Abstract
Phosphotransacetylase (EC 2.3.1.8) catalyzes the reversible transfer of the acetyl group from acetyl phosphate to coenzyme A (CoA): CH3COOPO32− + CoASH ⇆ CH3COSCoA + HPO42−. The role of arginine residues was investigated for the phosphotransacetylase from Methanosarcina thermophila. Kinetic analysis of a suite of variants indicated that Arg 87 and Arg 133 interact with the substrate CoA. Arg 87 variants were reduced in the ability to discriminate between CoA and the CoA analog 3′-dephospho-CoA, indicating that Arg 87 forms a salt bridge with the 3′-phosphate of CoA. Arg 133 is postulated to interact with the 5′-phosphate of CoA. Large decreases in kcat and kcat/Km for all of the Arg 87 and Arg 133 variants indicated that these residues are also important, although not essential, for catalysis. Large decreases in kcat and kcat/Km were also observed for the variants in which lysine replaced Arg 87 and Arg 133, suggesting that the bidentate interaction of these residues with CoA or their greater bulk is important for optimal activity. Desulfo-CoA is a strong competitive inhibitor of the enzyme, suggesting that the sulfhydryl group of CoA is important for the optimization of CoA-binding energy but not for tight substrate binding. Chemical modification of the wild-type enzyme by 2,3-butanedione and substrate protection by CoA indicated that at least one reactive arginine is in the active site and is important for activity. The inhibition pattern of the R87Q variant indicated that Arg 87 is modified, which contributes to the inactivation; however, at least one additional active-site arginine is modified leading to enzyme inactivation, albeit at a lower rate.
Phosphotransacetylase (EC 2.3.1.8) catalyzes the reversible transfer of the acetyl group from acetyl phosphate to coenzyme A (CoA): CH3COOPO32− + CoASH ⇆ CH3COSCoA + HPO42−. Phosphotransacetylase and acetate kinase are important components of the energy-yielding pathway in most anaerobic microbes from the domain Bacteria. In the archaeon Methanosarcina thermophila, sequential reactions catalyzed by acetate kinase and phosphotransacetylase initiate the pathway of acetoclastic methanogenesis by activating acetate to acetyl-CoA (1). Though acetyl transfer has been studied in other enzymes, very little is known concerning the catalytic mechanism of phosphotransacetylase. Until recently, the only biochemical study reported was in 1976 on the enzyme from Clostridium kluyveri (2). Genes encoding phosphotransacetylase from at least 34 organisms have been reported and all share 51 to 79% deduced sequence similarity to the M. thermophila enzyme (BLAST search results), suggesting similar mechanisms. Recently, the M. thermophila phosphotransacetylase was overexpressed in Escherichia coli, allowing the use of site-directed mutagenesis to investigate the mechanism (6, 8). Thus, this enzyme can serve as a model for all phosphotransacetylases. Chemical modification studies using N-ethylmaleimide implicated a cysteine as an essential active-site residue in the C. kluyveri phosphotransacetylase (2); however, recent site-directed mutagenesis studies on the M. thermophila enzyme showed that the reactive cysteine (Cys 312), though present in the active site, is nonessential for catalysis (8). A second cysteine (Cys 159) was identified as being important for structural stability and possibly for catalysis. Based on preliminary kinetic analyses of a single variant it was postulated that Arg 87 and Arg 133 may be important for binding of CoA (8); however, the roles for these residues were not investigated further. Here we report on the analysis of a suite of Arg 87 and Arg 133 variants using inhibitors and CoA analogs that establish roles for these residues and identify specific interactions with CoA. Inhibitor studies of the wild-type enzyme indicated that at least one arginine other than Arg 87 and Arg 133 is present in the active site and is important for catalysis.
MATERIALS AND METHODS
Site-directed mutagenesis.
Mutagenesis was performed according to the manufacturer's instructions using the Quikchange mutagenesis kit (Stratagene, La Jolla, Calif.), which employs a PCR-based in vitro mutagenesis technique (5). The substitutions were verified by automated double-stranded DNA sequence analysis using the dideoxy chain termination method (10). Oligonucleotides were obtained from Integrated DNA Technologies, Inc. (Coralville, Iowa).
Heterologous production and purification of phosphotransacetylase.
