Abstract
The Stanford Integrated Psychosocial Assessment for Transplant (SIPAT) is a standardized psychosocial evaluation tool used in liver transplantation (LT) evaluation. We assessed the impact of the SIPAT score and subdomains on transplant waitlisting decisions and post-LT outcomes including immunosuppression (IS) nonadherence, biopsy-proven rejection, andmortality/graft failure. We conducted a single-center observational cohort study of 1430 patients evaluated for LT. Patients were divided in 2 groups based on a SIPAT cutoff score of <21 or ≥21 (higher SIPAT scores indicate higher psychosocial risk). Regression models assessed relationships between total SIPAT score and domain scores and waitlisting decisions, IS nonadherence, allograft rejection, and death/graft failure. Elevated total SIPAT and SIPAT domain scores were associated not being added to the waitlist (total SIPAT core ≥21 adjusted odds ratio [aOR], 1.78 [95% confidence interval, CI, 1.36–2.33]; readiness score ≥5 aOR, 2.01 [95% CI, 1.36–2.76]; social support score ≥4aOR, 1.50 [95% CI, 1.15–1.94]; psychopathology score ≥7 aOR, 1.45 [95% CI, 1.07–1.94]; lifestyle/substance abuse score ≥12 aOR, 1.72 [95%CI, 1.23–2.39]) and were more likely to experience IS nonadherence as measured by the tacrolimus coefficient of variation (CoV) (total SIPAT score ≥21 aOR, 2.92 [95% CI, 1.69–5.03]; readiness score ≥5 aOR, 3.26 [95% CI, 1.63–6.52]; psychopathology score ≥7 aOR, 1.88 [95% CI, 1.00–3.50]; lifestyle substance abuse score ≥12 aOR, 3.03 [95% CI, 1.56–5.86]). SIPAT readinessscore ≥5 was associated with biopsy-proven allograft rejection (aOR, 2.66; 95% CI, 1.20–5.91). The SIPAT score was independently associated with LT listing decisions and IS nonadherence, and the readiness domain was associated with the risk of allograft rejection. These findings offer insights into higher risk recipients who require additional support before and aftertransplantation.
Psychosocial assessments are an essential component of liver transplantation (LT) evaluation; however, they can be difficult to execute in a standardized manner.(1,2) Psychosocial evaluations and transplant candidacy/listing decisions are not uniform across all transplant centers.(1–3) As a result, there are no standardized guidelines for LT psychosocial evaluation.(4,5)
Scoring systems such as the Stanford Integrated Psychosocial Assessment for Transplant (SIPAT) offer a standardized psychosocial evaluation for patients being considered for LT.(2) The SIPAT was first published by Maldonado et al. in 2012.(2) This tool assigns patients a score from 0 to 110 (higher scores indicating higher psychosocial risk) and encompasses the following 4 domains: (1) Patient’s Readiness Level, (2) Social Support System, (3) Psychological Suitability and Psychopathology, and (4) Lifestyle and Effect of Substance Use.(2) Initial studies show that the SIPAT is significantly associated with overall outcomes after LT.(2) Higher scores are associated with posttransplant hospitalizations, organ rejection, failure of social support systems, and adverse psychiatric and psychosocial outcomes among all organ transplant recipients.(6) Prior exploratory analyses have shown that higher SIPAT scores are also associated with post-LT alcohol relapse in patients transplanted for alcohol-related liver disease (ALD).(7)
Although the SIPAT is anecdotally used by transplant centers for psychosocial assessment, the association between SIPAT scores and the decision to list for LT is not well understood. Furthermore, the relationship between SIPAT scores and post-LT outcomes is not well reported and warrants further investigation.(2,6) Currently, research has focused on total SIPAT scores,(2,6) and the impact of specific SIPAT domains on waitlisting and post-LT outcomes has not yet been examined. To fill these gaps, we aimed to assess the relationship between total SIPAT score and specific domain scores on (1) waitlisting decisions for LT and (2) posttransplant outcomes including immunosuppression (IS) nonadherence, biopsy-proven allograft rejection, and patient/graft survival.
Patients and Methods
COHORT SELECTION AND DATA SOURCES
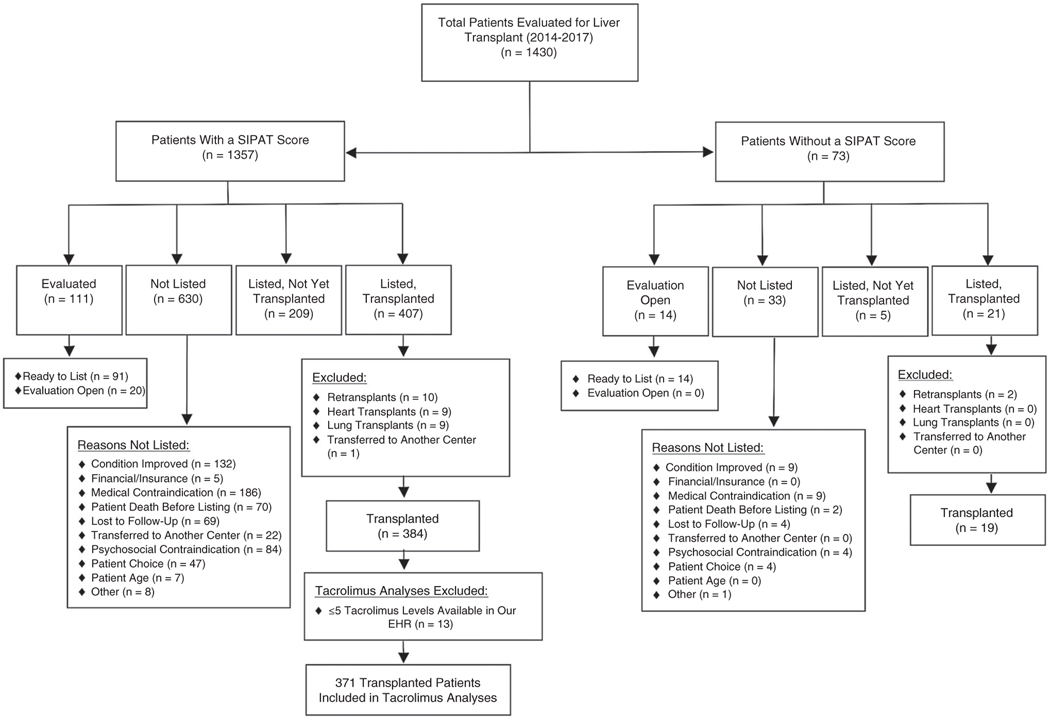
We conducted a single-center observational cohort study of 1430 patients evaluated for LT between 2014 and 2017 with follow-up through 2018 at a large, urban transplant center. The study was approved by the local institutional review board. A detailed flowchart of study participants is shown in Fig. 1. Of the 1430 patients evaluated for LT during the study time frame, 1357 (95%) were administered the SIPAT as part of the standard LT evaluation and were included in further analyses.
FIG. 1.
Study flow diagram.
Of the 428 patients who underwent LT, 407 (95%) were administered the SIPAT. For post-LT outcomes analyses, 23 patients were excluded for retransplantation (n = 10) or concomitant heart or lung transplant (n = 12) or were transferred to another center (n = 1), leaving 384 patients for the analysis of posttransplant outcomes. The IS nonadherence analyses included 371 patients because of the lack of available laboratory data among 13 patients.(8) SIPAT data were then merged with clinical data from the electronic health record (EHR) and United Network for Organ Sharing (UNOS) center-Specific Transplant Analysis and Research (STAR) file.
