Abstract
Background
The coronavirus pandemic resulted in many changes which had the potential to impact mortality related to opioid agonist therapy (OAT; methadone, buprenorphine), including changes in the prescribing and dispensing of OAT and patterns of drug availability and use. We aimed to assess the impact of the first lockdown (initiated March 23rd 2020) on methadone- and buprenorphine-related deaths in England in people both prescribed and not prescribed OAT using data from the National Programme on Substance Abuse Deaths.
Methods
This was a retrospective post-mortem toxicology study of OAT-related deaths which occurred in the 3-month period March 23rd to June 22nd in the years 2016-2020. Provisional data regarding numbers accessing treatment for opioid use disorder was provided by the National Drug Treatment Monitoring System.
Results
We found a 64% increase in methadone-related deaths in March to June 2020 compared to March to June 2019 (2019 n = 96; 2020 projected n = 157). There were increases in the mortality rate of both in-treatment decedents (22% increase; 2019 n = 45; an exponential smoothing model of the 2016-19 trend [α=0.5] predicted 44 deaths in 2020, 55 were reported) and decedents not prescribed methadone (74% increase; 2019 n = 46; 2016-19 trend predicted 43 deaths in 2020, 80 were reported). There was no increase in buprenorphine-related deaths (2019 n = 9/529; 2020 n = 11/566). There were no changes in the numbers of deaths where other opioids or multiple substances were detected, or in methadone levels detected. Numbers of people accessing treatment for opioid use disorder in 2020 did not decrease relative to previous years (p >0.05).
Conclusions
Methadone-related deaths in non-prescribed individuals, but not prescribed individuals, increased considerably above the annual trend forecast for 2020 during the first COVID-19 lockdown in England. Further studies are thus needed to understand this difference.
Keywords: Methadone, Buprenorphine, Opioid agonist therapy, COVID-19, Drug-related death, Mortality
Graphical abstract
Introduction
The COVID-19 pandemic was associated with reported increases in drug-related deaths in the United States and the United Kingdom (Jones et al., 2022, Office For National Statistics 2022). Deaths related to opioid agonist treatment (OAT) have historically contributed substantially to drug-related deaths (Strang et al., 2010, Strang, 2015) and thus the effect of the COVID-19 pandemic on rates of OAT-related death is of interest to prescribers and those involved in the design and provision of services. Data from the United States indicates an increase in methadone-involved deaths during March 2020 but that these were related to an increase in deaths primarily attributable to co-administered fentanyl (Jones et al., 2022). In-treatment cohort studies have reported no change in rates of overdose during the COVID-19 pandemic (Amram et al., 2021, Gomes et al., 2022). However, no studies to date have considered mortality related to OAT in those who have not been prescribed it, despite decedents not prescribed OAT accounting for the majority of OAT-related deaths in the UK (Claridge and Goodair, 2015). Given that harms related to OAT to those who are not prescribed it are difficult to study - and may thus remain hidden and unconsidered - it is important to address this gap.
The COVID pandemic resulted in numerous changes to the circumstances of people who use opioids including changes in drug supply and quality (Schofield et al., 2020, Kesten et al., 2021, Jacka et al., 2021, Grebely et al., 2020), changes in drug use and use context (Schofield et al., 2020, Kesten et al., 2021, Grebely et al., 2020, Otiashvili et al., 2022), and barriers to treatment (Schofield et al., 2020, Kesten et al., 2021, Jacka et al., 2021, Krawczyk et al., 2021) and harm reduction, which could all contribute to the increased drug-related mortality seen during the COVID-19 pandemic (Jones et al., 2022). Barriers to treatment were mitigated to an extent in some countries by changes to the delivery of treatment which aimed to facilitate access to OAT. In the UK, such changes included the widespread introduction of telemedicine, an effort to induct new patients onto OAT rapidly, and increased provision of take-home doses of OAT covering weeks rather than a requirement for daily directly supervised consumption (Kesten et al., 2021, Public Health England 2020). While qualitative research has considered the experience of people who are not in drug treatment as well as those who are (Schofield et al., 2020, Kesten et al., 2021, Otiashvili et al., 2022, Krawczyk et al., 2021), over-arching themes common to both have been reported, rather than separate consideration of the experience of those who not engaged with treatment. Quantitative research regarding outcomes including safety during the COVID-19 pandemic have focussed on those in treatment, with reassuring results regarding retention, stability and rate of overdose in a number of countries (Amram et al., 2021, Gomes et al., 2022, Roncero et al., 2020, Trujols et al., 2020, Meteliuk et al., 2021).
