Abstract
Prenatal exposure to stress increases risk for suboptimal child and adult mental and physical health outcomes, hypothesized to occur via fetal exposure to maternal stress hormones that alter growth and development. One proposed pathway through which stress exposure in utero could affect the offspring is by accelerating cellular aging in the form of telomere attrition. We tested this hypothesis in a cohort of 111 mother-child dyads, where mothers were assessed over 6 or more years, beginning prior to conception, and later during pregnancy, postpartum, and when the children were 3 to 5 years old. Adjusting for child age and concurrent maternal stress, we found that higher maternal perceived stress in the 3rd trimesters of pregnancy was predictive of shorter child buccal telomere length (bTL) (β = −.24, p< .05), while maternal preconception and postpartum maternal stress were not associated with bTL (all p’s > .42). These findings suggest a vulnerable time period in pregnancy when maternal stress influences offspring telomere length, suggesting the early embedding of adult disease might occur through biological aging pathways.
Keywords: maternal, perceived stress, pregnancy, child outcomes, telomere length, prenatal
INTRODUCTION
Prenatal exposure to maternal stress increases risk for suboptimal child and adult health outcomes such as cardiovascular and metabolic disease.1–3 This in utero or fetal programming of adult disease, first characterized by Barker4, is thought to occur through exposure of the fetus to a less than optimal in utero environment that alters growth and development. One proposed pathway through which exposure to stressors and resultant stress hormones could increase risk for suboptimal health outcomes is through accelerating cellular age via telomere attrition.1,5,6 Telomere length is a repetitive sequence of DNA at the chromosomal ends that can be lost during clonal expansion,7 a process occurring across tissues during fetal growth. Telomere length is a marker of biological aging,8 with shortened telomere length predictive of increased risk for numerous health outcomes9,10 and earlier mortality.11,12 Rapid growth of fetal cells can be accompanied by activated telomerase, an enzyme that rebuilds the telomere;13,14 however, stress may interfere with this process.15,16 In particular, cortisol has been shown to down-regulate the activity of telomerase,16 thereby offering a pathway through which stress hormone exposure may accelerate aging by shortening telomere length in the fetus, referred to as the fetal programming of telomere biology hypothesis.17
Stress, particularly during early life, and also chronic stress in adulthood, have been associated with shorter telomere length and accelerated telomere shortening.5,18–20 Fetal exposure to maternal stress has also been linked to offspring telomere length.21–23 Maternal recall of a major stressful life event occurring during the pregnancy was associated with shorter telomere length in offspring in adulthood, although the trimester in which the stressful event occurred was not identified.24 Alternatively, several studies have reported newborn cord blood telomere length to be shorter in offspring whose mothers reported high stress during either the first trimester22 or the third trimester21,23 of pregnancy, but neither study examined multiple trimesters. To date research has been limited by considering only a single time point of prenatal stress. Likewise, investigators have derived telomere length estimates from cord blood of newborns, or adult offspring, but have not as yet observed this effect in the offspring during young childhood. Preconception stress might also have an impact on child health,25 yet no research to date has examined the relationship of stress prior to conception and offspring telomere length. Theoretically, timing of stress exposure may be critically important to clarification of the fetal origins hypothesis.
The present study followed mothers who had previously given birth at least once in the year prior to conception of a subsequent child, then followed through the next pregnancy with assessments in second and third trimester, and at 1-month postpartum. In a follow-up study, child telomere length was measured from DNA extracted from buccal samples obtained at an in home visits occurring when the child was 3–5 years of age. We hypothesized that higher maternal stress would predict shorter child buccal telomere length (bTL). We further explored whether there were sensitive periods when maternal stress had a greater impact on telomere length –specifically, preconception, second trimester or third trimester of pregnancy, and postpartum/early life.