PRETRANSPLANT AND POSTTRANSPLANT CLINICAL PROTOCOLS
All patients in the study underwent thorough medical and psychosocial evaluations before LT. Initial psychosocial assessments and SIPAT administrations were performed by trained transplant social workers. The SIPAT was only administered to patients who were alert and oriented, and thus patients with encephalopathy and altered mental status did not receive SIPAT scores. Patients were further evaluated by transplant psychiatry if they had a substance use disorder within the 2 years preceding LT evaluation, significant psychiatric comorbidities, or if otherwise recommended by the transplant team. Transplant psychiatrists did not administer the SIPAT at our institution, and scores were not regenerated after transplant psychiatrists became involved in patient care. After the initial evaluation, transplant candidacy was determined by a multidisciplinary committee including transplant hepatology, transplant surgery, psychiatry, social work, and registered dietitians on a case-by-case basis.
Routine posttransplant monitoring included weekly follow-ups for the first 6 weeks, biweekly follow-ups from 6 weeks to 3 months, and follow-ups every 1 to 3 months at months 3 to 12 after LT. Subsequent follow-up was individualized based on patient health status and medical complications, with follow-up at least annually. Protocols were stable throughout the study period.
STUDY VARIABLES
Exposure Variable
The main study exposure variables were total SIPAT scores and individual SIPAT domain scores. The SIPAT assigns a score between 0 and 119 and is depicted in Supporting Table 1. Patients receive a score in 4 different domains: (1) Patient’s Readiness Level, (2) Social Support System, (3) Psychological Suitability and Psychopathology, and (4) Lifestyle and Effect of Substance Use. The Readiness domain encompasses a patient’s understanding of medical illness and the process of transplantation, willingness/desire for treatment, treatment adherence/compliance, and lifestyle habits. The Social Support domain encompasses availability and function of social support systems and appropriateness of physical living space and environment. The Psychopathology domain encompasses presence of psychopathology, neurocognitive impairment, personality disorders, and truthfulness/deceptive behavior. The Lifestyle and Effect of Substance Use domain encompasses alcohol, nicotine, and other substance use dependence and risk for relapse.
Previous literature shows that higher scores in the total SIPAT and specific SIPAT domains are associated with a higher psychosocial risk. A total score of 0 to 6 is termed an “excellent candidate,” 7 to 20 a “good candidate,” 21 to 39 a “minimally acceptable candidate,” 40 to 69 a “poor candidate,” and ≥70 a “high risk candidate.”(2) This study’s institution used SIPAT to aid in the psychosocial assessments for LT; however, no absolute thresholds were considered acceptable or unacceptable for transplant waitlisting because each candidate was considered on an individual basis.
Covariates
Clinical variables obtained included sex, age, race, liver disease etiology, Model for End-Stage Liver Disease-sodium (MELD-Na) at evaluation (<25, 25–34, >34), primary insurance (private, Medicaid, or Medicare), level of education, and zip code. Zip code was subsequently used to derive each patient’s Community Health Score (CHS), which has been used in prior studies as an indicator of socioeconomic status and community health resources.(9) The CHS has 2 composite scores: health outcomes and health factors.(10) The CHS scores are both continuous variables; higher scores indicate higher psychosocial and community risk for poor health outcomes. For patients who received a transplant, we also obtained from the UNOS STAR file the Karnofsky Performance Status and MELD-Na at time of transplant and whether the patients had simultaneous kidney transplants.
Outcomes
Medication nonadherence after LT was assessed using the tacrolimus coefficient of variation (CoV), a validated surrogate measure of medication nonadherence.(11,12) Tacrolimus CoV is calculated by dividing the standard deviation of tacrolimus levels by the mean. Prior research has shown that higher tacrolimus CoV correlates with medication nonadherence and rejection.(11,12) We employed a tacrolimus CoV ≥ 0.45 to measure medication nonadherence, which is consistent with prior studies.(11) Because of the high variability in the early postoperative period, tacrolimus levels were excluded for the first 90 days after LT and only outpatient levels were included.
Allograft rejection was defined as any biopsy-proven acute and/or chronic rejection, as determined by the final pathology reports available in the EHR. Biopsy reports were personally reviewed by study investigators. Rejection episodes in the first 90 days were excluded because very early rejection may be associated with other immunological factors.(13,14)
STATISTICAL ANALYSIS
Analyses were performed using Stata 15 (StataCorp, College Station, TX). Patients were categorized into 2 groups based on a SIPAT score <21 (signifying a “good” or “excellent” transplant candidate) or ≥21 (“minimally acceptable” to “poor” transplant candidate), which created a binary total SIPAT score variable.(2) Subsequently, each SIPAT domain was converted into a binary variable based on the highest 20% of scores in our study population, with higher scores representing higher psychosocial risk: Patient’s Readiness Level, <5/≥5; Social Support System, <4/≥4; Psychological Suitability and Psychopathology, <7/≥7; and Lifestyle and Effect of Substance Use, <12/≥12. Missing data were handled by case exclusion. Baseline characteristics and patient outcomes were initially compared using t tests, Wilcoxon rank sum tests, and chi-squared tests as appropriate.
Multivariable logistic regression assessed the association between the SIPAT score and its domains on the decision to waitlist for LT adjusting for age, sex, race, MELD-Na evaluation, liver disease etiology, education, and CHS. Multivariable logistic regression models were also fit to assess the association between the total SIPAT score and its 4 separate domains for the IS nonadherence (tacrolimus CoV >0.45), adjusting for the same covariates. Multivariable Cox proportion hazards models assessed the association between the total SIPAT score and its specific domains and time to allograft rejection and patient death/graft failure.
Results
PATIENT CHARACTERISTICS
Of the 1357 evaluated patients with SIPAT scores, 65% were men, 73% identified as White, 16% identified as Black/African American, and 6% identified as Hispanic/Latinx. About a third (31%) of patients had a SIPAT score of ≥21 (<21 considered “good/excellent” candidate). Compared with patients with SIPAT<21, those with a SIPAT score of ≥21 were younger (57 versus 60 years) and were more likely to identify as Black/African American (21% versus 14%). Patients with a SIPAT score of ≥21 were also more likely to have ALD (46% versus 19%), hepatitis C virus (HCV; 40% versus 29%), and less likely to have nonalcoholic steatohepatitis (NASH)/cryptogenic liver disease (6.1% versus 23%). Patients with a SIPAT score ≥21 were more likely to have Medicaid as their primary insurance (20% versus 5%), have not graduated from high school (16% versus 7.8%), and had a higher risk of health outcome CHS (18 versus 13; Table 1). Characteristics of the 73 patients evaluated for transplant without available SIPAT scores are shown in Supporting Table 2. Patients without SIPAT scores available were younger (56 years versus 59 years) and more likely to have a MELD-Na score >35 at evaluation, suggesting urgent inpatient evaluations where SIPAT scores could not be obtained (15% versus 3%).
TABLE 1.