In this study we have investigated methadone- and buprenorphine-related deaths in people prescribed OAT and in those not prescribed OAT which occurred in the 3-month period following the first COVID-19 lockdown (March 23rd to June 22nd) in 2020 in England and compared trends to those observed in the same 3-month period in the years 2016–2019 using data from the National Programme on Substance Abuse Deaths (NPSAD). NPSAD is a database which records drug-related deaths based on date of death and for which it is possible to access information about prescribing status. In order to gauge the concurrent impact of the COVID-19 lockdown on access to OAT, unofficial estimates of adult patients in treatment for opioid use disorder during the same period were obtained from the National Drug Treatment Monitoring System (NDTMS) provided by the National Drug Evidence Centre (NDEC), at the University of Manchester.
Method
National drug treatment monitoring system (NDTMS)
All services that provide structured drug and alcohol treatment in England are asked to submit data regarding clinical and demographic information about patients and interventions offered to the NDTMS (Public Health England 2020). NDTMS data on the number of adult patients in treatment for opioid use disorder during the months of interest in 2016-2020 were provided by the NDEC at University of Manchester. The counts supplied are unofficial estimates of the number of adults in contact with treatment services for opioid use disorder during each month. This means that in at least one triage appointment during the episode of treatment in the reporting month, the patient cited problematic use of at least one opioid substance.
The national programme on substance abuse deaths
NPSAD receives reports from over 85% of English coronial jurisdictions (n = 70/82) for deaths related to psychoactive drug use other than nicotine or caffeine (Yoganathan et al., 2020). Coronial inquest files typically include the coroner's decision as to cause of death, statements from witnesses, family and friends, first responders (e.g. police, emergency services), general practitioner (GP) records, hospital records, and post-mortem and toxicology results. Toxicology tests are requested dependent upon individual case circumstances at the discretion of the coroner and/or consulting pathologist.
NPSAD Ethics
The King's College London Biomedical & Health Sciences, Dentistry, Medicine and Natural & Mathematical sciences Research Ethics Sub-Committee confirmed in November 2020 that NPSAD does not require ethics review as all subjects are deceased.
NPSAD Case identification
We retrospectively identified cases by first extracting all cases occurring January 2016–June 2020 which had been reported to NPSAD by 1st November 2021. We then selected those which occurred between March 23rd and June 22nd for years 2016-2020 for further analysis. The date March 23rd 2020 was selected as the initiation date for the period as it was the date following which prescribing practice in community drug services changed following a meeting chaired by Public Health England and the guidance provided in the meeting to service providers. Where possible, people prescribed OAT were to be on a 14 day take-home script which means that instead of presenting daily to receive directly supervised consumption of their OAT, they would be provided enough of either methadone or buprenorphine to last them for 14 days.
The cause of death fields were used to assign cases to one of three categories: 1. Those with no opioids implicated in causing death; 2. Those with buprenorphine and/or methadone implicated; 3. Those with alternative opioid implicated (cases where methadone and/or buprenorphine were co-implicated with alternative opioids were assigned as methadone and/or buprenorphine cases). Cases were screened for the prescribing of methadone and buprenorphine for non- OAT indications and were removed prior to analysis (n = 1). Information regarding whether the methadone or buprenorphine was prescribed is typically submitted by coroners to NPSAD and is sourced from the standardised NHS GP health record summary (methadone: 7% of cases with missing prescribing status, n = 37/504; buprenorphine: 26% of cases with missing prescribing status, n = 9/35). Prior to the COVID-19 pandemic, patients prescribed OAT in England were typically reviewed by their keyworker who conferred with the prescribing physician every 14 days to approve a repeat prescription, with each reissuance of the OAT prescription valid for 14 days of OAT dispensed most commonly as daily supervised consumption. During COVID-19 the majority received a 2-week take-home supply. All decedents listed as on their day of death were considered as individuals prescribed OAT for analysis.
NPSAD Data analysis
Software: Analysis of trends over time, of co-administered substances, and of post-mortem blood concentrations with accompanying statistics (Chi squared [X 2], Student's t-test) was carried out using IBM® SPSS™ Statistics for Windows version 25 and Microsoft Excel 365.
2020 projection: The average time between date of death and conclusion of coronial inquest, whereupon cases are reported to NPSAD, is 7-10 months for a drug-related death. Whilst identification of cases reported to NPSAD took place over 16 months after the end of the study period, based on previous jurisdiction reporting trends of methadone- and buprenorphine-related deaths (2016-2019) it is estimated that by November 1st in a given year, 92% of deaths from the previous year were reported. The total number of deaths anticipated to be reported which occurred in 2020 was therefore projected. Such projections have been made in other studies using NPSAD data (e.g. for antihistamine- (Oyekan et al., 2021) and ketamine-related deaths (Corkery et al., 2021)) which upon re-analysis when all cases for a given year were likely reported, proved to be accurate.
Exponential smoothing models (α=0.5) were used to extrapolate the number of deaths which could have been anticipated to be received in March to June 2020 based upon the March to June 2016-2019 trends, with confidence bounds calculated using a 95% confidence interval (rounded to 0 d.p.).
