Abstract
Thiols easily react with [1.1.1]propellane to give sulfur-substituted bicyclo[1.1.1]pentanes in radical reactions, but this reactivity is not replicated in the case of heterocyclic thiols. Herein, we address this issue by electrophilically activating [1.1.1]propellane to promote its iodo-sulfenylation with 10 classes of heterocyclic thiols in two protocols that can be conducted on a multigram scale without exclusion of air or moisture.
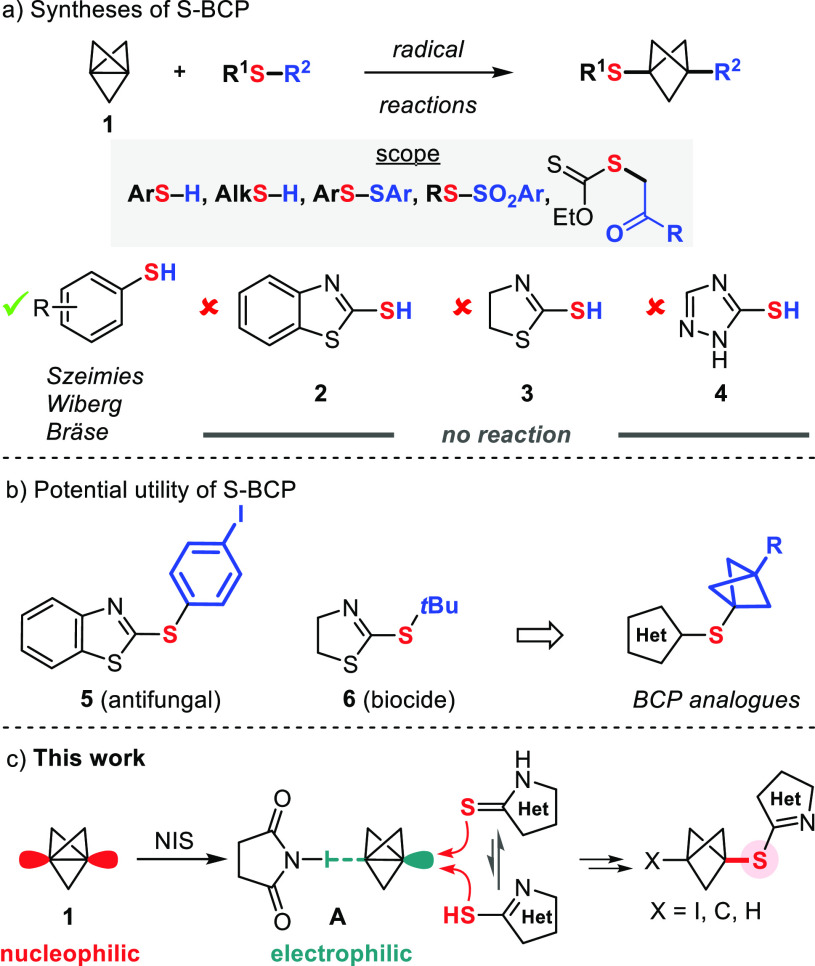
Bicyclo[1.1.1]pentanes (BCPs) often improve the potency, metabolic stability, and water solubility of bioactive compounds.1 These valuable properties have spurred the recent emergence of numerous methods for the synthesis of BCPs from [1.1.1]propellane.2,3 Although sulfur is the third most abundant heteroelement in drugs after nitrogen and oxygen,4 sulfur-substituted BCPs (S-BCPs) are strikingly scarce in the patent literature.5 The synthesis of S-BCPs has been reported by radical reactions of [1.1.1]propellane 1 with thiols,6 disulfides,7 xanthates,8 thiosulfonates,9 or sulfones (Figure 1a).10 Moreover, BCP sulfones and sulfonamides can be accessed from BCP sulfinates.11 However, although the addition of aromatic thiols to 1 has been known for several decades to be facile at room temperature, their heterocyclic counterparts 2–4 fail to react with 1 under the same conditions.12 These limitations restrict the exploration of the potential benefits of S-BCPs as bioisosteric replacements of para-substituted benzene rings and tert-butyl group in bioactive compounds, as for example antifungal 5(13) and biocide 6 (Figure 1b).14
Figure 1.
Sulfur-substituted bicyclo[1.1.1]pentanes (S-BCPs). (a) Previous syntheses of S-BCPs and failure of 2-mercapto-azoles and thiazoline. (b) Potential S-BCP analogues of bioactive compounds. (c) Iodo-sulfenylation of [1.1.1]propellane (this work).
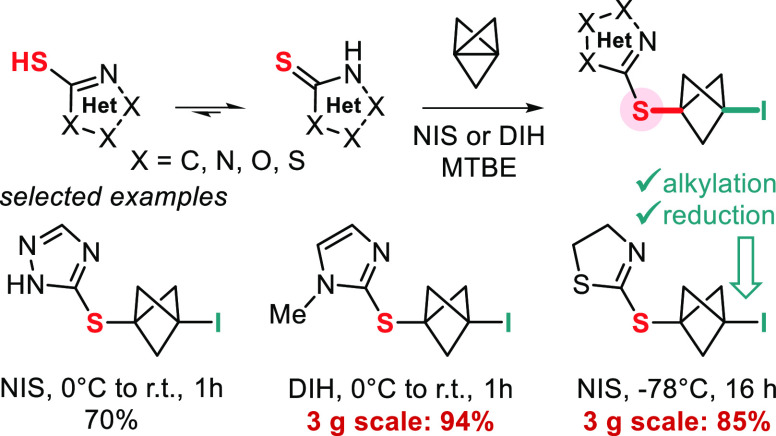
The reaction of thiols with 1 has been suggested to proceed by the reversible addition of a thiyl radical and the transfer of a hydrogen atom to the resulting bicyclo[1.1.1]pentyl radical.15 The reported rates of addition of thiyl radicals to olefins suggest that the apparent lack of reactivity of 2–4 with 1 in radical reactions is unlikely due to a slower addition of those thiyl radicals to 1(16) or differences in bond dissociation energies.16c Instead, it might be imputable to a polarity mismatch in the hydrogen atom transfer between heterocyclic thiol and the bicyclo[1.1.1]pentyl radical intermediate,17 because heterocyclic thiols are less hydridic than aryl or alkyl thiols.18 Alternatively, or in addition to this reasoning, the low concentration of heterocyclic thiols in solution created by the predominance of the thione tautomer19 would decrease the rates of addition of the thiyl radical to 1 and of the transfer of a hydrogen atom to the bicyclo[1.1.1]pentyl radical.
