Abstract
Objectives
To determine the effectiveness of Rituximab (RTX) therapy as the first therapeutic choice for the long-term prevention of secondary relapse in children with AIND that had relapse after primary treatment with immunosuppressive agents other than RTX.
Materials & Methods
We conducted a single-center retrospective study of 9 consecutive pediatric patients (≤ 18 years old) registered on Autoimmune and Demyelinating Disorders Database (ADDD) of Mofid Children Hospital, from 2012 to 2016 and experienced relapse following therapeutic interventions with immunosuppressive agents other than RTX.
Result
A remarkable reduction of 94.13% (p=0.015) occurred in annualized relapse rate (ARR) as a clinical indicator of therapeutic efficacy comparing before and after initiating RTX therapy.
Conclusion
Rituximab is an effective drug in relapse prevention of AIND when administrated to patients for whom initial treatment with other immunosuppressive agents fail.
POWER OF EVIDENCE: This study represents Class IV evidence that RTX therapy significantly reduces ARR in pediatric AIND including DDCNS.
Key Words: Rituximab, CNS demyelinating diseases, Immunological disease, efficacy
Introduction
Autoimmune neurological diseases (AIND) including demyelinating disease of the central nervous system (DDCNS) can involve any region of the central and / or peripheral nervous system including brain, spinal cord, nerve roots, peripheral nerves, neuromuscular junction, and muscle with mild to devastating manifestations. Therefore, it is important to determination of an autoimmune process as the pathogenesis of clinical presentation, as it may result in early diagnosis of disease and timely initiation of immunotherapy with subsequently improved long-term prognosis.
Materials & Methods
1) Research objective and study power: The main objective of the present study was to determine the effectiveness of an anti-B-cell therapy with rituximab (RTX) as the first therapeutic choice for the long-term prevention of secondary relapse after the first relapse in the pediatric autoimmune and inflammatory CNS diseases. Given the retrospective nature of our study and the lack of a control group, the evidence represents Class IV (10).
2) Patient and study design: This single-center retrospective study was conducted on consecutive pediatric patients aged ≤18 years who had been registered on the Autoimmune and Demyelinating Disorders Database (ADDD) of Mofid Children Hospital, Tehran, Iran, during 2012-2016 and experienced relapse following therapeutic interventions with immunosuppressive agents other than RTX. The parents of subjects signed informed consent for this board-approved study. The research was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1397.1331).
3) Diagnosis criteria: We defined NMO/NMOSD and ON according to the revised Wingerchuk criteria (11, 12) and International Pediatric Multiple Sclerosis Study Group criteria (13), respectively. Although there are no definitive diagnostic tests for OMS, we considered expert diagnostic criteria for diagnosing OMS (14).
4) Planning a first-treatment course: Before the initiation of treatment, we performed pre-treatment workup for blood tests, including whole blood count, liver function tests, and serology for hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV). Moreover, varicella-zoster virus (VZV) serology was performed if there was no history of primary infection. For latent tuberculosis (TB) in high-risk patients, QuantiFERON-TB Gold(made by QIAGEN Company,USA) or tuberculin skin testing was performed, followed by a chest radiograph if indicated. Therefore, we excluded contraindications of RTX therapy, including:
1) Active and/or severe infection (e.g., TB, sepsis, and opportunistic infections)
2) History and/or clinical findings of immunodeficiency state
5) Administration and dosing: There is no validated dosing strategy for RTX in neuroinflammatory diseases, and there is considerable diversity in the articles. However, two dosing regimens are suggested more commonly by experts:
1) A dosage of 375 mg/body surface area given once weekly for 4 weeks (adopted from hemato-oncology)
2) Two infusions of 500-1000 mg given a fortnight apart (adopted from clinical trials in rheumatoid arthritis). Following two 1000 mg infusions, the mean half-life of RTX is 20.8 days with a range of 8.58-35.9 days.
We identified nine patients with NMO/NMOSD and ON who received RTX courses (<14 years at first dose). We employed the dosage of 375 mg/body surface area weekly for 4 weeks. It is infused over 3-6 h intravenously. Premedication with corticosteroids and antihistamines was administered. Immunization with influenza vaccine was performed in all patients except one because of parents' refusal. Furthermore, pneumococcal vaccination was recommended for all patients if possible. We repeated treatment courses at regular 6-month intervals even if there were no clinical or laboratory relapses (CD19˃0.5%) (15).
6) Data collection: Demographic, clinical, and paraclinical data of patients were extracted from ADDD. Data collection focused on the relationship between RTX administration (timing, dose, the number of courses, and adverse reactions) and relapses. The data included age, gender, birth and development history, and preceding events. Clinical profile details, including the age of onset, duration, symptoms and signs, investigations, follow-up duration, relapses, and response to treatment, were noted. In addition, the results of MRI sequences and other paraclinical modalities, such as Visual Evoked Potential(VEP) and antibody levels, were reviewed and assessed for the presence of worsening or improvements over time. Disease duration before RTX therapy was defined as the time between the onset (first event) and initiation of RTX therapy. RTX treatment duration was defined as the time between RTX initiation and last follow-up (for patients with ongoing RTX therapy). We used annualized relapse rate (ARR) as a clinical indicator of therapeutic efficacy by comparing the ARR pre- and post-RTX therapy. ARR is defined as the number of relapses with onset occurring during a specific period of time, adjusted to a one-year period. Patient-level ARR was calculated by the number of relapses experienced by that patient divided by the number of days the patient participated in the study, and the ratio multiplied by 365.25;it only included patients who were followed up after treatment for at least 6 months. Relapses were analyzed 15 months before the initiation of therapy and the duration of therapy.