Results
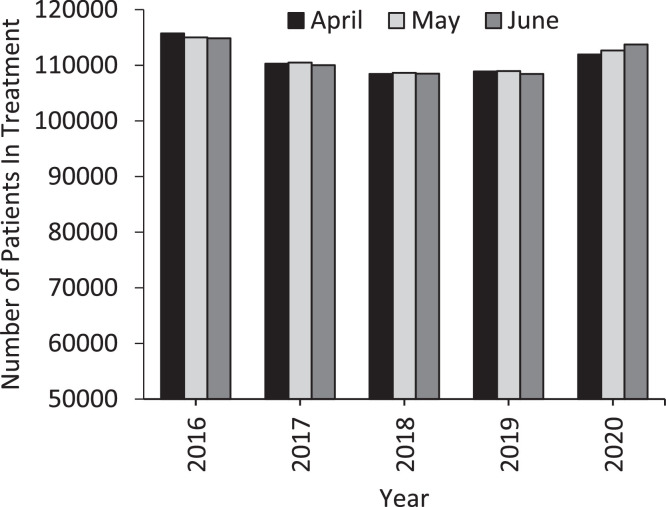
According to NDTMS data, the average number of patients registered with drug services for treatment of opioid dependence significantly increased in April-June 2020 in comparison to April-June 2019 (2019 mean average patients n = 108,764; 2020 mean average patients n = 112,778, Student's t-test p < 0.05; Fig. 1 ), although this increase is not evident when comparing 2020 to the overall 2016-2019 average (2016-2019 mean average patients n = 110,689; 2020 mean average patients n = 112,778, Student's t-test p > 0.05; Fig. 1).
Fig. 1.
Number of patients registered as in treatment for opioid use disorder in England by NDTMS in the April, May, and June of 2016-2020.
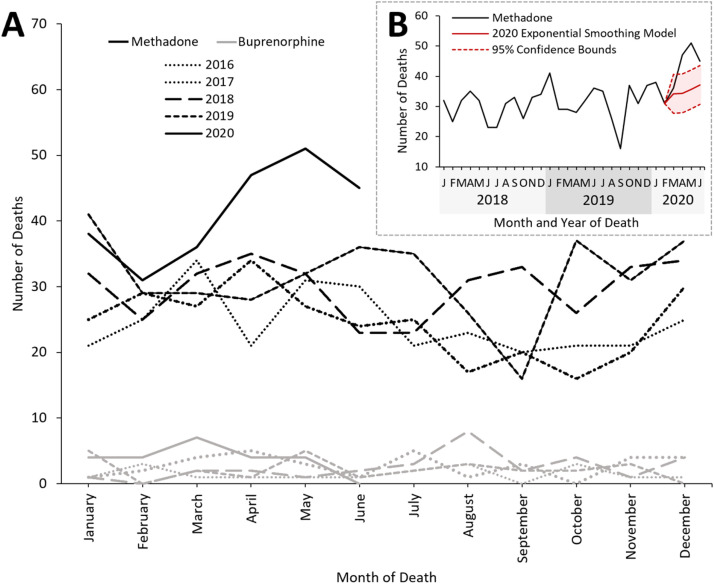
There is a clear increase in deaths where methadone, but not buprenorphine, was implicated in causing death following the instigation of the first UK lockdown on March 23rd, 2020 (Fig. 2 ). Further analysis was performed on deaths which occurred during the 3 months following this date (March 23rd to June 22nd) in the years 2016-2020.
Fig. 2.
