.666 | 0.19 |
| IL-6 | 5.28 (10.83) | 1.07 (0.98) | 1.15 (1.75) | 0.013 | 0.059 | 0.024 |
| IL-8 | 10.72 (24.3) | 4.78 (2.88) | 4.85 (2.06) | 0.031 | 0.129 | 0.023 |
| IL-10 | 0.75 (0.7) | 0.87 (0.44) | 1.03 (1.45) | 0.249 | 0.175 | 0.078 |
| MCP | 148.65 (98.74) | 23.63 (25.2) | 20.14 (14.52) | 0 | 0.271 | 0 |
| TNFα | 3.98 (0.83) | 2.27 (0.14) | 2.09 (0.11) | 0 | 0 | 0 |
| EGF | 67.50 (206.01) | 15.71 (21.24) | 21.41 (14.67) | 0.006 | 0.416 | 0.01 |
| IFN-y | 1.33 (1.87) | 0.44 (0.18) | 0.48 (0.55) | 0.088 | 0.695 | 0.144 |
| Placebo→curcumin group | ||||||
| IL1-α | 0.67 (1.34) | 0.49 (0.25) | 0.55 (0.2) | 0.592 | 0.702 | 0.603 |
| IL1-β | 0.76 (4.49) | 0.62 (0.65) | 0.74 (0.64) | 0.126 | 0.628 | 0.228 |
| IL-2 | 3.16 (17.37) | 3.57 (2.25) | 2.30 (0.99) | 0.259 | 0.824 | 0.271 |
| IL-4 | 1.94 (0.6) | 1.95 (0.67) | 1.79 (0.92) | 0.097 | 0.753 | 0.296 |
| IL-6 | 4.07 (4.88) | 1.38 (2.82) | 1.94 (0.89) | 0.106 | 0.804 | 0.057 |
| IL-8 | 4.95 (4.75) | 14.21 (22.29) | 4.65 (2.43) | 0.076 | 0.079 | 0.544 |
| IL-10 | 1.10 (1.69) | 081 (0.22) | 0.76 (0.4) | 0.268 | 0.588 | 0.445 |
| MCP-1 | 82.63 (39.47) | 157.75 (121.21) | 26.10 (29.78) | 0.162 | 0.005 | 0.032 |
| TNF-α | 4.32 (0.6) | 2.99 (0.2) | 2.10 (0.03) | 0 | 0 | 0 |
| EGF | 162.20 (180.59) | 43.22 (72.32) | 21.83 (22.83) | 0.238 | 0.118 | 0.053 |
| IFN-y | 0.55 (0.33) | 0.67 (1.06) | 0.58 (0.56) | 0.266 | 0.675 | 0.206 |
Values are expressed as median (interquartile range)
Serum cytokine levels are expressed as pg/ml
P1: P-value of the comparison between before and after the first course of treatment (baseline and week 5)
P2: P-value of the comparison between before and after the second course of treatment (weeks 5 and 11)
P2: P-value of the comparison between before and after the whole study period (baseline and week 11)
IL-1α: interleukin-1α, IL-1β: interleukin-1β, IL-2: interleukin-2, IL-4: interleukin-4, IL-6: interleukin-6, IL-8: interleukin-8, IL-10: interleukin-10, MCP1: monocyte chemoattractant protein, TNF-α: tumor necrosis factor-α, EGF: epidermal growth factor, IFN-γ: interferon-γ
Discussion
Our study assessed the effects of curcumin with a high bioavailability as an add-on therapy for intractable pediatric epilepsy. We found that nano-curcumin reduced the number of seizure attacks in patients with intractable epilepsy. This was the first randomized controlled trial assessing the effects of curcumin in patients with epilepsy. Other AEDs possess various health risks and can be a major source of disability and mortality. Moreover, these agents lead to the early discontinuation of treatment in a quarter of cases (17).
It has been shown that curcumin causes no critically adverse effects (18) and can improve the side effects of other AEDs (19), making it an ideal choice for treating epilepsy in cases that do not respond to current treatment options. We also found a significant decrease in the levels of two inflammatory cytokines after receiving nano-curcumin in both of our study groups. Furthermore, the concentration of three other cytokines declined after treatment in the first group. This can explain the physiological mechanism of curcumin in treating intractable epilepsy. Various inflammatory cytokines are over-expressed in seizure-generating areas of the brain in experimental models (9).