The variant and wild-type phosphotransacetylase genes were subcloned into the pT7-7 overexpression vector. Both the variant and wild-type phosphotransacetylases were overproduced in E. coli BL21(DE3). The cells were grown, and overexpression was induced with 1% Bacto lactose (Difco, Detroit, Mich.) as previously described (6). Proteins were purified from inclusion bodies by a modification of the procedures of Latimer and Ferry (6) and Rasche et al. (8). All steps were performed aerobically at ambient temperature (23°C) unless otherwise noted. Cells (20 to 50 g [wet weight]) were suspended in a volume of buffer (25 mM Tris [pH 7.6], 180 mM KCl, 2 mM dithiothreitol) sufficient to produce a cell density of 1 g/ml. The cells were then disrupted by passage through a French pressure cell at 20,000 lb/in2 and centrifuged at 4°C (25 min, 5,000 × g). The pellet was rinsed in 50 ml of the buffer described above and centrifuged at 4°C (25 min, 5,000 × g). The final pellet was resuspended in 10 ml of buffer, and the inclusion bodies were denatured by the addition of 20 ml of 9 M urea in the buffer described above. The mixture was incubated for 30 min at 13°C and then diluted with 240 ml of buffer and 30 ml of glycerol. The protein was allowed to renature for 16 h at 13°C. The renatured protein was filtered through a 0.45-μm syringe filter and separated on a 50-ml Q-Sepharose anion-exchange column (Amersham Pharmacia Biotech, Piscataway, N.J.) equilibrated with buffer A (25 mM Tris [pH 7.2], 2 mM dithiothreitol). The proteins were eluted using a 300-ml linear gradient of 100 to 700 mM KCl at a flow rate of 3 ml/min. The fractions containing phosphotransacetylase with the highest specific activities were pooled and dialyzed overnight against 4 liters of buffer C (50 mM Tris [pH 7.6], 2 mM dithiothreitol). The dialyzed protein was then separated on a 10-ml Mono-Q anion-exchange column (Amersham Pharmacia Biotech) equilibrated with buffer C. The protein was eluted with a 100-ml linear gradient of 100 to 600 mM KCl at a flow rate of 2 ml/min. The fraction eluting between 250 and 300 mM KCl contained phosphotransacetylase with high specific activity (8,000 to 10,000 μmol of acetyl-CoA/min/mg of protein for wild-type phosphotransacetylase). The enzymes were judged to be homogeneous by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein concentrations were determined using the dye-binding assay of Bio-Rad with bovine serum albumin as the standard.
Enzyme assay.
Phosphotransacetylase activity was quantified by measuring the change in absorbance at 340 nm (extinction coefficient = 4.66 mM−1 cm−1) due to the formation of NADH as described previously by Whiteley and Pelroy (11). The standard reaction mixture (500 μl) contained 25 mM Tris (pH 8.3), 2 mM dithiothreitol, 20 mM KCl, 4 mM acetyl phosphate (lithium-potassium salt), 0.3 mM CoA (lithium salt), 5 mM NAD, 5 mM malic acid, 1.95 U of citrate synthase (porcine heart), and 3.7 U of malic dehydrogenase (pigeon breast muscle). Kinetic constants were determined by nonlinear regression to fit the data to the Michaelis-Menten equation using the program Kaleidagraph (Synergy Software, Reading, Pa.).
Inhibition by 2,3-butanedione.
Inactivation of phosphotransacetylase by 2,3-butanedione was performed by a modification of a previously published procedure (7). Concentrations of 2,3-butanedione (up to 10 mM) were added to the enzyme in a final volume of 1 ml in 50 mM sodium borate, pH 9.0, at 23°C. Aliquots (20 μl) were removed at fixed times to measure phosphotransacetylase activity. Substrate protection experiments were performed by preincubating the enzyme for 5 min with various concentrations of CoA, pH 7.5, at 23°C, and diluting the enzyme in the inactivation mixture containing 10 mM 2,3-butanedione (final concentration) and the same concentration of CoA as the preincubation mix.
Inhibition by inorganic phosphate or desulfo-CoA.
Phosphotransacetylase activity was measured as usual except that K2HPO4 (up to 10 mM [final concentration]) or desulfo-CoA (up to 25 μM [final concentration]) was present in the standard assay mix.
Chemicals.
All chemicals were obtained from Sigma-Aldrich (St. Louis, Mo.) or Fisher Scientific (Pittsburgh, Pa.).
RESULTS
Initial characterization of Arg 87 and Arg 133 variants.