A. Methadone- (black) and buprenorphine- (grey) related deaths reported to NPSAD from England which occurred January 2016 – June 2020. B. Linear trend of methadone-related deaths January 2018 – June 2020. The exponential smoothing model for the number of deaths which could have been anticipated in March – June 2020 is indicated (red) along with the lower and upper confidence (95%) bounds. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
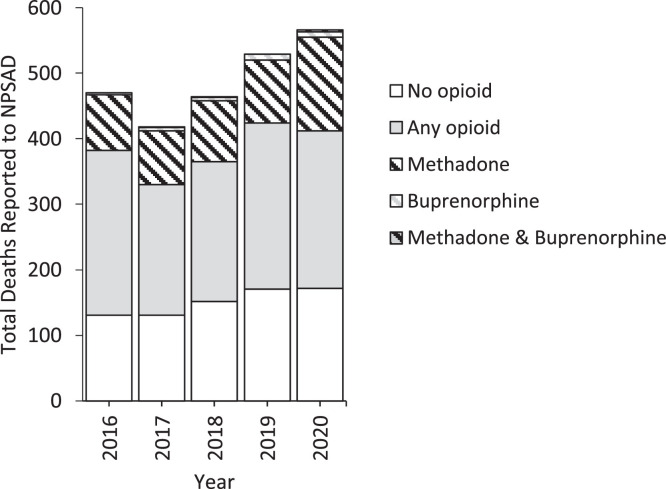
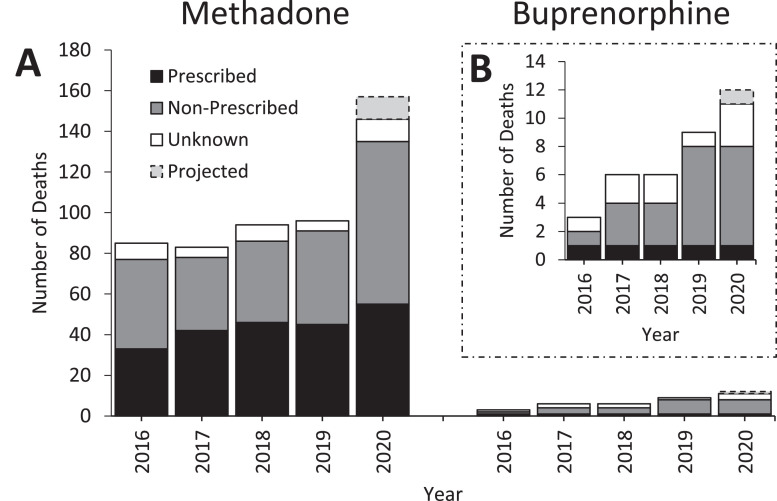
A total of 2,447 deaths were reported to the NPSAD between March 23rd–June 22nd in the years 2016-2020, 504 of which had methadone implicated in causing death, and 35 with buprenorphine implicated (in 5 cases both methadone and buprenorphine were co-detected). 1,156 deaths were reported where opioids other than methadone and buprenorphine were implicated, and 757 deaths where drugs other than opioids were implicated. When normalised against total NPSAD reporting, methadone-related deaths significantly increased by 8% in 2020 when compared to 2019 (2019 n = 96/529; 2020 n = 146/566, X 2 p < 0.05, Fig. 3 ). Buprenorphine-related deaths did not increase beyond that which could be anticipated, nor did the proportion of total cases where buprenorphine was implicated increase (2019 n = 9/529; 2020 n = 11/566 X 2 p > 0.05, Fig. 3). The proportion of deaths with opioids other than methadone and buprenorphine implicated decreased (5% decrease in 2020; 2019 n = 253/529; 2020 n = 240/566) Fig. 3), as did those with non-opioid drugs implicated (2% decrease in 2020; 2019 n = 171/529; 2020 n = 172/566) although not significant statistically (X 2 both p > 0.05, Fig. 3).
Fig. 3.
Total number of deaths reported to NPSAD from England which occurred 23rd March - 22nd June 2016-2020 delineated by opioid detection.
Using an exponential smoothing model (α=0.5), 96 methadone-related deaths could be anticipated to have occurred March 23rd- June 22nd 2020 (lower confidence bound n = 90, upper confidence bound n = 103). However, by November 1st 2021 NPSAD had received 146 reports of methadone-related deaths, with a further 11 reports projected to be received because of reporting delays (see Methods, 2020 projection). Methadone-related deaths in 2020 are therefore estimated to increase by 64% of 2019 numbers (2019 n = 96; 2020 n = 157; Fig. 4 ).
Fig. 4.
A. Number of deaths reported to NPSAD from England which occurred 23rd March - 22nd June 2016-2020 by methadone and buprenorphine obtainment source. The source of OAT was delineated from the GP summary for each decedent registered with a GP. Number of deaths anticipated to be reported which occurred in 2020 were projected (see Methods). B. Adjusted Y-axis range for deaths with buprenorphine detections to enable clearer inspection.
Coroner's data includes in-life prescribing details of decedents, which enabled examination of the relative contributions of prescribed and non-prescribed methadone to methadone-related mortality. The 22% increase in methadone-related deaths observed between 2019-2020 of prescribed individuals is only marginally higher than what could have been anticipated: an exponential smoothing model (α=0.5) of the 2016-2019 data estimates 44 deaths would be reported in 2020 (lower confidence bound n = 37, upper confidence bound n = 50), when 55 were reported at time of writing (2019 n = 45; Fig. 4). However, the 74% increase in deaths observed between 2019-20 where the implicated methadone had not been prescribed is greater than what could have been anticipated based upon the trend observed in the preceding years: the exponential smoothing model (α=0.5) of the 2016-2019 data estimates 43 deaths would be reported in 2020 (lower confidence bound n = 33, upper confidence bound n = 53), when 80 had been reported at time of writing (2019 n = 46; Fig. 4).
Post-mortem blood concentrations of methadone were examined to assess any changes in the amount of methadone ingested in 2020 which had led to death. The median methadone concentration in decedents who had consumed non-prescribed methadone tended to be lower than the level in those who had consumed prescribed methadone, although these differences were not statistically significant (Student's t-test p > 0.05). The highest median level was detected in patients prescribed methadone who died in 2020 (Table 1 ).