A review by Erfani et al. reported that 16 out of 17 preclinical studies showed the effectiveness of curcumin in treating epilepsy (18). Kaur et al. showed that curcumin significantly decreased pro-inflammatory cytokines in the hippocampus and cortex of rats after pentylenetetrazole-induced seizures. Overall, curcumin was effective for chronic epilepsy by attenuating glial activation (20). This result was consistent with our study. However, research conducted by Drion et al. found no significant association between curcumin and the frequency or duration of seizures in rats. The mentioned finding was probably due to the fast metabolization of curcumin in the blood. They found no trace of curcumin in the plasma 2 h after administering a high dose of curcumin (400 mg/kg) (21). We addressed this problem with nano-curcumin, which raises the bioavailability of curcumin by five times compared to simple powder. (4)
The benefits of curcumin have been reported for many disorders, such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, Huntington's disease, diabetes, and cardiovascular diseases (22). In a review by Tang et al., curcumin was suggested to be more influential than the current treatments for Alzheimer’s disease if the bioavailability issue was resolved (23). Curcumin has also been suggested as a potential treatment for cancers, such as pancreatic, lung, ovarian, oral, colorectal, and breast carcinomas and even melanoma (24). However, the low bioavailability of curcumin has limited its effectiveness as a treatment (25). Using nano-curcumin instead of simple curcumin powder could resolve this issue and open a new treatment path for numerous disorders.
Several studies have demonstrated the strong regulatory effect of curcumin on gene expression (26). Therefore, a washout period of two weeks should be considered a limitation of our study.
We recommend further studies with an extended period to a minimum of 21 days. The current investigation was a pilot study, and intractable epilepsy has a low prevalence. Therefore, another limitation of our study was its small sample size. Future studies with larger study groups are recommended. Although we found no detectable side effects for nano-curcumin, other studies with more extensive follow-ups could further assess the safety of this treatment. Moreover, nano-curcumin with much higher bioavailability than powder curcumin for other disorders can prove curcumin to be a better treatment than currently available.
As mentioned before, this was the first randomized controlled trial regarding the effects of curcumin on epilepsy which showed the potential of nano-curcumin in treating intractable epilepsy. If confirmed by future research, nano-curcumin can help people with epilepsy with fewer side effects and a wider efficiency range. It may also treat patients with other neurological disorders or cancer due to high bioavailability.
Authors' Contribution
Mina Erfani: Student doing her thesis on PBL. She contacted the patients and collected the data.
Farah Ashrafzadeh: Neurologist, corresponding author. Developed the study concept. Responsible for the decision to treat and how to treat the patients and involved in the drafting and revising of the manuscript.
Hamid Reza Rahimi: Preparing the medicine.
Seyed Ali Ebrahimi: Drafting and revising of the manuscript.
Keivan Kalali: drafting and revising of the manuscript.
Mehran Beiraghi-Toosi: Referred patients, involved in the drafting and revising the manuscript.
Elnaz Faraji Rad: Drafting and revising of the manuscript. Final manuscript approval for submission and publication.
All authors read and approved the final manuscript.
Conflict of interest
All authors declare no financial or personal relationships with other people or organizations that could inappropriately influence (bias) this work.
Acknowledgment
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (IRCT20130811014330N7).