METHODS
Procedures
Data were collected from participants in the Community Child Health Network, comprised of five sites (Washington, D.C., Baltimore, Maryland, Los Angeles County, California, Lake County, Illinois, and seven counties in North Carolina) that enrolled 2,510 Latina, White, and African-American women. Study design and sample characteristics are reported elsewhere.26 Women were recruited to the CCHN study after the birth of a child and followed for two years. Mothers were interviewed during the inter-pregnancy interval or preconception up to 4 times, M(SD) = 3.44(2.7) months, 2nd trimester, M(SD) = 20.6(3.9) weeks, 3rd trimester, M(SD) = 33.1(3.3) weeks, and post-partum, M(SD) = 12.7(5) weeks. A subset of 242 women in three sites participating in the follow-up study had a subsequent pregnancy and participated in at least one interview during the pregnancy. Of those who could be located, 127 agreed to participate in a follow-up home visit with their offspring at 3 of the 5 original sites (eastern North Carolina, Washington, DC, and Lake County IL). Of these, 111 had children who provided buccal samples using a cheek swab from which telomere length was determined (see participant flowchart Figure 1).1 Mothers were instructed to avoid feeding their child prior to the visit, and children were asked to rinse their mouth with water prior to sample collection. Buccal cell samples were collected from the inside of each child’s cheek by rotating a cytology brush (one for each cheek), and this swab was then placed in buccal cell lysis provided as part of the Gentra Puragene buccal cell extraction kit (Qiagen, Germantown, MD).
Figure 1.
Flow diagram for data collection at 3 Preconception study sites
Measures
Perceived Stress Scale.
Maternal stress was measured using the Perceived Stress Scale (PSS), a validated, reliable instrument of perception of stress over the past month (Cohen & Williamson, 1988). The 10-item questionnaire includes items assessing the general unpredictability and uncontrollability of stressors (e.g., In the past month, how often have you found that you could not cope with all the things that you had to do?) and mothers answered on a 5-point Likert scale ranging from never (0) to almost always (4). Mothers completed the PSS up to three times in the year prior to conception (α range .77 to .83), during the 2nd trimester (α = .87), 3rd trimester (α = .86), and 1-month post-partum (α = .70). Higher scores indicate higher levels of perceived stress. Preconception stress using the PSS was assessed within 12 months of conception.
Buccal Telomere Length (bTL).
Children provided buccal cell samples when they were 3 – 5 years old. Genomic DNA was extracted from buccal cells using the Gentra Puragene buccal cell extraction kit (Qiagen, Germantown, MD). DNA quality was determined using NanoDrop full-spectrum spectrophotometer, and quantity verified using high sensitivity Invitrogen Quant-iT dsDNA assay kit.
Telomere length values were estimated using a standard real-time quantitative polymerase chain reaction (qPCR) methodology as reported previously.27 Values are expressed as the ratio of the estimated concentration generated by PCR of the telomere gene (T) divided by the hemoglobin single (S) copy gene = (T/S). Primer used were Tel1b [CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT], Tel2b [GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT], hgb1 [GCTTCTGACACAACTGTGTTCACTAGC], and hgb2 [CACCAACTTCATCCACGTTCACC]. Samples were run in triplicate and then assessed for reliability, with acceptable intra-assay (range: 0.1 – 6%; average = 1%) and inter-assay variability for both T and S plates (range: 0.9– 4%; average = 2%; ICC = .995). Assays were completed in two batches, with a subset of samples run in each batch (ICC = .988). Telomere estimates between batches were similar (R2=.96) with no significant differences in estimates. Batch was included as a covariate in all models.
Demographic and Medical Factors.
Interviews and medical charts provided data on child and mother demographics, father age, birth outcomes, and maternal medical conditions. Treatment of covariates is described in detail the plan of analyses.
Statistical Analyses.
Sample descriptives and bivariate correlations were analysed using IBM SPSS Statistics version 25. Regression analyses were run using MPlus version 7 employing full information maximum likelhood to account for missing data. Multivariate outlier analyses, using DFFITS, DFBETAS, Cook’s distance as criteria,28 were conducted to identify potential influential cases. Tolerance and variance inflation factors were checked to account for potential issues with multicollinearity. Tolerance values ranged from .41 to .65 and Variance Inflation Factors were all less than 2.5, suggesting minimal influence of multicollinearity.29 Outlier analyses revealed no influential cases; therefore, all participants were included in analyses. Child bTL values identified as outliers (± 3 SD from the Mean) were winsorized. Child bTL values were then z-transformed for analyses to improve comparability across studies, as has been recommended.30
Preliminary analyses examined sample characterstics and bivariate correlations of child buccal telomere length with child demographic (child age, sex, race as African-American vs. other; ethnicity as Hispanic vs. other), mother demographic (years education, per capita income adjusted for cost of living in the site), father age, mother health (pre-pregnancy BMI, smoking during pregnancy, gestational diabetes) and pregnancy outcome variables (child birth weight, gestational length) to identify potential covariates given previous links to these factors and child telomere length.31–38 Any variable significantly associated with child telomere length was included as a covariate in follow-up analyses. In addition, child age was included in all analyses given its established relationship with telomere length. PSS was measured multiple times before preconception. For these analyses, we used the PSS measure most proximal to timing of conception (range 0 months – 12 months) to represent “preconception maternal stress.”