Baseline Characteristics of Patients Evaluated for LT by SIPAT† Score
| SIPAT Score |
||||
|---|---|---|---|---|
| Characteristic | Total (n = 1357) | <21 (n = 930) | ≥21 (n = 427) | P Value |
|
| ||||
| Sex | 0.94 | |||
| Female | 481 (35) | 329 (35) | 152 (36) | |
| Male | 876 (65) | 601 (65) | 275 (64) | |
| Age at evaluation | 59 (52–64) | 60 (52–65) | 57 (51–62) | <0.001* |
| Race/ethnicity | <0.001* | |||
| White/Caucasian | 992 (73) | 684 (74) | 308 (72) | |
| Black/African American | 217 (16) | 128 (14) | 89 (21) | |
| Hispanic/Latinx | 80 (6) | 56 (6) | 24 (6) | |
| Asian | 50 (4) | 45 (5) | 5 (1) | |
| Other | 18 (1) | 17 (2) | 1 (0) | |
| Etiology of liver disease | <0.001* | |||
| ALD | 374 (28) | 177 (19) | 197 (46) | |
| HCV | 445 (33) | 274 (29) | 171 (40) | |
| HBV | 32 (2) | 28 (3) | 4 (1) | |
| NASH/cryptogenic | 243 (18) | 217 (23) | 26 (6.1) | |
| PSC | 61 (5) | 58 (6) | 3 (1) | |
| Autoimmune | 38 (3) | 30 (3) | 8 (2) | |
| PBC | 38 (3) | 34 (4) | 4 (1) | |
| Other | 126 (9) | 112 (12) | 14 (3) | |
| MELD-Na at evaluation‡ | 0.001* | |||
| <25 | 1103 (81) | 778 (84) | 325 (76) | |
| 25–34 | 210 (16) | 130 (14) | 90 (19) | |
| >35 | 42 (3) | 21 (2) | 21 (5) | |
| Education§ | <0.001* | |||
| <High school | 140 (10) | 72 (7.8) | 68 (16) | |
| High school/GED | 503 (37) | 320 (35) | 183 (43) | |
| Some college/technical school | 279 (21) | 202 (22) | 77 (18) | |
| Associate’s/bachelor’s degree | 296 (22) | 227 (24) | 69 (16) | |
| Graduate degree | 128 (10) | 100 (11) | 28 (7) | |
| Primary insurance¶ | <0.001* | |||
| Private insurance | 749 (55) | 549 (59) | 200 (47) | |
| Medicare | 481 (35) | 338 (36) | 143 (33) | |
| Medicaid | 126 (9) | 42 (5) | 84 (20) | |
| CHS|| | ||||
| Health outcomes | 13 (5–34) | 13 (5–27) | 18 (5–34) | 0.01* |
| Health factors | 12 (3–26) | 12 (3.5–26) | 12 (3–33) | 0.14 |
NOTE: Data are provided as n (%) or median (interquartile range).
P < 0.05.
Higher scores indicate higher psychosocial risk. A score <21 is a “good or excellent candidate” and a score ≥21 is a “minimally acceptable candidate.”
A total of 2 missing values.
A total of 11 missing values.
A total of 1 missing value.
A total of 2 missing values.
SIPAT AND WAITLISTING
Table 2 depicts waitlist outcomes stratified by SIPAT score. Of the patients, 650 (48%) were not listed for transplant after evaluation. The most common reasons for not being listed for transplant were medical contraindications (n = 186, 30%), candidate condition improved/too early for transplant (n = 132, 21%), and psychosocial contraindications (n = 84, 13%). Patients with a SIPAT score ≥21 were less likely to be added to the waiting list (42% compared with 57%). Of those added to the waiting list, patients with a SIPAT score ≥21 had a longer duration of time from evaluation to waitlisting (89 days compared with 67 days). Compared with patients with SIPAT scores <21, those with SIPAT scores ≥21 were more likely to have psychosocial contraindications to waitlisting (30% versus 3%) and to have no further transplant center follow-up, including loss to follow-up or receiving care elsewhere (15% versus 8%).
TABLE 2.
Transplant Evaluation and Listing Outcomes by SIPAT† Score
| SIPAT Score |
||||
|---|---|---|---|---|
| Characteristic | Total (n = 1357) | <21 (n = 930) | ≥21 (n = 427) | P Value |
|
| ||||
| Evaluation outcome | <0.001* | |||
| Listed, transplanted | 407 (30) | 308 (33) | 99 (23) | |
| Listed, not transplanted | 209 (15) | 158 (17) | 51 (12) | |
| Evaluated, ineligible | 630 (46) | 391 (42) | 239 (56) | |
| Evaluated, ready to list‡ | 91 (7) | 60 (7) | 31 (7) | |
| Evaluation still open | 20 (2) | 13 (1) | 7 (2) | |
| Transplant listing decision | <0.001* | |||
| Yes§ | 707 (52) | 526 (57) | 181 (42) | |
| No | 650 (48) | 404 (43) | 246 (57) | |
| Time in days from evaluation to waitlist | 616 | 466 | 150 | 0.05 |
| 72 (24–146) | 67 (23–137) | 89 (31–177) | ||
| Reason for not listing | 630 | 391 | 239 | <0.001* |
| Candidate condition improved/transplant not medically necessary at time of evaluation | 132 (21) | 97 (25) | 35 (15) | |
| Financial/insurance issues | 5 (1) | 3 (1) | 2 (1) | |
| Medical contraindications | 186 (30) | 129 (33) | 57 (24) | |
| Patient death prior to listing | 70 (11) | 50 (13) | 20 (8) | |
| No further transplant center follow-up | 69 (11) | 32 (8) | 37 (15) | |
| Transferred to another center | 22 (4) | 20 (5) | 2 (1) | |
| Psychosocial contraindications | 84 (13) | 13 (3) | 71 (30) | |
| Patient choice | 47 (8) | 35 (9) | 12 (5) | |
| Patient age | 7 (1) | 6 (2) | 1 (0) | |
| Other | 8 (1) | 6 (2) | 2 (1) | |
| Waitlisted | 707 (52) | 526 (57) | 181 (42) | <0.001* |
| Delisted for transplant | 123 (17) | 90 (17) | 33 (18) | 0.73 |
| Reason delisted for transplant | 0.18 | |||
| Candidate condition deteriorated | 39 (32) | 29 (32) | 10 (30) | |
| Candidate condition improved | 11 (9) | 7 (8) | 4 (12) | |
| Financial/insurance issues | 4 (3) | 2 (2) | 2 (6) | |
| Medical contraindications | 7 (6) | 6 (7) | 1 (3) | |
| Other | 10 (8) | 7 (8) | 3 (9) | |
| Patient choice | 5 (4) | 3 (3) | 2 (6) | |
| Patient death | 31 (25) | 24 (27) | 7 (21) | |
| Psychosocial contraindications | 6 (5) | 2 (2) | 4 (12) | |
| Transplanted at another institution | 10 (8) | 10 (11) | 0 | |
NOTE: Data are provided as n, n (%), or median (interquartile range).
P < 0.05.
Higher scores indicate higher psychosocial risk. A score <21 is a “good or excellent candidate” and a score ≥21 is a “minimally acceptable candidate.”
Patients who are “ready to list” have been deemed appropriate psychosocial transplant candidates but are not medically ready for transplant.
Listed includes patients deemed “ready to list,” as these patients were deemed acceptable transplant candidates.