Table 1.
Methadone levels in prescribed and non-prescribed cases.
| Median post-mortem blood concentration of detected methadone (mg/l) |
|||||
|---|---|---|---|---|---|
| Year | 2016 | 2017 | 2018 | 2019 | 2020 |
| Prescribed | 0.77 | 0.51 | 0.78 | 0.67 | 0.88 |
| Non-prescribed | 0.50 | 0.60 | 0.54 | 0.38 | 0.42 |
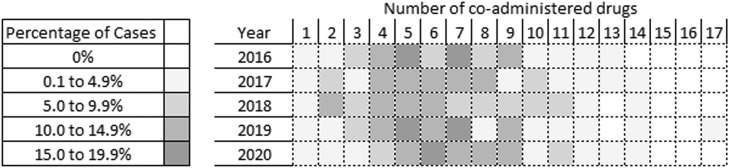
The median number of drugs co-administered with methadone did not significantly change in 2020 in comparison to 2016-2019 (2016 n = 7, 2017 n = 6, 2018 n = 6, 2019 n = 6, 2020 n = 7, Fig. 5 , Student's t-test p > 0.05), nor did the median number of drugs co-administered when comparing 2020 decedents prescribed and not prescribed methadone (prescribed n = 6, not prescribed n = 7, Student's t-test p > 0.05). However, in individuals prescribed methadone a significantly greater proportion had administered methadone alone in 2020 than in 2016-2019 ((X 2 p < 0.05, Table 2 ), which corelates with a reduction in the co-administration of heroin/morphine specifically (as heroin is rapidly metabolised to 6-monoacetylmorphine and morphine (Brunton et al., 2011), it cannot be determined whether heroin or morphine was administered if heroin-specific markers are not tested for - as such, heroin and morphine are combined in the NPSAD database). No such changes were observed in individuals who were not prescribed methadone (Table 2). Accordingly, there was a significant increase in the number of cases where methadone alone was implicated in causing death in 2020 (2016 n = 15; 2017 n = 12; 2018 n = 12; 2019 n = 9; 2020 n = 26; (X 2 p < 0.001).
Fig. 5.
Percentage of cases each year with co-detections of one or more drugs.
Table 2.
Co-administration of additional opioids in prescribed and non-prescribed methadone cases.
| Prescribed Methadone Administered: |
Non-Prescribed Methadone Administered: |
|||||
|---|---|---|---|---|---|---|
| Year(s) | Alone (%) | With heroin/morphine (%) | With an alternate opioid (%) | Alone (%) | With heroin/morphine (%) | With an alternate opioid (%) |
| 2016-2019 | n = 40/166 (24%) | n = 109/166 (66%) | n = 17/166 (10%) | n = 45/166 (27%) | n = 107/166 (64%) | n = 14/166 (8%) |
| 2020 | n = 25/55 (45%) | n = 24/55 (44%) | n = 6/55 (11%) | n = 21/80 (26%) | n = 52/80 (65%) | n = 7/80 (9%) |
There were no significant changes in the proportion of cases where a benzodiazepine (2016-2019 62% of cases, n = 223/358; 2020 64% of cases, n = 93/146, X 2 p > 0.05) or alcohol (2016-2019 21% of cases, n = 76/358; 2020 28% of cases, n = 41/146, X 2 p > 0.05) had been co-administered with methadone (cases where alcohol was attributed to post-mortem production by the consulting pathologist [≤10mg/dl] (O'Neal and Poklis, 1996) were excluded).
Discussion
This is the first UK study exploring methadone- and buprenorphine-related deaths following the first COVID-19 lockdown with a focus on decedents who were prescribed OAT and those who were not. The increase in methadone-related deaths in March to June 2020 occurred primarily in decedents who were not in receipt of a prescription for methadone. The levels detected were unchanged in both groups relative to previous years. Opioids other than methadone were less likely to be detected in decedents prescribed methadone. There were no changes in the proportion of decedents with co-detected benzodiazepines or alcohol. There were no changes in deaths related to buprenorphine, either prescribed or non-prescribed.
There are multiple factors that could account for the increase in methadone-related deaths in those not prescribed OAT. NDTMS data for the same period indicates that the number of patients in treatment for opioid use disorder increased in April-June 2020 compared to 2019. However, difficulties in accessing telephones or other digital technology may have presented barriers to entering treatment as could fear of contracting COVID-19 during face to face visits, and the loss of privacy and stigma related to queueing outside a pharmacy or drug service (Schofield et al., 2020, Kesten et al., 2021). Reduction in the provision of harm reduction measures such as naloxone via needle exchanges or outreach work to those not in treatment (Kesten et al., 2021, Grebely et al., 2020, Whitfield et al., 2020) may also have contributed to overdose risk for this group. Psychological support for people engaged with treatment services moved to digital or telephone-based formats, which may have mitigated the isolation and personal distress experienced during lockdown conditions. In summary, being prescribed OAT may have been protective, as the large increase in methadone-associated death seen in those out of treatment did not occur in those receiving a prescription. This is consistent with evidence prior to the COVID-19 pandemic that risk of death is lower for patients receiving OAT than those who have left treatment (Sordo et al., 2017) and consistent with treatment cohort findings of no increase in overdose death during the pandemic (Amram et al., 2021, Kitchen et al., 2022). Other healthcare factors such as increased response times of emergency responders (Goddard, 2022), reduced staffing to provide emergency treatment (Propper et al., 2020), and reluctance to attend hospitals for fear of contracting COVID-19 (Hughes et al., 2020), may have all increased the proportion of opioid overdoses which ultimately proved fatal but would arguably have affected both groups.
