The development of hepatic encephalopathy (HE) marks a significant transition in the natural history of cirrhosis. Following a diagnosis of HE, the median survival for persons with cirrhosis is foreshortened substantially to 2 years,1 1 year if over 65 years old.2 HE occurs in as many as 40% of patients with cirrhosis. Although it is more common among those with portal hypertension and alcohol-related liver disease (ALD), it is also the most common first decompensation event among those with nonalcoholic fatty liver disease (NAFLD).3,4 As the epidemiology of cirrhosis shifts to reflect a higher prevalence of NAFLD and its comorbidities as well as ALD, these changes will transform the contemporary epidemiology of HE. Herein, we examine the prevalence of HE and how shifts in the etiology of cirrhosis will affect the burden and clinical course of this debilitating disease (Figure 1).
Figure 1.
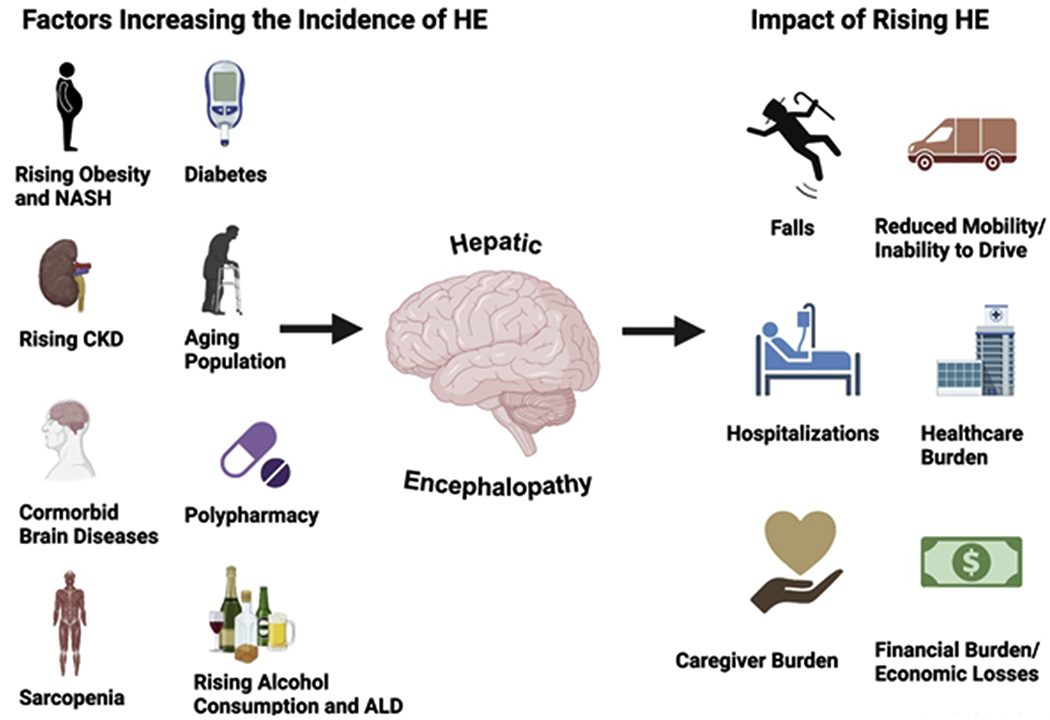
Contributors to the epidemiology of HE and their impact. Many comorbidities and lifestyle behaviors contribute to the development of HE. The impact of HE is broad and has consequences for the individual, their household and community, and the health care system. CKD, Chronic kidney disease; NASH, nonalcoholic steatohepatitis.
The Spectrum of HE
HE occurs along a spectrum that can broadly be dichotomized into covert HE (CHE) and overt HE (OHE). The former involves subclinical neurocognitive deficits identified on psychometric testing, whereas the latter involves varying degrees of disorientation and alertness.5 Across the HE spectrum, HE is challenging due to its unpredictability and its ability to impair multiple facets of physical, social, and mental functioning.