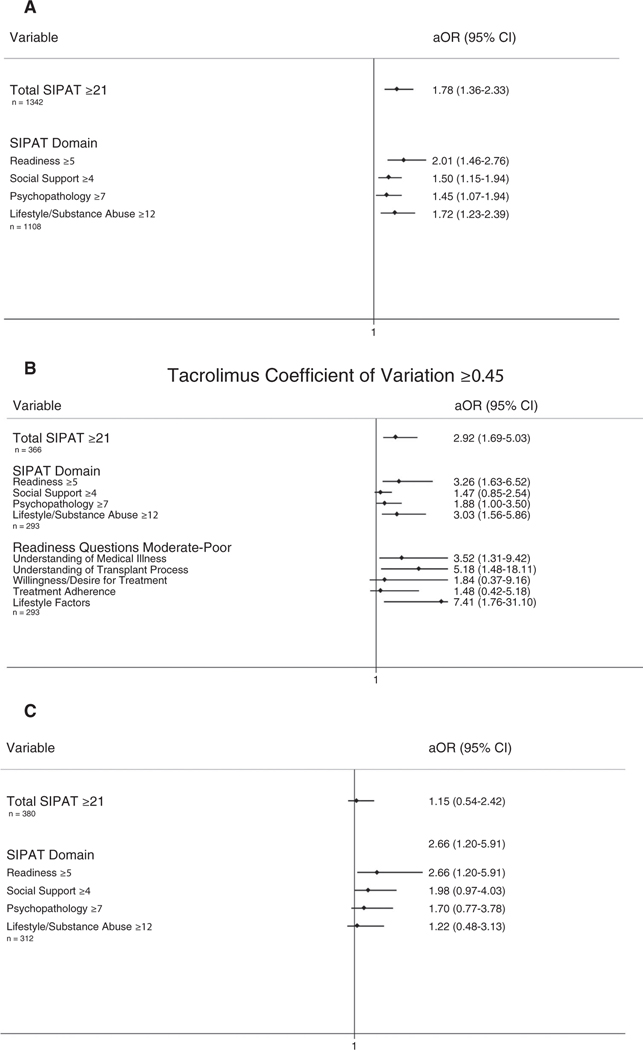
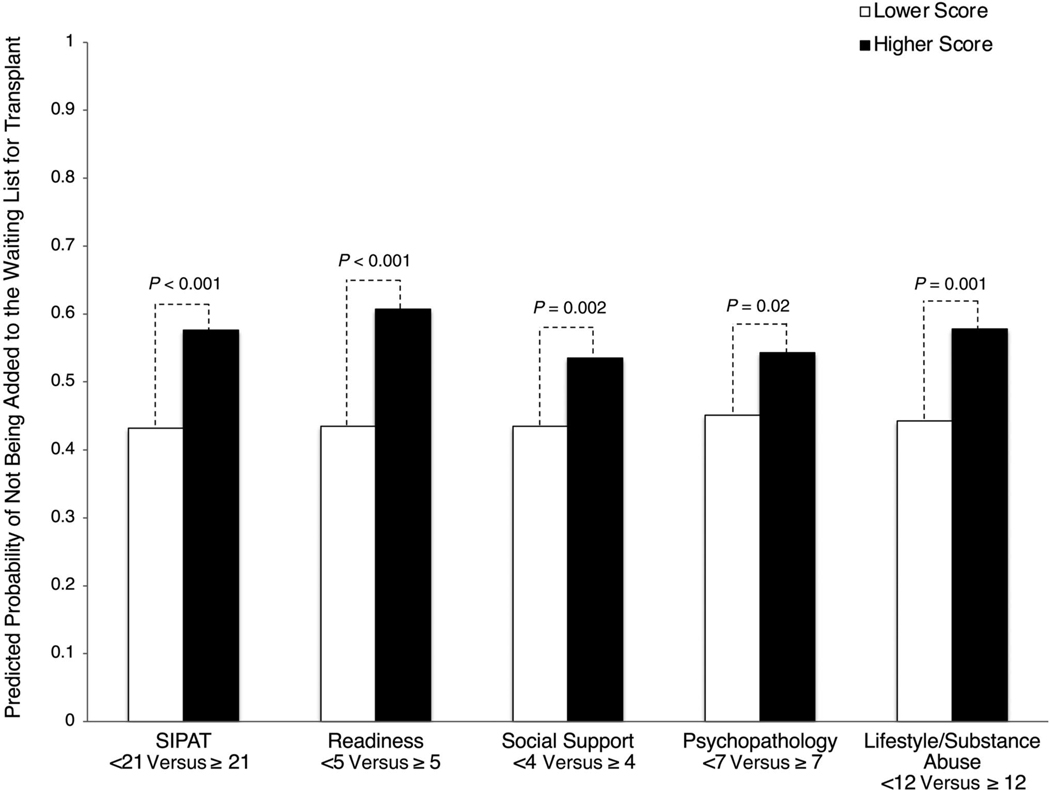
In multivariable analyses, patients with higher total SIPAT and SIPAT domain scores were less likely to be added to the waiting list for transplant: total SIPAT score ≥21 adjusted odds ratio (aOR), 1.78 (95% confidence interval [CI], 1.36–2.33); readiness score ≥5 aOR, 2.01 (95% CI, 1.46–2.76); social support score ≥4 aOR, 1.50 (95% CI, 1.15–1.94); psychopathology score ≥7 aOR, 1.45 (95% CI, 1.07–1.94); and lifestyle/substance abuse score ≥12 aOR, 1.72 (95% CI, 1.23–2.39) (Fig. 5A). Predicted probabilities of the decision not to add a patient to the waiting list for transplant are shown in Fig. 2.
FIG. 5.
Summary of significant relationships: (A) not being added to the waiting list for transplant, (B) tacrolimus CoV ≥0.45, (C) posttransplant biopsy-proven allograft rejection.
FIG. 2.
Adjusted impact of SIPAT total score and SIPAT domain scores on patients not being added to the waiting list for transplant. Higher scores indicate higher psychosocial risk. See Supporting Table 1 for individual items in each domain. Models adjusted for age, sex, race, MELD-Na at evaluation, liver disease diagnosis, education, and CHS. P values are derived from individual logistic regressions.
To further explore transplant listing, we investigated factors associated with a higher likelihood of transplant listing in patients with a SIPAT score ≥21. Among patients with a SIPAT score ≥21, factors that were associated with a higher likelihood of transplant listing were male sex (aOR, 1.86; 95% CI, 1.16–2.99), higher MELD-Na scores (25–34 score aOR, 2.64; 95% CI, 1.52–4.58; ≥35 score aOR, 3.13; 95% CI, 1.14–8.59), and higher educational attainment (compared with less than high school: high school graduate/General Educational Development [GED] aOR, 3.51 [95% CI, 1.75–7.02]; some college/technical school aOR, 2.90 [95% CI, 1.30–6.48]; associate’s/bachelor’s degree aOR, 5.08 [95% CI, 2.21–11.68]; graduate degree aOR, 4.10 [95% CI 1.46–11.54]; Supporting Table 3).
SIPAT AND POSTTRANSPLANT OUTCOMES
Table 3 depicts the baseline characteristics of the 384 patients who received a transplant included in the posttransplant outcomes analyses. The 99 patients (26%) with a SIPAT score ≥21 were more likely to have HCV (47% versus 32%) or ALD (39% versus 20%). Although the median transplant MELD-Na scores were not significantly different by SIPAT score, compared with candidates with SIPAT scores <21, those with SIPAT scores ≥21 were more likely to have MELD-Na evaluation scores of >35 (10% versus 4%) and 25–34 (37% versus 25%).
TABLE 3.
Liver Transplant Recipient Characteristics
| SIPAT Score† |
||||
|---|---|---|---|---|
| Characteristic | Total (n = 384) | <21 (N = 285), 74% | ≥21 (n = 99), 26% | P Value |
|
| ||||
| Sex | 0.21 | |||
| Female | 108 (28) | 85 (30) | 23 (23) | |
| Male | 276 (72) | 200 (70) | 76 (77) | |
| Age, years | 59 (52–64) | 59 (52–64) | 57 (52–63) | 0.07 |
| Race/ethnicity | 0.31 | |||
| White/Caucasian | 289 (75) | 214 (75) | 75 (76) | |
| Black/African American | 54 (14) | 36 (13) | 18 (18) | |
| Asian | 16 (4) | 14 (5) | 2 (2) | |
| Hispanic/Latinx | 22 (6) | 18 (6) | 4 (4) | |
| Other | 3 (1) | 3 (1) | 0 | |
| Primary insurance | 0.16 | |||
| Private | 273 (71) | 207 (73) | 66 (67) | |
| Medicaid | 10 (3) | 5 (2) | 5 (5) | |
| Medicare | 101 (26) | 73 (26) | 28 (28) | |
| Education‡ | 0.27 | |||
| Less than high school | 10 (3) | 7 (3) | 3 (3) | |
| High school/GED | 154 (41) | 106 (38) | 48 (49) | |
| Some college/technical school | 83 (22) | 67 (24) | 16 (16) | |
| Associate’s/bachelor’s degree | 87 (23) | 65 (23) | 22 (22) | |
| Post–college graduate degree | 46 (12) | 37 (13) | 9 (9) | |
| CHS | ||||
| Health outcomes | 13 (5–34) | 11 (5–32) | 15 (5–34) | 0.34 |
| Health factors | 12 (3–26) | 11 (3–24) | 12 (3–33) | 0.29 |
| Karnofsky Performance Status | 0.68 | |||
| 10%−40% | 126 (33) | 90 (32) | 36 (36) | |
| 50%−70% | 161 (42) | 122 (43) | 39 (39) | |
| 80%−100% | 97 (25) | 73 (26) | 24 (24) | |
| MELD-Na at transplant | 0.38 | |||
| <25 | 242 (63) | 184 (65) | 58 (59) | |
| 25–34 | 91 (24) | 67 (24) | 24 (24) | |
| >35 | 51 (13) | 34 (12) | 17 (17) | |
| Evaluation MELD-Na§ | 0.002* | |||
| <25 | 273 (67) | 203 (71) | 52 (53) | |
| 25–34 | 111 (27) | 71 (25) | 36 (37) | |
| >35 | 22 (5) | 11 (4) | 10 (10) | |
| Liver disease etiology | <0.001* | |||
| HCV | 137 (36) | 90 (32) | 47 (47) | |
| ALD | 95 (25) | 56 (20) | 39 (39) | |
| HBV | 14 (4) | 12 (4) | 2 (2) | |
| NASH/cryptogenic | 58 (15) | 54 (19) | 4 (4) | |
| PSC | 30 (8) | 29 (10) | 1 (1) | |
| Autoimmune | 14 (4) | 12 (4) | 2 (2) | |
| PBC | 17 (4) | 16 (6) | 1 (1) | |
| Other | 17 (5) | 16 (6) | 3 (3) | |
| Simultaneous kidney transplant | 18 (5) | 13 (5) | 5 (5) | 0.84 |
| Body mass index, kg/m2 | 27 (24–31) | 26 (23–31) | 28 (25–31) | 0.14 |
| Patient deaths | 32 (8) | 23 (8) | 9 (9) | 0.75 |
| Allograft rejection | 42 (11) | 30 (11) | 12 (12) | 0.66 |
| Tacrolimus CoV¶ | 0.38 (0.30–0.50) | 0.36 (0.29–0.46) | 0.46 (0.35–0.57) | <0.001* |
| ≥0.45 | 134 (36) | 81 (30) | 53 (54) | <0.001* |
NOTE: Data are provided as n (%) or median (interquartile range).