The COVID-19 lockdown had varying reported effects on availability of drugs and drug use patterns, with changes in drug use due to lack of access to preferred drug (Kesten et al., 2021, Grebely et al., 2020, Zvolensky et al., 2020). Changes in drug availability, particularly fentanyl and other synthetic opioids, have been important contributors to drug-related death rates in the United States during COVID-19 (Jones et al., 2022). However, in our sample, the proportion of cases where opioids were co-detected fell in those prescribed OAT and remained constant in those not prescribed OAT. Fentanyl detections were extremely low (<10 cases in the total dataset). People who use drugs in the UK report that heroin was of lower quality and in some places, more expensive during COVID-19 (Schofield et al., 2020, Kesten et al., 2021). The increase in deaths where methadone was the only opioid detected in the prescribed group perhaps reflects reduced access to heroin or difficulty meeting with dealers, as well as difficulty in self-regulating consumption (Schofield et al., 2020).
Alcohol use, which can contribute to OAT-related death, increased in heavy drinkers in the UK during the pandemic (Rossow et al., 2021), but in this sample there were no differences in the frequency of alcohol co-detection in either group. Benzodiazepines are a growing factor in drug-related deaths in Scotland and the frequency of detections parallels the increase in drug-related death (Mcauley et al., 2022), but no such increase in co-detections was found in our sample when comparing 2020 with previous years. The increase in methadone-related death seen in patients not prescribed it raises the possibility that an important change to the drug market that occurred during the COVID-19 pandemic in England was an increased availability of methadone.
This possibility raises the question of diversion. Evidence from the early 2000s suggests that diversion was relatively commonplace (Fountain et al., 2000). With the widespread increase in take-home prescriptions enacted to facilitate continuation of OAT supply and minimise risk of coronavirus exposure during the COVID-19 pandemic, diversion was a concern (Hunter, Dopp, Ober, & Uscher-pines, 2021). Few patients self-reported diversion of take-home supply during the pandemic (Otiashvili et al., 2022, Figgatt et al., 2021) although they reported that others diverted OAT (Schofield et al., 2020). There was no increase in early requests for methadone prescriptions in Spain (Trujols et al., 2020). Studies comparing proportion of methadone-negative urine samples in treatment populations pre- and post-prescribing change during the pandemic yielded mixed results but the overall proportion with methadone-negative samples in both cohorts was low (Ezie et al., 2022, Bart, Wastvedt, Hodges, & Rosenthal, 2022). However diversion of part of, rather than the whole of, the prescribed supply would not be detected using this method, and may be more common (Fountain et al., 2000). Prior to the pandemic, a systematic review (Saulle et al., 2017) found only one trial reporting self-disclosed diversion, which occurred rarely in patients on supervised and unsupervised OAT dosing (Holland et al., 2014). The same systematic review found no difference in methadone-related mortality or all-cause mortality between patients receiving supervision relative to those who were not, but did not include studies which could evaluate harms to those not in treatment (Saulle et al., 2017).
Buprenorphine-related death was comparatively infrequent despite the potential for it to enter the drug market in the same way as methadone. Buprenorphine is a partial agonist at opioid receptors so is less rewarding and has less of a respiratory depressant effect (Comer et al., 2008). Self-reported non-prescribed use appears to be rare (Bach et al., 2022, Kleinman et al., 2022) and those who use non-prescribed buprenorphine report using it to manage withdrawal symptoms rather than for euphoriant effect, which may involve lower amounts (Kleinman et al., 2022, Carlson et al., 2020). Frequent use of non-prescribed buprenorphine has been associated with a lower risk of overdose perhaps because of its relative safety compared with full agonists (Carlson et al., 2020). Our finding is consistent with previous reports of a higher general population risk of methadone-related mortality relative to that associated with buprenorphine (Marteau et al., 2015) and not merely because methadone prescriptions outnumber buprenorphine prescriptions (EBM DataLab University of Oxford 2021).