Prevalence of Covert HE
The prevalence of CHE is high but variable between studies and settings for multiple reasons. First, the prevalence depends on the testing strategy. Most are classified as psychometric tests. These include the gold-standard timed paper-pencil Psychometric Hepatic Encephalopathy Score (PHES) test, the 1-minute Animal Naming test, and the computerized EncephalApp Stroop.6 The prevalence of CHE, based on PHES testing, is 20.3% to 37% in persons with cirrhosis.7–9 However, prevalence increases to 54% when minimal hepatic encephalopathy (MHE) is diagnosed according to the performance on the Stroop EncephalApp.9 Second, CHE is more prevalent in later stages of cirrhosis. The prevalence is 27%, 42%, and 60% in patients with Child A, B, and C cirrhosis, respectively.10 Third, despite the wide prevalence of MHE, the full burden of disease is likely underestimated. Although 84% of providers recognize the clinical importance of CHE, 38% have never performed diagnostic testing for MHE.11 Fourth, tests which rely on psychometric performance can be directly affected by social determinants of health, smoking, diabetes, and alcohol use, which vary from region to region.12
Incidence of Overt Hepatic Encephalopathy
The incidence of HE varies according to the stage of disease at enrollment and the means of outcome ascertainment. Large databases provide the most generalizable estimates but rely on indirect outcome assessments. However, HE-related International Classification of Diseases (ICD) codes and HE-specific medications (lactulose/rifaximin prescription) are highly specific to identify OHE.13 For example, in patients enrolled in Medicare with Part D prescription coverage followed for a median 5.3 years, HE-related ICD codes and prescription use found that the incidence rate of HE was 11.6 per 100 patient-years.3 A multistate study of 1979 patients from the Veterans Administration showed an incidence of HE of 43.7% over 5 years.14 Prospective studies enhance rigor using clinically confirmed OHE as an outcome, but selected cohorts lack generalizability. The incidence proportion of patients with Child A cirrhosis developing a first-time episode of OHE is 10% at 1 year; increasing to 25% in patients with Child B cirrhosis.15 In patients with NAFLD, the incidence rate of hepatic encephalopathy in persons with F4 fibrosis was 2.39 per 100 person-years during a median follow-up of 4 years.4
Contributors to the Prevalence of HE
The key clinical factors that impact the prevalence of HE include the disease etiology and their related extrahepatic comorbidities. HE predicts mortality independent of Model for End-Stage Liver Disease because it is a biomarker of disordered homeostasis, capturing effects unmeasured by the Model for End-Stage Liver Disease such as sarcopenia, malnutrition, frailty, portal hypertension, and cognitive reserve. We address these factors below.
NAFLD
By 2030, it is estimated that the overall proportion of liver transplant waiting list entry NAFLD cirrhosis will increase by more than 50% of the 2016 level.16 This has important implications on the prevalence and natural history of HE. In a prospective multicenter study of patients with biopsy-proven NAFLD, HE was the most frequent first decompensating event.4 Although ascites is traditionally identified as the most frequent decompensating event,17 multiple mechanisms may promote the development of HE in NAFLD cirrhosis over other decompensating events.
Diabetes.
The prevalence of diabetes is as high as 42% in persons with NAFLD.4 Independent of the presence of NAFLD, diabetes and glycemic control is a risk factor for the development of HE in patients with cirrhosis.18 In a cohort of 862 patients with cirrhotic ascites, Jepsen et al observed a higher 1-year risk of incident OHE in those with diabetes compared with those without (26% vs 18.1%).19 Furthermore, comorbid diabetes was associated with an 86% increased risk of developing HE.19 There are at least 3 reasons diabetes raises the risk of HE. First, diabetes causes autonomic neuropathy. This can slow intestinal transit, promoting constipation and bacterial overgrowth.20 Bacterial overgrowth along with increased bacterial translocation can further exacerbate the role of inflammation in the pathophysiology of HE.21,22 Second, diabetes causes chronic kidney disease. In addition to the liver and skeletal tissue, the kidneys are an important site for ammonia metabolism to glutamine; therefore, renal impairment promotes hyperammonemia and the risk of HE.23 Third, the neurocognitive consequences of diabetes, including increased permeability of the blood brain barrier and neuroinflammation related to hyperglycemia, can act synergistically with similar changes seen in HE.24
Obesity and Sarcopenia.
Obesity contributes to the metabolic syndrome that is associated with NAFLD. Several important body compositional changes can occur in the setting of obesity that promote HE. Emerging evidence suggests both NAFLD and obesity are independently associated with sarcopenia.25 NAFLD increases the odds of sarcopenic obesity by more than 6 times.26 Furthermore, fat deposition within muscle, or myosteatosis, is positively correlated to total body adiposity.27 In advanced chronic liver disease, sarcopenia and myosteatosis impair the ability of skeletal tissue to serve as a major site of ammonia metabolism, leading to an increased risk of HE.28,29
Aging.
The mean age of persons with newly diagnosed with cirrhosis is rising, which reflects the shift in cirrhosis etiology towards NAFLD. A statewide analysis found an increase in the mean age at diagnosis from 56.0 years in 2004 to 59.1 years in 2014.30 This trend is also reflected in the rising average age of patients listed for liver transplantation nationally (51.2 years to 55.7 years from 2002 to 2014).31 Furthermore, the prevalence of HE in this population is rising. From 2004 to 2014, there was a 1.10% per year percent change in the prevalence of HE in patients 65 years of age and older.30
The interaction between non-cirrhotic age-related cognitive changes and the presence of HE is an emerging area of study. The cognitive consequences of comorbidities – which increase with age – can increase susceptibility to and often overlap with the attention and psychomotor impairments seen in HE.32 Notably, although memory is typically preserved in CHE, the memory deficits associated with aging can synergistically interact with the neurocognitive dysfunction of HE, worsening health-related quality of life. Beyond neurocognitive changes, there are age-related body compositional changes that impact HE. Similar to skeletal muscle changes seen in obesity, myosteatosis and sarcopenia are more prevalent in older age and serves as an additional risk factor for the development of HE in this population.27,33
Hepatitis C Infection
Another link between cirrhosis etiology and the risk of OHE is seen with Hepatitis C infection. Sustained virologic response (SVR) after direct-acting antivirals for HCV treatment leads to a 59% decreased incidence of OHE and an additional 41% decreased risk in HE-related hospitalizations.34 Indeed, SVR was associated with a reduced risk of HE irrespective of the treatment regimen. These findings extend into psychometric testing for MHE. PHES testing performance improved significantly with SVR in a prospective study of patients with HCV with cirrhosis achieving SVR.35 Combined with efforts to screen and treat HCV prior to the development of cirrhosis,36 the landscape of HCV-related HE risk has been significantly modified in the direct-acting antiviral era.
