Abstract
Coronavirus disease-2019 (COVID-19) had become a global pandemic since March 2020. Although, the most common presentation is of pulmonary involvement, hepatic abnormalities can be encountered in up to 50% of infected individuals, which may be associated with disease severity, and the mechanism of liver injury is thought to be multifactorial. Guidelines for management in patients with chronic liver disease during COVID-19 era are being regularly updated. Patients with chronic liver disease and cirrhosis, including liver transplant candidates and liver transplant recipients are strongly recommended to receive SARS-CoV-2 vaccination because it can reduce rate of COVID-19 infection, COVID-19–related hospitalization, and mortality.
Keywords: COVID-19, SARS-CoV-2, Cirrhosis, Chronic liver disease, Acute liver disease, Abnormal liver biochemistries, Liver transplantation, Vaccination
Key points
-
•
Hepatic biochemical test abnormalities in patients with Coronavirus disease-2019 (COVID-19) can be encountered in up to 50% of infected individuals; the pattern of liver injury is mostly hepatocellular, whereas the mechanism of liver injury is thought to be multifactorial. Chronic hepatobiliary manifestation of cholangiopathy is being increasingly recognized.
-
•
Underlying chronic liver disease is not uncommon in patients with COVID-19 infection, and such patients with cirrhosis have higher and increasing mortality with liver disease severity as assessed by Child-Pugh class.
-
•
Because of the high rate of hepatic decompensation in patients with cirrhosis following COVID-19 infection, early diagnosis and early admission should be emphasized.
-
•
Although response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination may be suboptimal in immunosuppressed and immunocompromised patients, patients with cirrhosis receiving SARS-CoV-2 vaccination can result in a reduction of COVID-19 infection, COVID-19–related hospitalization, and mortality; thus, patients with chronic liver disease and particularly patients with cirrhosis, liver-transplant candidates and liver transplant recipients are strongly recommended for COVID-19 vaccination.
Introduction
Coronavirus disease-2019 (COVID-19), the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China, in December 2019, and has become a global pandemic since March 2020, leading to significant morbidity and mortality in humans. Although, it most commonly presents with pulmonary manifestations, hepatic abnormalities can be encountered in up to 50% of infected individuals, which can vary in severity from asymptomatic to severe liver injury.1 Chronic liver disease (CLD) is not uncommon in the background of patients hospitalized with COVID-19 infection, which is, in itself, associated with more severe COVID-19 disease and higher mortality,2 especially in patients with cirrhosis.3 This review summarizes hepatic manifestations in patients with COVID-19 infection and outcome in those with CLD and addresses vaccination and management of patients with CLD during the ongoing COVID-19 pandemic.
Prevalence of Liver Dysfunction and Hepatobiliary Manifestation in SARS-CoV-2–Infected Patients
The incidence of elevated liver biochemistries in hospitalized COVID-19 infected patients ranges from 14% to 83%.2 More commonly, an elevation of aspartate transaminase (AST) has been reported in 8% to 83%, and an elevation of alanine transaminase (ALT) in 10% to 61%; however, mild elevation of bilirubin has been reported in 3% to 23%, of ALP in 1% to 22%, and of gamma-glutamyl transferase in 13% to 54% of patients with COVID-19 infection.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Abnormalities in liver biochemistries are reported with similar frequencies regardless of the presence of preexisting liver disease.26 The pattern of liver injury is mostly hepatocellular rather than cholestatic.1 Mild AST and ALT elevations of 1 to 2 times the upper limit of normal (ULN) are commonly observed early in the disease course. AST is usually higher than ALT, and this may increase with COVID-19-associated-disease severity and mortality, which could possibly reflect nonhepatic injury.2 , 27 One retrospective cohort of patients with COVID-19 infection from the United States (n = 3381) noted mild liver injury in 45%, moderate in 21%, and severe acute liver injury in 6.4% of hospitalized patients with COVID-19.28 Usually the hepatic biochemical test abnormalities return to normal values within 2 to 3 weeks without specific treatment.18 Liver injury is more commonly observed in severe COVID-19 cases than in mild cases, and COVID-19 infection in patients with elevated liver biochemistries (especially with AST and ALT elevation greater than 5 times ULN) was associated with higher mortality.29 Hypoalbuminemia at hospital admission has also been a marker of COVID-19 severity.30, 31, 32 When assessing COVID-19 patients with elevated hepatic biochemical tests, other causes unrelated to COVID-19 such as viral hepatitis should be considered.2 Further, pregnant patients with COVID-19 infection have also been reported to have AST or ALT elevation in up to 21% to 22%, suggesting that appropriate monitoring of hepatic biochemical tests is needed in this population.33 Notably, a recent study reported that patients with COVID-19 had underlying CLD in around 2% to 11%.14 Patients with more advanced liver disease had higher mortality after COVID-19 infection, with the highest mortality among patients with cirrhosis and with the rate increasing with more severe liver disease, as assessed by Child-Pugh class.2 , 34
Mechanisms of Liver Injury from Coronavirus Disease-2019 Infection
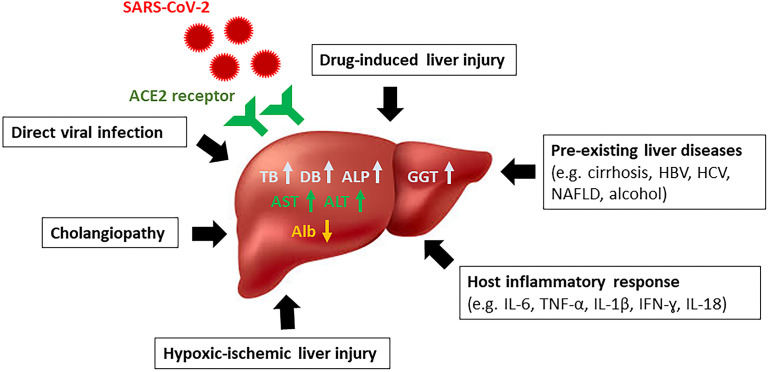
SARS-CoV-2 is a single, positive-stranded RNA virus that replicates using a virally encoded RNA-dependent RNA polymerase.2 There are several potential mechanisms and causes of liver injury in patients with COVID-19 infection, some of which may be virus specific and others nonspecific. Liver histologic findings at autopsy35 have noted one or more features of microvesicular/macrovesicular steatosis, mixed lobular necroinflammation and portal inflammation, focal necrosis, and porto-venous/sinusoidal microthrombosis (Fig. 1 ).