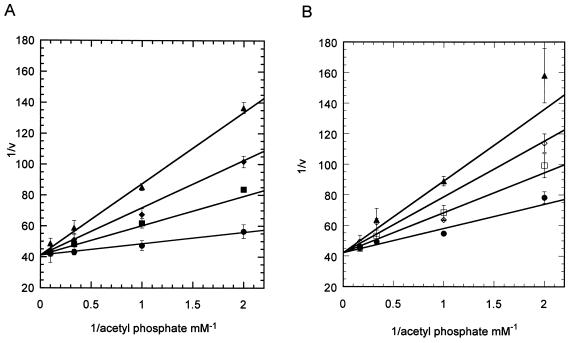
All variants of the M. thermophila phosphotransacetylase were produced in inclusion bodies as observed previously with the wild type (6). The variants were purified by a three-step protocol comprising the solubilization of inclusion bodies and the refolding of the protein followed by ion-exchange chromatography. All of the variants had the same chromatographic properties during purification to homogeneity, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, with yields that were 65 to 90% of those of the wild-type enzyme. These results indicate no global conformational changes for any of the variants compared to the wild type. The inhibition patterns with inorganic phosphate for the variants R87Q and R133Q were determined to ensure that substitutions of Arg 87 or Arg 133 did not disrupt the active site and kinetic mechanism. Both variants showed patterns of competitive inhibition between acetyl phosphate and inorganic phosphate (Fig. 1) that were identical to the inhibition pattern for the wild-type enzyme (data not shown). The Kis of inorganic phosphate for the variants (0.52 and 0.32 mM for R87Q and R133Q, respectively) were similar to that for the wild type (0.81 mM). These results indicate that changes in the properties of the Arg 87 and Arg 133 variants relative to the wild type are not attributable to changes in the active site unrelated to the specific roles for Arg 87 and Arg 133.
FIG. 1.
Inorganic phosphate inhibition of R87Q and R133Q phosphotransacetylases. K2HPO4 inhibition was determined using the coupled assay in the presence of various levels of acetyl phosphate and a fixed level of CoA (2 mM). Data were plotted as 1/v versus 1/concentration of acetyl phosphate. Ki was determined using the equation for a competitive inhibitor: v = Vmax[Km/(concentration of acetyl phosphate) (1 + [I]/Ki) + 1]. (A) Inhibition of R87Q with no added phosphate (●), 3 mM phosphate (■), 6 mM phosphate (⧫), and 10 mM phosphate (▴). (B) Inhibition of R133Q with no added phosphate (●), 2 mM phosphate (□), 4 mM phosphate (◊), and 6 mM phosphate (▴).
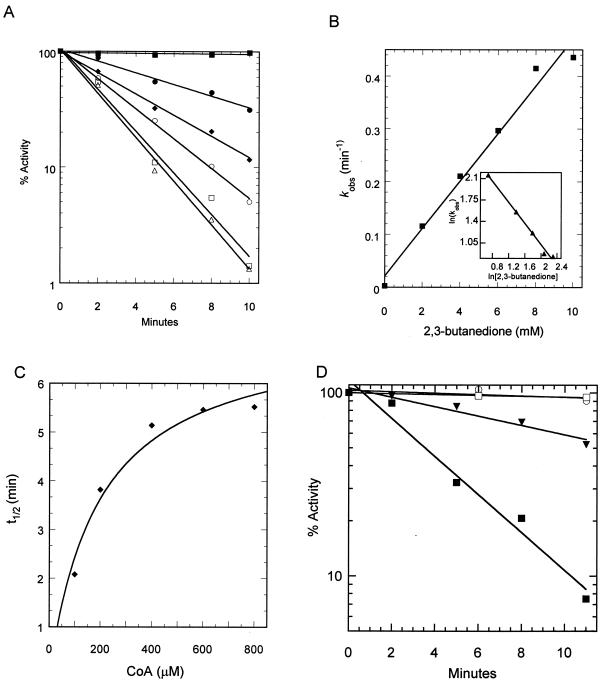
Inactivation of wild-type phosphotransacetylase and the R87Q and R133Q variants by 2,3-butanedione.