P < 0.05.
A cutoff score of 21 chosen to distinguish good/excellent candidates (<21) from minimally acceptable to high-risk candidates (≥21).
A total of 4 missing values.
A total of 1 missing value.
n = 371. Higher tacrolimus CoV indicates higher tacrolimus level variability and serves as a marker of possible medication nonadherence. We only included outpatient tacrolimus values >3 months after LT.
Supporting Table 4 shows comparisons of the baseline characteristics of LT recipients with and without SIPAT data. Patients with missing SIPAT scores were generally younger, were more likely to have MELD-Na scores >35 at evaluation and transplant, and were more likely to have biopsy-proven allograft rejection.
IS NONADHERENCE
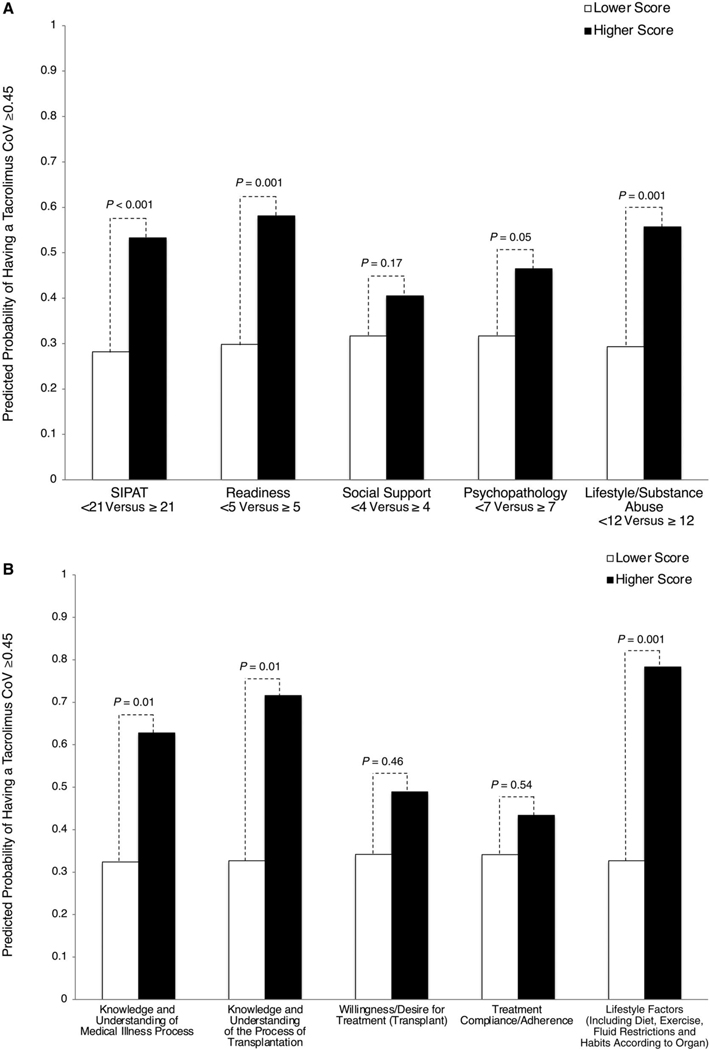
Figure 3 shows the results of multivariable models examining the impact of total SIPAT and SIPAT domain scores (Fig. 3A) and individual readiness domain questions (Fig. 3B) on the predicted probability of having a tacrolimus CoV ≥0.45. A SIPAT score ≥21 (aOR, 2.92; 95% CI, 1.69–5.03), a readiness score ≥5 (aOR, 3.26; 95% CI, 1.63–6.52), a psychopathology score ≥7 (aOR, 1.88; 95% CI, 1.00–3.50), and a lifestyle/substance abuse score of ≥12 (aOR, 3.03; 95% CI, 1.56–5.86) were all associated with having a tacrolimus CoV ≥0.45. Of the 5 individual readiness questions, scoring moderate to poor on knowledge/understanding of medical illness (aOR, 3.52; 95% CI, 1.31–9.42), knowledge/understanding of the process of transplantation (aOR, 5.18; 95% CI, 1.48–18.11), and lifestyle factors (aOR, 7.41; 95% CI, 1.76–31.10) were all associated with having a tacrolimus CoV ≥0.45 (Fig. 5B).
FIG. 3.
Adjusted impact of total SIPAT, SIPAT domains, and individual readiness questions on tacrolimus CoV ≥0.45. (A) Total SIPAT and SIPAT domain scores. (B) Individual readiness domain questions. Higher scores indicate higher psychosocial risk. See Supporting Table 1 for individual items in each domain. Models adjusted for age, sex, race, transplant MELD-Na, liver disease diagnosis, education, and CHS. P values are derived from individual logistic regressions.