Fig. 1.
Proposed mechanisms of liver injury from SARS-CoV-2 infection.
Direct hepatic infection by SARS-CoV-2
SARS-CoV-2 binds to target cells through angiotensin-converting enzyme 2 (ACE2) entry receptors. ACE2 is present in both hepatocytes and cholangiocytes; therefore, liver is a potential target for infection and may be the pathogenesis of SARS-CoV-2–related liver injury. ACE2 expression in healthy liver is found in the cholangiocytes (59.7%), and this rate is much higher than in the hepatocytes (2.6%)36; thus, liver injury may result from direct viral damage to bile duct epithelial cells, which has been known to be significant in liver regeneration and immune response,37 although the exact mechanism is still unclear. Multiple levels of evidence, using autopsy samples, suggest SARS-CoV-2 liver tropism, including the detection of SARS-CoV-2 viral RNA by PCR in up to 55% to 69% of liver samples,38 , 39 successful isolation of infectious SARS-CoV-2 particles, and identification of transcription-based, proteomic-based, and transcription factor-based activity profiles in hepatic autopsy samples.39 For example, transcriptomic profiling confirmed the expression of known SARS-CoV-2 entry receptors and proteins that included ACE2, transmembrane protease serine 2 (TMPRSS2), procathepsin L (CTSL), Ras-related protein Rab-7a (RAB7A), and the high-density lipoprotein scavenger receptor B type 1 (SR-B1)39 and with relative upregulation of type-I, type-II, and type-III interferons (IFNs), JAK/STAT (Janus kinase/signal transducerik and activator of transcription) and metabolic signaling in the RT-PCR-positive livers.40
Host inflammatory response to SARS-CoV-2
Following SARS-CoV-2 infection, the host immune response can be triggered, which can cause excessive release of inflammatory mediators such as IL-6, IL-10, IL-2, and IFN gamma in parallel with disease severity and which in turn may lead to a cytokine storm.41 To support this hypothesis, studies have noted that COVID-19 patients in an ICU setting with multiorgan failure have features of severe hepatic dysfunction associated with higher inflammatory markers.6 , 18 Global proteomic profiling in hepatic tissues has noted significant upregulation of type I and II IFN responses after SARS-CoV-2 infection.39
Drug-induced liver injury
Medications used during treatment of COVID-19 include antibiotics, antiviral agents, corticosteroids, and immunomodulators, which can variably cause liver injury. Cai Q reported that the use of lopinavir/ritonavir increased the risk of liver injury by 4-fold.17 Remdesivir (a nucleoside analog inhibitor of viral RNA polymerase) has been associated with a 23% increase in hepatic biochemical levels.42 Transaminase elevations have been observed in patients treated with tocilizumab (IL-6 inhibitors).43 A systematic review reported the pooled incidence of drug-induced liver injury in patients with COVID-19 at 25.4% (95%CI 14.2–41.4).29 Furthermore, some drugs used in combination such as acetaminophen, nonsteroidal anti-inflammatory drugs, and Chinese herbal medicines may also account for hepatotoxicity. A large global series noted that transaminase elevation was preferentially caused by antiviral drugs administered empirically due to their known therapeutic efficacy for other viral infections. Often a hepatocellular pattern has been encountered as opposed to cholestatic or mixed injury. Outcome was favorable in most patients and fatality attributable to a drug was rare.2 , 14
Preexisting liver diseases
About 2% to 11% of patients with COVID-19 have underlying CLD.14 Data on preexisting liver diseases in COVID-19 from 2 international registries (SECURE-Cirrhosis and COVID-Hep; n = 745) reported causes including nonalcoholic fatty liver disease (NAFLD) 43%, alcohol-related liver disease (ALD) 24%, chronic hepatitis B (HBV) infection 12%, and chronic hepatitis C (HCV) infection 13%. In this cohort, 48% had CLD without cirrhosis and 52% had cirrhosis.3 Corticosteroids or other immunosuppressive agents for COVID-19 treatment may facilitate HBV reactivation in patients with occult or chronic HBV infection.35 Further, patients with more advanced liver disease may be at increased risk of infection due to cirrhosis-associated immune dysfunction.44
Cholangiopathy/secondary sclerosing cholangitis
Several case series have reported delayed-onset and progressive cholestasis as a unique clinical entity in patients following severe COVID-19 infection.45, 46, 47, 48 Cholestasis is present early in the disease course and cholangiopathy occurs later. A retrospective study from a single US center45 reported 12 patients who experienced progressive biliary injury after recovering from severe COVID-19, characterized by marked elevation in serum ALP accompanied by evidence of biliary tree abnormalities on imaging. Median time from COVID-19 diagnosis to onset of cholangiopathy was 118 days. Magnetic resonance cholangiopancreatography (MRCP) findings included beading of intrahepatic ducts, multifocal strictures, and dilation of the biliary tree. Liver biopsy has noted features of acute and/or chronic bile duct obstruction without ductopenia. The pathogenesis is still unclear. These manifestations may represent changes due to biliary tree ischemia, which may reflect a continuum of secondary sclerosing cholangitis in critically ill patients (SSC-CIP), and/or may also be a consequence of direct infection of SARS-CoV-2 of the liver and biliary tract.46 , 49 Furthermore, this complication may be more frequently encountered in patients with preexisting CLD.48
Hypoxic-ischemic liver injury
In critically ill patients, hemodynamic instability may cause liver injury from a hypoxic-ischemic process, which causes an increase in aminotransferases in the setting of shock or cardiac failure.41 Ischemic hepatitis and hepatic congestion related to cardiomyopathy is a common consequence of COVID-19 infection, occurring in 33% of individuals in 1 US series.22 Further, venous and arterial thromboses are currently recognized as a feature of COVID-19, including hepatic involvement.50
COVID-19 and Patients with Chronic Liver Diseases
In a cohort of 2780 multicenter US patients with COVID-19 (CLD 9%), CLD was associated with significantly higher mortality (RR = 2.8, 95%CI 1.9–4.0). Mortality was higher in patients with cirrhosis (RR = 4.6, 95%CI 2.6–8.3). Fatty liver disease and nonalcoholic steatohepatitis (NASH) were the most common causes among the patients with CLD, and the mortality was independent of risk factors of body mass index, hypertension, and diabetes.26 Another large cohort from an International Registry (SECURE-Cirrhosis and COVID-Hep) in patients with CLD and cirrhosis (n = 745) noted 32% mortality in patients with cirrhosis versus 8% in those without cirrhosis (P < .001); and mortality in patients with cirrhosis increased according to liver disease severity based on Child-Pugh classification.3 Studies on COVID-19 outcome and mortality in patients with CLD and cirrhosis are described in Table 1 .