Alcohol-related Liver Disease
The changing landscape of alcohol use and ALD in the United States will affect the development and course of HE. Alcohol consumption has increased in recent years, and more significantly since the COVID-19 pandemic.37,38 These trends have been mirrored by a rising prevalence of ALD and ALD-related mortality over the past decade.39,40 A recent modeling study estimated that the 1-year increase in alcohol consumption observed during the COVID-19 pandemic could account for 8000 additional ALD-related deaths and 18,700 additional cases of decompensated cirrhosis.41
Independent of the presence or absence of cirrhosis, chronic alcohol use is associated with negative neurologic sequelae.42 Longstanding alcohol consumption is associated with the development of Wernicke encephalopathy, Korsakoff syndrome, and alcohol-related dementia.42 Compared with patients with nonalcohol-related cirrhosis, patients with alcohol-related cirrhosis appear to have more brain atrophy,43 brain edema, and cortical damage.44 Other studies have shown increased benzodiazepine receptor affinity in alcohol-related cirrhosis, which could theoretically decrease the threshold for developing HE.45 Alcohol use disorder may also increase the development of known precipitants of HE, such as hypokalemia and hyponatremia.46 Altogether, alcohol’s effects on the brain and electrolyte homeostasis may increase the risk for the development of HE.
Heavy alcohol consumption in ALD impacts the risk of HE. The incidence and prevalence of overt HE is higher in patients with alcohol-related cirrhosis than cirrhosis from other causes.3,47 In a large study of Medicare patients, alcohol-related cirrhosis was most associated with incident HE.3 The relationship between alcohol-related cirrhosis and CHE is less clear. In one study involving comprehensive neuropsychological testing, patients with chronic alcohol use and cirrhosis exhibited worse working memory and executive function when compared with chronic alcohol misuse or cirrhosis alone, suggesting a possible synergistic effect.48 Another study found that CHE was more common in ALD (using both psychometrics and critical clicker frequency).49 Nonetheless, other studies have demonstrated no interaction between psychometric testing and alcohol use.50–52
Medication Use (and Misuse)
Proton Pump Inhibitors.
From 2002 to 2017, the percent of Americans overall using a proton pump inhibitor (PPI) rose from 5.70% to 6.73%.53 PPI use in cirrhosis is highly prevalent with greater than 40% of outpatients with an active prescription; often without a clear indication.54 In a sample of Medicare recipients enrolled in Part D prescription coverage, PPI use was not only common, but an independent predictor of the development of HE on follow-up.3 PPI use is also more common in those with than without CHE55; although multiple confounders exist. Nevertheless, these data relating PPI use with a potential increased risk of HE in cirrhosis should remind clinicians to deprescribe when safe.56
Opioids.
Psychoactive medications, including opioids, are widely used in both the inpatient and outpatient settings.57,58 Data from the Veterans Health Administration identified 47% of patients with cirrhosis prescribed an opioid for short- or long-term (54%) use in 2014; up from 36% in 2005.59 Forty-seven percent of patients with cirrhosis were prescribed an opioid during a 1-year period between 2015 and 2016 in a large single-center retrospective.60 Along with a myriad of adverse outcomes associated with long-term opioid use that are beyond the scope of this review, opioid use modifies the risk of HE. An analysis of 6451 privately insured patients with compensated cirrhosis found a significant increase in the incidence of HE with either short-term or long-term use of opioids compared with no opioid use.58 Results were similar in a cohort of older adults (median age, 65 years), where the occurrence of HE was 24% higher with opioid use.57 Though interestingly, no significant association between opioid use and HE risk was found in a Veterans Administration-based study when adjusting for liver disease severity, which may suggest population-level predictors needing further study.14 Similarly, benzodiazepines have been significantly linked with incident HE but not after adjusting for confounders.14,15,58 It is challenging to disentangle confounding by indication from these associations and therefore causality remains unclear.