Main analyses tested whether maternal stresss in preconception, 2nd trimester, 3rd trimester, and post-partum predicted child telomere length at age 3 to 5 years in 4 separate multiple regression equations, controlling for child age, batch (1 or 2) when telomere samples were assayed, and pre-pregnancy BMI. All timepoints of maternal stress were then inputed into one model to examine which time point predicted child telomere length over and above the others. Finally, regression analyses were re-run, controlling for concurrent maternal stress to check if results remained consistent.
RESULTS
Table 1 shows sample characteristics of the mother-child dyads in the current analyses. Nearly half (46.8%) of children were of Hispanic ethnicity, while the remaining were non-Hispanic Caucasian (28.8%), African-American (18.4%), and mutiracial (9.2%). Child bTL was not significantly associated with child race (African American vs. all others; p = 0.25) or ethnicity (Hispanic vs. all others; p = 0.95), child sex (p = 0.29), child BMI (p = .61), mother age (p = .90), father age (p = .55), mother education (p = .81), mother adjusted per capita income (p = 0.79), mother gesational diabetes (N=7; p = .38), mother smoking history (N=7, p = .55), gestational length (p = .13) or child birth weight (p = 0.26). Mother pre-pregnancy BMI was positively associated with child bTL (r = .26, p = .02). Child age was negatively associated with bTL (r = −.22, p = .02). Children BMIs were normal, average household income was middle class, and 55% were female.
Table 1.
Participant Demographics and Medical Factors.
| Variable | Telomere Length, r or M(SD), p value | |||
|---|---|---|---|---|
|
| ||||
| M | SD | Range | ||
|
| ||||
| Mother Age at T1 | 27.10 | 5.26 | 18.19–39.32 | r = −.01, p = .90 |
| Father Age at T1 | 28.58 | 6.26 | 18.68–51.00 | r = −.07, p = .55 |
| Maternal Education (years) | 12.76 | 3.39 | 6 – 21 | r = .02, p = .81 |
| Household Income at T1, Per capita | 16,424.28 | 26,747.27 | 0 – 241, 80.04 | r = −.03, p = .79 |
| Pre-pregnancy Body Mass Index (BMI) | 29.95 | 7.31 | 18.00 – 54.00 | r = .26, p = .02 |
| Perceived Stress | ||||
| Preconception | 13.18 | 5.34 | 1 – 26 | r = −.13, p = .24 |
| 2nd Trimester | 14.30 | 5.88 | 0 – 29 | r = −.21, p = .11 |
| 3rd Trimester | 17.61 | 5.73 | 5 – 33 | r = −.25, p = .03 |
| Postpartum | 17.04 | 6.13 | 4 – 39 | r = −.13, p = .24 |
|
| ||||
| Category | N | % | ||
|
| ||||
| Maternal Ethnicity | F = .37, p = .77 | |||
| Hispanic | 54 | 48.6 | 1.71(.99) | |
| Non-Hispanic Caucasian | 36 | 32.4 | 1.62 (.75) | |
| African American | 19 | 17.1 | 1.48 (.65) | |
| Multiracial | 2 | 1.8 | 1.80 (.85) | |
|
| ||||
| M | SD | Range | ||
|
| ||||
| Child Age at SC1 | 3.84 | 0.41 | 3.35–5.48 | r = −.22, p = .02 |
| Child BMI | 16.25 | 1.64 | 12–25 | r = .05, p = .61 |
| Gestational Length (weeks) | 38.84 | 1.74 | 29.71–42.00 | r = −.15, p = .13 |
| 3,225.13 | 590. 19 | 1,247 – 4,750 | r = −.12, p = .26 | |
| Birth weight (g) | ||||
| Category | N | % | ||
| Child Sex | t = −1.28, p = .20 | |||
| Female | 61 | 55.0 | 1.64 (.64) | |
| Male | 50 | 45.0 | 1.49 (.64) | |
| Child Ethnicity | F = .46, p = .72 | |||
| Hispanic | 52 | 46.8 | 1.57 (.63) | |
| Caucasian | 32 | 28.8 | 1.66 (.65) | |
| African American | 18 | 16.2 | 1.50 (.66) | |
| Multiracial | 9 | 8.1 | 1.42 (.46) | |
|
| ||||
| Child Buccal Telomere Length | 1.57 | .63 | .17 – 3.00 | -- |
Note. Household income is yearly income adjusted for cost of living. Child buccal telomere length is non-transformed values.