Table 1.
Studies on coronavirus disease-2019 outcome and mortality in patients with chronic liver disease and cirrhosis
| Study | Number | Country | Pre-Existing Liver Diseases | Findings |
|---|---|---|---|---|
| Yadav DK, et al.,86 2020 (meta-analysis) | 2115 | China | 4% (mostly cirrhosis and HBV) |
|
| Sarin SK, et al,87 2020 (The APCOLIS study) | 228 | 13 Asian countries | 185 CLD patients including 43 with cirrhosis (NAFLD in 55%, and viral hepatitis in 30%) |
|
| Mallet V, et al,68 2020 | 15,476 COVID-19 patients with chronic liver disease | France | Chronic liver disease (alcohol-induced 23%, HBV 5%, HCV 4.6%, HCC 4.6%, LT 2.1%) |
|
| Butt AA, et al,53 2021 (ERCHIVES database) | SARS-CoV-2 with HCV = 975 SARS-CoV-2 without HCV = 975 |
United States | HCV |
|
| Verhelst X, et al,88 2021 | 110 | Belgium | Autoimmune hepatitis |
|
| Di Giorgio A, et al,89 2020 | 148 | Italy | Autoimmune liver diseases (AILD) |
|
| Marjot T, et al,54 2021(ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis) | 932 patients with CLD and COVID-19 (70 with AIH) | International registry | Autoimmune hepatitis |
|
| Younossi ZM, et al,90 2021 | 4835 patients with COVID-19 (NAFLD = 553) | United States | NAFLD |
|
| Kim D, et al,67 2021(The COLD study) | 867 CLD = 620 (71.5%) Cirrhosis = 227 (26.2%) ALD = 94 NAFLD = 456 HBV = 62 HCV = 190 HCC = 22 |
US multicenter | Chronic liver disease and cirrhosis |
|
| Jin Ge, et al,91 2021(The National COVID Cohort Collaborative (N3C) study) | 220,727 patients with CLD and known SARS-CoV-2 test status: 58% noncirrhosis/negative, 13% noncirrhosis/positive, 24% cirrhosis/negative 4% cirrhosis/positive SARS-CoV-2 test |
United States | Chronic liver disease and cirrhosis |
|
| Marjot T, et al,3 2021(SECURE -cirrhosis and COVID-Hep) | 745 ALD = 179 NAFLD = 322 HBV = 96 HCV = 92 HCC = 48 |
International registry | Chronic liver disease and cirrhosis |
|
| Lavarone M, et al,92 2020 | 50 | Italy | Cirrhosis |
|
| Clift AK, et al,93 2020 (population-based cohort study) | 11,865 patients with cirrhosis (0.2% of total cohort) | United Kingdom | Cirrhosis |
|
| Bajaj JS, et al,62 2021 |
|
North America and Canada | Cirrhosis |
|
| Ioannou GN, et al,94 2021 (Veterans Affairs national healthcare system) | 305 cirrhosis with SARS-CoV-2 | United States | Cirrhosis |
|
Abbreviations: AIH, autoimmune hepatitis; FIB-4, fibrosis-4; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; LT, liver transplant; MELD, Model for End-Stage Liver Disease.
Viral Hepatitis
A retrospective cohort from Hong Kong (n = 5639, 6.3% current HBV infection, 6.4% past HBV infection) demonstrated that current or past HBV infection was not associated with more severe liver injury or mortality from COVID-19.51 Similarly, a large retrospective cohort from China (n = 2073 patients with COVID-19) found that HBV infection was not associated with the risk of poor COVID-19 outcomes.52 Notably an appropriate use of antiviral therapy for HBV during corticosteroid therapy for COVID-19 should be considered to minimize the risk of HBV reactivation. In parallel, data from the Electronically Retrieved Cohort of HCV infected Veterans (ERCHIVES; including 975 HCV-positive and 975 propensity score matched HCV-negative persons with SARS-CoV-2 infection) demonstrated similar mortality in patients with versus without HCV infection.53
Autoimmune Liver Diseases
De novo autoimmune hepatitis may rarely occur following SARS-CoV-2 infection.49 Data on patients with autoimmune hepatitis (AIH) and SARS-CoV-2 infection from 3 international registries (ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis; n = 932 CLD with SARS-CoV-2, including 70 with AIH) demonstrated that patients with AIH were not at increased risk of adverse outcomes and mortality despite receiving immunosuppressants.54 Another retrospective study on patients with AIH and COVID-19 from an international multicenter study (110 patients with AIH) revealed that patients with AIH were not at risk for worse outcomes following COVID-19. Cirrhosis was an independent predictor of severe COVID-19 in patients with AIH (odds ratio [OR] = 17.46; 95%CI 4.22–72.13, P < .001), and maintenance of immunosuppression during COVID-19 was not associated with an increased risk of severe COVID-19 but could lower the risk of new-onset liver injury.55 This finding should reassure clinicians not to routinely reduce immunosuppression in such patients following COVID-19 infection.