Previously, we established in collaboration with the Duarte group that electrophilic activation of 1 in halogen bond complex A (Figure 1c),20 formed between propellane 1 and electrophilic reagents such as N-iodosuccinimide (NIS), is a viable method for promoting reactions of the interbridgehead bond of 1 with weak nucleophiles. We therefore wondered whether a similar strategy, which does not rely on a radical mechanism, could be applicable to heterocyclic thiols and thus overturn their lack of reactivity with 1 in radical reactions. Herein, we describe the successful deployment of this strategy for the iodo-sulfenylation of 1 with 10 classes of heterocyclic thiols under conditions that do not require dry reagents and solvents or an inert atmosphere (Figure 1c).
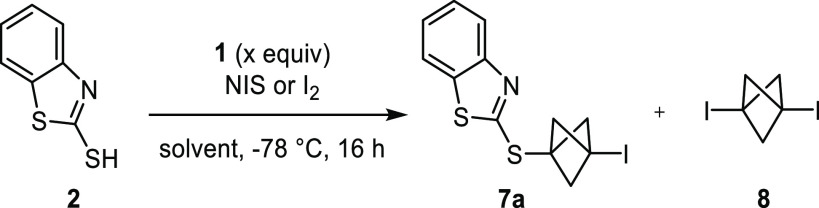
Following our previous report on the reaction of anilines with propellane 1 and NIS in acetone,20 we examined these conditions with 2 (Table 1, entry 1). The desired adduct 7a, a direct bioisosteric analogue of antifungal 5,13 was obtained as a bench-stable solid, and its structure was also confirmed by X-ray crystallography. However, we were surprised to observe the formation of 1,3-bis-iodo-BCP 8 in large amounts. Among the solvents examined (entries 1–6), ethers (entries 5 and 6) were best for keeping the 7a/8 ratio at an optimal level. Decreasing the stoichiometry of propellane 1 and NIS further decreased the amount of unwanted 8 (entries 8 and 9). Conversely, the extent of formation of 8 was increased when molecular iodine was used instead of NIS (entry 10). Similarly, the conditions previously reported by Zarate and co-workers for the attack of 1 by 4-iodo-pyrazole in the presence of I2 and Cs2CO3 in MeCN21 led to unfavorable 7a/8 ratios when applied to 2 (Table S1). Finally, attempts to extend this electrophilic activation with N-bromo- and N-chlorosuccinimide did not afford the expected BCP products.
Table 1. Optimization of the Reaction Conditionsa,b.
| run | xc | iodination reagent | solvent | yield of 7a (%) | yield of 8 (%) |
|---|---|---|---|---|---|
| 1 | 1.5 | NIS (1.5 equiv) | acetone | 80 | 28 |
| 2 | 1.5 | NIS (1.5 equiv) | CH2Cl2 | 77 | 11 |
| 3 | 1.5 | NIS (1.5 equiv) | EtOAc | 80 | 18 |
| 4 | 1.5 | NIS (1.5 equiv) | toluene | 0 | 0 |
| 5 | 1.5 | NIS (1.5 equiv) | Et2O | 98 | 10 |
| 6 | 1.5 | NIS (1.5 equiv) | MTBE | 99 | 12 |
| 7 | 1.5 | NIS (1.1 equiv) | MTBE | 99 | 7 |
| 8 | 1.1 | NIS (1.1 equiv) | MTBE | 99 | 2 |
| 9 | 1.1 | NIS (1.0 equiv) | MTBE | 99 | 2 |
| 10 | 1.5 | I2 (0.75 equiv) | MTBE | 36 | 42 |
Reactions conducted with 0.2 mmol of 2 (0.2 M) and using a 0.85–1.10 M solution of 1 in Et2O.
Yields determined by 1H NMR with CH2Cl2 as the internal standard. MTBE denotes methyl tert-butyl ether.
Number of equivalents of 1.