We found that the arginine-modifying reagent phenylglyoxal weakly inhibited the enzyme; thus, we used the arginine-modifying agent 2,3-butanedione in order to test for the presence of an arginine residue in the active site of the M. thermophila phosphotransacetylase. The reagent inactivated the wild-type enzyme in a time- and concentration-dependent manner (Fig. 2A). Nearly all enzyme activity was lost after exposure to 10 mM 2,3-butanedione for 10 min. A plot of the pseudo-first-order inactivation rate constant (kobs) versus 2,3-butanedione concentration yielded a bimolecular rate constant of 0.75 M−1s−1 (Fig. 2B). A double log plot of kobs versus the inhibitor concentration was linear with a slope of 0.85 (Fig. 2B, inset), indicating that the modification of a single arginine is sufficient to inactivate the enzyme. There was a corresponding decrease in inhibition when the enzyme was preincubated for 5 min with increasing concentrations of CoA (100 μM or higher) before the addition of 2,3-butanedione, demonstrating substrate protection (Fig. 2C). The Ki of protection (194 μM) is in the range of the Km(CoA) (70 μM) (Table 1). These results indicate that one or more reactive arginines, important for activity, are in the active site. Preincubation of the enzyme with the indicated CoA concentrations (Fig. 2C) was performed in 25 mM Tris, pH 7.5. An aliquot of this mixture was then diluted in the inactivation mixture containing 50 mM borate (pH 9.0), 10 mM 2,3-butanedione, and the indicated concentration of CoA. The protection effect of CoA against the inhibitor was not observed if the preincubation step was performed at pH 9.0, suggesting that substrate binding is impaired at this pH value. Acetyl phosphate (Km = 323 μM) up to 1 mM afforded no protection against inactivation, indicating that these active-site arginines are not in the vicinity of the acetyl phosphate binding site.
FIG. 2.
Inactivation of phosphotransacetylase by 2,3-butanedione. (A) Wild-type phosphotransacetylase was incubated with various concentrations of 2,3-butanedione and 50 mM sodium borate (pH 9) in a final volume of 1 ml as described in Materials and Methods. At the indicated time points, 20 μl of the solution was removed and the phosphotransacetylase activity was measured. The concentrations of 2,3-butanedione used were 0 (■), 2 (●), 4 (⧫), 6 (○), 8 (□), and 10 (▵), mM. (B) Rate of inactivation of phosphotransacetylase (kobs) obtained from panel-A plotted against the concentration of 2,3-butanedione. (C) CoA protection of wild-type phosphotransacetylase against 2,3-butanedione inactivation. The wild-type enzyme was inactivated by 10 mM 2,3-butanedione. Half-lives were obtained from plots of percent activity remaining versus time at each CoA concentration. Ki(CoA) was calculated by fitting the data to the hyperbola equation, t1/2 = t1/2(max)[CoA]/(Ki + [CoA]). (D) Inactivation of R87Q and R133Q phosphotransacetylases by 10 mM 2,3-butanedione. Phosphotransacetylase was incubated with 0 or 10 mM 2,3-butanedione. The symbols represent R87Q with no 2,3-butanedione (○), R87Q with 10 mM 2,3-butanedione (▾), R133Q with no 2,3-butanedione (□), and R133Q with 10 mM 2,3-butanedione (■).
TABLE 1.
Kinetic properties of the wild type and Arg 87 and Arg 133 variants
| Enzyme | CoA
|
dCoA
|
[kcat/Km](CoA) [kcat/Km](dCoA) | ||||
|---|---|---|---|---|---|---|---|
| kcat-(s−1)a | Km (mM)a | kcat/Km (mM−1s−1) | kcat (s−1)a | Km (mM)a | kcat/Km (mM−1s−1) | ||
| Wild type | 4,880 ± 448 | 0.07 ± 0.01 | 70,000 | 272 ± 14 | 1.4 ± 0.1 | 198 | 350 |
| R87A | 1,225 ± 109 | 0.9 ± 0.2 | 1,400 | 184 ± 38 | 6 ± 2 | 31 | 45 |
| R87Q | 1,390 ± 64 | 1.1 ± 0.1 | 1,250 | 98 ± 23 | 1.3 ± 0.7 | 76 | 17 |
| R87K | 373 ± 25 | 0.57 ± 0.09 | 650 | 41 ± 7 | 3.9 ± 0.9 | 10 | 65 |
| R87E | 17 ± 1 | 0.27 ± 0.06 | 63 | 23 ± 2 | 0.8 ± 0.1 | 30 | 2 |
| R133A | 631 ± 28 | 0.36 ± 0.05 | 1,700 | 0.75 ± 0.08 | 1.3 ± 0.4 | 0.6 | 2,800 |
| R133Q | 1,146 ± 55 | 0.7 ± 0.1 | 1,700 | 28 ± 4 | 4 ± 1 | 7 | 250 |
| R133K | 418 ± 36 | 0.11 ± 0.03 | 3,800 | 8 ± 0.4 | 0.8 ± 0.1 | 10 | 380 |
| R133E | 44 ± 5 | 0.7 ± 0.1 | 60 | 0.28 ± 0.02 | 1 ± 0.2 | 0.3 | 200 |
Values are given as means ± standard deviations.