Nonalcoholic Fatty Liver Disease
Patients with NAFLD are at increased overall risk of developing severe COVID-19, which may be contributed by the presence of other high-risk comorbidities such as obesity, diabetes mellitus, and hypertension.49 A retrospective study from China (202 patients with COVID-19, including 37.6% with NAFLD, demonstrated that NAFLD was associated with COVID-19 progression (OR = 6.4, 95%CI 1.5–31.2), and patients with NAFLD had a longer viral shedding time (17.5 ± 5.2 days vs 12.1 ± 4.4 days, P < .0001) compared with patients without NAFLD.56 Patients with NAFLD are more likely to develop liver injury when having COVID-19 infection but no patient developed severe liver-related complications during hospitalization in one cohort from China (280 COVID-19 patients including 30% with NAFLD).57 Moreover, patients with NAFLD, with noninvasive fibrosis scores (fibrosis-4 index and NAFLD fibrosis score) seemed to correlate with a higher likelihood of developing severe COVID-19, irrespective of metabolic comorbidities.58 A large US multicenter study (n = 363, NAFLD 15.2%) demonstrated that NAFLD was independently associated with ICU admission (OR = 2.30, 95%CI 1.27–4.17) and mechanical ventilation (OR = 2.15, 95%CI 1.18–3.91); and presence of cirrhosis was an independent predictor of mortality (OR = 12.5, 95%CI 2.16–72.5).59 Additionally, a systematic review and meta-analysis on clinical outcomes in NAFLD patients with COVID-19 (14 studies including 1851 NAFLD patients) found an increased risk of severe COVID-19 and admission to ICU due to COVID-19 in patients with underlying NAFLD; however, no difference in mortality was observed between NAFLD versus non-NAFLD patients.60
Alcohol-Related Liver Disease
ALD has been reported as an independent risk factor for mortality (OR = 1.79, 95%CI 1.03–3.13) in CLD patients with COVID-19.3 The frequency of ALD has rapidly increased since the beginning of COVID-19 pandemic. Data from United Network for Organ Sharing (UNOS) demonstrated a significant increase in ALD listing (+7.26%; P < .001) during the COVID-19 pandemic, and ALD (40.1%) accounted for more listings than those due to HCV (12.4%) and NASH (23.4%) combined.61 The greatest increase in ALD listing has been among young adults aged 18 to 34 years and aged 35 to 50 years (plus 35%) and among patients with severe alcohol-associated hepatitis (plus 50%). This increase in alcoholism may be due to COVID-19–related stressors, such as unemployment or increased health risks due to the pandemic.
Cirrhosis
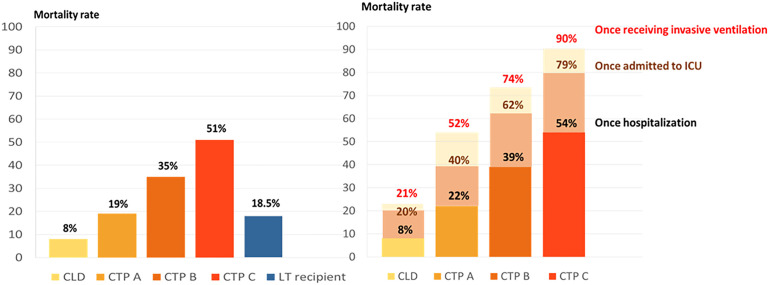
Patient with cirrhosis have high rates of hepatic decompensation, acute-on-chronic liver failure (ACLF), and death from respiratory failure following SARS-CoV-2 infection.50 Data from an International Registry (SECURE-Cirrhosis and COVID-Hep) on COVID-19–infected patients with CLD and cirrhosis (n = 745, including 386 patients with cirrhosis, 359 with noncirrhotic CLD from 21 countries in 4 continents reported mortality in COVID-19-infected patients with cirrhosis at 32% versus mortality in CLD without cirrhosis at 8% (P < .001). Mortality increased according to Child-Pugh class (mortality in classes A [19%], B [35%], and C [51%]) in patients with cirrhosis with respiratory failure being the main cause of death (71%). In this study, there was also an increase in mortality following hospitalization, admission to ICU, and invasive ventilation, with Child-Pugh class C patients having 90% mortality in those requiring mechanical ventilation (Fig. 2 ).3 Acute hepatic decompensation occurred in 46% and half of these patients had ACLF, and around 21% of patients with cirrhosis infected with SARS-CoV-2 lacked respiratory symptoms; hence, patients with new onset of hepatic decompensation or ACLF should be tested for SARS-CoV-2 even in the absence of respiratory symptoms. This large cohort also demonstrated that age, baseline liver disease stage (especially Child-Pugh classes B and C), and ALD were independent risk factors for mortality from COVID-19.3 The possible pathogenesis linking cirrhosis and severe COVID-19 lung disease is likely multifactorial and likely related to factors such as increased systemic inflammation, cirrhosis-associated immune dysfunction, coagulopathy, and intestinal dysbiosis.50 A multicenter-matched cohort study from North America compared mortality in those with cirrhosis and COVID-19 (n = 37) versus cirrhosis alone (n = 127) versus COVID-19 alone (n = 108) and reported that patients with cirrhosis and COVID-19 had higher mortality compared with COVID-19 alone (30% vs 13%, P = .03) but comparable to cirrhosis alone (30% vs 20%, P = .16); in those with ACLF, the mortality was similar regardless of COVID-19 (55% vs 36%, P = .25).62 Recent meta-analysis of 63 studies revealed a pooled OR for all-cause mortality of 2.48 (95% CI: 2.02–3.04) in patients with cirrhosis and COVID-19.63 Accordingly, patients with cirrhosis infected with SARS-CoV-2 should have their COVID-19 vaccination prioritized due to their high mortality.
Fig. 2.
Mortality in patients with COVID-19 infection in chronic liver disease, cirrhosis (Child-Turcotte-Pugh classes A, B, and C) and liver-transplant recipients, and stepwise increment of mortality in patients with chronic liver disease and cirrhosis following hospitalization, admission to ICU and invasive ventilation.
(Data from Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. Journal of hepatology. 2021;74(3):567-577; and Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. The Lancet Gastroenterology & Hepatology. 2020;5(11):1008-1016.)
Postliver Transplant
As opposed to patients with cirrhosis, liver-transplant recipients do not seem to have an increased mortality following SARS-CoV-2 infection compared with the matched general population.50 A prospective nationwide study conducted by the Spanish Society of Liver Transplantation (SETH) reported the incidence of COVID-19 to be higher in LT patients but mortality (around 18%) was lower than in the matched general population; in this cohort, mycophenolate was associated with a risk of developing severe COVID-19 in a dose-dependent manner.64 Another large multicenter study from 2 international registries (COVID-Hep and SECURE-Cirrhosis), including 151 LT recipients with COVID-19 infection, found that LT was not associated with increased mortality (rate = 18.5%; see Fig. 2), whereas increased age and presence of comorbidities (such as elevated creatinine level and nonliver cancer) were associated with mortality among LT-recipients.65 Such data are consistent with data from the European Liver and Intestine Transplantation Association (ELITA)/the European Liver Transplant Registry (ELTR) multicenter COVID-19 registry (149 LT centers, 243 COVID-19-infected LT recipients) where the mortality was 20%, with mortality being higher in patients aged older than 70 years and with comorbidities (such as impaired renal function or diabetes mellitus); contrariwise, tacrolimus use was associated with an improved survival.66 Studies on COVID-19 outcomes in patients with postliver transplantation are reported in Table 2 .