References
- 1.Ezz HSA, Khadrawy YA, Noor NA. The neuroprotective effect of curcumin and Nigella sativa oil against oxidative stress in the pilocarpine model of epilepsy: a comparison with valproate. Neurochemical research. 2011;36(11):2195. doi: 10.1007/s11064-011-0544-9. [DOI] [PubMed] [Google Scholar]
- 2.Sander JW. The epidemiology of epilepsy revisited. Current opinion in neurology. 2003;16(2):165–70. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 3.Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. The Lancet Neurology. 2008;7(6):514–24. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- 4.Forozanfar F, Majeed M, Jamialahmad T, Sahebkar A. (e book) Part of the advances in exprimental medicine and bioloy, book series(PMISB, volum 1291) Curcumin: A review of its effects on epilepsy. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer research. 2003;23(1/A):363–98. [PubMed] [Google Scholar]
- 6.Eigner D, Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. Journal of ethnopharmacology. 1999;67(1):1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 7.Mohseni M, Sahebkar A, Askari Gh, Johnston Th, Alikiaii B, Bagherniya M. The clinical use of curcumin on neurological disorders: An updated systematc review of clinical trials. Phyti Res. 2021;35:6862–6882. doi: 10.1002/ptr.7273. [DOI] [PubMed] [Google Scholar]
- 8.Bavarsad K, Barreto GE, Hadizadeh MR, Sahebkar A. Protective effects of curcumin against ischemic-reperfusion injury in the nervous system. Mole Neuro. 2019;56:1391–1404. doi: 10.1007/s12035-018-1169-7. [DOI] [PubMed] [Google Scholar]
- 9.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain, behavior, and immunity. 2008;22(6):797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Smith AJ, Oertle J, Prato D. Multiple actions of curcumin including anticancer, anti-inflammatory, antimicrobial and enhancement via cyclodextrin. Journal of cancer therapy. 2015;6(03):257. [Google Scholar]
- 11.Dhir A. Curcumin in epilepsy disorders. Phytotherapy Research. 2018;32(10):1865–75. doi: 10.1002/ptr.6125. [DOI] [PubMed] [Google Scholar]
- 12.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Molecular pharmaceutics. 2007;4(6):807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 13.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) The Journal of Alternative & Complementary Medicine. 2003;9(1):161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 14.Bertoncello KT, Aguiar GPS, Oliveira JV, Siebel AM. Micronization potentiates curcumin’s anti-seizure effect and brings an important advance in epilepsy treatment. Scientific reports. 2018;8(1):2645. doi: 10.1038/s41598-018-20897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagheri H, Ghasemi F, Barreto GE, Rafiee R, Sathyapalan TH, Sahebkar A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors . 2019;46:5–20. doi: 10.1002/biof.1566. [DOI] [PubMed] [Google Scholar]
- 16.Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366:l4378. doi: 10.1136/bmj.l4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheless JW, Clarke DF, McGregor AL, Pearl PL, Ng Y-T. Epilepsy in Children and Adolescents. John Wiley & Sons; 2012. pp. 92–175. [Google Scholar]
- 18.Keshvarzi Z, Shakeri F, Barreto GE, Bibak B. Sathyapalan T, Sahebkar A. Medicinal plants in traumatic brain injury: neuroprotective mechanisms revisited. Biofators ;45:517–535. doi: 10.1002/biof.1516. [DOI] [PubMed] [Google Scholar]
- 19.Yılmaz BD, Eren B, Sağır D, Eren Z, Gökçe AB. Stereological examination of curcumin's effects on hippocampal damage caused by the antiepileptic drugs phenobarbital and valproic acid in the developing rat brain. Acta Histochemica. 2019;121(4):430–6. doi: 10.1016/j.acthis.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Kaur H, Patro I, Tikoo K, Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochemistry international. 2015;89:40–50. doi: 10.1016/j.neuint.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Drion CM, Borm LE, Kooijman L, Aronica E, Wadman WJ, Hartog AF, et al. Effects of rapamycin and curcumin treatment on the development of epilepsy after electrically induced status epilepticus in rats. Epilepsia. 2016;57(5):688–97. doi: 10.1111/epi.13345. [DOI] [PubMed] [Google Scholar]
- 22.Bhat A, Mahalakshmi AM, Ray B, Tuladhar S, Hediyal TA, Manthiannem E, et al. Benefits of curcumin in brain disorders. BioFactors. 2019;45(5):666–89. doi: 10.1002/biof.1533. [DOI] [PubMed] [Google Scholar]
- 23.Tang M, Taghibiglou C. The mechanisms of action of curcumin in Alzheimer’s disease. Journal of Alzheimer's disease. 2017;58(4):1003–16. doi: 10.3233/JAD-170188. [DOI] [PubMed] [Google Scholar]
- 24.Vallianou NG, Evangelopoulos A, Schizas N, Kazazis C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer research. 2015;35(2):645–51. [PubMed] [Google Scholar]
- 25.Feng T, Wei Y, Lee RJ, Zhao L. Liposomal curcumin and its application in cancer. International journal of nanomedicine. 2017;12:6027. doi: 10.2147/IJN.S132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashemian M, Anissian D, Ghasemi-Kasman M, Akbari A, Khalili-Fomeshi M, Ghasemi S, et al. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017;79:462–71. doi: 10.1016/j.pnpbp.2017.07.025. [DOI] [PubMed] [Google Scholar]