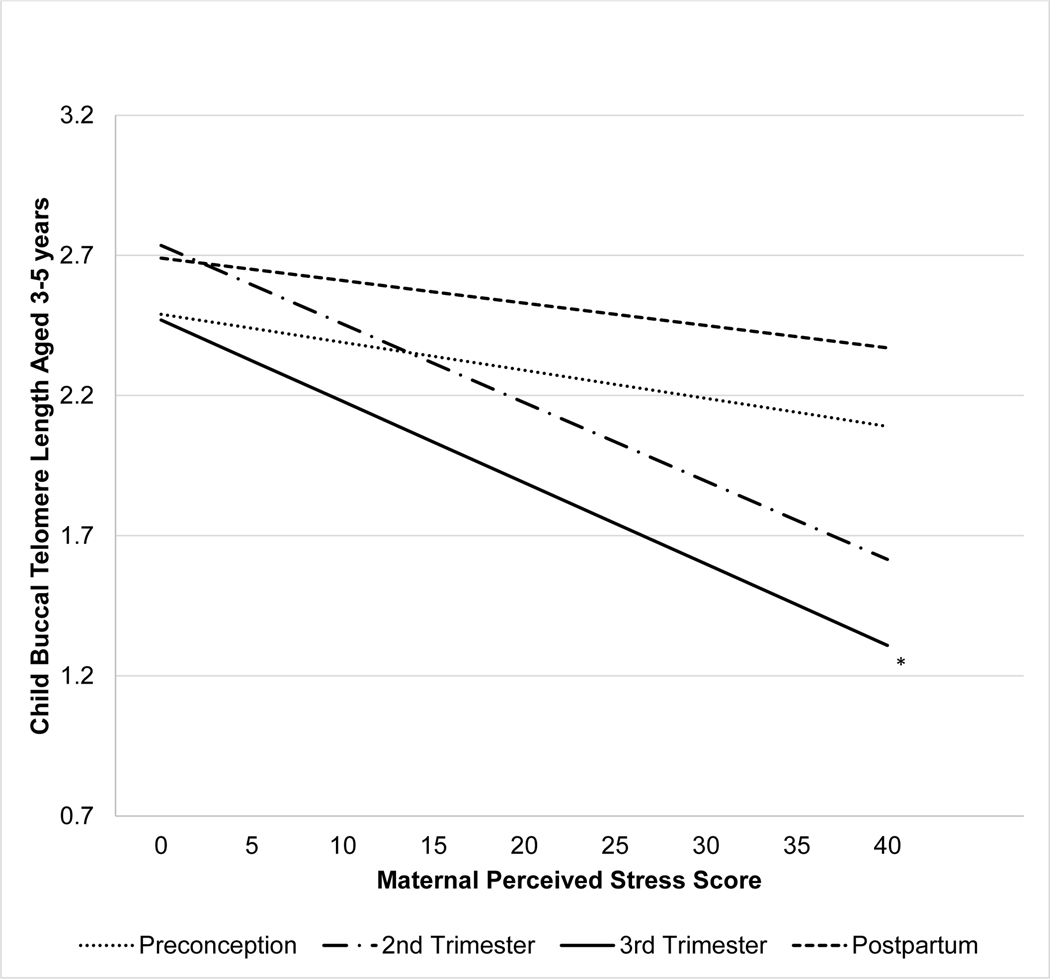
PSS scores at preconception, second and third trimester and postpartum were significantly related over time at p < .01 (r values range .39 - .47). In a first step, each PSS score was tested for an association with child bTL, adjusting for child age and batch of telomere assay (see Table 2). Results indicate that maternal stress in the 2nd trimester, and 3rd trimester each had a modest relationship to bTL, although only 3rd trimester stress met the p<.05 threshold for significance (b (SE) = −.04 (.02), β = −.25, p< .05; See Figure 2). Maternal stress preconception and postpartum did not significantly relate to child telomere length (all p’s > .12). Sensitivity analyses were performed to determine if the 3rd trimester was the most sensitive in predicting child telomere length with maternal stress from all other time points entered simultaneously, controlling for child age and batch (Table 2). Analyes revealed a similar effect size of maternal stress in the 3rd trimester on offspring bTL (b (SE) = −.04 (.02), β = −.24, p = .06), while all other time points were non-significant when included in the same regression model, with an overall R2 = .19, p = .012. Additional adjustment for concurrent maternal stress did not modify these results (individual model third trimester, b (SE) = −.04 (.02), β = −.24, p = .033; simultaneous model third trimester, b (SE) = −.04 (.02), β = −.23, p = .078). Secondary analyses controlling for gestational length did not modify the results. In this simultaneous regression model the tolerance, VIF, and standard errors were within normal limits and do not suggest multicollinearity was a problem.