Table 2.
Studies on coronavirus disease-2019 outcome and mortality in liver transplant recipients
| Study | Numbers of LT Recipients with SARS-CoV-2 Infection | Country | Findings |
|---|---|---|---|
| Rabiee A, et al,95 2020 | 112 | United States |
|
| Colmenero J, et al,64 2021 (SETH registry) | 111 | Spain |
|
| Kates OS, et al,96 2021 | 73 | United States |
|
| Ravanan R, et al,97 2020 (UK National Health Service Blood and Transplant registry) | 64 | England |
|
| Webb GJ, et al,65 2020 (SECURE-cirrhosis and COVID-Hep) | 151 | International registry |
|
| Belli LS, et al,66 2021 (ELITA/ELTR COVID-19 registry) | 243 | Europe |
|
| Polak WG, et al,98 2020 (ELITA/ELTR COVID-19 registry) | 57 SARS-CoV-2–infected LT candidates 272 SARS-CoV-2–infected LT recipients |
Europe |
|
Abbreviations: HR, hazard ratio; LT, liver transplant; N, number of patients; SOT, solid organ transplant.
Hepatocellular Carcinoma
In a US multicenter study of adult patients with CLD and COVID-19 (n = 867), hepatocellular carcinoma (HCC) was found to be a factor associated with higher overall mortality (hazard ratio = 3.31, 95%CI 1.53–7.16) independent of ALD and decompensated cirrhosis.67 Concomitantly, in another study from France on COVID-19 patients with CLD (15,476 CLD patients with COVID-19), 30-day mortality was associated with primary liver cancer (OR = 1.38, 95%CI 1.17–1.62, P < .001), CLD (OR = 1.79, 95%CI 1.71–1.87, P < .001), decompensated cirrhosis (OR = 2.21, 95%CI 1.94–2.51, P < .001), and alcohol use disorders (OR = 1.11, 95%CI 1.05–1.17, P < .001).68 HCC is often associated with liver cirrhosis, suggesting that impaired immunity may increase the risk of developing severe COVID-19.69
Notably, COVID-19 may exacerbate preexisting liver disease and thus complicate HCC management.70 An experience from a multicenter study from France in patients with HCC (n = 670, 293 with SARS-CoV-2 infection and 377 without infection) demonstrated fewer patients with HCC presenting to the multidisciplinary tumor board, especially with their initial HCC diagnosis. Treatment strategy was modified in 13.1% of patients, and patients experienced significant treatment delay (≥1 month) in 2020 compared with 2019 (21.5% vs 9.5%, P < .001). Around 7.1% of HCC patients had a diagnosis of active COVID-19 infection (52.4% hospitalized, 19.1% mortality).71 Summaries of recommendations from the AASLD Expert Panel Consensus Statement1 and the EASL-ESCMID position article34 , 72 on the management of patients with CLDs during COVID-19 era are reported in Table 3 .
Table 3.
Guideline recommendations for patients with chronic liver diseases during coronavirus disease-2019 pandemic
| Chronic Liver Diseases | AASLD recommendation1 and EASL-ESCMID Position article34,49,72 |
|---|---|
| Chronic viral hepatitis (HBV and HCV) |
|
| Autoimmune liver diseases |
|
| NAFLD |
|
| ALD |
|
| Cirrhosis |
|
| Liver transplant recipients |
|
| HCC |
|
Abbreviations: AIH, autoimmune hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; IL, interleukin; JAK, Janus kinase; LT, liver transplant.
COVID-19 Vaccination in Patients with Chronic Liver Disease and Liver-Transplant Recipients
Adult CLD patients, particularly those with cirrhosis, are strongly recommended to receive COVID-19 vaccination.73 A large cohort study of patients with cirrhosis from the Veterans Administration on the clinical outcome of mRNA vaccines compared with unvaccinated patients reported that patients with CLD who received at least one dose of an mRNA vaccine (n = 20,037) had a 64.8% reduction in SARS-CoV-2 infections and 100% protection against hospitalization or death at 28 days after the initial dose.74 The rate of reduction of SARS-CoV-2 infection after the first dose in those with decompensated cirrhosis was 50.3% and in those with compensated cirrhosis was 66.8%. Receiving a second dose of the vaccine was associated with a 78.6% reduction in COVID-19 infection and 100% reduction in COVID-19–related hospitalization or death after 7 days.74 Another retrospective study among US veterans demonstrated that some patients with cirrhosis developed breakthrough COVID-19 infection after full or partial vaccination; however, these infections were associated with reduced mortality compared with those without vaccination.75
A case series (n = 40, including 21 with CLD and 19 with LT) from the SECURE-Cirrhosis and COVID-Hep international registries reported that vaccination against SARS-CoV-2 seems to result in favorable outcomes, as demonstrated by the absence of the need for mechanical ventilation, the need for ICU care, or death among fully vaccinated patients.76 Risk factors for lower serologic response to immunization included older age, use of antimetabolite drugs, time from transplantation, and use of B cell–depleting therapies.73 A study on immunogenicity of the first and second doses of the mRNA SARS-CoV-2 vaccine among solid organ transplant recipients demonstrated low levels of detectable antibody around 17% at 20 days after the first dose77 and 54% at a median of 29 days after the second dose.78 The French National Authority for Health recommends administration of a third dose of vaccine in immunosuppressed patients based on the data on 3 doses of the BNT162b2 mRNA COVID-19 vaccine (manufactured by Pfizer-BioNTech) in solid organ transplant recipients (n = 101) that reports significant improvement in anti-SARS-CoV-2 antibody response (up to 68% at 4 weeks after the third dose).79 A fourth dose of SARS-CoV-2 vaccine was associated with slightly improved humoral response among patients with a weak response after 3 doses but no improvement among those with no response after 3 doses, although, no breakthrough infections were observed during follow-up.80
Additionally, a prospective cohort study that compared the SARS-CoV-2-specific humoral and T-cell immune response after the second mRNA vaccination in patients with cirrhosis and in LT recipients (n = 194 including 141 LT and 53 cirrhosis Child-Pugh classes A–C) demonstrated that after the second dose, seroconversion was achieved in 63% of LT recipients and 100% of patients with cirrhosis and controls using the anti-S trimer assay (P < .001).81 Spike-specific T-cell response rates were 36.6%, 65.4%, and 100% in LT recipients, cirrhosis, and controls, respectively. Around 28% of LT recipients did not develop both humoral and T-cell responses after the second vaccination. These data, therefore, support the potential role for a third vaccine dose, especially in LT recipients with low or absent prior vaccine responses. In this cohort, predictors of absent or low humoral response were age greater than 65 years (OR = 4.57, 95%CI 1.48–14.05) and arterial hypertension (OR = 2.50, 95%CI 1.10–5.68). In contrast, failure was less likely with calcineurin inhibitor monotherapy versus other immunosuppressive regimens (OR = 0.36, 95%CI 0.13–0.99).81 Guideline recommendations for COVID-19 vaccination in CLD and liver-transplant recipients73 , 82 , 83 are described in Box 1 . The Centers for Disease Control recommendations on vaccinations are periodically updated and can be accessed by the link of https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html.