7) Statistical analysis: Data were analyzed by the SPSS statistical software version 22. In this study, descriptive statistics included range, mean, median, standard deviation, number, and percentage. The number of pre-and post-RTX clinical events was compared using the Wilcoxon test. Moreover, Spearman’s rank correlation coefficient (non-parametric) was used to assess the relationship between the number of relapses and treatment duration. P<0.05 was considered statistically significant.
Results
Demographics: We identified a total of nine children with autoimmune neurological diseases (AIND) hospitalized at our center during 2012-2016. There were five female (56%) and four male (44 %) patients. All patients met the published clinical criteria for individual AINDs (11-14). The mean and median age of disease onset was 7.6±3.4 and 9.5 years (range: 3-12 years), respectively.
2) Clinical presentation : There were seven patients (78%) with NMO/NMOSD, one (11%) with chronic relapsing idiopathic ON (CRION), and one (11%) with OMS. Several clinical presentations occurred in every relapse episode before and during RTX therapy, with hemiparesis being the most common sign detected in five patients (55.6%). Other presentations are shown in Table 1.
Table 1.
Clinical and paraclinical features and first-line and second-line immune treatments administered before Rituximab therapy
| Patient | Gender | Diagnosis | Age at disease onset (yr) | VEP | MRI findings | NMO antibody |
MOG
antibody |
Type of clinical event | Prior immunosuppressive medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | NMOSD | 10 | Abnormal | Abnormal | - | - | Hemiparesis, vision disorder | IVMP, OP, PE, IVIG, AZA |
| 2 | F | NMOSD | 3.5 | Abnormal | Abnormal | + | - | Hemiparesis | IVMP, OP, PE, IVIG, AZA |
| 3 | F | NMOSD | 9.5 | Abnormal | Abnormal | + | - | Hemiparesis, blurred vision | IVMP, OP, PE, IVIG, AZA |
| 4 | F | NMOSD | 10 | Abnormal | Abnormal | + | - | Hemiparesis, fever, speech problem, vision disorder, convulsion, dLOC | IVMP, OP, PE, IVIG, AZA |
| 5 | F | NMOSD | 9.5 | Abnormal | Abnormal | - | - | Paraplegia, Neck pain, Urine retention | IVMP, OP, IVIG |
| 6 | M | NMOSD | 7.5 | Abnormal | Abnormal | - | - | Speech problem, Dysphagia, Hemiparesis, Urine retention | IVMP, PE, OP, IVIG, AZA |
| 7 | F | CRION | 12 | Abnormal | Abnormal | Not performed | Not performed | Blurred vision | IVMP, PE, OP, IVIG, AZA |
| 8 | M | OMS | 3.5 | Not done | Normal | Not performed | Not performed | OMS | IVMP, OP, IVIG, AZA |
| 9 | M | NMOSD | 3 | Abnormal | Abnormal | - | - | Paraplegia | IVMP, OP, IVIG, AZA |
Abbreviations: VEP=Visual Evoked Potential; MRI=Magnetic Resonance Imaging; NMO=Neuromyelitis Optica; MOG=Myelin Oligodendrocyte Glycoprotein; M=Male; F=Female; OMS=Opsoclonus-Myoclonus Syndrome; NMOSD=Neuromyelitis Optica Spectrum Disorders; CRION=Chronic Relapsing Idiopathic Optic Neuritis; -=Negative; +=Positive; IVMP=IV Methylprednisolone; OP=Oral Prednisolone; AZA=Azathioprine; PE=Plasmapheresis; dLOC=Decreased Level of Conscious
3) Paraclinical findings: Brain and/or spinal MRI as well as a visual evoked potential (VEP) were performed in eight cases (89%) with suspected NMO/NMOSD and CRION;these subjects represented abnormal MRI and VEP findings consistent with the suspected conditions. All nine patients had normal immunoglobulins before treatment. The NMO-IgG levels (i.e., an autoantibody against AQP4) were measured in seven patients with suspected NMO/NMOSD (Table 1).
4) Immune therapies before RTX therapy: The administrated medications and/or therapeutic interventions before RTX therapy included first-line (intravenous methylprednisolone, oral prednisolone, plasmapheresis, and IVIG) and second-line (azathioprine) immune treatments (Table 1). Before RTX, all patients received IV methylprednisolone (mean of 2.9±1 courses per patient, range: 2-4), followed by oral prednisolone. Plasmapheresis was administered in six patients (mean of 7.2±2.2 cycles per patient, range: 5-10). IVIG was administered in all patients (mean of 4.6±1 cycles per patient, range: 3-6). Moreover, eight patients (89%) received azathioprine, and eight cases (89%) were vaccinated against varicella before receiving RTX.
5) Rituximab administration: A total of 59 RTX courses were administered to nine patients. The mean and median age at the initiation of RTX therapy was 8.6±3.4 and 10 years (range: 4-13.1 years), respectively. The mean interval between disease onset and RTX therapy was 12.1±3 months, with a range of 6-18 months. The mean duration of RTX therapy was 16.6±9.7 months, with a range of 4-30 months. (Table 2)
Table 2.