Chemical modification using 2,3-butanedione was attempted for the Arg 87 and Arg 133 variants to determine if either residue was the reactive arginine. Variant R87Q (kcat = 1,390 ± 64) was susceptible to inhibition, although at a lower rate than the wild type, losing 50% of enzyme activity on exposure to a 10 mM concentration of the inhibitor for 10 min (Fig. 2D). Preincubation with CoA afforded partial protection against inactivation of R87Q; however, this protection required greater than 500 μM CoA and did not follow hyperbolic saturation kinetics (data not shown). The R133Q variant was inactivated by 2,3-butanedione at a rate similar to that for the wild-type enzyme, indicating that this residue is not modified in the wild-type enzyme. This variant was also partially protected from inactivation by preincubation with CoA. The protection required greater than 200 μM CoA and did not follow saturation kinetics (data not shown). As with the wild type, the R87Q and R133Q variants were not protected against 2,3-butanedione inactivation by acetyl phosphate at concentrations of up to 1 mM (data not shown).
Kinetic analyses of the wild type and arginine variants.
All of the Arg 87 and Arg 133 variants showed substantial decreases in kcat(CoA) (4- to 287-fold) and the catalytic efficiency with CoA (50- to 1,100-fold), indicating that both Arg 87 and Arg 133 are important, although not essential, for enzyme activity (Table 1). The R133E variant showed the smallest catalytic efficiency and R133K showed the largest catalytic efficiency among the Arg 133 variants, a result which indicates that the positive charge of Arg 133 is important for enzyme activity.
All of the Arg 87 and Arg 133 variants showed increases in Km(CoA) (4- to 15-fold) compared to the wild-type enzyme (Table 1), suggesting that Arg 87 and Arg 133 are important for interactions with CoA. Among the Arg 133 variants, R133E and R133Q showed the largest increases (10-fold), while R133K showed the smallest increase (1.6-fold). The R133A variant displayed an intermediate value. These results indicate that the positive charge of Arg 133 plays a role in interactions with CoA, consistent with the kcat results which indicated that the positive charge of Arg 133 is important for enzyme activity.
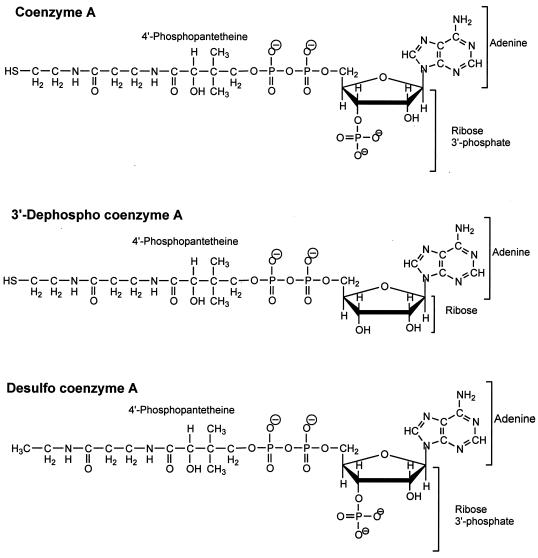
The question of either Arg 87 or Arg 133 forming a salt bridge with the 3′-phosphate of CoA was addressed in experiments utilizing 3′-dephospho CoA (dCoA) (Fig. 3), since no such interaction can occur with this substrate analog. The wild-type enzyme exhibited a Km(dCoA) value of 1.4 mM, which is 20-fold greater than the Km(CoA), a result suggesting a specific interaction of the enzyme with the 3′-phosphate of CoA. Thus, the kinetic properties of the variant enzymes were analyzed using either CoA or dCoA as the substrate and then compared with the wild type to determine if either Arg 87 or Arg 133 interacts with the 3′-phosphate of CoA (Table 1). All of the Arg 87 variants showed either no increase (R87E, R87Q) or relatively modest increases (R87A, R87K) in Km(dCoA) compared to the wild-type enzyme. The