Box 1. Guideline recommendations for coronavirus disease-2019 vaccination in chronic liver disease and liver transplant recipients.
- Guideline recommendations for COVID-19 vaccination in CLD
-
•Patients with CLD who are receiving antiviral treatment of HBV or HCV or medical treatment of PBC or AIH should continue their medications while receiving the COVID-19 vaccines
-
•Patients with HCC undergoing locoregional or systemic therapy should be considered for vaccination without treatment interruption
-
•An additional third dose of an mRNA vaccine is recommended at least 28 d after the second dose of an mRNA COVID-19 vaccine in all immunosuppressed patients, HCC, and CLD patients receiving prednisone, antimetabolites, or biological therapies with a booster 3 mo after the third dose
-
•It is not recommended to withhold immunosuppression before or after COVID-19 vaccine
-
•
- Guideline recommendations for COVID-19 vaccination in liver-transplant recipients
-
•COVID-19 vaccination is recommended for all LT recipients.
-
•LT candidates should receive a COVID-19 vaccine before transplantation whenever possible, if not, the optimal time to administer the COVID-19 vaccine is ≥ 3 mo post-LT. However, immunization may be initiated as early as 4 wk posttransplant, especially for high-risk individuals
-
•A reduction in immunosuppression is not recommended in LT recipients when receiving COVID-19 vaccine due to the risk of ACR
-
•COVID-19 vaccination should be avoided in LT recipients with active ACR
-
•Potential live liver donors and recipients should be vaccinated ≥2 weeks before transplantation if feasible
-
•Family members and caregivers of LT recipients should also be vaccinated against SARS-CoV-2
-
•LT recipients who recover from COVID-19 infection should still complete COVID-19 vaccine series
-
•
Abbreviations: ACR, acute cellular rejection; AIH, autoimmune hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; LT, liver transplant; PBC, primary biliary cholangitis.
Acute Liver Injury after Coronavirus Disease-2019 Vaccination
A large epidemiologic study from Europe reported no increment in new AIH cases diagnosed during the widespread use of COVID-19 vaccination, and these data do not support the assumption that COVID-19 vaccination induces AIH.84 However, de novo AIH-like liver injury occurring after COVID-19 vaccination has been reported in case series in which data from 18 countries demonstrated liver injury after SARS-CoV-2 vaccination (n = 87, 63% women).85 Liver injury was diagnosed at a median of 15 (range 3–65) days after vaccination, attributed to the Pfizer-BioNTech (BNT162b2) vaccine in 59%, the Oxford-AstraZeneca (ChAdOX1 nCoV-19) vaccine in 23%, and the Moderna (mRNA-1273) vaccine in 18%. The liver injury was predominantly hepatocellular (84%) and 57% of patients had features of immune-mediated hepatitis (positive autoantibodies and elevated immunoglobulin G levels). Corticosteroids were administered to 53% of patients and resulted in complete biochemical resolution without a relapse after corticosteroid withdrawal. Outcome was generally favorable, except for one patient who developed fulminant liver failure.85 Of note, the mechanisms leading to acute liver injury after COVID-19 vaccination have not been fully elucidated, and it is difficult to establish a definite causal relationship between COVID-19 vaccination and hepatitis. Furthermore, these events are extremely rare and respond well to corticosteroid treatment, and the overall benefits of vaccination outweigh the risks of liver injury; thus, this side effect should not represent a barrier to SARS-CoV-2 vaccination.49
Summary
COVID-19 infection has had a major impact on people across the world since December 2019, causing up to 6.5 million deaths globally until now in 2022. Patients with CLD, especially cirrhosis, and liver-transplant recipients are particularly vulnerable to severe COVID-19. These populations are, therefore, strongly recommended to receive COVID-19 vaccination to reduce their morbidity and mortality. Data on vaccine safety and efficacy is emerging but several issues remain unresolved, such as prevalence of breakthrough infection after vaccinations and adequate doses and timing of vaccination in those receiving immunosuppressants or in transplant recipients. Management of immunosuppressive agents in post-LT patients with severe COVID-19 infection requires further study. Because the COVID-19 pandemic rapidly evolves in different regions due to the emergence of mutant strains, early diagnosis and treatment of COVID-19 in patients with advanced liver disease deserves a special focus to minimize the risk of hepatic decompensation. The pandemic has been further associated with increased alcohol consumption, unhealthy eating behaviors, and interruptions of hepatology care, which may lead to an increase in severity of liver disease; therefore, clinicians should strongly recommend alcohol cessation and provide health education to their patients with liver diseases.
Clinics care points
-
•
Early admission in patients with cirrhosis and COVID-19 infection is recommended due to high rate of hepatic decompensation.
-
•
SARS-CoV-2 vaccination is strongly recommended for all patients with chronic liver disease including those with cirrhosis, liver-transplant candidates and recipients.
-
•
Areduction of immunosuppression in patients with autoimmune hepatitis and liver-transplant recipients without evidence of COVID-19 infection, is not recommended.