Transjugular Intrahepatic Portosystemic Shunts
Transjugular intrahepatic portosystemic shunts (TIPS) are an important tool in the treatment refractory ascites and variceal bleeding. Unfortunately, post-TIPS portosystemic encephalopathy (PSE) can be a debilitating consequence. Some early studies revealed high rates (> 50%) of PSE following TIPS placement61; however, contemporary analyses underscore a changing epidemiology of post-TIPS PSE. Smaller diameter TIPS result in less PSE with equivalent ascites and bleeding control.62 A smaller (8 mm) diameter TIPS is associated with lower risk of PSE (27%) than 10-mm TIPS (43%).62 Furthermore, post-TIPS PSE occurred in 11% vs 29.5% of patients receiving a 6-mm vs an 8-mm diameter TIPS.63 Additionally, post-TIPS PSE risk can be reduced pharmacologically. A recent randomized controlled trial of 186 French patients showed that pre-TIPS, and thereafter, use of rifaximin leads to a 50% decrease in the odds of OHE (driven in part by patients whose lactulose was discontinued pre-TIPS).64 For these reasons, a prior history of OHE alone should not preclude the decision to pursue TIPS placement.65
The Public Health Burden of HE
Hospitalizations
Hospitalizations for HE are frequent, increasing, and costly.66 In 2006, national data from the Healthcare Cost and Utilization Project found a rate of 6.8 HE-related (ICD-9 code 572.2) hospital discharges per 100,000 persons; by 2015, the rate was 18.3.67 From 2010 to 2014, a 25% increase in hospitalizations for HE was found in an analysis using the National Inpatient Sample; an estimate of nearly 700,000 HE-related hospitalizations.68 During the same period, the cost of HE-related hospitalizations increased as well from 1.8 billion United States dollars (USD) to 2.33 billion USD in 2007 and 2014, respectively. A recent analysis by Volk et al using 2 large private insurance databases found an average cost of a hospital stay for HE of USD $29,063 to $34,810 during the study period (2014–2019).69 Readmissions multiply the burden of hospitalization. HE is the strongest risk factor for readmission.70 Ninety-day readmission rates in persons with cirrhosis range from 21% to 53%.71,72 Data from the North American Consortium for the Study of End-Stage Liver Disease identified nearly one-quarter of all 90-day readmissions in cirrhosis were related to HE.72 Using a large multistate inpatient database, the presence of hepatic encephalopathy was found to increase the odds of a 30- and 90-day liver-related readmission by 3-fold.71
Among cirrhosis complications, HE is also a uniquely modifiable risk factor for readmission. However, barriers to widespread use have predominantly included costs leading to inadequate access to medications and deficits in prescribing behaviors.2,73 A 30-day supply of rifaximin can cost more than $2500 USD, depending on insurance coverage.74 There are additional pharmacoequity concerns75 regarding disparities in access to this disease-modifying medication. In a recent study of United States Medicare enrollees, rifaximin use was lower among Black patients, who additionally paid more per prescription than White patients and were less likely to fill prescriptions as medication costs rose.76 Still, it is cost-effective after factoring in a significant reduction in overall health care costs, mainly related to less hospitalizations.69,77 Indeed, the number needed to treat with rifaximin, a nonabsorbable antibiotic approved for recurrent HE, to avoid one HE-related admission is 9.78 Despite the evidence-based effectiveness of rifaximin, implementation gaps exist that are frequently related to prescribing behaviors. In-hospital interventions, however, that optimize HE therapy prescription using electronic decision supports can help reduce readmissions.79,80 Additionally, remote patient monitoring can be leveraged for the purposes of monitoring medication adherence and symptoms to allow for intervention prior to a preventable HE-related readmission,81 although uptake of remote patient monitoring tools for HE is not universal and dependent on opinions and comfort with technology.82
Falls
Falls in cirrhosis are highly prevalent and associated with significant morbidity and mortality. One-half of all patients with decompensated cirrhosis will experience a fall during a 3-year period, of which 16.5% will result in injury.83 Both CHE and OHE contribute to fall risk. The observed increased risk of falls related to HE reflects the associated neuromuscular deficits of the condition. Ambulation requires an interplay of postural control, positional/sensory awareness, and ability to adjust and react to unexpected shifts; all altered in HE.84,85 In one retrospective study of 130 patients with predominantly Child A and B cirrhosis, 40% of patients with CHE reported a fall in the preceding year compared with only 12.9% of patients without CHE.10 Additionally, falls in those with CHE were associated with a higher need for primary care physician visits or hospitalization.10 A prospective at the same center further illustrated a higher adjusted risk of falls in persons with CHE (40% vs 6.2%) over follow-up.86 Not unexpectedly, falls in patients with comorbid OHE are 26% more likely to result in severe injury than in patients without a history of OHE.87
Socioeconomic and Health-related Quality of Life Consequences of HE
Employment/Income.
Cognitive impairment in cirrhosis is associated with unemployment, a reduced earning potential, and financial toxicity. A multicenter study of 243 patients with cirrhosis found a relationship between cognitive impairment and unemployment.88 Furthermore, the degree of cognitive impairment was significantly related to income. A trend towards decreased yearly income between persons with, compared to without, OHE has been shown ($35,000–$50,000 vs $50,000–$75,000).89 Updated analyses are needed, but the socioeconomic consequences of HE appear more pronounced in ‘blue-collar’ jobs where psychomotor skills are required.90 As a consequence of HE-associated cognitive impairment, cirrhosis care is associated with a significant financial toxicity, with approximately 50% of families caring for a person with cirrhosis unable to contribute to their savings and experience mounting debt.91
Driving.
Perhaps an additional contributor to socioeconomic stability and earning ability is the impairment of driving skills observed in HE. Performance on driving simulations, and on actual driving assessments, is impaired in the presence of CHE and OHE.92–94 Yet, most patients are often unaware of their driving deficits and have confidence in driving ability that is discordant with their performance and the observed performance reported by family or friends.93,94 Driving performance deteriorates incrementally in those with CHE and low-grade OHE, with only 48% and 39% of these patients, respectively, deemed capable of operating a vehicle by a driving instructor.94 Consequently, patients with HE have an increased risk of motor vehicle collisions and traffic violations.92
Health-related Quality of Life.