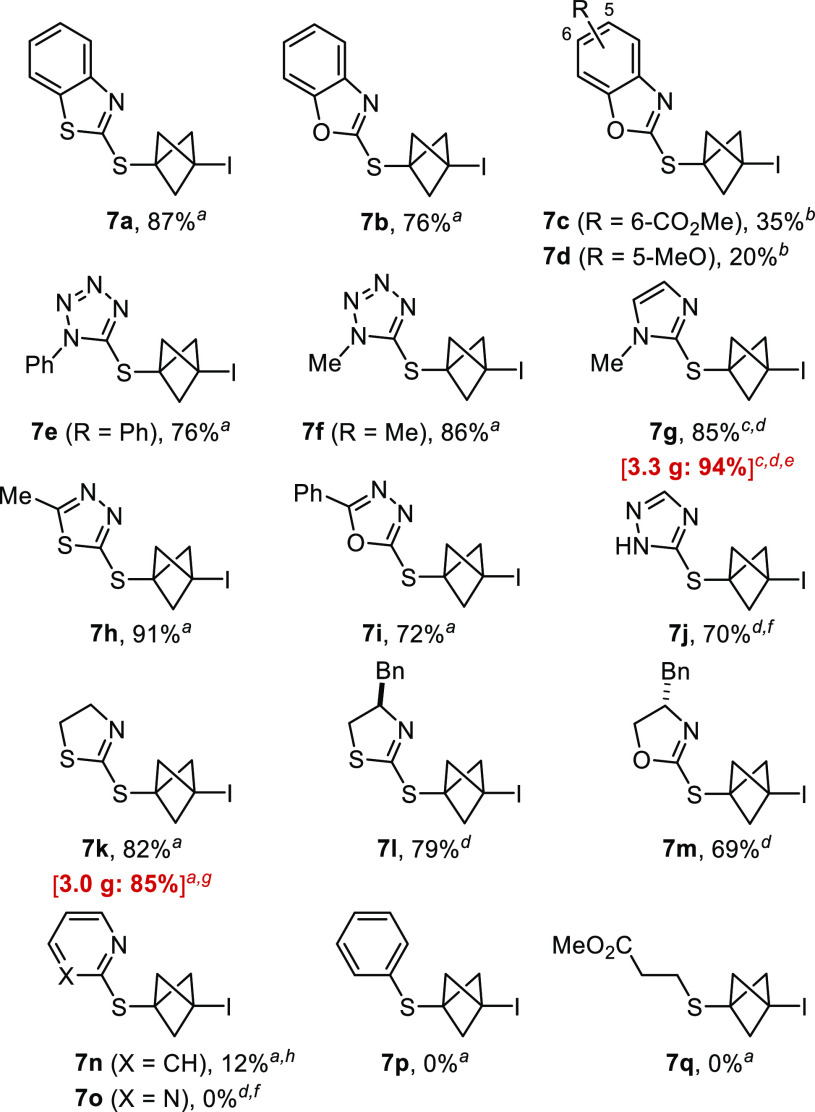
With the optimized conditions in hands, we examined the generality of the reaction with a set of diverse mercapto reagents and were delighted to obtain 7a–n in 11–94% yields as air-stable compounds (Figure 2).22 Hence, mercapto reagents 2–4, which previously failed to react with propellane 1 without an electrophilic activating reagent,12 gave 7a, 7j, and 7k, respectively, readily in the presence of NIS. It is noteworthy that the reaction does not require any dry reagents or solvents. In the case of 7g, it was necessary to use 1,3-diiodo-hydantoin (DIH) instead of NIS for ease of purification, and the reaction was conducted at room temperature after adding the reagents at −10 °C because of the poor solubility of the starting material at −78 °C. These conditions and the conditions optimized in entry 9 of Table 1 were compatible with reactions conducted on a multigram scale, as shown by the excellent yields of 7g (94%) and 7k (85%) thus obtained. It is also noteworthy that the clean conversion of the starting materials to these compounds allowed for purification by simple filtration of the crude material over a short pad of silica gel. The stoichiometry of the mercapto reagent in the reaction leading to 7j was slightly increased compared to that under the optimized conditions due to the poor solubility of this starting material.
Figure 2.
Iodo-sulfenylation of propellane 1. Yields of pure isolated products. aSame reaction conditions as in entry 9 of Table 1, except as otherwise noted. bIn acetone. cDIH (0.50 equiv) instead of NIS. dAt −10 °C for 10 min and then room temperature for 1 h. eOn 11.4 mmol of mercapto reagent. fMercapto reagent (1.5 equiv), NIS (1.1 equiv), and 1 (1.0 equiv). gOn 11.2 mmol of mercapto reagent. hMercapto reagent (1.0 equiv), NIS (1.0 equiv), and 1 (2.0 equiv).
In contrast to the 10 classes of heterocyclic thiols that showed the desired reactivity to give 7a, 7b, and 7e–m, electronic variation of the benzo[d]oxazole ring led to decreased yields in the case of 7c and 7d (Scheme 1). In these two cases, the solubility of the starting thiols was low in MTBE and we switched the solvent to acetone. However, the solubility remained problematic, which led to incomplete conversion and the isolation of 1,3-bisiodo-BCP 8 as a side product in 27% and 29% yields. Moreover, 2-mercaptopyridine gave 7n in only low yield, whereas 2-mercaptopyrimidine, thiophenol, and an alkyl thiol failed to give 7o–q entirely. The disulfides resulting from the oxidation of the thiols were the major components of the crude mixtures in these four cases.
Scheme 1. Functional Group Tolerance.
Yields of isolated products.
Additive recovered in >80% yield (see the Supporting Information).
With 8 (31%).
At a 4/1 7a/8 ratio (crude 1H NMR).
With 8 (23%).
Recovery of 15 not attempted.