-
•
Treatment should not be interrupted in those with HCC and without COVID-19 infection, while similarly liver transplantation should be pursued as needed.
Disclosure
The authors declare no conflicts of interest relevant to this publication.
References
- 1.Fix O.K., Hameed B., Fontana R.J., et al. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72(1):287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical best practice advice for hepatology and liver transplant providers during the covid-19 pandemic: Aasld Expert Panel Consensus Statement. https://www.aasld.org/sites/default/files/2021-11/AASLD%20COVID-19%20Expert%20Panel%20Consensus%20Statement%20Update%2011.02.2021.pdf Available at: Accessed November 2, 2021. [DOI] [PMC free article] [PubMed]
- 3.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74(3):567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X.-W., Wu X.-X., Jiang X.-G., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ (Clinical research ed) 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q., Huang D., Ou P., et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 11.Cao B., Wang Y., Wen D., et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Z., Chen L., Li J., et al. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Z., Chen L., Li J., et al. Clinical features of COVID-19 related liver damage. medRxiv. 2020;2020:2026. doi: 10.1016/j.cgh.2020.04.002. 20026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y., Yang R., Xu Y., et al. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020;2020 2002.2027.20029009. [Google Scholar]
- 16.Cao M., Zhang D., Wang Y., et al. Clinical Features of Patients Infected with the 2019 Novel Coronavirus (COVID-19) in Shanghai, China. medRxiv. 2020;2020 2003.2004.20030395. [Google Scholar]
- 17.Cai Q., Huang D., Yu H., et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Zheng L., Liu L., et al. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40(9):2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 19.Vespa E., Pugliese N., Piovani D., et al. Liver tests abnormalities in COVID-19: trick or treat? J Hepatol. 2020;73(5):1275–1276. doi: 10.1016/j.jhep.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with sars-cov-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cholankeril G., Podboy A., Aivaliotis V.I., et al. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology. 2020;159(2):775–777. doi: 10.1053/j.gastro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arentz M., Yim E., Klaff L., et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. jama. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang C., Zhang K., Wang W., et al. Clinical Characteristics of 20,662 Patients with COVID-19 in mainland China: A Systemic Review and Meta-analysis. medRxiv. 2020;2020 2004.2018.20070565. [Google Scholar]
- 25.Hundt M.A., Deng Y., Ciarleglio M.M., et al. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatol. 2020;72(4):1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S., Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159(2):768–771.e763. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei F., Liu Y.M., Zhou F., et al. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72(2):389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phipps M.M., Barraza L.H., LaSota E.D., et al. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72(3):807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni A.V., Kumar P., Tevethia H.V., et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52(4):584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W., Tao Z.W., Wang L., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira M.R., Mohan S., Cohen D.J., et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L., Liu J., Lu M., et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L., Li Q., Zheng D., et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382(25):e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boettler T., Marjot T., Newsome P.N., et al. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2(5):100169. doi: 10.1016/j.jhepr.2020.100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Xiao S.-Y. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92(9):1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 36.Chai X., Hu L., Zhang Y., et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv. 2020;2020:931766. [Google Scholar]
- 37.Banales J.M., Huebert R.C., Karlsen T., et al. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16(5):269–281. doi: 10.1038/s41575-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagana S.M., Kudose S., Iuga A.C., et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33(11):2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanner N., Andrieux G., Badia-i-Mompel P., et al. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4(3):310–319. doi: 10.1038/s42255-022-00552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes E. Infection of liver hepatocytes with SARS-CoV-2. Nat Metab. 2022;4(3):301–302. doi: 10.1038/s42255-022-00554-4. [DOI] [PubMed] [Google Scholar]
- 41.Bertolini A., van de Peppel I.P., Bodewes F.A.J.A., et al. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72(5):1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grein J., Ohmagari N., Shin D., et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marra F., Smolders E.J., El-Sherif O., et al. Recommendations for Dosing of Repurposed COVID-19 Medications in Patients with Renal and Hepatic Impairment. Drugs R D. 2021;21(1):9–27. doi: 10.1007/s40268-020-00333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bajaj J.S., Kamath P.S., Reddy K.R. The Evolving Challenge of Infections in Cirrhosis. N Engl J Med. 2021;384(24):2317–2330. doi: 10.1056/NEJMra2021808. [DOI] [PubMed] [Google Scholar]
- 45.Faruqui S., Okoli F.C., Olsen S.K., et al. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116(7):1414–1425. doi: 10.14309/ajg.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 46.Roth N.C., Kim A., Vitkovski T., et al. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116(5):1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 47.Bütikofer S., Lenggenhager D., Wendel Garcia P.D., et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41(10):2404–2417. doi: 10.1111/liv.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartl L., Haslinger K., Angerer M., et al. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology. 2022 doi: 10.1002/hep.32582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marjot T., Eberhardt C.S., Boettler T., et al. Impact of COVID-19 on the liver and on the care of patients with chronic liver disease, hepatobiliary cancer, and liver transplantation: an updated EASL position paper. J Hepatol. 2022;77(4):1161–1197. doi: 10.1016/j.jhep.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marjot T., Webb G.J., Barritt ASt, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18(5):348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yip T.C., Wong V.W., Lui G.C., et al. Current and Past Infections of HBV Do Not Increase Mortality in Patients With COVID-19. Hepatology. 2021;74(4):1750–1765. doi: 10.1002/hep.31890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding Z.Y., Li G.X., Chen L., et al. Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74(6):1295–1302. doi: 10.1016/j.jhep.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butt A.A., Yan P., Chotani R.A., et al. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int. 2021;41(8):1824–1831. doi: 10.1111/liv.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marjot T., Buescher G., Sebode M., et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74(6):1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Efe C., Dhanasekaran R., Lammert C., et al. Outcome of COVID-19 in Patients With Autoimmune Hepatitis: An International Multicenter Study. Hepatology. 