In this study post-mortem levels of methadone remained consistent over time within group (prescribed, not prescribed). Indeed, whilst the methadone level associated with mortality can vary according to individual tolerance (Giorgetti et al., 2021), the amount of methadone which would be fatal in a tolerant (receiving a daily dose) or non-tolerant individual (taking non-prescribed methadone more sporadically) would not have changed over the course of the study (Moffat et al., 2011, Baselt, 2011). There were however differences between groups, with those prescribed methadone generally having higher levels detected than those not prescribed it. This disparity may be due to lower relative tolerance and fluctuations in tolerance in those without a daily prescribed supply. Fluctuating or lowered tolerance is thought to underpin spikes in opioid-related death following, for example, prison release (Strang, 2015) and this risk is mitigated by in-prison exposure to OAT (Marsden et al., 2017).
Our findings contrast with studies from North America, two of which found no increase in methadone-related death in a treatment cohort (Amram et al., 2021, Gomes et al., 2022), and one of which found an increase in methadone detections following drug-related death which appeared part of a more general increase in drug-related death (Jones et al., 2022). This may reflect study methodology as these two studies concerned people prescribed OAT only. Differences in drug markets may also have contributed: in the United States fentanyl accounted for a greater proportion of deaths during early 2020 (Macmadu et al., 2021), whereas in our sample fentanyl detections remained very rare. Differences in treatment approaches could also have impacted: in the United States methadone supply was reduced in some states (Chen and Powell, 2022) and a significant minority of services stopped taking new patients (Joudrey et al., 2021) whereas in the UK treatment facilitating access to OAT was emphasised (Public Health England, 2020) and there is some evidence that it could be accessed more quickly during the pandemic than prior to COVID-19 (Schofield et al., 2020, Kesten et al., 2021).
To understand the increase in deaths following non-prescribed methadone use, further studies are needed. The key demographic captured in this study are a hard to reach group–those who were not in treatment. Retrospective analysis of near-fatal opioid overdose patients who were admitted to hospital during the first lockdown could provide further insight into this demographic, as could interrogation of individual police crime scene reports in fatal cases to determine the circumstances surrounding the sourcing and use of the non-prescribed methadone. Qualitative interviews with people who use drugs exploring possible reasons for the observed increase may also generate testable hypotheses regarding contributing causes.
This study's strengths lie in its use of a large post-mortem dataset with rich clinical information which is based on date of death, rather than date of registration of death, and provides more detailed data which is more accurate in terms of timing of death. During 2020 and 2021, the median length of time between date of death and date of registration in England was 189 days and 205 days (Office For National Statistics 2022) making it difficult to use ONS data to track deaths with the temporal granularity that is possible using NPSAD. The source–coroner's data–means that deaths attributable to OAT in decedents not receiving an OAT prescription can be detected; this enables comparison with deaths of individuals prescribed OAT. Limitations also relate to the data source, as the number of deaths where OAT was implicated over the study period are likely under-reported: for example, sufficient post-mortem samples are not always available for comprehensive toxicology testing. Furthermore, as NPSAD is reported to voluntarily (see Methods), post-mortems with toxicology tests are not carried out for all deaths, and the pandemic has presented additional challenges and delays to post-mortem reporting (Justice Committee 2021) - the figures presented here, whilst indicative of an overall trend, are likely conservative. The use of current prescriptions as a marker of prescribed methadone detected post-mortem does not exclude the possibility that decedents in the non-prescribed group may have previously-prescribed methadone detected in their bodily fluids, owing to its long half-life.
For patients in treatment, the increase in methadone-related deaths in 2020 did not far exceed the annual forecast based on the 2016-2019 trend. There was no increase in buprenorphine-related deaths in decedents who were or were not prescribed it. However, methadone-related deaths in people not prescribed methadone substantially increased beyond the 2020 annual forecast based on previous trends. This happened despite an apparent increase in numbers of people accessing opioid treatment services during the lockdown period relative to the same period in previous years, and no significant change when 2020 was considered relative to 2016-2019. Further investigation is thus needed to understand the increase in deaths attributable to methadone in people not prescribed it.
Ethics
The King's College London Biomedical & Health Sciences, Dentistry, Medicine and Natural & Mathematical sciences Research Ethics SubCommittee confirmed in November 2020 that NPSAD does not require ethics review as all subjects are deceased.
Declarations of Interest
D Aldabergenov – None.
L Reynolds - None.
J Scott – is a University based researcher and clinician with a voluntary sector drug treatment provider.
J Strang – JSt is a researcher and clinician in the university and NHS and has also worked with pharmaceutical companies to investigate new or improved medications, but they do not have a direct relationship to the study and findings reported here. This has included research grant support and consultancy payments to JS's employer (King's College London) from, past 3 years, MundiPharma, Camurus, Molteni/Accord. For updated information see John Strang's info on Departmental website at http://www.kcl.ac.uk/ioppn/depts/addictions/people/hod.aspx
CS Copeland – Reports personal fees from Transport Research Laboratory (TRL), outside the submitted work.