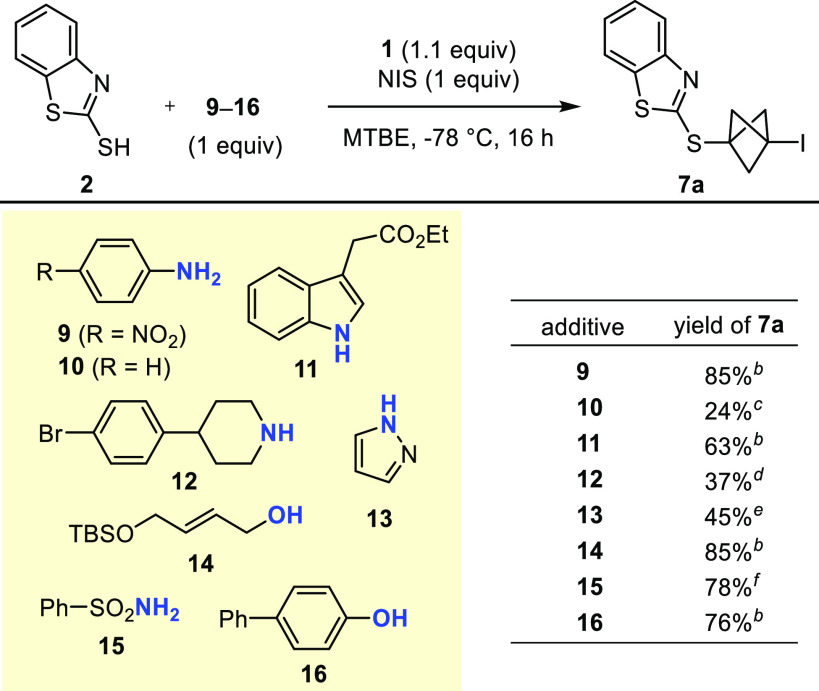
The functional group tolerance of the reaction was evaluated with 2-mercaptobenzothiazole 2 in the presence of nucleophilic additives 9–16 (Scheme 2). The expected BCP 7a was obtained in all cases, albeit in varied yields. Importantly, no BCP adduct was formed from 9–16 in those reactions, even in cases in which the yield of isolated 7a was lower than in the absence of those additives. Thus, whereas electron-poor aniline 9 reacted smoothly with propellane 1 and NIS at −78 °C to give a stable iodinated BCP when no other nucleophile was present,20 treating an equimolar mixture of 2 and 9 under similar conditions left 9 intact and gave 7a exclusively. Other nucleophiles, i.e., indole 11, alcohol 14, sulfonamide 15, and phenol 16, were also perfectly well tolerated to give good to high yields of 7a. In contrast, adding electron-neutral aniline 10, amine 12, and pyrazole 13 led to a decreased yield of 7a and a sizable amount of 1,3-bisiodo-BCP 8.
Scheme 2. Control Reactions.
(a) Reaction of 2-mercaptobenzothiazole with NIS and treatment of the crude thus obtained with [1.1.1]propellane and (b) reactions in the presence of radical inhibitors. All yields determined by 1H NMR with an internal standard. BHT denotes 2,6-bis(tert-butyl)-4-methylphenol, and TEMPO 2,2,5,5-tetramethyl-4-piperidin-1-oxyl.
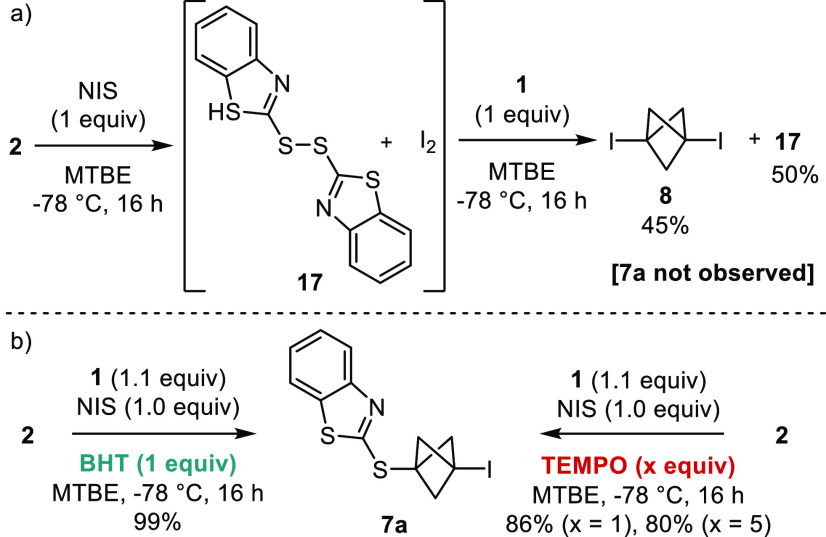
To gain insight into the mechanism of this reaction, we treated 2-mercaptobenzothiazole 2 with NIS in the absence of propellane 1, which led to a mixture of disulfide 17 and molecular iodine (Scheme 2a). Importantly, when this crude mixture was treated with 1, only 1,3-bis-iodo-BCP 8 (45%) and 17 (50%) were obtained, whereas S-BCP 7a was not observed. In addition, treating 8 with 2 did not lead to the formation of 7a (see the Supporting Information). These results suggest that a hypoiodothioite intermediate, or a S···I bond complex23 formed between NIS and the thione tautomer of the mercapto reagent, is not involved in the formation of S-BCPs 7a–m. Moreover, the reactions of 1 with 2 and NIS under the optimized conditions but in the presence of radical inhibitors BHT and TEMPO led to the formation of the expected S-BCP 7a in excellent to quantitative yields (Scheme 2b). Taken together, these results make a radical mechanism for the iodo-sulfenylation of 1 with 2-mercapto-azoles and NIS less likely.