2021;73(6):2099–2109. doi: 10.1002/hep.31797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji D., Qin E., Xu J., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73(2):451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang R., Zhu L., Wang J., et al. Clinical Features of Patients With COVID-19 With Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2020;4(12):1758–1768. doi: 10.1002/hep4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Targher G., Mantovani A., Byrne C.D., et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69(8):1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 59.Hashemi N., Viveiros K., Redd W.D., et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40(10):2515–2521. doi: 10.1111/liv.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh A., Hussain S., Antony B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: A comprehensive systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15(3):813–822. doi: 10.1016/j.dsx.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cholankeril G., Goli K., Rana A., et al. Impact of COVID-19 Pandemic on Liver Transplantation and Alcohol-Associated Liver Disease in the USA. Hepatology. 2021;74(6):3316–3329. doi: 10.1002/hep.32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajaj J.S., Garcia-Tsao G., Biggins S.W., et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70(3):531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Middleton P., Hsu C., Lythgoe M.P. Clinical outcomes in COVID-19 and cirrhosis: a systematic review and meta-analysis of observational studies. BMJ open Gastroenterol. 2021;8(1):e000739. doi: 10.1136/bmjgast-2021-000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colmenero J., Rodriguez-Peralvarez M., Salcedo M., et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webb G.J., Marjot T., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belli L.S., Fondevila C., Cortesi P.A., et al. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients With Covid-19: Results From the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160(4):1151–1163.e1153. doi: 10.1053/j.gastro.2020.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim D., Adeniji N., Latt N., et al. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19(7):1469–1479.e1419. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mallet V., Beeker N., Bouam S., et al. Prognosis of French COVID-19 patients with chronic liver disease: A national retrospective cohort study for 2020. J Hepatol. 2021;75(4):848–855. doi: 10.1016/j.jhep.2021.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kudo M., Kurosaki M., Ikeda M., et al. Treatment of hepatocellular carcinoma during the COVID-19 outbreak: The Working Group report of JAMTT-HCC. Hepatol Res. 2020;50(9):1004–1014. doi: 10.1111/hepr.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan S.L., Kudo M. Impacts of COVID-19 on Liver Cancers: During and after the Pandemic. Liver Cancer. 2020;9(5):491–502. doi: 10.1159/000510765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amaddeo G., Brustia R., Allaire M., et al. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. 2021;3(1):100199. doi: 10.1016/j.jhepr.2020.100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boettler T., Newsome P.N., Mondelli M.U., et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3):100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.AASLD expert panel consensus statement: vaccines to prevent covid-19 in patients with liver disease. https://www.aasld.org/sites/default/files/2022-03/AASLD%20COVID-19%20Vaccine%20Document%20Update%203.28.2022.1%20FINAL.pdf Available at: Accessed March 28, 2022.
- 74.John B.V., Deng Y., Scheinberg A., et al. Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis. JAMA Intern Med. 2021;181(10):1306–1314. doi: 10.1001/jamainternmed.2021.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.John B.V., Deng Y., Schwartz K.B., et al. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022;76(1):126–138. doi: 10.1002/hep.32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moon A.M., Webb G.J., García-Juárez I., et al. SARS-CoV-2 Infections Among Patients With Liver Disease and Liver Transplantation Who Received COVID-19 Vaccination. Hepatol Commun. 2022;6(4):889–897. doi: 10.1002/hep4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamar N., Abravanel F., Marion O., et al. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamar N., Abravanel F., Marion O., et al. Assessment of 4 Doses of SARS-CoV-2 Messenger RNA–Based Vaccine in Recipients of a Solid Organ Transplant. JAMA Netw Open. 2021;4(11):e2136030. doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruether D.F., Schaub G.M., Duengelhoef P.M., et al. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin Gastroenterol Hepatol. 2022;20(1):162–172.e169. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html Available at: Accessed August 11, 2022. [PubMed]
- 83.Fix O.K., Blumberg E.A., Chang K.-M., et al. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74(2):1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rüther D.F., Weltzsch J.P., Schramm C., et al. Autoimmune hepatitis and COVID-19: No increased risk for AIH after vaccination but reduced care. J Hepatol. 2022;77(1):250–251. doi: 10.1016/j.jhep.2022.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Efe C., Kulkarni A.V., Terziroli Beretta-Piccoli B., et al. Liver injury after SARS-CoV-2 vaccination: Features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022 doi: 10.1002/hep.32572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yadav D.K., Singh A., Zhang Q., et al. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2020;70(4):807–809. doi: 10.1136/gutjnl-2020-322072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarin S.K., Choudhury A., Lau G.K., et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14(5):690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verhelst X., Somers N., Geerts A., et al. Health status of patients with autoimmune hepatitis is not affected by the SARS-CoV-2 outbreak in Flanders, Belgium. J Hepatol. 2021;74(1):240–241. doi: 10.1016/j.jhep.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Di Giorgio A., Nicastro E., Speziani C., et al. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73(3):702–705. doi: 10.1016/j.jhep.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Younossi Z.M., Stepanova M., Lam B., et al. Independent Predictors of Mortality Among Patients With NAFLD Hospitalized With COVID-19 Infection. Hepatol Commun. 2021 doi: 10.1002/hep4.1802. n/a(n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ge J., Pletcher M.J., Lai J.C., et al. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161(5):1487–1501.e1485. doi: 10.1053/j.gastro.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iavarone M., D'Ambrosio R., Soria A., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73(5):1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clift A.K., Coupland C.A.C., Keogh R.H., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ (Clinical research ed) 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ioannou G.N., Liang P.S., Locke E., et al. Cirrhosis and Severe Acute Respiratory Syndrome Coronavirus 2 Infection in US Veterans: Risk of Infection, Hospitalization, Ventilation, and Mortality. Hepatology. 2021;74(1):322–335. doi: 10.1002/hep.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rabiee A., Sadowski B., Adeniji N., et al. Liver Injury in Liver Transplant Recipients With Coronavirus Disease 2019 (COVID-19): U.S. Multicenter Experience. Hepatology. 2020;72(6):1900–1911. doi: 10.1002/hep.31574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kates O.S., Haydel B.M., Florman S.S., et al. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin Infect Dis. 2021;73(11):e4090–e4099. doi: 10.1093/cid/ciaa1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ravanan R., Callaghan C.J., Mumford L., et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: A national cohort study. Am J Transplant. 2020;20(11):3008–3018. doi: 10.1111/ajt.16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polak W.G., Fondevila C., Karam V., et al. Impact of COVID-19 on liver transplantation in Europe: alert from an early survey of European Liver and Intestine Transplantation Association and European Liver Transplant Registry. Transpl Int. 2020;33(10):1244–1252. doi: 10.1111/tri.13680. [DOI] [PMC free article] [PubMed] [Google Scholar]