Table 2.
Results of linear regression analyses testing the direct effect of each time point of maternal stress on child buccal telomere length (z-transformed).
| Individual | Simultaneous | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| b (SE) | β | p | b (SE) | β | P | |
| Covariates | ||||||
| Child Age | −.042(.02) | −.210 | .027 | |||
| Batch | .196 (.24) | .077 | .405 | |||
| Maternal Pre-Pregnancy BMI | .030 (.01) | .233 | .031 | |||
| Predictors | ||||||
| Preconception (< 12 months) | −.029 (.02) | −.156 | .126 | −.003 (.003) | −.127 | .221 |
| 2nd Trimester | −.045 (.02) | −.258 | .062 | −.012 (.03) | −.070 | .730 |
| 3rd Trimester | −.042 (.02) | −.254 | .016 | −.041 (.02) | −.243 | .062 |
| Postpartum | −.015 (.02) | −.091 | .360 | .003 (.02) | .017 | .900 |
Note: Covanate regression effects from an empty model. Individual: Regression models were run separately for each predictor. Simultaneous: Regression model run with all predictors entered simultaneously.
Figure 2. Slope of the individual linear regression equation for perceived maternal stress predicting child buccal telomere length (T/S).
Note: Estimated slopes derived from regression models adjusting for child age, batch, and pre-pregnancy BMI, with perceived stress score predicting non-transformed child buccal telomere length. *p<.05
DISCUSSION
The current study examined whether maternal stress reported in the past month at four different critical periods, preconception, second trimester, third trimester, and postpartum, was prospectively related to child buccal telomere length at 3 to 5 years old. The stress measure used (PSS) is a well validated standardized scale that assesses properties of stress such as uncontrollability, inability to cope, and other aspects reflecting chronic stress from any and all sources in life.39 Analyses examined and controlled for many maternal, birth, and child factors. Additional analyses controlling for maternal stress at the time of the buccal collection and for length of gestation did not alter these results. Our findings indicate that the strength of the association is strongest for third trimester prenatal maternal stress, while a similar coefficient (moderate effect) for second trimester prenatal maternal stress was marginally significant. In the model where all time points were entered simultaneously, only third trimester maternal stress predicted bTL of the child.
Notably, we did not observe effects of maternal stress on offspring telomere length when the stress was measured within 12 months prior to conception, nor when stress was assessed in the postpartum period. These results are consistent with the fetal origins hypothesis arguing that maternal stress is most impactful on offspring health when it occurs in utero, and less impactful when there is not a shared biological milieu. Likewise, our findings are consistent with prior research indicating that maternal stress during pregnancy relates to newborn telomere length21–23, and extend these into early childhood.