ALLOGRAFT REJECTION AND PATIENT/GRAFT SURVIVAL
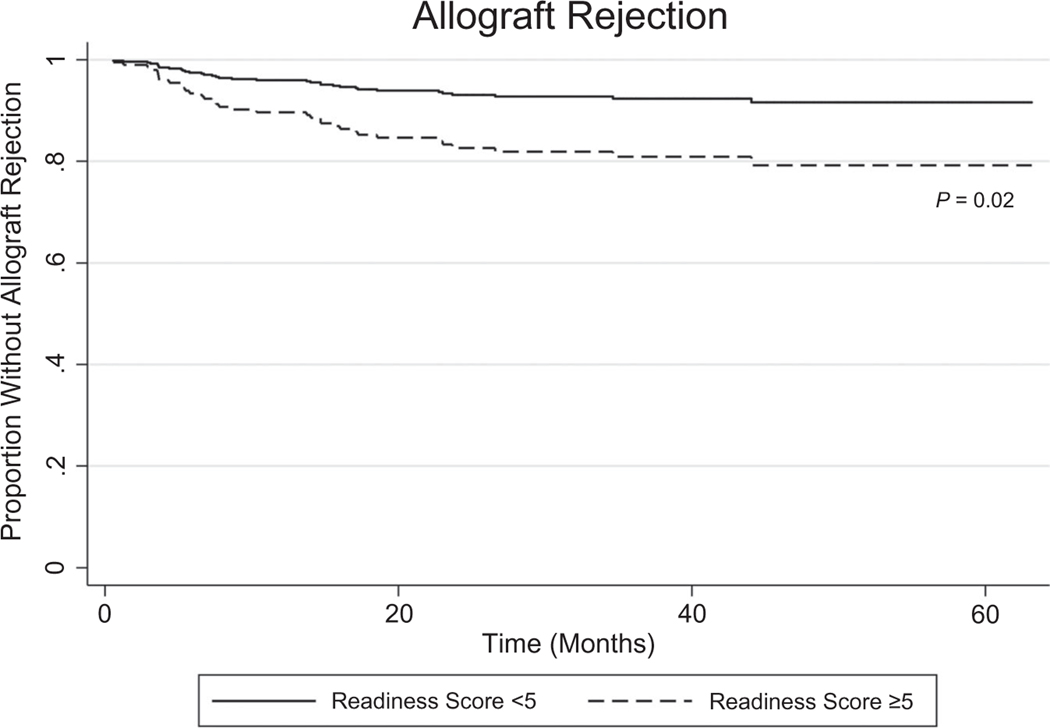
A SIPAT readiness score of ≥5 was associated with a higher risk of biopsy-proven allograft rejection after 3 months (adjusted hazard ratio, 2.66; 95% CI, 1.20–5.91; Figs. 4 and 5C). A total SIPAT score ≥21, a social support score ≥4, a psychopathology score ≥7, and a lifestyle and effect of substance use score ≥12 were not significantly related to posttransplant allograft rejection or death/graft failure.
FIG. 4.
Adjusted impact of SIPAT readiness domain score ≥5 on time to allograft rejection. Higher scores indicate higher psychosocial risk. Readiness score encompasses patient’s understanding of medical illness and the process of transplantation, willingness/desire for treatment, treatment adherence/compliance, and lifestyle habits. Model adjusted for age, sex, race, liver disease diagnosis, education, and CHS.
Discussion
In a large, urban tertiary care transplant center, we found that patients with higher risk SIPAT scores (≥21, categorized as minimally acceptable, poor, or high-risk candidates) were less likely to be added to the waiting list for LT compared with patients with a SIPAT score <21 (categorized as good or excellent candidates). The most commonly cited reason for ineligibility in these patients was “psychosocial contraindications,” which includes lack of social support, active/high-risk substance use, and treatment nonadherence among others. Higher scores (top 20% for each category) in every SIPAT domain (Patient’s Readiness Level, Social Support System, Psychological Suitability and Psychopathology, and Lifestyle and Effect of Substance Use) were also associated with not being added to the waiting list for transplant. Although prior research has shown that low health literacy can reduce the likelihood of LT listing,(15) to our knowledge, the impact of total SIPAT and SIPAT domain scores on waitlisting have not previously been explored in detail in the LT population.
Our study further investigated the role of total SIPAT and specific SIPAT domain scores on post-LT outcomes. Elevated total SIPAT score and SIPAT readiness, psychopathology, and lifestyle/substance abuse domain scores were associated with an increased risk of IS nonadherence as measured by a tacrolimus CoV ≥0.45. The readiness domain was most strongly associated with IS nonadherence, and 3 questions that comprised this domain were individually associated with IS nonadherence (understanding of medical illness, transplant process, and lifestyle factors). The SIPAT readiness domain was also associated with an increased risk of post-LT biopsy-proven allograft rejection. Total SIPAT scores and domain scores were not significantly associated with patient death or graft failure, possibly as a result of shorter interval follow-up.
Among our transplant evaluation cohort, patients with elevated SIPAT scores (≥21) were more likely to have ALD or HCV, identify as Black/African American, have a MELD-Na score ≥25 at evaluation, and were younger and more socially disadvantaged with lower education and higher CHS scores. We expected ALD/HCV to be more common among patients with elevated SIPAT scores because the SIPAT includes questions that explore the history of alcohol and substance use. We acknowledge, however, that alcohol use/alcohol history likely had a significant impact on patient psychosocial risk and medical illness among patients with diagnoses other than ALD.
Higher MELD-Na scores among patients with elevated SIPAT scores may reflect that patients with higher psychosocial risk profiles present later in their disease process for medical evaluation. This is consistent with research in other disease processes that have shown that lack of social support and low financial resources can cause delays in seeking care.(16,17) Patients who are socially disadvantaged with lower social support were less likely to undergo waitlisting and may require enhanced transplant navigation and support. Finally, the higher prevalence of Black/African American patients among patients with elevated SIPAT scores may reflect higher rates of socioeconomic deprivation/chronic stress that culminates in higher psychosocial risk. Racial differences in SIPAT scores have not been closely examined in the literature. Ways to mitigate sociodemographic and racial disparities in transplantation are vastly understudied and present an important area of future research.
To further investigate these associations, we analyzed SIPAT scores by race and educational status and found that after controlling for education and CHS, having a SIPAT score ≥21 did not differ between White, Black, and Latinx patients, but patients identifying as Asian and “other” were less likely to have elevated SIPAT scores. We also observed that patients who had not attended college were more likely to have a SIPAT score ≥21, suggesting that SIPAT scores differ across education (Supporting Table 5, Supporting Figs. 1 and 2). Although we observed these associations, we included race, education, and CHS as covariates in our regression models examining listing decisions and post-LT outcomes to account for these relationships.
The results indicated that a minority of patients who were evaluated (31%) or transplanted (26%) received a SIPAT score ≥21 despite a total maximum score of 110. We believe this may in part be secondary to the transplant evaluation process. Patients who exhibit very high-risk psychosocial profiles may have not been offered transplant evaluation or may not have been able to overcome barriers to accessing outpatient transplant evaluation. As such, the evaluated patient population may be somewhat preselected. Our study does not have information, however, on patients with severe liver disease who were not evaluated for transplantation, although this should be a future area of study.
Further analyses demonstrated that among patients who had a SIPAT score ≥21, factors associated with transplant waitlisting were sex (with men more likely to be listed than women), higher MELD-Na scores, and higher educational attainment. This coincides with prior research showing that the majority of patients transplanted for alcohol-associated hepatitis have been men,(18) and although rates of alcohol use disorder are higher among men than women, that gap is closing, and this does not entirely account for the disparities we have seen in transplantation.(19) Sex disparities in LT warrants further investigation. Higher educational attainment may coincide with level of health literacy, and previous research has shown that lower health literacy is associated with lower likelihood of transplant listing.(15) This may reflect difficulty navigating the health care system and the extensive requirements patients must complete prior to listing.
Our study’s findings on SIPAT and IS nonadherence are consistent with prior studies showing that low medical literacy,(20) mood disorders,(21) lack of social support,(21) and pretransplant nonadherence(22) are associated with post-LT medication nonadherence. One prior study with a smaller sample size demonstrated a relationship between total SIPAT score and IS nonadherence as measured by the tacrolimus CoV, but did not examine specific SIPAT domain scores.(7) Our results did not show that the SIPAT social support domain was significantly associated with medication nonadherence, which may reflect differences in social support measures because no other studies have studied SIPAT domains specifically.
SIPAT and posttransplant outcomes have been examined in other studies, but because of sample size have included a composite positive versus negative outcome (which have included treatment nonadherence and allograft rejection, but also other negative outcomes such as substance relapse and graft failure)(2,6) and included all solid-organ transplants.(2,6) Of note, a recently published article demonstrated an association between elevated SIPAT scores and posttransplant alcohol relapse and included an overlap of 63 patients in the current study who had ALD.(7) The observed relationship between the SIPAT readiness domain score and posttransplant allograft rejection in our study is a novel finding and suggests the potential impact of medical literacy on posttransplant IS adherence.