Duration of disease, frequency of clinical events, and side effects of Rituximab
| Patient |
Disease duration
pre-RTXT (m) |
Age at RTXT (yr) | RTXT duration (m) | RTXT courses (no.) | CEF before RTXT (no.) | CEF during RTXT (no.) | ARR before RTXT | ARR during RTXT | Side effects of RTX therapy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 11 | 22 | 8 | 6 | 2 | 6 | 1.09 | Tachycardia, vomiting |
| 2 | 12 | 4.5 | 18 | 7 | 5 | 1 | 5 | 0.67 | None |
| 3 | 12 | 10.5 | 15 | 6 | 5 | 1 | 5 | 0.80 | Dyspnea, abdominal pain, skin allergy |
| 4 | 12 | 11 | 17 | 7 | 5 | 1 | 5 | 0.71 | None |
| 5 | 6 | 10 | 7 | 5 | 3 | 0 | 6 | 0 | Dyspnea, hypoxemia |
| 6 | 18 | 9 | 30 | 9 | 5 | 0 | 6 | 0 | None |
| 7 | 13 | 13.1 | 30 | 8 | 5 | 0 | 4.6 | 0 | Fever and chills |
| 8 | 12 | 4.5 | 6 | 5 | 4 | 0 | 4 | 0 | Chickenpox |
| 9 | 12 | 4 | 4 | 4 | 3 | 0 | 3 | 0 | None |
| Mean±SD | 12.1±3.02 | 8.6±3.4 | 16.6±9.7 | 6.6±1.7 | 4.6±1.01 | 0.56±0.73 | 4.96±1.01 | 0.36±0.45 | |
| Median | 12 | 10 | 17 | 7 | 5 | 0 | 5 | 0 | |
| Range | 6-18 | 4-13.1 | 4-30 | 4-9 | 3-6 | 0-2 | 3-6 | 0-1.09 |
Abbreviations: RTXT=Rituximab therapy; CEF=Clinical Events Frequency; ARR=Annualized Relapse Rate
6) Infusion reactions and adverse events: Infusion reactions to treatment and/or adverse events occurred in five patients (55.6%). One individual who did not have a history of varicella-zoster immunization and had not received a related vaccine before treatment was affected by chickenpox (Table 2).
7) Rituximab efficacy and relapse episodes : Five (55.6 %) patients (patients 5-9) were relapse-free during RTX therapy in the follow-up of 6-12 months. In the four relapsing patients (44.4 %), five relapse episodes occurred during RTX therapy, while 21 relapse episodes occurred in these cases before RTX therapy with a long disease course (15-22 months). The mean number of relapse episodes before and during RTX therapy was 4.6±1.01 (range: 3-6 episodes) and 0.56±0.73 (range: 0-2 episodes), respectively. There was a statistically significant reduction in ARR after initiating RTX therapy when the first events were included (P<0.008) (Table 2).
The mean of ARR during 15 months before initiating RTX therapy was 4.6. After the median therapy duration of 29 months (range: 13-48 months), the mean ARR declined to 0.56, with a remarkable reduction of 88.7%. The mean of relapse episodes was 4.3 months, with a range of 2-8 months after the last RTX therapy course.
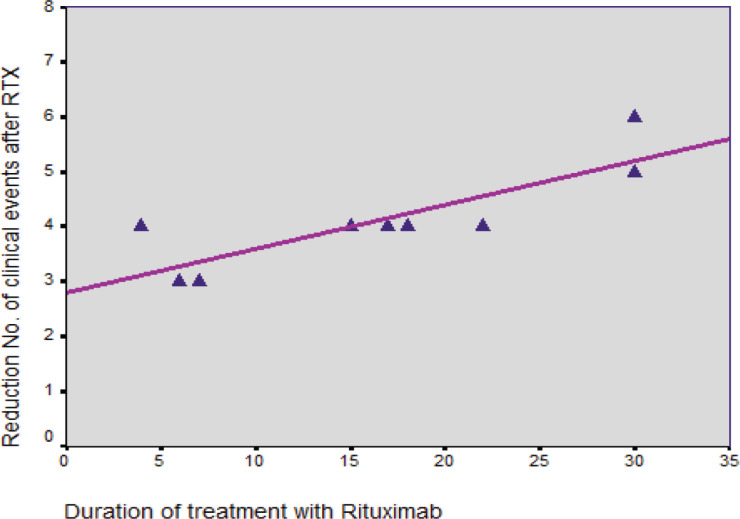
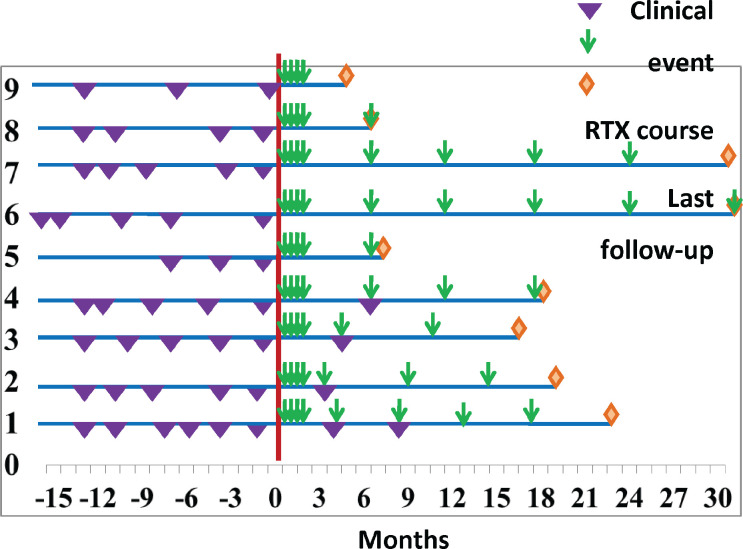
As presented in Figure 1, the results revealed a significant relationship between the duration of RTX therapy and a reduced number of episodes (P=0.005, r=0.837). However, the relationship of RTX therapy with gender was not significant (P˃0.05). Figure 2 shows the distribution of relapse episodes before and during treatment with RTX, along with the median number of RTX therapy courses in this period. In our study, RTX therapy was generally initiated after ≥3 relapse episodes.