The clinical symptoms of HE directly contribute to impairments in multiple health-related quality of life domains. Sleep disturbances and functional impairments are frequently endorsed by persons with HE.95 However, the psychological consequences of HE are perhaps more pervasive and extend to involve those who care for the patient. The development of HE is associated with a profound emotional burden related to feelings of fear, anxiety, and other mood disturbances for both patients and their caregivers.96 Beyond these patient-reported outcome measures, impairments in quality of life lead to increased hospitalizations and death.97 Recognition of the physical and mental consequences of HE is paramount as pharmacologic (lactulose and rifaximin) and mindfulness strategies lead to meaningfulness improvements.98,99
Conclusion
The next several years will bring about dynamic shifts in the etiology of chronic liver disease and demographic composition of those with cirrhosis. These changes will have profound implications on the development and natural history of HE, necessitating more novel and holistic approaches to the management of this debilitating illness.
Acknowledgments
This paper is part of a supplement supported by an independent educational grant from Salix Pharmaceuticals, a division of Bausch Health US, LLC. Salix Pharmaceuticals had no involvement in the creation of the content, including author selection, manuscript development or peer review. The views and opinions expressed in the articles are those of the authors.
Funding
Elliot Tapper receives funding from the National Institutes of Health, United States through National Institute of Diabetes and Digestive and Kidney Diseases, United States (1K23DK117055, U01DK130113). Jeremy Louissaint is a recipient of the American Association for the Study of Liver Diseases Advanced Hepatology Award 2021. Sasha Deutsch-Link was supported in part by National Institutes of Health grant T32DK007634.
Conflicts of interest
This author discloses the following: Elliot Tapper has served as a consultant to Norvartis, Axcella, and Allergan; has served on advisory boards for Mallinckrodt, Bausch Health, Kaleido, and Novo Nordisk; and has received unrestricted research grants from Gilead and Valeant. The remaining authors disclose no conflicts.
Abbreviations used in this paper:
- ALD
alcohol-related liver disease
- CHE
covert hepatic encephalopathy
- HE
hepatic encephalopathy
- ICD
International Classification of Diseases
- MHE
minimal hepatic encephalopathy
- NAFLD
nonalcoholic fatty liver disease
- OHE
overt hepatic encephalopathy
- PHES
Psychometric Hepatic Encephalopathy Score
- PPI
proton pump inhibitor
- PSE
portosystemic encephalopathy
- SVR
sustained virologic response
- TIPS
transjugular intrahepatic portosystemic shunts
- USD
United States dollars.
Footnotes
Continuing Medical Education
The CME Activity for this article can be found at the following link: https://agau.gastro.org/diweb/catalog/item/eid/Pubs-Missing-the-Mark-01-Changing
References
- 1.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 2.Tapper EB, Aberasturi D, Zhao Z, et al. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Aliment Pharmacol Ther 2020;51:1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapper EB, Henderson JB, Parikh ND, et al. Incidence of and risk factors for hepatic encephalopathy in a population-based cohort of Americans with cirrhosis. Hepatol Commun 2019;3:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Van Natta ML, Clark J, et al. NASH Clinical Research Network (CRN). Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 6.Tapper EB, Parikh ND, Waljee AK, et al. Diagnosis of minimal hepatic encephalopathy: a systematic review of point-of-care diagnostic tests. Am J Gastroenterol 2018;113:529–538. [DOI] [PubMed] [Google Scholar]
- 7.Ampuero J, Montoliú C, Simón-Talero M, et al. Minimal hepatic encephalopathy identifies patients at risk of faster cirrhosis progression. J Gastroenterol Hepatol 2018;33:718–725. [DOI] [PubMed] [Google Scholar]
- 8.Labenz C, Toenges G, Schattenberg JM, et al. Outcome prediction of covert hepatic encephalopathy in liver cirrhosis: comparison of four testing strategies. Clin Transl Gastroenterol 2020;11:e00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allampati S, Duarte-Rojo A, Thacker LR, et al. Diagnosis of minimal hepatic encephalopathy using Stroop EncephalApp: a multicenter US-based, norm-based study. Am J Gastroenterol 2016;111:78–86. [DOI] [PubMed] [Google Scholar]
- 10.Román E, Córdoba J, Torrens M, et al. Minimal hepatic encephalopathy is associated with falls. Am J Gastroenterol 2011;106:476–482. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj JS, Etemadian A, Hafeezullah M, et al. Testing for minimal hepatic encephalopathy in the United States: an AASLD survey. Hepatology 2007;45:833–834. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Matos MC, Jiang ZG, Tapper EB. Factors that affect results of psychometric tests to identify patients with minimal hepatic encephalopathy. Clin Gastroenterol Hepatol 2018;16:1836–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom PP, Tapper EB. The use of administrative data to investigate the population burden of hepatic encephalopathy. J Clin Med 2020;9:3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapper EB, Parikh ND, Sengupta N, et al. A risk score to predict the development of hepatic encephalopathy in a population-based cohort of patients with cirrhosis. Hepatology 2018;68:1498–1507. [DOI] [PubMed] [Google Scholar]