Our study had several limitations. This was a single-center, observational cohort study with the potential for unmeasured confounding and limited generalizability to other transplant programs. Although a small proportion of patients had missing SIPAT assessments, we compared demographic and clinical characteristics between those with complete and missing data. Despite this, our study sample was sociodemographically and ethnically diverse, highlighting key risk factors for not being added to the waiting list and adverse posttransplant outcomes.
We must also acknowledge that the SIPAT, although standardized, requires some interpretation by those who administer it and can be subjective and vulnerable to bias. Our study lacked data on interrater reliability on our study’s SIPAT administrations; however, previous studies have demonstrated excellent interrater reliability with a Pearson correlation coefficient of 0.853.(2) Furthermore, transplant social workers who administered SIPATs at our institution were not assigned to patients with any sort of trend or bias, so different administration styles should be randomly distributed throughout the sample. Despite these limitations, we believe our study of the SIPAT was performed under pragmatic clinical conditions and mirrors clinical practice.
Another potential limitation of this study involves ascertainment bias. Although SIPAT scores are discussed and known at time of transplant listing, the study’s institution does not include specific SIPAT cutoff scores in transplant evaluation, and each patient is evaluated individually by a multidisciplinary committee. Furthermore, SIPAT scores are not routinely incorporated into posttransplant care and do not trigger specific postmonitoring protocols. Every post-LT patient is monitored in a careful, standardized manner, which would hopefully capture as many negative outcomes as possible and thus minimize ascertainment bias.
Our study could be further improved on by including more detailed psychosocial and clinical data. For instance, our study lacked data on toxicology reports and more detailed psychiatric history and outcomes such as hospitalizations, prior substance use disorder treatment, or psychiatric medications, which would have added more depth to SIPAT analyses. Our data also lacked information on mixed etiologies of liver disease, as only primary etiology of liver disease was available in the institution’s UNOS database. We acknowledge this information could have offered a more accurate understanding of SIPAT score distributions across liver disease. Finally, the study interval follow-up was relatively short because of the relatively recent adoption of the SIPAT in 2014 and may not capture enough later complications such as graft failure or patient death. Future studies should examine these relationships prospectively in multicenter and diverse transplant populations.
The significant clinical ramifications of listing decisions highlight the importance of comprehensive transplant evaluation. Although our article demonstrates several important relationships between SIPAT score and listing decisions and outcomes, it should not replace thoughtful multidisciplinary evaluations. For instance, of the patients with a SIPAT score <21 who were not listed for transplanted, 3.3% were not listed secondary to psychosocial contraindications. As such, psychosocial contraindications to transplant may not be entirely captured by the SIPAT. Furthermore, scoring tools need to be understood across diverse populations and centers to ensure they are appropriate and do not introduce bias that may negatively impact certain patient populations.
FUTURE DIRECTIONS
We believe the results of this study will help clinicians better assess psychosocial risk early in transplant-related care and provide avenues for targeted intervention to improve outcomes before and after transplant. Although pretransplant interventions to improve medical literacy and psychosocial risk factors in the LT population have not been extensively studied,(15) we can examine research done in other solid-organ transplants and chronic diseases to explore potential avenues to improve psychosocial risk in patients undergoing LT evaluation.
In the kidney transplant population, an intervention using a mobile web-based decision tool demonstrated effectiveness in improving pretransplant health literacy and knowledge.(23) In another study among heart transplant candidates, patients underwent psychosocial evaluation including the SIPAT, and then if deemed at risk, received an intervention called the “Psychosocial Treadmill,” which included a set of collaborative interventions to improve psychosocial risk, including mandated substance use treatment, random substance screening (urine and blood), rigorous monitoring of appointment attendance, referrals for neurocognitive and psychological evaluation, financial and disability counseling, support groups, family meetings, and regular social work meetings.(24)
Other studies have shown effective interventions to improve health literacy in patients with hypertension through targeted and interactive clinic-based teaching,(25) family medical literacy/social support in patients with heart failure through a family-based intervention(26) and psychopathology/mental health with a stress-relief physical activity and nutrition-based intervention among cancer survivors.(27) These areas are vastly understudied in LT.
LT recipients with elevated pretransplant SIPAT scores, particularly in the readiness domain, may be at higher risk for nonadherence and posttransplant rejection and should receive enhanced resources and interventions to improve medication adherence after LT. Prior effective interventions to improve adherence have included regular appointments with a dedicated pharmacist,(28,29) increased and systematic monitoring after transplant(30,31) and enhanced educational efforts.(32) The concept of a patient-centered medical home has gained increased attention in the end-stage renal disease and renal transplant populations and may be applicable to the pre-LT and post-LT population(33,34)
As such, we propose that the SIPAT be incorporated as a tool to improve patient outcomes in the pretransplant, peritransplant, and posttransplant time periods. Patients undergoing LT evaluation with a SIPAT score ≥21 should be targeted for enhanced psychosocial resources that could improve their psychosocial candidacy for transplant and enhance the likelihood of waitlisting. Previously, we discussed interventions including a web-based tool to improve pretransplant medical literacy(23) and the “Psychosocial Treadmill” to improve multiple psychosocial factors pretransplant,(24) the latter of which involves comprehensive multidisciplinary care. These interventions could be expanded on and tailored to the LT population. We also believe there could be a role for SIPAT reassessment after an intervention to see if this could be a valid standardized tool to evaluate the effectiveness of these interventions in individual patients.
After LT, patients with a SIPAT score ≥21 should also be targeted for enhanced resources and support. Perhaps a “high-risk” clinic/or clinic day could be coordinated in a post-LT clinic in which multiple resources (medical, social work, case management, mental health, nutrition, pharmacy) are colocated in a post-LT clinic simultaneously, further reducing barriers to accessing these valuable resources. This model has been effective in patients with substance use disorders, including pregnant women(35) and patients with human immunodeficiency virus/acquired immune deficiency syndrome or tuberculosis and substance use disorders.(36) These models highlight the paramount importance of multidisciplinary care. Ultimately, we hope the study’s findings provide an impetus for targeted interventions to improve pre-LT and post-LT outcomes and give rise to further research on the role of SIPAT in facilitating such improvements.
Supplementary Material
Acknowledgments
Marina Serper is supported by the National Institute of Diabetes, Digestive and Kidney Diseases Award 1K23DK115897-01.
Marina Serper consults for Gilead.
Additional supporting information may be found in the online version of this article.