Figure 1.
Relationship between the mean duration of RTX therapy and reduced number of episodes
Figure 2.
Distribution of relapse episodes before and during treatment with RTX along with the median number of RTX therapy courses in this period
Discussion
A) Autoimmune neurological diseases with and without CNS demyelination
Autoimmune diseases of the pediatric nervous system constitute a diverse group of uncommon disorders that can involve any region of the central and / or peripheral nervous system including brain, spinal cord, nerve roots, peripheral nerves, neuromuscular junction, and muscle. Accordingly, children of any age may present with various symptoms and signs with acute or subacute course in onset. It is important to determination of an autoimmune process as the pathogenesis of clinical presentation, as it may result in early diagnosis of disease and timely initiation of immunotherapy with subsequently improved long-term prognosis (16). The antigen–antibody interactions are the basic mechanism in the primary pathogenesis of autoimmune neurological diseases that can lead to nervous system involvement with demyelination (e.g. NMOSD, ON and MS) or without demyelination (e.g. OMS) (17). In this study, there were 8 patients (89 %) with demyelinating disorders including NMOSD and chronic relapsing idiopathic ON, and one patient (11%) with OMS, non- demyelinating autoimmune nervous system disorder.
1) NMOSD/NMO: Neuromyelitis optica spectrum disorder (Devic’s disease) is one of the DDCNS, notably involving the optic nerves and spinal cord (18). The characteristic features include recurrent episodes of longitudinally extensive transverse myelitis (LETM), extending ≥ 3 vertebral segments, as well as optic neuritis. Some patients with NMOSD/NMO may have an autoantibody against aquaporin-4 (AQP4) in serology (19-21).
Available literature about pediatric NMOSD/NMO originates some case series. In children and adults, the incidence and prevalence of NMOSD/NMO range from 0.05 to 4 and from 0.52 to 4.4, respectively but up to 3-5% of cases have childhood onset and between 4-10% of cases occur before the age of 18 years (22-25).Interestingly, NMOSD/NMO have been reported in children as young as 12-16 months; however, the typical age of onset is 10 to 12 years (26). In Japan, the incidence of children NMOSD/NMO was reported as 0.06 per 100,000 children (27). In our country, there is no epidemiologic study determining the incidence and prevalence of children NMOSD/NMO, although the current study showed that 78 percent of patients had NMOSD/NMO.
Females are more likely to be affected than males at a ratio of 3:1 in the pediatric population but up to 9:1 in adults (28). In many regions of the world, there is a pattern of female predominance in adults and children: east Brazil 5:1, Iran 5.9:1, 2.8:1 in Germany, 7:1 in Pediatric patients treated at Mayo Clinic, Spain 2.4:1, Southern Denmark 2.8:1, and in Japan 6.4:1. The disease predilection for females in NMOSD/NMO is stronger than in MS, in which gender ratio varies from 1.1:1 to 3.4:1 in Europe and 3.1:1 in United States. Due to the high prevalence in females, some investigators suggest the possibility of hormones influencing the development of NMOSD/NMO (29).
This ratio was 1.25: 1 in our study that was not similar to results of other studies, indicating more studies with more cases needed to be done. In a similar Iranian study conducted on adult NMOSD/NMO, female/male ratio was 5.4:1.
In our study, the mean age at the initiation of RTX therapy was 8.6 ± 3.4 years (range of 4 to 13 years). In similar studies of children NMOSD/NMO, the mean age at Rituximab administration was 9.6 (30), 9.9 (31), and 13.7 (32) years, suggesting comparable ages at the initiation of RTX therapy.