- 15.Tapper EB, Zhao L, Nikirk S, et al. Incidence and bedside predictors of the first episode of overt hepatic encephalopathy in patients with cirrhosis. Am J Gastroenterol 2020;115:2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology 2019;70:487–495. [DOI] [PubMed] [Google Scholar]
- 17.Ginés P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987;7:122–128. [DOI] [PubMed] [Google Scholar]
- 18.Labenz C, Nagel M, Kremer WM, et al. Association between diabetes mellitus and hepatic encephalopathy in patients with cirrhosis. Aliment Pharmacol Ther 2020;52:527–536. [DOI] [PubMed] [Google Scholar]
- 19.Jepsen P, Watson H, Andersen PK, et al. Diabetes as a risk factor for hepatic encephalopathy in cirrhosis patients. J Hepatol 2015;63:1133–1138. [DOI] [PubMed] [Google Scholar]
- 20.Nightingale JMD, Paine P, McLaughlin J, et al. Small Bowel and Nutrition Committee and the Neurogastroenterology and Motility Committee of the British Society of Gastroenterology. The management of adult patients with severe chronic small intestinal dysmotility. Gut 2020;69:2074–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel VC, White H, Støy S, et al. Clinical science workshop: targeting the gut-liver-brain axis. Metab Brain Dis 2016;31:1327–1337. [DOI] [PubMed] [Google Scholar]
- 22.Trebicka J, Macnaughtan J, Schnabl B, et al. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol 2021;75(Suppl 1):S67–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc 2015;90:646–658. [DOI] [PubMed] [Google Scholar]
- 24.Cheon SY, Song J. The association between hepatic encephalopathy and diabetic encephalopathy: the brain-liver axis. Int J Mol Sci 2021;22:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y-H, Jung KS, Kim SU, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol 2015;63:486–493. [DOI] [PubMed] [Google Scholar]
- 26.Carias S, Castellanos AL, Vilchez V, et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 2016;31:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 2005;81:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nardelli S, Lattanzi B, Merli M, et al. Muscle alterations are associated with minimal and overt hepatic encephalopathy in patients with liver cirrhosis. Hepatology 2019;70:1704–1713. [DOI] [PubMed] [Google Scholar]
- 29.Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int 2018;12:377–386. [DOI] [PubMed] [Google Scholar]
- 30.Orman ES, Roberts A, Ghabril M, et al. Trends in characteristics, mortality, and other outcomes of patients with newly diagnosed cirrhosis. JAMA Netw Open 2019;2:e196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su F, Yu L, Berry K, et al. Aging of liver transplant registrants and recipients: trends and impact on waitlist outcomes, post-transplantation outcomes, and transplant-related survival benefit. Gastroenterology 2016;150:441–453.e6; quiz: e16. [DOI] [PubMed] [Google Scholar]
- 32.Weissenborn K Minimal/covert hepatic encephalopathy - impact of comorbid conditions. J Clin Exp Hepatol 2019;9:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebadi M, Montano-Loza AJ. Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis. Dig Liver Dis 2019;51:1493–1499. [DOI] [PubMed] [Google Scholar]
- 34.Tapper EB, Parikh ND, Green PK, et al. Reduced incidence of hepatic encephalopathy and higher odds of resolution associated with eradication of HCV Infection. Clin Gastroenterol Hepatol 2020;18:1197–1206.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibáñez-Samaniego L, Rapado-Castro M, Cabrero L, et al. Hepatitis C eradication improves cognitive function in patients with or without cirrhosis: a prospective real-life study. Eur J Neurol 2022;29:400–412. [DOI] [PubMed] [Google Scholar]
- 36.Patel AA, Bui A, Prohl E, et al. Innovations in hepatitis C screening and treatment. Hepatol Commun 2021;5:371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spillane S, Shiels MS, Best AF, et al. Trends in alcohol-induced deaths in the United States, 2000-2016. JAMA Network Open 2020;3:e1921451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Network Open 2020;3:e2022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon AM, Yang JY, Barritt AS, et al. Rising mortality from alcohol-associated liver disease in the United States in the 21st century. Am J Gastroenterol 2020;115:79–87. [DOI] [PubMed] [Google Scholar]
- 40.Rutledge SM, Schiano TD, Florman S, et al. COVID-19 after-shocks on alcohol-associated liver disease: an early crosssectional report from the U.S. epicenter. Hepatol Commun 2021;5:1151–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Julien J, Ayer T, Tapper EB, et al. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-related liver disease: a modeling study. Hepatology 2022;75:1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis BC, Bajaj JS. Effects of alcohol on the brain in cirrhosis: beyond hepatic encephalopathy. Alcohol Clin Exp Res 2018;42:660–667. [DOI] [PubMed] [Google Scholar]
- 43.Amodio P, Pellegrini A, Amistà P, et al. Neuropsychological-neurophysiological alterations and brain atrophy in cirrhotic patients. Metab Brain Dis 2003;18:63–78. [DOI] [PubMed] [Google Scholar]
- 44.Ahluwalia V, Wade JB, Moeller FG, et al. The etiology of cirrhosis is a strong determinant of brain reserve: a multimodal magnetic resonance imaging study. Liver Transpl 2015;21:1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jalan R, Turjanski N, Taylor-Robinson S, et al. Increased availability of central benzodiazepine receptors in patients with chronic hepatic encephalopathy and alcohol related cirrhosis. Gut 2000;46:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med 2017;377:1368–1377. [DOI] [PubMed] [Google Scholar]