Maternal stress may impact fetal programming by altering cellular age, as indexed by buccal telomere length, with implications for risk of later health problems in offspring, including metabolic and cardiovascular disease.40–43 If stress experienced by the mother influences fetal development via programming the offspring’s systems, the pathways through which this drives telomere loss are multiplicative. Stress hormones, including cortisol and catecholamines, both have plausible mechanistic roles. Prenatal stress has previously been associated with elevated maternal inflammation44–46 and stress hormones.47,48 Indeed, recent findings have demonstrated a connection between a pro-inflammatory state in pregnancy with shorter newborn leukocyte telomere length,49 suggesting a mechanism through which maternal stress might alter child outcomes. Cortisol exposure in utero may contribute to decreased telomerase activity, which would reduce the capacity of rapidly reproducing stems cells from elongating telomere length in the fetus. In combination with this, catecholamines directly activate inflammatory pathways within immune cells,50 promoting cellular replication that can drive telomere loss. Catecholamines also stimulate cellular metabolic activity, a source of oxidative stress known to damage telomeric ends,51 and potentially reduce telomere length if unrepaired before cell replication. The developing fetus has rapidly expanding cell populations and there may be times when cells are particularly vulnerable to stress hormone exposure that induces telomere loss. Stress in late pregnancy could exert its effects on offspring telomere length via multiple biological pathways beyond the ones outlined above,17,48 and further research is warranted to better understand these dynamics.
Of note, a prior report examining cord blood and placental telomere length in newborns with maternal pre-pregnancy BMI in a large sample from Belgium38 report a correlation of r=−.11, suggesting increasing BMI is associated with shorter newborn telomere length. In our smaller sample of multi-racial cohort residing in the United States of America, analyses show longer buccal telomere length in offspring ages 3–5 with higher maternal pre-pregnancy BMI. Our study differs in several ways, including a different population, with variability in diets, a greater distribution in BMI, and telomere length measured in young childhood rather than at birth. Our findings should be replicated in a larger sample of women designed to assess the impact of maternal BMI on offspring telomere length.
Limitations and Future Directions
The sample size varied by time point, and although we used statistical models that included all available data points, it remains possible that the strength of the effects observed would be altered if we had a complete dataset. Larger sample size would improve statistical power, which may result in effects such as second trimester stress and child bTL reaching statistical significance. Thus, the effect estimates generated for each time point when stress was assessed should be considered, irrespective of statistical significance. In addition, the timing of the assessment of preconception stress was on average six months prior to conception. Future research may consider the proximity of stress to conception as an important factor that might influence risk for shortened telomere length in the offspring. A more proximal assessment of stress in relation to conception such as within three months before conception may yield alternative findings. Child telomere length was captured using buccal samples, which has been shown to be highly correlated to circulating leukocyte telomere length;52 Nevertheless, bTL estimates may be less predictive of adult health risk than leukocyte telomere length values, and further work is warranted to determine the difference in risk prediction between these two sample sources. Additional limitations to the current analyses include an absence of biological indices of maternal stress (e.g., cortisol, catecholamines) that provide an additional way to examine the connection between stress and offspring telomere length, no measure of maternal telomere length during pregnancy which could relate to offspring telomere length, nor a measure of telomere length at birth to replicate prior reports on newborn telomere length. In addition, we did not replicate prior reports linking child sex and race with telomere length,53,54 although we were underpowered to properly test interactions. Despite these shortcomings, the study has several strengths. First, the prospective design allowed us to assess stress at four distinct times and. Unlike life events measures, the assessments were for a short recent time period (past month), providing for more accurate estimates of all stress exposures. Secondly, the timing of child assessments is novel and extends prior findings about telomere length at birth. Finally, the study sample is ethnically, racially, and socioeconomically diverse, giving better representation of these underrepresented groups.
Conclusion
In conclusion, we report that maternal stress in late pregnancy is prospectively associated with reduced child buccal telomere length at 3 to 5-years of age, independent of postpartum and concurrent maternal reported stress. These findings are consistent with the hypothesis of fetal programming of lifelong risk for disease and point to a mechanism by which fetal programming occurs, in this case potentially through shortened telomere length, a marker of biological aging.
Highlights:
Maternal stress reported during pregnancy was prospectively associated with shortened buccal cell telomere length (an indicator of cellular aging) in the offspring at 3–5 years of age.
This study supports the theory that accelerated biological aging is a mechanism through which in utero exposure to stress may increase risk for aging-related disease and earlier death, and these findings extend previous reports by documenting that the effects of maternal stress on offspring aging biology are detectable in early childhood.
Acknowledgements
Financial Support for The Preconception and Prenatal Stress: Pathways to Child Biology and Behavior Study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD;5R01HD072021-05). Support also provided to JEC and CDS from the Cousins Center for Psychoneuroimmunology, and funding for the Community Child Health Network (CCHN) was supported through cooperative agreements with the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; U HD44207, U HD44219, U HD44226, U HD44245, U HD44253, U HD54791, U HD54019, U HD44226-05S1, U HD44245-06S1, R03 HD59584) and the National Institute for Nursing Research (NINR; U NR008929).