NMO-IgG (AQP4) is an antibody directed against the aquaporin-4 water channel located on astrocyte end feet. This serum biomarker has a sensitivity of 73% and a specificity of 91% in differentiating NMO from other demyelinating disorders, such as MS, in adult NMO patients and is detected in up to 78% of children with relapsing NMO (33). Approximately 65% of pediatric patients with NMOSD are AQP4 antibody seropositive; however, seropositivity may not occur at the time of the initial attack but up to 4 years later. Approximately 10% to 15% of cases of pediatric NMOSD are MOG antibody seropositive, which has implications for both prognosis and treatment, and they may be considered separately from AQP4 antibody–seropositive cases. Dual seropositivity has not been reported in children. Approximately 15% of pediatric NMOSD cases are both AQP4 and MOG antibody seronegative. Moreover, seropositivity may not occur at the time of the initial attack but up to 4 years later. Therefore, serial testing is recommended for highly suspicious cases (34). In a study of 37 patients with NMOSD in the U.S. Network of Pediatric MS Centers, Serum testing for NMO-IgG was positive in 60% of those tested. The timing of testing showed that, of the seropositive patients, 57% tested positive within 12 months of disease onset, 13% at 12 to 23 months, 13% at 24 to 35 months, and 17% at more than 36 months after disease onset (25). It has been recognized for some time that a proportion of individuals with an NMO phenotype repeatedly test negative for NMO-IgG even when sensitive cell-based assays are employed. A large retrospective study of 175 Caucasian NMO patients reported that compared with seronegative patients, seropositive patients were more likely to be female, to harbor additional serum autoantibodies, to have more severe clinical attacks, and to have a greater spinal cord lesion load, including an increased likelihood of having spinal lesions greater than six spinal segments in length. In this same study, seronegative patients were more likely to have bilateral ON or simultaneous ON and TM at presentation and were more likely to experience a monophasic (as opposed to relapsing) disease course (35).
In our study, 43% of cases were AQP4 antibody seropositive that was inconsistent with results of similar studies; this can be due to poor assay techniques or reduced number of patients. Also, it can be due to measurement of antibody during seronegativity period. A subset of children and adults with clinical NMO who are seronegative for NMO-IgG display serum anti-MOG antibodies. The two antibodies rarely coexist in the same patient, suggesting that anti-MOG antibodies are not simply a reflection of heightened autoimmunity in NMO patients (36-38).In our study, all patients were anti-MOG seronegative.
Visual-evoked potentials are frequently abnormal in NMO patients and typically display a prolongation or absence of the P100 response, in keeping with anterior visual pathway involvement. In adults with NMO, markedly abnormal or absent visual-evoked potential responses at baseline are associated with worse longer-term visual outcomes. Delayed P100 responses may be observed in the absence of a clear history of ON, suggesting that optic nerve involvement may be subclinical for some patients (39, 40). In current study, VEP was abnormal in all patients.
2) ON: Optic neuritis is one of the most common presentations of acquired
demyelinating syndromes in childhood and should be considered in the differential
diagnosis of any child presenting with acute or subacute visual.
3) OMS: Opsoclonus-myoclonus syndrome (OMS) is a rare and primarily immune-mediated non-demyelinating disease in children and adults. The main symptoms include opsoclonus, myoclonus and ataxia. In children, the symptoms also include irritability, and, over a long-term course, learning and behavioral disturbances. OMS can be idiopathic, parainfectious or occur as a paraneoplastic (tumor-associated) syndrome. Paraneoplastic OMS in children is almost exclusively associated with neuroblastoma, whereas in adults, small cell lung cancer and breast cancer are the main underlying tumors. An autoimmune pathophysiology is suspected because childhood OMS patients have functionally active autoantibodies, proinflammatory changes in the cytokine network and immunotherapy responses. Children appear to respond regularly to immunosuppressive treatment. However, although the neurological symptoms show a good response, most children continue to show neuropsychological disturbances (42). In present study, one patient (11%) had OMS.
B) Rituximab therapy in autoimmune neurological diseases
1) Overview: Similarly to systemic autoimmune diseases, autoimmune neurological diseases may be mediated by all elements of the immune system, including B cells (43). Rituximab (Mabthera, Rituxan) is a chimeric human/murine monoclonal antibody against CD-20 surface antigen expressed on B-cells. Rituximab, by causing B-cell depletion, appears to be effective in several autoimmune disorders (44). Rituximab induces both apoptotic and cytotoxic (ADCC and CDC) cell death in CD20-expressing B cells (43). A thereby triggered reduction of antibody formation is presumably the RTX mechanism of action (45).
2) RTX and NMOSD: NMO is a relapsing disease with a high early mortality
rate. More than 50% of patients with NMO will be functionally blind or will progress to wheelchair dependence within 5 years without employing appropriate immunosuppressant treatment (46,47). Treatment options for NMO are based on case series and expert opinion; among which, immunosuppressive therapy is the main method used to prevent recurrence and disability. Successful use of RTX has been widely reported in NMO. However, randomized controlled trials in NMO are relatively few, and no established guidelines have been established for RTX treatment. Although RTX is expensive, it can offset the cost of recurrence and plasma exchange due to its good therapeutic effect (48, 49)
In 2005, an open label study described for the first time a significant reduction in disease activity in eight NMO patients treated with RTX (50). In this study, RTX was infused weekly at 375 mg/m2 for 4 weeks followed by 1,000 mg reinfusion if CD19+ B cells were detectable in the peripheral blood. Positive curative effect was observed in all cases. Since then, an increasing number of patients were treated with RTX. However, to date only a few prospective studies investigating the effect of RTX on NMOSD exist (45).
The therapeutic effects of RTX last weeks to begin due to its long half-life which include observed benefits such as reduced relapse rates and maintained or improved patients’ neurological health (50, 51). Despite these claims, not all patients respond favorably to this medication, as several cases report relapse episodes after therapy initiation (51, 52). This can happen due to a sudden increment of the anti AQP4 antibodies expressed by the patients after a 2 week period (52). In another 5-year prospective study, researchers reported that 26 of 30 patients benefited from the treatment (53).