- 47.Long L, Li H, Deng G, et al. Impact of hepatic encephalopathy on clinical characteristics and adverse outcomes in prospective and multicenter cohorts of patients with acute-on-chronic liver diseases. Front Med (Lausanne) 2021;8:709884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campagna F, Montagnese S, Schiff S, et al. Confounders in the detection of minimal hepatic encephalopathy: a neuropsychological and quantified EEG study. Liver Int 2015;35:1524–1532. [DOI] [PubMed] [Google Scholar]
- 49.Jagtap N, Bhakhar P, Miftahussurur M, et al. Minimal hepatic encephalopathy in patients with alcohol related and non-alcoholic steatohepatitis related cirrhosis by Psychometric Hepatic Cephalopathy Score and critical Flicker frequency. Acta Med Indones 2021;53:24–30. [PubMed] [Google Scholar]
- 50.Tarter RE, Switala J, Lu S, et al. Abstracting capacity in cirrhotic alcoholics: negative findings. J Stud Alcohol 1995;56:99–103. [DOI] [PubMed] [Google Scholar]
- 51.Edwin D, Flynn L, Klein A, et al. Cognitive impairment in alcoholic and nonalcoholic cirrhotic patients. Hepatology 1999;30:1363–1367. [DOI] [PubMed] [Google Scholar]
- 52.Tarter RE, Van Thiel DH, Arria AM, et al. Impact of cirrhosis on the neuropsychological test performance of alcoholics. Alcohol Clin Exp Res 1988;12:619–621. [DOI] [PubMed] [Google Scholar]
- 53.Mishuk AU, Chen L, Gaillard P, et al. National trends in prescription proton pump inhibitor use and expenditure in the United States in 2002-2017. J Am Pharm Assoc (2003) 2020: Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Thomson MJ, Lok ASF, Tapper EB. Appropriate and potentially inappropriate medication use in decompensated cirrhosis. Hepatology 2021;73:2429–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nardelli S, Gioia S, Ridola L, et al. Proton pump inhibitors are associated with minimal and overt hepatic encephalopathy and increased mortality in patients with cirrhosis. Hepatology 2019;70:640–649. [DOI] [PubMed] [Google Scholar]
- 56.Williams S, Louissaint J, Nikirk S, et al. Deprescribing medications that may increase the risk of hepatic encephalopathy: a qualitative study of patients with cirrhosis and their doctors. United European Gastroenterol J 2021;9:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tapper EB, Risech-Neyman Y, Sengupta N. Psychoactive medications increase the risk of falls and fall-related injuries in hospitalized patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:1670–1675. [DOI] [PubMed] [Google Scholar]
- 58.Moon AM, Jiang Y, Rogal SS, et al. Opioid prescriptions are associated with hepatic encephalopathy in a national cohort of patients with compensated cirrhosis. Aliment Pharmacol Ther 2020;51:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogal SS, Beste LA, Youk A, et al. Characteristics of opioid prescriptions to veterans with cirrhosis. Clin Gastroenterol Hepatol 2019;17:1165–1174.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agbalajobi OM, Gmelin T, Moon AM, et al. Characteristics of opioid prescribing to outpatients with chronic liver diseases: a call for action. PLoS One 2021;16:e0261377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tripathi D, Stanley AJ, Hayes PC, et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut 2020;69:1173–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, Lv Y, Bai M, et al. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol 2017;67:508–516. [DOI] [PubMed] [Google Scholar]
- 63.Liu J, Ma J, Zhou C, et al. Potential benefits of underdilation of 8-mm covered stent in transjugular intrahepatic portosystemic shunt creation. Clin Transl Gastroenterol 2021;12:e00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bureau C, Thabut D, Jezequel C, et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled trial. Ann Intern Med 2021;174:633–640. [DOI] [PubMed] [Google Scholar]
- 65.Saab S, Zhao M, Asokan I, et al. History of hepatic encephalopathy is not a contraindication to transjugular intrahepatic portosystemic shunt placement for refractory ascites. Clin Transl Gastroenterol 2021;12:e00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elsaid MI, John T, Li Y, et al. The health care burden of hepatic encephalopathy. Clin Liver Dis 2020;24:263–275. [DOI] [PubMed] [Google Scholar]
- 67.Agency for Healthcare Research and Quality; Healthcare Cost and Utilization Project. HCUPnet. Available at: https://hcupnet.ahrq.gov/. Accessed January 16, 2022. [PubMed]
- 68.Hirode G, Vittinghoff E, Wong RJ. Increasing burden of hepatic encephalopathy among hospitalized adults: an analysis of the 2010-2014 National Inpatient Sample. Dig Dis Sci 2019;64:1448–1457. [DOI] [PubMed] [Google Scholar]
- 69.Volk ML, Burne R, Guérin A, et al. Hospitalizations and healthcare costs associated with rifaximin versus lactulose treatment among commercially insured patients with hepatic encephalopathy in the United States. J Med Econ 2021;24:202–211. [DOI] [PubMed] [Google Scholar]
- 70.Seraj SM, Campbell EJ, Argyropoulos SK, et al. Hospital readmissions in decompensated cirrhotics: factors pointing toward a prevention strategy. World J Gastroenterol 2017;23:6868–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin Gastroenterol Hepatol 2016;14:1181–1188.e2. [DOI] [PubMed] [Google Scholar]
- 72.Bajaj JS, Reddy KR, Tandon P, et al. North American Consortium for the Study of End-Stage Liver Disease. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology 2016;64:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orr JG, Currie CJ, Berni E, et al. The impact on hospital resource utilisation of treatment of hepatic encephalopathy with rifaximin-α. Liver Int 2016;36:1295–1303. [DOI] [PubMed] [Google Scholar]
- 74.Rifaximin Prices, Coupons & Savings Tips. GoodRx. Available at: https://www.goodrx.com/rifaximin. Accessed February 12, 2022.