Footnotes
The 111 women in the current sample were older (t(242) = −4.91, p < .001) and had higher incomes (t (241) = −2.93, p < .01) compared to the 131 women who had subsequent pregnancies but did not participate. There were no significant ethnic/racial or educational differences (p’s > .29).
Disclosures/Conflicts of Interest
All authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: Concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes 2010; 17: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellman LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Dev Psychobiol 2008; 50: 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunkel Schetter C. Psychological Science on Pregnancy : Stress Processes , Biopsychosocial Models , and Emerging Research Issues. Annu Rev Psychol 2010. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJP. In utero programming of chronic disease. Clin. Sci 1998; 95: 115–128. [PubMed] [Google Scholar]
- 5.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 2013; 38: 1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coimbra BM, Carvalho CM, Moretti PN, Mello MF, Belangero SI. Stress-related telomere length in children: A systematic review. J Psychiatr Res 2017; 92: 47–54. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn E. Structure and function of telomeres. Nature 1991.http://adsabs.harvard.edu/abs/1991Natur.350..569B. [DOI] [PubMed] [Google Scholar]
- 8.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res 2011; : 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol 2008; 28: 1379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 2007; 165: 14–21. [DOI] [PubMed] [Google Scholar]
- 11.Bakaysa SL, mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B et al. Telomere length predicts survival independent of genetic influences. Aging Cell 2007; 6: 769. [DOI] [PubMed] [Google Scholar]
- 12.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber R a. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003; 361:393–6. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn EH. Telomere states and cell fates. Nature 2000; 408: 53–6. [DOI] [PubMed] [Google Scholar]
- 14.Forsyth NR, Wright WE, Shay JW. Telomerase and differentiation in multicellular organisms: Turn it off, turn it on, and turn it off again. Differentiation. 2002; 69: 188–197. [DOI] [PubMed] [Google Scholar]
- 15.Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci 2009; 1172: 34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun 2008; 22: 600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Entringer S, de Punder K, Buss C, Wadhwa PD. The fetal programming of telomere biology hypothesis: An update. Philos. Trans. R. Soc. B Biol. Sci 2018; 373. doi: 10.1098/rstb.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epel E, Blackburn E, Lin J, Dhabhar F, Adler N, Morrow J et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci 2004; 101: 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 2012. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rentscher KE, Carroll JE, Mitchell C . Psychosocial Stressors and Telomere Length: A Current Review of the Science. Annu Rev Public Health. [DOI] [PubMed] [Google Scholar]
- 21.Marchetto NM, Glynn RA, Ferry ML, Ostojic M, Wolff SM, Yao R et al. Prenatal stress and newborn telomere length. Am J Obstet Gynecol 2016. doi: 10.1016/j.ajog.2016.01.177. [DOI] [PubMed] [Google Scholar]
- 22.Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH et al. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol 2013; 208: 134.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Send TS, Gilles M, Codd V, Wolf I, Bardtke S, Streit F et al. Telomere Length in Newborns is Related to Maternal Stress During Pregnancy. Neuropsychopharmacology 2017; 42: 2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH et al. PNAS Plus: Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci 2011; 108: E513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keenan K, Hipwell AE, Class QA, Mbayiwa K. Extending the developmental origins of disease model: Impact of preconception stress exposure on offspring neurodevelopment. Dev Psychobiol 2018; 60: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramey SL, Schafer P, DeClerque JL, Lanzi RG, Hobel C, Shalowitz M et al. The Preconception Stress and Resiliency Pathways Model: A Multi-Level Framework on Maternal, Paternal, and Child Health Disparities Derived by Community-Based Participatory Research. Matern Child Health J 2015; 19: 707–719. [DOI] [PubMed] [Google Scholar]
- 27.Carroll JE, Esquivel S, Goldberg A, Seeman TE, Effros RB, Dock J et al. Insomnia and Telomere Length in Older Adults. Sleep 2016; 39: 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neter J, Wasserman W, Kutner MH. Applied Linear Models. 2nd ed. Irwin: Boston, 1989. [Google Scholar]