decreases in catalytic efficiencies with dCoA were also modest (2- to 19-fold). A comparison of the catalytic efficiencies (Table 1) shows that the wild-type enzyme has a 350-fold preference for CoA over dCoA. All Arg 87 variants showed a weakening of the preference for CoA over dCoA as measured by the ratio of kcat/Km for CoA to kcat/Km for dCoA. The R87E variant showed only a 2-fold preference for CoA over dCoA, which represents a 175-fold decrease in specificity for CoA with respect to the wild-type enzyme; however, R87K retained a 65-fold preference for CoA. These results indicate that the positive charge of Arg 87 is important for the interaction with the 3′-phosphate of CoA. When the neutral residue alanine or glutamine replaced Arg 87, the decrease in specificity was intermediate, a result consistent with the above inference. The Km(dCoA) values for Arg 133 variants were between 0.8 and 4 mM, a result comparable to that of the wild-type enzyme. All of the Arg 133 variants showed similar decreases in catalytic efficiency towards CoA and dCoA compared to the wild type. Consequently, all Arg 133 variants retained at least a 200-fold preference for CoA over dCoA. These results indicate that the positive charge of Arg 133, although important for CoA binding, does not interact with the 3′-phosphate of CoA. The Km values for acetyl phosphate for all Arg 87 and Arg 133 variants were found to be in the same range as that of the wild type (Table 2), a result suggesting that neither arginine interacts with this substrate.
FIG. 3.
Structures of CoA and analogs used.
TABLE 2.
Km(acetyl phosphate) values for wild-type and variant phosphotransacetylases
| Enzyme | Km(acetyl phosphate) (μM)a |
|---|---|
| Wild type | 323 ± 26 |
| R87A | 230 ± 44 |
| R87Q | 297 ± 27 |
| R87K | 445 ± 89 |
| R87E | 68 ± 21 |
| R133A | 308 ± 50 |
| R133Q | 297 ± 37 |
| R133K | 202 ± 37 |
| R133E | 192 ± 59 |
Values are given as means ± standard deviations.
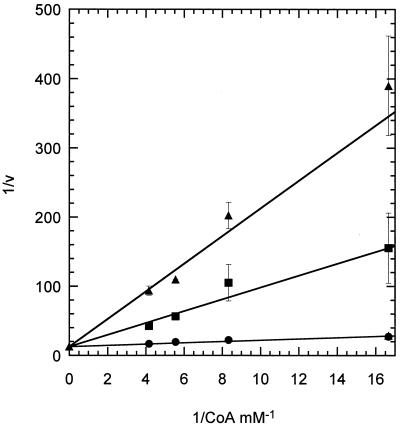
Inhibition by desulfo-CoA.
The effect of desulfo-CoA (Fig. 3) on the phosphotransacetylase reaction was investigated to determine the role of the sulfhydryl group of CoA in substrate binding to phosphotransacetylase. Desulfo-CoA was a strong competitive inhibitor with respect to CoA (Ki = 1 μM) (Fig. 4); in fact, the enzyme shows greater affinity towards desulfo-CoA than towards CoA (Ki of substrate protection = 194 μM). A Gibbs free energy change of 3.1 kcal mol−1 in favor of desulfo-CoA binding was calculated using the expression ΔΔG = −RT ln Ki(CoA)/Ki(desulfo-CoA), where ΔΔ G is the incremental free energy change, R is the gas constant (0.00198 kcal mol−1K−1), and T is the temperature, 296 K.
FIG. 4.
Desulfo-CoA inhibition of phosphotransacetylase. Desulfo-CoA inhibition was determined by the coupled assay in the presence of various levels of CoA and a fixed level of acetyl phosphate. Data were plotted as 1/v versus 1/[CoA]. Ki was determined using the equation for a competitive inhibitor, v = Vmax{Km/[CoA](1 + [I]/Ki) + 1}. The symbols represent no added desulfo-CoA (●), 10 μM desulfo-CoA (■), and 25 μM desulfo-CoA (▴).