- 75.Essien UR, Dusetzina SB, Gellad WF. A policy prescription for reducing health disparities-achieving pharmacoequity. JAMA 2021;326:1793–1794. [DOI] [PubMed] [Google Scholar]
- 76.Tapper EB, Essien UR, Zhao Z, et al. Racial and ethnic disparities in rifaximin use and subspecialty referrals for patients with hepatic encephalopathy in the United States. J Hepatol March 2022:Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 77.Jesudian AB, Ahmad M, Bozkaya D, et al. Cost-effectiveness of rifaximin treatment in patients with hepatic encephalopathy. J Manag Care Spec Pharm 2020;26:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071–1081. [DOI] [PubMed] [Google Scholar]
- 79.Tapper EB, Finkelstein D, Mittleman MA, et al. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Louissaint J, Grzyb K, Bashaw L, et al. An electronic decision support intervention reduces readmissions for patients with cirrhosis. Am J Gastroenterol 2022;117:491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ganapathy D, Acharya C, Lachar J, et al. The patient buddy app can potentially prevent hepatic encephalopathy-related readmissions. Liver Int 2017;37:1843–1851. [DOI] [PubMed] [Google Scholar]
- 82.Louissaint J, Lok AS, Fortune BE, et al. Acceptance and use of a smartphone application in cirrhosis. Liver Int 2020;40:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tapper EB, Nikirk S, Parikh ND, et al. Falls are common, morbid, and predictable in patients with cirrhosis. J Hepatol 2021;75:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Urios A, Mangas-Losada A, Gimenez-Garzó C, et al. Altered postural control and stability in cirrhotic patients with minimal hepatic encephalopathy correlate with cognitive deficits. Liver Int 2017;37:1013–1022. [DOI] [PubMed] [Google Scholar]
- 85.Murphy SL, Tapper EB, Blackwood J, et al. Why do individuals with cirrhosis fall? A mechanistic model for fall assessment, treatment, and research. Dig Dis Sci 2019;64:316–323. [DOI] [PubMed] [Google Scholar]
- 86.Soriano G, Román E, Córdoba J, et al. Cognitive dysfunction in cirrhosis is associated with falls: a prospective study. Hepatology 2012;55:1922–1930. [DOI] [PubMed] [Google Scholar]
- 87.Ezaz G, Murphy SL, Mellinger J, et al. Increased morbidity and mortality associated with falls among patients with cirrhosis. Am J Med 2018;131:645–650.e2. [DOI] [PubMed] [Google Scholar]
- 88.Bajaj JS, Riggio O, Allampati S, et al. Cognitive dysfunction is associated with poor socioeconomic status in patients with cirrhosis: an international multicenter study. Clin Gastroenterol Hepatol 2013;11:1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011;106:1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis 2001;16:37–41. [DOI] [PubMed] [Google Scholar]
- 91.Volk ML. Burden of cirrhosis on patients and caregivers. Hepatol Commun 2020;4:1107–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lauridsen MM, Thacker LR, White MB, et al. In patients with cirrhosis, driving simulator performance is associated with real-life driving. Clin Gastroenterol Hepatol 2016;14:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajaj JS, Saeian K, Hafeezullah M, et al. Patients with minimal hepatic encephalopathy have poor insight into their driving skills. Clin Gastroenterol Hepatol 2008;6:1135–1139; quiz: 1065. [DOI] [PubMed] [Google Scholar]
- 94.Kircheis G, Knoche A, Hilger N, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009;137:1706–1715. e1–9. [DOI] [PubMed] [Google Scholar]
- 95.Montagnese S, Bajaj JS. Impact of hepatic encephalopathy in cirrhosis on quality-of-life issues. Drugs 2019;79(Suppl 1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fabrellas N, Moreira R, Carol M, et al. Psychological burden of hepatic encephalopathy on patients and caregivers. Clin Transl Gastroenterol 2020;11:e00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kok B, Whitlock R, Ferguson T, et al. Health-related quality of life: a rapid predictor of hospitalization in patients with cirrhosis. Am J Gastroenterol 2020;115:575–583. [DOI] [PubMed] [Google Scholar]
- 98.Orr JG, Homer T, Ternent L, et al. Health related quality of life in people with advanced chronic liver disease. J Hepatol 2014;61:1158–1165. [DOI] [PubMed] [Google Scholar]
- 99.Bajaj JS, Ellwood M, Ainger T, et al. Mindfulness-based stress reduction therapy improves patient and caregiver-reported outcomes in cirrhosis. Clin Transl Gastroenterol 2017;8:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]