Generally, as mentioned in methods, there are two different regimens (15). In most studies, Rituximab was administered at regular 6-month intervals beginning with four weekly doses of 375 mg/m2 followed by two biweekly doses of 1 g (50, 53-55). Other studies used more frequent infusions (usually 1 g every 12 months) (56, 57) or administration depending on circulating B-cell numbers (54-57). In each study, patients treated with Rituximab demonstrated a significant stabilization of disability.
In a retrospective analysis, 25 patients with Devic’s disease, who were unresponsive to other treatments, showed significant improvement with RTX therapy. Patients were followed for 19 months after receiving the standard regimen of 2g in 1 month. This study although retrospective and uncontrolled showed that up to 80% of patients with Devic’s disease may benefit from RTX therapy (54).A Multicenter retrospective study of 16 children with NMO/NMOSD receiving ≥ 2 RTX courses showed that Rituximab is effective in relapse prevention (30). Similar to results of other studies(30,50-57), our patients had a high relapse rate (41 episodes) and a long disease course (6-18 months) before RTX therapy but both reduced significantly following treatment initiation, suggesting competent preventive effectiveness of Rituximab.
All patients received immunosuppressive therapies before RXT therapy. All received IV Methylprednisolone and IVIG, 89% received Azathioprine and 67% received plasma exchange. History of immunosuppressive therapy before RTX therapy in our study was comparable to other studies (54-58). In present study, Rituximab was generally initiated after ≥ 3 relapse episodes while in the most studies it was initiated after ≥ 1-2 relapse episodes (50-58).This delay in the initiation of RXT therapy compared to similar pediatric studies can be due to a variety of factors including loss of adapted guideline in Iran, poor socioeconomic state of patients' family, misdiagnosis of affected patients in the early visits and inadequate drug deposit and / or drug delivery in Iran, emphasizing evaluation of the causes of delayed treatment in another study and solving problems based on the results.
3) RTX and OMS: The first successful treatment of pediatric OMS patients using Rituximab was done by M. Pranzatelli in 2005 (64). The same investigator showed the efficiency of Rituximab in a group of 16 children with OMS with an increased subset of B-cells in the CSF after unsuccessful treatment with ACTH, IVIG or both (59).
In a study mainly investigated the safety of Rituximab in pediatric neurological autoimmune diseases, which included more than 30 pediatric OMS patients. Whereas all OMS patients had a modified ranking scale (MRS) of 3 that was worse before treatment, Rituximab led to a significant improvement (60). Other studies confirm the beneficial effects of Rituximab (59, 42, and 61).
In Conclusion
RTX has acceptable tolerance, reduces the relapse frequency, and improves disability in most patients with NMO/NMOSD and other AINDs such as OMS. Future studies should focus on reducing the health-care costs, improving the functional outcomes, and reducing the adverse effects associated with RTX treatment.
Author’s Contribution
Conception and design of the work: Mohammad Mahdi Nasehi
Data collection: Bakhtyar Khosravi
Data analysis and interpretation: Fatemeh Abdollah Gorji
Drafting the article: Aydin Tabrizi and Bakhtyar Khosravi
Critical revision of the article: Mohammad Mahdi Nasehi
Final approval of the version to be published: Mohammad Ghofrani
Conflict of interest
None
Acknowledgment
The authors thank the Pediatric Neurology Research center and Mofid Clinical research for logistical support in order to conduct the study.
All authors declare that they have no conflicts of interest.
The research was approved by the Ethics Committee
of Shahid Beheshti University of Medical Sciences
(IR.SBMU.RETECH.REC.1397.1331)
References
- 1.Eibel H, Kraus H, Sic H, Kienzler AK, Rizzi M. B cell biology: an overview. Curr Allergy Asthma Rep. 2014;14:434. doi: 10.1007/s11882-014-0434-8. [DOI] [PubMed] [Google Scholar]
- 2.Krumbholz M, E Meinl. "B cells in MS and NMO: pathogenesis and therapy. emin Immunopathol. 2014;36(3):339–350. doi: 10.1007/s00281-014-0424-x. [DOI] [PubMed] [Google Scholar]
- 3.Seyfizadeh N. A molecular perspective on rituximab: A monoclonal antibody for B cell non Hodgkin lymphoma and other affections. Crit Rev Oncol Hematol. 2016;97:275–290. doi: 10.1016/j.critrevonc.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Cerny T, Borisch B, Introna M, Johnson P, Rose AL. Mechanism of action of rituximab. Anticancer Drugs. 2002;13(2):3–10. doi: 10.1097/00001813-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 6.Quartier P, Brethon B, Philippet P, et al. Treatment of childhood auto-immune hemolytic anemia with rituximab. Lancet. 2001 doi: 10.1016/s0140-6736(01)06573-4. [DOI] [PubMed] [Google Scholar]
- 7.Faurschou M, Hasselbalch HC, Nielsen OJ. Sustained remission of platelet counts following monoclonal anti-CD20 antibody therapy in two cases of idiopathic autoimmune thrombocytopenia and neutrope-nia. Eur J Haematol. 2001;66:408–411. doi: 10.1034/j.1600-0609.2001.066006408.x. [DOI] [PubMed] [Google Scholar]