DISCUSSION
Residues Arg 87 and Arg 133 of the M. thermophila phosphotransacetylase are completely conserved in the 34 phosphotransacetylase sequences from the domains Bacteria and Archaea that are reported in the databases. In order to determine the role of Arg 87 and Arg 133, a suite of variants was generated by site-directed mutagenesis and analyzed using inhibitors and substrate analogs. The increased Km(CoA) values for all of the variants relative to the wild type provided an initial indication that Arg 87 and Arg 133 are important for binding CoA. The higher concentrations of CoA required for protection of the R87Q and R133Q variants from 2,3-butanedione inhibition support this interpretation. Thus, the interaction of Arg 87 and Arg 133 with CoA was investigated further with the CoA analogs dCoA and desulfo-CoA. The predicted properties of a salt bridge variant would include a decreased affinity for CoA but no significant change in the affinity for dCoA. The results obtained with the suite of Arg 87 variants strongly indicate that the positive charge of Arg 87 forms a salt bridge with the 3′-phosphate of CoA. Although the Km(CoA) values for the Arg 133 variants indicated that the positive charge of Arg 133 is important for binding CoA, the results obtained with dCoA indicate that a salt bridge is not formed with the 3′-phosphate of CoA. The simplest interpretation is that the positive charge of Arg 133 forms a salt bridge with one of the two 5′-phosphate groups of CoA or participates in a hydrogen bond with another part of the CoA molecule. This is in agreement with a kinetic analysis of the C. kluyveri phosphotransacetylase leading to the proposal that a group with a pKa near 8.9 is involved in interactions with the phosphopantetheine side chain of CoA (3). The finding that CoA protects the enzyme from 2,3-butanedione inactivation when CoA treatment is performed at pH 7.5, but not at pH 9.0, is consistent with the existence of salt bridges between the enzyme and the substrate. The observation that the Arg 87 and Arg 133 variants studied had Km(acetyl phosphate) values similar to those of the wild type and the inability of acetyl phosphate to protect the enzyme from 2,3-butanedione inhibition suggest that acetyl phosphate is bound at a site remote from the proposed CoA binding site.
A weak competitive inhibition between CoA and desulfo-CoA would imply a role for the sulfhydryl of CoA as a hydrogen bond donor to the enzyme (9). When desulfo-CoA was used as an inhibitor of the phosphotransacetylase reaction, a strong competitive inhibition with respect to CoA was observed for the wild-type enzyme. The finding that phosphotransacetylase binds desulfo-CoA nearly 200 times more tightly than CoA suggests that the enzyme selectively destabilizes the substrate CoA, utilizing the binding energy to increase the rate of the reaction rather than to cause tight binding (4). The presence of the sulfhydryl group may be important for this destabilization effect; thus, CoA is destabilized by 3.1 kcal mol−1 compared to desulfo-CoA, which differs only in the substitution of a proton for the sulfhydryl group.
The large decreases in kcat and kcat/Km for all of the Arg 87 and Arg 133 variants suggest that these residues are also important, although not essential, for catalysis. Variant R87Q was less susceptible to inhibition by 2,3-butanedione than was the wild type, which is consistent with an influence of this residue on optimum activity. Variant R133Q, however, was inhibited similarly to the wild type, suggesting that the inhibitor cannot access Arg 133; indeed, the inability of the bulkier phenylglyoxal to inhibit the wild-type enzyme is consistent with a constricted active site. Although the results indicate that the positive charges of Arg 87 and Arg 133 are important for maximum enzyme activity, variants containing lysine at these two positions also showed large decreases in kcat and kcat/km which were similar to other variants. This finding suggests that a single positive charge cannot perform the function of these arginines and that a bidentate interaction with CoA or the greater bulk of arginine is required for maximum activity. The two-point interaction of Arg 87 and Arg 133 with CoA may be important to optimally position the substrate for nucleophilic attack of the thiolate anion of CoA on the carbonyl carbon of acetyl phosphate.
Inhibition of the wild-type enzyme and protection from inhibition by CoA indicated that at least one active-site arginine is important for catalysis or substrate binding and is modified by 2,3-butanedione. The results indicate that Arg 87 resides in the active site and its modification contributes to inactivation of the enzyme. Inactivation of the wild-type enzyme is most likely due to the addition of bulk to Arg 87, eliminating substrate binding. The finding that a fivefold-higher CoA concentration is required to protect the R87Q variant from 2,3-butanedione inactivation, compared to that of the wild-type enzyme, is strongly supportive of the idea that the variant has a reduced affinity for CoA. Variant R133Q (kcat = 1,146 ± 55) was inactivated at a rate similar to that of the wild type (Fig. 2D), suggesting that the modification of this residue does not contribute to the inactivation of the wild-type enzyme by 2,3-butanedione.