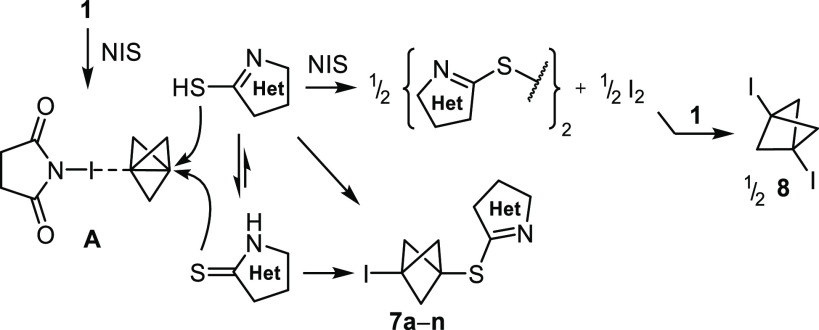
Accordingly, we propose that the formation of S-BCPs 7a–m proceeds by the electrophilic activation of propellane 1 in halogen bond complex A formed with the electrophilic N-iodo reagent (Scheme 3). As previously established,20 the analysis of Fukui’s dual descriptor24 indicates that the nucleophilic interbridgehead bond of propellane 1 is rendered electrophilic in A, which is a true minimum with a binding energy of −4.5 kcal mol–1. The high yields of formation of 7a–m contrast with the absence of S-BCPs 7o and 7p when model aryl and alkyl thiols were used. These opposite results might be explained by the predominance of the thione tautomer of the 2-mercapto-azoles in solution.19 Thus, the low concentration of the thiol tautomer of the 2-mercapto-azoles would contribute to the high yields of 7a–m as it would favor the selective reaction of NIS with 1 to give A over the reaction of NIS with the thiol. The latter pathway leads to the formation of disulfides and molecular iodine, and eventually 1,3-bis-iodo-BCP 8, and is therefore detrimental to the formation of 7a–m. This unproductive pathway was followed by aryl and alkyl thiols that failed to give 7p and 7q because a tautomeric equilibrium toward a thione is not possible. In agreement with this interpretation, treating an equimolar mixture of 2-mercapto-benzothiazole 2 and thiophenol under the optimized conditions led to the quantitative formation of phenyl disulfide and the recovery of 2 in 68% yield, whereas S-BCP 7a was not formed. Once A is formed selectively, it is not certain which of the thione or thiol tautomers of the 2-mercapto-azoles reacts with this intermediate to give 7a–m. In the case of 2-mercaptopyridine and 2-mercaptopyrimidine, we assume that the efficient formation of 7n and 7o could be hampered by either (i) lower oxidation potentials compared to those of the other 2-mercapto-azoles,25 (ii) greater aromatic character in both of its tautomeric forms that would decrease nucleophilicity,26 or (iii) a combination of the two.
Scheme 3. Plausible Mechanism.
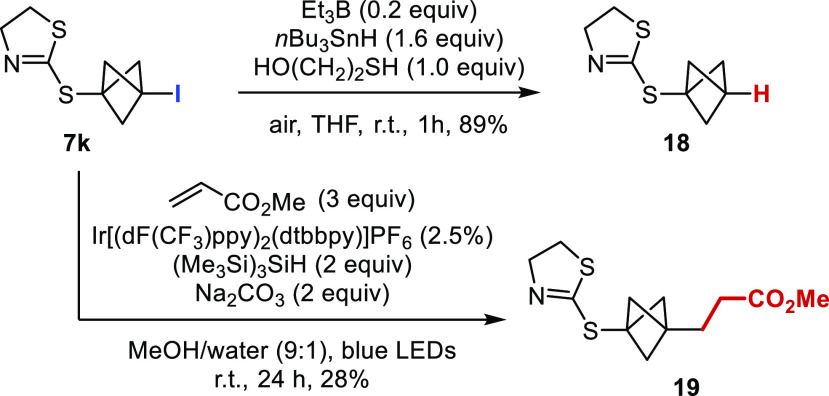
Finally, the conversion of the C–I bond of the S-BCP into other bonds under radical conditions proved to be challenging. Thus, for model substrates 7a, 7e, and 7g, attempts to reduce the C–I bond or to engage these compounds into a Giese reaction led to decomposition by cleavage of the C(sp3)–S bond of the starting material. However, thiazoline derivative 7k was more stable under the same reaction conditions (Scheme 4), and we could obtain the reduced S-BCP 18 in excellent yield. It is noteworthy that 18 is a direct bioisosteric analogue of biocide 6. Similarly, compound 19 was obtained after Giese reaction under the conditions recently described by Anderson and co-workers.3n The moderate yield of 19 is due to the need to perform a purification by preparative TLC of the material obtained after a first purification by flash chromatography.
Scheme 4. Conversion of the C–I Bond.
Yields of isolated product.
In conclusion, we have demonstrated that the electrophilic activation of [1.1.1]propellane with NIS or DIH can address the lack of reactivity of heterocyclic thiols for the synthesis of sulfur-substituted bicyclo[1.1.1]pentanes. The procedure can be conducted on a multigram scale and does not require exclusion of air or moisture. We anticipate that this method could benefit the future exploration of the potential benefits of S-BCPs in the optimization of the bioactivity of drugs and agrochemicals.
Acknowledgments
S.L. thanks the EPSRC for a studentship (EP/N509693/1) and AstraZeneca, Eli Lilly, Syngenta, and LiverpoolChiroChem for a CASE award. The authors thank Dr. John Ward (University of Liverpool) for giving us access to blue LED lamps and Dr. William Ashworth (AstraZeneca) for a DSC test.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c02875.