- 29.Fox J, Monette G. Generalized Collinearity Diagnostics. J Am Stat Assoc 1992; 87: 178. [Google Scholar]
- 30.Verhulst S. Improving comparability between qPCR-based telomere studies. Mol. Ecol. Resour 2020; 20: 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Song L, Zhang L, Wu M, Wang L, Cao Z et al. Prenatal second-hand smoke exposure and newborn telomere length. Pediatr Res 2019; : 1–6. [DOI] [PubMed] [Google Scholar]
- 32.Needham BL, Fernandez JR, Lin J, Epel ES, Blackburn EH. Socioeconomic status and cell aging in children. Soc Sci Med 2012. doi: 10.1016/j.socscimed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Tarik M, Ramakrishnan L, Sinha S, Sachdev HPS, Tandon N, Roy A et al. Association of birth outcomes and postnatal growth with adult leukocyte telomere length: Data from New Delhi Birth Cohort. Matern Child Nutr 2019; : e12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M et al. Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics 2016; 137: e20153927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Ye J, Wu Y, Zhang H, Luo Q, Han C et al. Reduced Fetal Telomere Length in Gestational Diabetes. PLoS One 2014; 9: e86161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ip P, Chung BHY, Ho FKW, Chan GCF, Deng W, Wong WHS et al. Prenatal Tobacco Exposure Shortens Telomere Length in Children. Nicotine Tob Res 2017; 19: 111–118. [DOI] [PubMed] [Google Scholar]
- 37.Akkad A, Hastings R, Konje J, Bell S, Thurston H, Williams B. Telomere length in small-for-gestational-age babies. BJOG An Int J Obstet Gynaecol 2006; 113: 318–323. [DOI] [PubMed] [Google Scholar]
- 38.Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot TS. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med 2016; 14: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav 1983; 24: 385. [PubMed] [Google Scholar]
- 40.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2014; 349: g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley M a. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One 2010; 5: e8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzpatrick AL, Kronmal R a, Kimura M, Gardner JP, Psaty BM, Jenny NS et al. Leukocyte Telomere Length and Mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 2011; 66: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S, Lin J, Matsuguchi T, Blackburn E, Yeh F, Best LG et al. Short leukocyte telomere length predicts incidence and progression of carotid atherosclerosis in American Indians: The Strong Heart Family Study. Aging (Albany NY) 2014; 6: 414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross KM, Cole SW, Carroll JE, Dunkel Schetter C. Elevated pro-inflammatory gene expression in the third trimester of pregnancy in mothers who experienced stressful life events. Brain Behav Immun 2019; 76: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun 2012; 26: 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun 2007; 21: 343–350. [DOI] [PubMed] [Google Scholar]
- 47.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev 2010; 81: 131–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rakers F, Rupprecht S, Dreiling M, Bergmeier C, Witte OW, Schwab M. Transfer of maternal psychosocial stress to the fetus. Neurosci Biobehav Rev 2017. doi: 10.1016/j.neubiorev.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Lazarides C, Epel ES, Lin J, Blackburn EH, Voelkle MC, Buss C et al. Maternal pro-inflammatory state during pregnancy and newborn leukocyte telomere length: A prospective investigation. Brain Behav Immun 2019; 80. doi: 10.1016/j.bbi.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole SW, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A 2010; 107: 5681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 2002; 27: 339–344. [DOI] [PubMed] [Google Scholar]
- 52.Gadalla SM, Cawthon R, Giri N, Baerlocher G, Lansdorp PM, Alter BP et al. Correlation of Telomere Length in Blood, Buccal Cells, and Fibroblasts From Patients with Inherited Bone Marrow Failure Syndromes. Blood 2009; 114.http://www.bloodjournal.org/content/114/22/1083?sso-checked=true (accessed 19 Apr2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drury SS, Esteves K, Hatch V, Woodbury M, Borne S, Adamski A et al. Setting the trajectory: Racial disparities in newborn telomere length. J Pediatr 2015; 166. doi: 10.1016/j.jpeds.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosquet Enlow M, Sideridis G, Bollati V, Hoxha M, Hacker MR, Wright RJ. Maternal cortisol output in pregnancy and newborn telomere length: Evidence for sex-specific effects. Psychoneuroendocrinology 2019; 102. doi: 10.1016/j.psyneuen.2018.12.222. [DOI] [PMC free article] [PubMed] [Google Scholar]