The inhibition pattern of R87Q also indicates that at least one arginine residue other than Arg 87 is present in the active site and is important for activity; however, it is modified at a lower rate than Arg 87. One of the probable candidates for this second arginine is Arg 133, which is not affected by 2,3-butanedione in the wild type but may be modified in the absence of Arg 87. As outlined above, Arg 133 is important in interactions with CoA and therefore its modification may preclude productive substrate binding, leading to the loss of enzyme activity. Arg 310, conserved among all phosphotransacetylases, is another candidate for 2,3-butanedione modification. This proposal is consistent with the finding that this residue is essential for activity based on a kinetic analysis of R310Q and the close proximity of Arg 310 to Cys 312, shown previously to be located in the active site (8). Although the function of this unidentified arginine is unknown, it is possible this residue may interact with the acetyl phosphate to orient the substrate or to polarize the carbonyl group. Moreover, it has been proposed that in the C. kluyveri enzyme, a residue with a pKa of >9 may function to polarize the carbonyl carbon of acetyl phosphate, facilitating a nucleophilic attack by CoA (3).
In summary, this is the first investigation of phosphotransacetylase using chemical inactivators and substrate analogs to identify Arg 87 and Arg 133 active-site residues and to further identify specific roles for these residues in substrate binding. Inhibitor studies indicated that an additional arginine, possibly Arg 310, is important for catalysis. Given the high sequence similarity among phosphotransacetylases, the results presented here for the M. thermophila enzyme likely apply to all phosphotransacetylases.
ACKNOWLEDGMENTS
This work was supported by Department of Energy grant DE-FG02-95ER20198 and National Institutes of Health grant GM44661.
We are grateful to J. M. Bollinger, P. C. Bevilacqua, and A. Gorrell for insightful comments and advice.
REFERENCES
- 1.Ferry J G. Fermentation of acetate. In: Ferry J G, editor. Methanogenesis. New York, N.Y: Chapman and Hall; 1993. pp. 304–334. [Google Scholar]
- 2.Henkin J, Abeles R H. Evidence against an acyl-enzyme intermediate in the reaction catalyzed by clostridial phosphotransacetylase. Biochemistry. 1976;15:3472–3479. doi: 10.1021/bi00661a012. [DOI] [PubMed] [Google Scholar]
- 3.Hibbert F, Kyrtopoulos S A, Satchell D P N. Kinetic studies with phosphotransacetylase. Biochim Biophys Acta. 1971;242:39–54. doi: 10.1016/0005-2744(71)90086-6. [DOI] [PubMed] [Google Scholar]
- 4.Jencks W P. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol. 1975;43:219–410. doi: 10.1002/9780470122884.ch4. [DOI] [PubMed] [Google Scholar]
- 5.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latimer M T, Ferry J G. Cloning, sequence analysis, and hyperexpression of the genes encoding phosphotransacetylase and acetate kinase from Methanosarcina thermophila. J Bacteriol. 1993;175:6822–6829. doi: 10.1128/jb.175.21.6822-6829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullan L M, Igarashi P, Noltmann E A. Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by phenylglyoxal and butanedione and in the protection by substrate analogs. Arch Biochem Biophys. 1983;221:489–498. doi: 10.1016/0003-9861(83)90167-4. [DOI] [PubMed] [Google Scholar]
- 8.Rasche M E, Smith K S, Ferry J G. Identification of cysteine and arginine residues essential for the phosphotransacetylase from Methanosarcina thermophila. J Bacteriol. 1997;179:7712–7717. doi: 10.1128/jb.179.24.7712-7717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raybuck S A, Bastian N R, Orme-Johnson W H, Walsh C T. Kinetic characterization of the carbon monoxide-acetyl-CoA (carbonyl group) exchange activity of the acetyl-CoA synthesizing CO dehydrogenase from Clostridium thermoaceticum. Biochemistry. 1988;27:7698–7702. doi: 10.1021/bi00420a019. [DOI] [PubMed] [Google Scholar]
- 10.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiteley H R, Pelroy R A. Purification and properties of phosphotransacetylase from Veillonella alcalescens. J Biol Chem. 1972;247:1911–1917. [PubMed] [Google Scholar]