Experimental details for the synthesis of 7a–n, control experiments, DSC data (7e), and characterization data of new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For selected examples, see:; a Stepan A. F.; Subramanyam C.; Efremov I. V.; Dutra J. K.; O’Sullivan T. J.; DiRico K. J.; McDonald W. S.; Won A.; Dorff P. H.; Nolan C. E.; Becker S. L.; Pustilnik L. R.; Riddell D. R.; Kauffman G. W.; Kormos B. L.; Zhang L.; Lu Y.; Capetta S. H.; Green M. E.; Karki K.; Sibley E.; Atchison K. P.; Hallgren A. J.; Oborski C. E.; Robshaw A. E.; Sneed B.; O’Donnell C. J. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 2012, 55, 3414. 10.1021/jm300094u. [DOI] [PubMed] [Google Scholar]; b Westphal M. V.; Wolfstädter B. T.; Plancher J. M.; Gatfield J.; Carreira E. M. Evaluation of tert-Butyl Isosteres: Case Studies of Physicochemical and Pharmacokinetic Properties, Efficacies, and Activities. ChemMedChem. 2015, 10, 461. 10.1002/cmdc.201402502. [DOI] [PubMed] [Google Scholar]; c Auberson Y. P.; Brocklehurst C.; Furegati M.; Fessard T. C.; Koch G.; Decker A.; La Vecchia L.; Briard E. Improving Nonspecific Binding and Solubility: Bicycloalkyl Groups and Cubanes as para-Phenyl Bioisosteres. ChemMedChem. 2017, 12, 590. 10.1002/cmdc.201700082. [DOI] [PubMed] [Google Scholar]; d Measom N. D.; Down K. D.; Hirst D. J.; Jamieson C.; Manas E. S.; Patel V. K.; Somers D. O. Investigation of a Bicyclo [1.1. 1] pentane as a Phenyl Replacement within an LpPLA2 Inhibitor. ACS Med. Chem. Lett. 2017, 8, 43. 10.1021/acsmedchemlett.6b00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent reviews, see:; a Kanazawa J.; Uchiyama M. Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. Synlett 2019, 30, 1. 10.1055/s-0037-1610314. [DOI] [Google Scholar]; b Ma X.; Nhat Pham L. Selected topics in the syntheses of bicyclo[1.1.1]pentane (BCP) analogues. Asian J. Org. Chem. 2020, 9, 8. 10.1002/ajoc.201900589. [DOI] [Google Scholar]; c He F.-S.; Xie S.; Yao Y.; Wu J. Recent advances in the applications of [1.1.1]propellane in organic synthesis. Chin. Chem. Lett. 2020, 31, 3065. 10.1016/j.cclet.2020.04.023. [DOI] [Google Scholar]
- For selected examples, see:Gianatassio R.; Lopchuk J. M.; Wang J.; Pan C.; Malins L. R.; Prieto L.; Brandt T. A.; Collins M. R.; Gallego G. M.; Sach N. W.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-release amination. Science 2016, 351, 241. 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kanazawa J.; Maeda K.; Uchiyama M. Radical Multicomponent Carboamination of [1.1.1]Propellane. J. Am. Chem. Soc. 2017, 139, 17791. 10.1021/jacs.7b11865. [DOI] [PubMed] [Google Scholar]; c Caputo D. F.; Arroniz C.; Dürr A. B.; Mousseau J. J.; Stepan A. F.; Mansfield S. J.; Anderson E. A. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. 2018, 9, 5295. 10.1039/C8SC01355A. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Shelp R. A.; Walsh P. J. Synthesis of BCP Benzylamines From 2-Azaallyl Anions and [1.1.1]Propellane. Angew. Chem., Int. Ed. 2018, 57, 15857. 10.1002/anie.201810061. [DOI] [PubMed] [Google Scholar]; e Nugent J.; Arroniz C.; Shire B. R.; Sterling A. J.; Pickford H. D.; Wong M. L.; Mansfield S. J.; Caputo D. F.; Owen B.; Mousseau J. J.; Duarte E. A.; Anderson E. A. A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal. 2019, 9, 9568. 10.1021/acscatal.9b03190. [DOI] [Google Scholar]; f Hughes J. M. E.; Scarlata D. A.; Chen A. C.; Burch J. D.; Gleason J. L. Aminoalkylation of [1.1.1]Propellane Enables Direct Access to High-Value 3-Alkylbicyclo[1.1.1]pentan-1-amines. Org. Lett. 2019, 21, 6800. 10.1021/acs.orglett.9b02426. [DOI] [PubMed] [Google Scholar]; g Trongsiriwat N.; Pu Y.; Nieves-Quinones Y.; Shelp R. A.; Kozlowski M. C.; Walsh P. J. Reactions of 2-Aryl-1, 3-Dithianes and [1.1.1]Propellane. Angew. Chem., Int. Ed. 2019, 58, 13416. 10.1002/anie.201905531. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Kondo M.; Kanazawa J.; Ichikawa T.; Shimokawa T.; Nagashima Y.; Miyamoto K.; Uchiyama M. Silaboration of [1.1.1]propellane: a storable feedstock for bicyclo[1.1.1]pentane derivatives. Angew. Chem., Int. Ed. 2020, 59, 1970. 10.1002/anie.201909655. [DOI] [PubMed] [Google Scholar]; i Nugent J.; Shire B. R.; Caputo D. F.; Pickford H. D.; Nightingale F.; Houlsby I. T.; Mousseau J. J.; Anderson E. A. Synthesis of all-carbon disubstituted bicyclo[1.1.1]pentanes by iron-catalyzed Kumada cross-coupling. Angew. Chem., Int. Ed. 2020, 59, 11866. 10.1002/anie.202004090. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhang X.; Smith R. T.; Le C.; McCarver S. J.; Shireman B. T.; Carruthers N. I.; MacMillan D. W. C. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 2020, 580, 220. 10.1038/s41586-020-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Kim J. H.; Ruffoni A.; Al-Faiyz Y. S. S.; Sheikh N. S.; Leonori D. Divergent Strain-Release Amino-Functionalization of [1.1.1]Propellane with Electrophilic Nitrogen-Radicals. Angew. Chem., Int. Ed. 2020, 59, 8225. 10.1002/anie.202000140. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Shin S.; Lee S.; Choi W.; Kim N.; Hong S. Visible-LightInduced 1,3-Aminopyridylation of [1.1.1]Propellane with N-Aminopyridinium Salts. Angew. Chem., Int. Ed. 2021, 60, 7873. 10.1002/anie.202016156. [DOI] [PubMed] [Google Scholar]; m Pickford H. D.; Nugent J.; Owen B.; Mousseau J. J.; Smith R. C.; Anderson E. A. J. Am. Chem. Soc. 2021, 143, 9729. 10.1021/jacs.1c04180. [DOI] [PubMed] [Google Scholar]; n Wong M. L.; Sterling A. J.; Mousseau J. J.; Duarte F.; Anderson E. A. Direct catalytic asymmetric synthesis of α-chiral bicyclo[1.1.1]pentane. Nat. Commun. 2021, 12, 1644. 10.1038/s41467-021-21936-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Huang W.; Keess S.; Molander G. A. Dicarbofunctionalization of [1.1.1]Propellane Enabled by Nickel/Photoredox Dual Catalysis: One-Step Multicomponent Strategy for the Synthesis of BCP-Aryl Derivatives. J. Am. Chem. Soc. 2022, 144, 12961. 10.1021/jacs.2c05304. [DOI] [PubMed] [Google Scholar]
- Smith B. R.; Eastman C. M.; Njardarson J. T. Beyond C, H, O, and N! Analysis of the Elemental Composition of U.S. FDA Approved Drug Architectures. J. Med. Chem. 2014, 57, 9764. 10.1021/jm501105n. [DOI] [PubMed] [Google Scholar]
- A SciFinder search (January 2022) revealed >400 patents for N-BCPs, >50 patents for O-BCPs, and six patents for S-BCPs among patents that disclose bioactive BCPs.