Abbreviations:
- ALD
alcohol-related liver disease
- aOR
adjusted odds ratio
- CHS
community health score
- CI
confidence interval
- CoV
coefficient of variation
- EHR
electronic health record
- GED
General Educational Development
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- IS
immunosuppression
- LT
liver transplantation
- MELD-Na
Model for End-Stage Liver Disease—sodium
- NASH
nonalcoholic steatohepatitis
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- SIPAT
Stanford Integrated Psychosocial Assessment for Transplant
- STAR
Specific Transplant Analysis and Research
- UNOS
United Network for Organ Sharing
REFERENCES
- 1).Dew MA, Switzer GE, DiMartini AF, Matukaitis J, Fitzgerald MG, Kormos RL. Psychosocial assessments and outcomes in organ transplantation. Prog Transplant 2000;10:239–261. [DOI] [PubMed] [Google Scholar]
- 2).Maldonado JR, Dubois HC, David EE, Sher Y, Lolak S, Dyal J, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics 2012;53:123–132. [DOI] [PubMed] [Google Scholar]
- 3).Olbrisch ME, Levenson JL. Psychosocial assessment of organ transplant candidates. Current status of methodological and philosophical issues. Psychosomatics 1995;36:236–243. [DOI] [PubMed] [Google Scholar]
- 4).Dobbels F, Verleden G, Dupont L, Vanhaecke J, De Geest S. To transplant or not? The importance of psychosocial and behavioural factors before lung transplantation. Chron Respir Dis 2006;3:39–47. [DOI] [PubMed] [Google Scholar]
- 5).Dobbels F, De Geest S, Cleemput I, Fischler B, Kesteloot K, Vanhaecke J, et al. Psychosocial and behavioral selection criteria for solid organ transplantation. Prog Transplant 2001;11:121–132. [DOI] [PubMed] [Google Scholar]
- 6).Maldonado JR, Sher Y, Lolak S, Swendsen H, Skibola D, Neri E, et al. The Stanford integrated psychosocial assessment for transplantation. Psychosom Med 2015;77:1018–1030. [DOI] [PubMed] [Google Scholar]
- 7).Deutsch-Link S, Weinrieb RM, Jones LS, Solga SF, Weinberg EM, Serper M. Prior relapse, ongoing alcohol consumption, and failure to engage in treatment predict alcohol relapse after liver transplantation. Dig Dis Sci 2020;65:2089–2103. [DOI] [PubMed] [Google Scholar]
- 8).Lieber SR, Helcer J, Leven E, Knight CS, Wlodarkiewicz C, Shenoy A, et al. Pretransplant psychosocial risk factors may not predict late nonadherence and graft rejection in adult liver transplant recipients. Exp Clin Transplant 2018;16:533–540. [DOI] [PubMed] [Google Scholar]
- 9).Ross K, Patzer RE, Goldberg DS, Lynch RJ. Sociodemographic determinants of waitlist and posttransplant survival among end-stage liver disease patients. Am J Transplant 2017;17: 2879–2889. [DOI] [PubMed] [Google Scholar]
- 10).Remington PL, Catlin BB, Gennuso KP. The county health rankings: rationale and methods. Popul Health Metr 2015;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Pizzo HP, Ettenger RB, Gjertson DW, Reed EF, Zhang J, Gritsch HA, et al. Sirolimus and tacrolimus coefficient of variation is associated with rejection, donor-specific antibodies, and nonadherence. Pediatr Nephrol 2016;31:2345–2352. [DOI] [PubMed] [Google Scholar]
- 12).Del Bello A, Congy-Jolivet N, Danjoux M, Muscari F, Lavayssière L, Esposito L, et al. High tacrolimus intra-patient variability is associated with graft rejection, and de novo donor-specific antibodies occurrence after liver transplantation. World J Gastroenterol 2018;24:1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Moreau A, Varey E, Anegon I, Cuturi MC. Effector mechanisms of rejection. Cold Spring Harb Perspect Med 2013;3:a015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Zhu Y, Zhou Y, Zhang L, Zhang J, Lin J. Efficacy of interventions for adherence to the immunosuppressive therapy in kidney transplant recipients: a meta-analysis and systematic review. J Investig Med 2017;65:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Bittermann T, Dwinnells K, Chadha S, Wolf MS, Olthoff KM, Serper M. Low health literacy is associated with frailty and reduced likelihood of liver transplant listing: a prospective cohort study. Liver Transpl 2020;26:1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Kummer S, Walter FM, Chilcot J, Scott S. Measures of psychosocial factors that may influence help-seeking behaviour in cancer: a systematic review of psychometric properties. J Health Psychol 2019;24:79–99. [DOI] [PubMed] [Google Scholar]
- 17).Li J, Xu R, Hu D, Zhang Y, Gong T, Wu X. Prehospital delay and its associated psychosocial factors in patients presenting with acute appendicitis in a southwestern city in China: a single-centre prospective observational study. BMJ Open 2019;9:23491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Lee BP, Mehta N, Platt L, Gurakar A, Rice JP, Lucey MR, et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology 2018;155:422–430.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).White A. Gender differences in the epidemiology of alcohol use and related harms in the United States. Alcohol Res Curr Rev 2020;40:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Serper M, Patzer RE, Reese PP, Przytula K, Koval R, Ladner DP, et al. Medication misuse, nonadherence, and clinical outcomes among liver transplant recipients. Liver Transpl 2015;21:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Rodrigue J, Nelson D, Hanto D, Reed A, Curry M. Patient-reported immunosuppression nonadherence 6 to 24 months after liver transplant: association with pretransplant psychosocial factors and perceptions of health status change. Prog Transplant 2013;23:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Telles-Correia D, Barbosa A, Mega I, Monteiro E. Psychosocial predictors of adherence after liver transplant in a single transplant center in Portugal. Prog Transplant 2012;22:91–94. [DOI] [PubMed] [Google Scholar]
- 23).Patzer RE, McPherson L, Basu M, Mohan S, Wolf M, Chiles M, et al. Effect of the iChoose Kidney decision aid in improving knowledge about treatment options among transplant candidates: a randomized controlled trial. Am J Transplant 2018;18:1954–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Newman L. The psychosocial treadmill: the road to improving high-risk behavior in advanced therapy candidates. Curr Heart Fail Rep 2018;15:70–74. [DOI] [PubMed] [Google Scholar]
- 25).Warren-Findlow J, Coffman MJ, Thomas EV, Krinner LM. ECHO: a pilot health literacy intervention to improve hypertension self-care. Heal Lit Res Pract 2019;3:e259–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Wu JR, Mark B, Knafl GJ, Dunbar SB, Chang PP, DeWalt DA. A multi-component, family-focused and literacy-sensitive intervention to improve medication adherence in patients with heart failure—a randomized controlled trial. Heart Lung 2019;48:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Golubic M, Schneeberger D, Kirkpatrick K, Bar J, Bernstein A, Weems F, et al. Comprehensive lifestyle modification intervention to improve chronic disease risk factors and quality of life in cancer survivors. J Altern Complement Med 2018;24:1085–1091. [DOI] [PubMed] [Google Scholar]
- 28).Chisholm MA, Mulloy LL, Jagadeesan M, Dipiro JT. Impact of clinical pharmacy services on renal transplant patients’ compliance with immunosuppressive medications. Clin Transplant 2001;15:330–336. [DOI] [PubMed] [Google Scholar]
- 29).Klein A, Otto G, Krämer I. Impact of a pharmaceutical care program on liver transplant patientsĝ€™ compliance with immunosuppressive medication: a prospective, randomized, controlled trial using electronic monitoring. Transplantation 2009;87:839–847. [DOI] [PubMed] [Google Scholar]
- 30).Shemesh E, Annunziato RA, Shneider BL, Dugan CA, Warshaw J, Kerkar N, et al. Improving adherence to medications in pediatric liver transplant recipients. Pediatr Transplant 2008;12:316–323. [DOI] [PubMed] [Google Scholar]
- 31).Promraj R, Dumronggittigule W, Sirivatanauksorn Y, Ruenrom A, Tovikkai C, Limsrichamrern S, et al. Immunosuppressive medication adherence in liver transplant recipients. Transplant Proc 2016;48:1198–1201. [DOI] [PubMed] [Google Scholar]
- 32).Annunziato RA, Emre S, Shneider BL, Dugan CA, Aytaman Y, McKay MM, et al. Transitioning health care responsibility from caregivers to patient: a pilot study aiming to facilitate medication adherence during this process. Pediatr Transplant 2008;12:309–315. [DOI] [PubMed] [Google Scholar]
- 33).Dubose TD, Behrens MT, Berns A, Davis C, Goldfarb S, Hostetter T, et al. The patient-centered medical home and nephrology. J Am Soc Nephrol 2009;20:681–682. [DOI] [PubMed] [Google Scholar]
- 34).Ertel AE, Kaiser T, Shah SA. Using telehealth to enable patient-centered care for liver transplantation. JAMA Surg 2015;150:674–675. [DOI] [PubMed] [Google Scholar]
- 35).Jansson LM, Svikis D, Lee J, Paluzzi P, Rutigliano P, Hackerman F. Pregnancy and addiction: a comprehensive care model. J Subst Abuse Treat 1997;13:321–329. [DOI] [PubMed] [Google Scholar]
- 36).Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy 2007;18:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.