- 8.Pranzatelli MR, Tate ED, Travelstead AL, Longee D. Immunologic and Clinical Responses to Rituximab in a Child With Opsoclonus-Myoclonus Syndrome. Pediatrics. 2005;115(1):115–119. doi: 10.1542/peds.2004-0845. [DOI] [PubMed] [Google Scholar]
- 9.Leandro MJ, Edwards JC, Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis . 2002;61:883–888. doi: 10.1136/ard.61.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence based medicine. Plast Reconstr Surg. 2011;128(1):305–310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:148–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 12.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 13.Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system Demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 14.Matthay KK, Blaes F, Hero B, et al. Opsoclonus myoclonus syndrome in neuroblastoma: a report· from a workshop on the dancing eyes syndrome at the advances in neuroblastoma meeting in Genoa , Italy , 2004. Cancer Letters . 2005;228:275–282. doi: 10.1016/j.canlet.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Whittam DH, Tallantyre EC, Jolles S, et al. Rituximab in neurological disease: principles , evidence and practice. Practical Neurology. 2019;19:5–20. doi: 10.1136/practneurol-2018-001899. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney M. Autoimmune Neurologic Diseases in Children. Semin Neurol. 2018;38:355–370. doi: 10.1055/s-0038-1660520. [DOI] [PubMed] [Google Scholar]
- 17.Lancaster E, Dalmau J. Neuronal autoantigens – pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banwell B, Tenembaum S, Lennon VA, et al. Neuromyelitis optica IgG in childhood inflammatory demyelinating CNS disorders. Neurology. 2008;70(05):344–352. doi: 10.1212/01.wnl.0000284600.80782.d5. [DOI] [PubMed] [Google Scholar]
- 19.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 20.Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etemadifar M, Nasr Z, Khalili B, Taherioun M, Vosoughi R. Epidemiology of neuromyelitis optica in the world: a systematic review and meta-analysis. Mult Scler Int. 2015;2015:174720. doi: 10.1155/2015/174720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandit L, Asgari N, Apiwattanakul M, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler. 2015;21(7):845–853. doi: 10.1177/1352458515572406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collongues N, Marignier R, Zéphir H, et al. Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology. 2010;74(09):736–742. doi: 10.1212/WNL.0b013e3181d31e35. [DOI] [PubMed] [Google Scholar]
- 25.Chitnis T, Ness J, Krupp L, et al. Clinical features of neuromyelitis optica in children: US Network of Pediatric MS Centers report. Neurology. 2016;86(03):245–252. doi: 10.1212/WNL.0000000000002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotze TE, Northrop JL, Hutton GJ, Ross B, Schiffman JS, Hunter JV. Spectrum of pediatric neuromyelitis optica. Pediatrics. 2008;122(05):e1039–e1047. doi: 10.1542/peds.2007-2758. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi Y, Torisu H, Kira R, et al. A nationwide survey of pediatric acquired demyelinating syndromes in Japan. Neurology. 2016;87(19):2006–2015. doi: 10.1212/WNL.0000000000003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenembaum S, Chitnis T, Nakashima I, et al. Neuromyelitis optica spectrum dsorders in children and adolescents. Neurology. 2016;87(2):59–66. doi: 10.1212/WNL.0000000000002824. [DOI] [PubMed] [Google Scholar]
- 29.Zarei S, Eggert J, Franqui-Dominguez L, et al. Comprehensive review of neuromyelitis optica and clinical characteristics of neuromyelitis optica patients in Puerto Rico. Surg Neurol Int . 2018;9:242. doi: 10.4103/sni.sni_224_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosadini M, Alper G, Riney CJ, et al. Rituximab monitoring and redosing in pediatric neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3(1):e188. doi: 10.1212/NXI.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dale RC, Brilot F, Duffy LV, et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology. 2014;83(2):142–150. doi: 10.1212/WNL.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longoni G, Banwell B, Filippi M, Yeh EA. Rituximab as a first-line preventive treatment in pediatric NMOSDs: preliminary results in 5 children. Neurol Neuroimmunol Neuroinflamm. 2014;1(4):e46. doi: 10.1212/NXI.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makhani N, Brenton JN, Banwell B. Acquired Disorders Affecting the White Matter. In: Swaiman KF, editor. Swaiman’s Pediatric Neurology: Principles and Practice. New York: Elsevier Inc; 2017. pp. e1734–e1736. [Google Scholar]
- 34.Chitnis T. Pediatric Central Nervous System Demyelinating Diseases. CONTINUUM: Multiple Sclerosis and other CNS Inflammatory Diseases. 2019;25(3):793–814. doi: 10.1212/CON.0000000000000730. [DOI] [PubMed] [Google Scholar]
- 35.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79(12):1273–1277. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 37.Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014;71(3):276–283. doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]