- a Semmler K.; Szeimies G.; Belzner J. Tetracyclo[5.1.0.01,6.02,7]octane, a [1.1.1]propellane derivative, and a new route to the parent hydrocarbon. J. Am. Chem. Soc. 1985, 107, 6410. 10.1021/ja00308a053. [DOI] [Google Scholar]; b Wiberg K. B.; Waddell S. T. Reactions of [1.1.1]propellane. J. Am. Chem. Soc. 1990, 112, 2194. 10.1021/ja00162a022. [DOI] [Google Scholar]; c Bär R. M.; Kirschner S.; Nieger M.; Bräse S. Alkyl and aryl thiol addition to [1.1.1]propellane: scope and limitations of a fast conjugation reaction. Chem. - Eur. J. 2018, 24, 1373. 10.1002/chem.201704105. [DOI] [PubMed] [Google Scholar]
- Bär R. M.; Heinrich G.; Nieger M.; Fuhr O.; Bräse S. Insertion of [1.1.1]propellane into aromatic disulfides. Beilstein J. Org. Chem. 2019, 15, 1172. 10.3762/bjoc.15.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout S. K.; Marghem G.; Lan J.; Leyssens T.; Riant O. A radical exchange process: synthesis of bicyclo[1.1.1]pentane derivatives of xanthates. Chem. Commun. 2019, 55, 14976. 10.1039/C9CC07610G. [DOI] [PubMed] [Google Scholar]
- a Wu Z.; Xu Y.; Wu X.; Zhu C. Synthesis of selenoether and thioether functionalized bicyclo[1.1.1]pentanes. Tetrahedron 2020, 76, 131692. 10.1016/j.tet.2020.131692. [DOI] [Google Scholar]; b Wu Z.; Xu Y.; Liu J.; Wu X.; Zhu C. A practical access to fluoroalkylthio(seleno)-functionalized bicyclo[1.1.1]pentanes. Sci. China Chem. 2020, 63, 1025. 10.1007/s11426-020-9733-y. [DOI] [Google Scholar]
- a Wu Z.; Xu Y.; Zhang H.; Wu X.; Zhu C. Radical-mediated sulfonyl alkynylation, allylation, and cyanation of propellane. Chem. Commun. 2021, 57, 6066. 10.1039/D1CC02249K. [DOI] [PubMed] [Google Scholar]; b Wei Y.; Chen Z.; Wu Z.; Xu Y.; Wu X.; Zhu C. Radical Carbosulfonylation of Propellane: Synthesis of Sulfonyl β-Keto-bicyclo[1.1.1]pentanes. Synthesis 2021, 53, 3325. 10.1055/a-1484-1028. [DOI] [Google Scholar]
- a Bär R. M.; Gross P. J.; Nieger M.; Bräse S. Sodium Bicyclo[1.1.1]pentanesulfinate: A Bench-Stable Precursor for Bicyclo[1.1.1]pentylsulfones and Bicyclo[1.1.1]pentanesulfonamides. Chem. - Eur. J. 2020, 26, 4242. 10.1002/chem.202000097. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kokhan S. O.; Valter Y. B.; Tymtsunik A. V.; Komarov I. V.; Grygorenko O. O. 3-Carboxy-/3-Aminobicyclo[1.1.1]pentane-Derived Sulfonamides and Sulfonyl Fluorides–Advanced Bifunctional Reagents for Organic Synthesis and Drug Discovery. Eur. J. Org. Chem. 2020, 2020, 2210. 10.1002/ejoc.202000303. [DOI] [Google Scholar]
- On the basis of our own observations for reagents 2 and 3 (see the Supporting Information). For 4, see:Donnelly K.; Baumann M. A continuous flow synthesis of [1.1.1]propellane and bicyclo[1.1.1]pentane derivatives. Chem. Commun. 2021, 57, 2871. 10.1039/D0CC08124H. [DOI] [PubMed] [Google Scholar]
- Herrera Cano N.; Ballari M. S.; Lopez A. G.; Santiago A. N. New synthesis and biological evaluation of benzothiazole derivates as antifungal agents. J. Agri. Food Chem. 2015, 63, 3681. 10.1021/acs.jafc.5b00150. [DOI] [PubMed] [Google Scholar]
- Yamashita M.; Sakai K.; Kondo S.. 2-Substituted thiothiazoline derivatives. JP60184070 A, 1985.
- Rates measured in Freon-113: ki = (6.2 ± 2.0) × 107 M–1 s–1 and k–i ≈ 6.8 × 107 s–1:McGarry P. F.; Johnston L. J.; Scaiano J. C. Addition of oxygen-and sulfur-centered radicals to [1.1.1]propellane. J. Org. Chem. 1989, 54, 6133. 10.1021/jo00287a033. [DOI] [Google Scholar]
- a Ito I.; Matsuda M. New Dual Parameters for Radical Reactivity of Vinyl Monomers. Prog. Polym. Sci. 1992, 17, 827. 10.1016/0079-6700(92)90011-M. [DOI] [Google Scholar]; b Lalevee J.; Allonas X.; Morlet-Savary F.; Fouassier J. P. Respective contributions of polar vs enthalpy effects in the addition/fragmentation of mercaptobenzoxazole-derived thiyl radicals and analogues to double bonds. J. Phys. Chem. A 2006, 110, 11605. 10.1021/jp062991u. [DOI] [PubMed] [Google Scholar]; c Lalevée J.; Morlet-Savary F.; Roz M. E.; Allonas X.; Fouassier J. P. Thiyl radical generation in thiol or disulfide containing photosensitive systems. Macromol. Chem. Phys. 2009, 210, 311. 10.1002/macp.200800566. [DOI] [Google Scholar]
- Roberts B. P. Chem. Soc. Rev. 1999, 28, 25. 10.1039/a804291h. [DOI] [Google Scholar]
- a As inferred from the lower pKa of heteroaromatic thiols:Yu H.-Z.; Yang Y.-M.; Zhang L.; Dang Z.-M.; Hu G. H. Quantum-Chemical Predictions of pKa’s of Thiols in DMSO. J. Phys. Chem. A 2014, 118, 606. 10.1021/jp410274n. [DOI] [PubMed] [Google Scholar]; b The transfer of the hydrogen atom to C-centered radicals is slower for more acidic thiols:Munar I.; Fındık V.; Degirmenci I.; Aviyente V. Solvent effects on thiol–ene kinetics and reactivity of carbon and sulfur radicals. J. Phys. Chem. A 2020, 124, 2580. 10.1021/acs.jpca.9b10165. [DOI] [PubMed] [Google Scholar]
- a Minkin V. I.; Garnovskii A. D.; Elguero J.; Katritzky A. R.; Denisko O. V. Tautomerism of heterocycles: five-membered rings with two or more heteroatoms. Adv. Heterocycl. Chem. 2000, 76, 157. 10.1016/S0065-2725(00)76005-3. [DOI] [Google Scholar]; b Moran D.; Sukcharoenphon K.; Puchta R.; Schaefer H. F.; Schleyer P. V. R.; Hoff C. D. 2-pyridinethiol/2-pyridinethione tautomeric equilibrium. A comparative experimental and computational study. J. Org. Chem. 2002, 67, 9061. 10.1021/jo0263768. [DOI] [PubMed] [Google Scholar]
- Livesley S.; Sterling A. J.; Robertson C. M.; Goundry W. R. F.; Morris J. A.; Duarte F.; Aïssa C. Electrophilic Activation of [1.1.1]Propellane for the Synthesis of Nitrogen-Substituted Bicyclo[1.1.1]pentanes. Angew. Chem., Int. Ed. 2022, 61, e202111291 10.1002/anie.202111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C.; Ardolino M.; Morriello G. J.; Logan K. M.; Kaplan W. P.; Torres L.; Li D.; Chen M.; Li H.; Su J.; Fuller P.; Maddess M. L.; Song Z. J. Development of Scalable Routes to 1-Bicyclo[1.1.1]pentylpyrazoles. Org. Process Res. Dev. 2021, 25, 642. 10.1021/acs.oprd.0c00446. [DOI] [Google Scholar]
- CAUTION: A DSC test shows that compound 7e is a potential explosive and has a high risk of being sensitive to shock (see the Supporting Information), even if we have not observed any adverse event with any of 7a–n in this study.
- a Lorpaiboon W.; Bovonsombat P. Halogen bond-induced electrophilic aromatic halogenations. Org. Biomol. Chem. 2021, 19, 7518. 10.1039/D1OB00936B. [DOI] [PubMed] [Google Scholar]; b Isaia F.; Aragoni M. C.; Arca M.; Demartin F.; Devillanova F. A.; Floris G.; Garau A.; Hursthouse M. B.; Lippolis V.; Medda R.; Oppo F.; Pira M.; Verani G. Interaction of Methimazole with I2: X-ray Crystal Structure of the Charge Transfer Complex Methimazole– I2. Implications for the Mechanism of Action of Methimazole-Based Antithyroid Drugs. J. Med. Chem. 2008, 51, 4050. 10.1021/jm8001857. [DOI] [PubMed] [Google Scholar]; c Tamilselvi A.; Mugesh G. Interaction of heterocyclic thiols/thiones eliminated from cephalosporins with iodine and its biological implications. Bioorg. Med. Chem. Lett. 2010, 20, 3692. 10.1016/j.bmcl.2010.04.087. [DOI] [PubMed] [Google Scholar]; d Daga V.; Hadjikakou S. K.; Hadjiliadis N.; Kubicki M.; Santos J. H. D.; Butler I. S. Synthesis, Spectroscopic and Structural Characterization of Novel Diiodine Adducts with the Heterocyclic Thioamides, Thiazolidine-2-thione (tzdtH), Benzothiazole-2-thione (bztzdtH) and Benzimidazole-2-thione (bzimtH). Eur. J. Inorg. Chem. 2002, 2002, 1718.. [DOI] [Google Scholar]
- Morell C.; Grand A.; Toro-Labbé A. New dual descriptor for chemical reactivity. J. Phys. Chem. A 2005, 109, 205. 10.1021/jp046577a. [DOI] [PubMed] [Google Scholar]
- Corban G. J.; Antoniadis C. D.; Hadjikakou S. K.; Kourkoumelis N.; Tyurin V. Y.; Dolgano A.; Milaeva E. R.; Kubicki M.; Bernhardt P. V.; Tiekink E. R. T.; Skoulika S.; Hadjiliadis N. Reactivity of di-iodine toward thiol: Desulfuration reaction of 5-nitro-2-mercapto-benzimidazole upon reaction with di-iodine. Heteroatom Chem. 2012, 23, 498. 10.1002/hc.21042. [DOI] [Google Scholar]
- Katritzky A. R.; Jug K.; Oniciu D. C. Quantitative Measures of Aromaticity for Mono-, Bi-, and Tricyclic Penta- and Hexaatomic Heteroaromatic Ring Systems and Their Interrelationship. Chem. Rev. 2001, 101, 1421. 10.1021/cr990327m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.