- 38.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe A, Matsushita T, Doi H, et al. Multimodality-evoked potential study of anti-aquaporin-4 antibody-positive and -negative multiple sclerosis patients. J Neurol Sci. 2009;281(1–2):34–40. doi: 10.1016/j.jns.2009.02.371. [DOI] [PubMed] [Google Scholar]
- 40.Ringelstein M, Kleiter I, Ayzenberg I, et al. Visual evoked potentials in neuromyelitis optica and its spectrum disorders. Mult Scler. 2014;20(5):617–620. doi: 10.1177/1352458513503053. [DOI] [PubMed] [Google Scholar]
- 41.Banwell B, Kennedy J, Sadovnick D, et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology. 2009;72(3):232–239. doi: 10.1212/01.wnl.0000339482.84392.bd. [DOI] [PubMed] [Google Scholar]
- 42.Blaes F, Dharmalingam B. Childhood opsoclonus-myoclonus syndrome: diagno-sis and treatment. Expert Rev Neurother. 2016;16(6):641–648. doi: 10.1080/14737175.2016.1176914. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos H, Biba1 A, Dalakas MS. Anti-B-Cell Therapies in Autoimmune Neurological Diseases:Rationale and Efficacy Trials. Neurotherapeutics. 2016;13:20–33. doi: 10.1007/s13311-015-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosmidis ML, Dalakas MS. Practical considerations on the use of rituximab in autoimmune neurological disorders. Ther Adv Neurol Disord. 2010;3(2):93–105. doi: 10.1177/1756285609356135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalakas MC. B-cells in the pathophysiology of autoimmune neurological disorders: a credible therapeutic target. Pharmacol Ther. 2006;112:57–70. doi: 10.1016/j.pharmthera.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Birnbaum J, Kerr D. Optic neuritis and recurrent myelitis in a woman with systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2008;4(7):381–386. doi: 10.1038/ncprheum0818. [DOI] [PubMed] [Google Scholar]
- 47.Jacob A, Matiello M, Weinshenker BG, Wingerchuk DM, Lucchinetti C, Shuster E, et al. Treatment of neuromyelitis optica with mycophenolate mofetil retrospective analysis of 24 patients. Arch Neurol . 2009;66(9):1128–1133. doi: 10.1001/archneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 48.Chay J, Donovan P, Cummins L, Kubler P, Pillans P. Experience with lowdose rituximab in off-label indications at two tertiary hospitals. Intern Med J. 2013;43(8):871–882. doi: 10.1111/imj.12207. [DOI] [PubMed] [Google Scholar]
- 49.Gao F, Chai B, Gu C·, Wu R, Dong T, Yao Y, Zhang Y. Effectiveness of rituximab in neuromyelitis optica: a meta-analysis. BMC Neurol . 2019; 19(1) doi: 10.1186/s12883-019-1261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borisow N, Mori M, Kuwabara S, Scheel M, Paul F. Diagnosis and treatment of NMO Spectrum Disorder and MOG-Encephalomyelitis. Front Neurol. 2018;23(9):888. doi: 10.3389/fneur.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cree BAC, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology . 2005;64:1270–1272. doi: 10.1212/01.WNL.0000159399.81861.D5. [DOI] [PubMed] [Google Scholar]
- 52.Pellkofer HL, Krumbholz M, Berthele A, Hemmer B, Gerdes LA, Havla J, et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology. 2011;76:1310–1315. doi: 10.1212/WNL.0b013e3182152881. [DOI] [PubMed] [Google Scholar]
- 53.Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison ofrelapse and treatment failure rates among patients with neuromyelitis optica: Multicenter study of treatment effcacy. JAMA Neurol . 2014;71:324–330. doi: 10.1001/jamaneurol.2013.5699. [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, Huh SY, Lee SJ, Joung A, Kim HJ. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110–1117. doi: 10.1001/jamaneurol.2013.3071. [DOI] [PubMed] [Google Scholar]
- 55.Jacob A, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol. 2008;65:1443–1448. doi: 10.1001/archneur.65.11.noc80069. [DOI] [PubMed] [Google Scholar]
- 56.Kim S. Repeated treatment with Rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412–1420. doi: 10.1001/archneurol.2011.154. [DOI] [PubMed] [Google Scholar]
- 57.Bedi G S, et al. Impact of rituximab on relapse rate and disability in neuromyelitis optica. Mult Scler. 2011;17:1225–1230. doi: 10.1177/1352458511404586. [DOI] [PubMed] [Google Scholar]
- 58.Greenberg B M, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler . 2012;18:1022–1026. doi: 10.1177/1352458511432896. [DOI] [PubMed] [Google Scholar]
- 59.Pranzatelli MR, Tate ED, Travelstead AL, Barbosa J, Bergamini RA, Civitello L, Franz DN, Greffe BS, Hanson RD, Hurwitz CA, Kalinyak KA, Kelfer H, Khakoo Y, Mantovani JF, Nicholson SH, Sanders JM, Wegner S. Rituximab Adjunctive Therapy for Opsoclonus Myoclonus syndrome. J Pediatr Hematol Oncol. 2006;28(9):585–593. doi: 10.1097/01.mph.0000212991.64435.f0. [DOI] [PubMed] [Google Scholar]
- 60.Dale RC, Brilot F, Duffy LV, Twilt M, Waldman AT, Narula S, et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology. 2014;83(2):142–150. doi: 10.1212/WNL.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pranzatelli MR, Tate ED, McGee NR. Demographic, clinical, and immunologic Features of 389 children with Opsoclonus Myoclonus syndrome: A cross-sectional study. Front Neurol. 2017;8:468. doi: 10.3389/fneur.2017.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]