MJ Kelleher - Dr Kelleher was Clinical Advisor to Public Health England's Alcohol Drugs Tobacco and Justice division and chaired the meeting on the 17th March 2020. In the last 3 years he has carried out research sponsored by Indivior and Camurus into long acting forms of buprenorphine and Mundipharma into naloxone. He has received honoraria from Camurus and Indivior.
NJ Kalk – is a researcher and clinician in the university and NHS. Her PhD which concerned unrelated work was supported by GSK via a Wellcome Trust GSK Translational Medicine Training Fellowship (2010-2013) and she attended unrelated educational meetings hosted by Lundbeck in 2010. She has served as an unpaid expert advisor for the Public Health England Alcohol Treatment Guidelines and for the Advisory Council on the Misuse of Drugs.
Acknowledgements
This paper represents independent research part funded by the National Institute for Health and Care Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The authors would also like to acknowledge the support of the coroners who contribute vital data to the dataset. With permission from the Office for Health Inequalities and Disparities (OHID) to use the National Drug Treatment Monitoring system (NDTMS) for the purpose, unofficial estimates of monthly counts, of adults (18+) who had been in-treatment for opioid use disorder, were provided by the National Drug Evidence Centre (NDEC), University of Manchester.
Footnotes
Acknowledgements: NK and JSt are supported by the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King's College London.
References
- Amram O, Amiri S., Panwala V., Lutz R, Joudrey P.J., Socias E. The impact of relaxation of methadone take-home protocols on treatment outcomes in the COVID-19 era. American Journal of Drug and Alcohol Abuse. 2021:722–729. doi: 10.1080/00952990.2021.1979991. [DOI] [PubMed] [Google Scholar]
- Bach P., Bawa M., Grant C., milloy M.J., Hayashi K. Availability and use of non-prescribed buprenorphine-naloxone in a Canadian setting 2014-2020. International Journal of Drug Policy. 2022;101 doi: 10.1016/j.drugpo.2021.103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Wastvedt S, Hodges JS, Rosenthal R. Did drug use increase following COVID-19 relaxation of methadone take-out regulations ? 2020 was a complicated year. Journal of Substance Abuse Treatment. 2022;133:108590. doi: 10.1016/j.jsat.2021.108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselt RC. 9th. Biomedical Publications; Seal Beach, CA: 2011. Disposition of toxic drugs and chemicals in man. [Google Scholar]
- Brunton L.L., Hilal-Dandan R., Knollman BC. Goodman and Gilman's the pharmacological basis of therapeutics. 2011 [Google Scholar]
- Carlson R.G., Daniulaityte R., Silverstein S.M., Nahhas R.W., Martins SS. Unintentional drug overdose: Is more frequent use of non-prescribed buprenorphine associated with lower risk of overdose? International Journal of Drug Policy. 2020;79 doi: 10.1016/j.drugpo.2020.102722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.Y., Powell D.SBD. Changes in buprenorphine and methadone supplies in the US during the COVID-19 pandemic. JAMA Network Open. 2022;5(7) doi: 10.1001/jamanetworkopen.2022.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge H., Goodair C. 2015. Drug-related deaths in England, Northern Ireland, the Channel Islands and the Isle of Man: January-December 2013. London. [Google Scholar]
- Comer SD, Sullivan MA, Whittington MD, Vosburg SK, Kowalczyk B. Relative abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008;64(12):2391–2404. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkery J.M., Hung W.C., Claridge H., Goodair C., Copeland C.S., Schifano F. Recreational ketamine-related deaths notified to the National Programme on Substance Abuse Deaths, England, 1997-2019. Journal of Psychopharmacology. 2021;35(11):1324–1348. doi: 10.1177/02698811211021588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBM DataLab University of Oxford. OpenPrescribing.net [Internet]. [cited 2021 Sep 29]. Available from: https://openprescribing.net/
- Ezie C, Badolato R, Rockas M, Nafiz R, Sands B, Wolkin A, et al. COVID 19 and the opioid epidemic: An analysis of clinical outcomes during COVID 19. Substance Abuse Treatment. 2022;16:1–8. doi: 10.1177/11782218221085590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figgatt MC, Salazar Z, Day E, Vincent L, Dasgupta N. Take-home dosing experiences among persons receiving methadone maintenance treatment during COVID-19. Journal of Substance Abuse Treatment. 2021;123 doi: 10.1016/j.jsat.2021.108276. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain J, Strang J, Gossop M, Farrell M, Griffiths P. Diversion of prescribed drugs by drug users in treatment: Analysis of the UK market and new data from London. Addiction. 2000;95(3):393–406. doi: 10.1046/j.1360-0443.2000.95339310.x. [DOI] [PubMed] [Google Scholar]
- Giorgetti A, Pascali J, Montisci M, Amico I, Bonvicini B, Fais P, et al. The role of risk or contributory death factors in methadone-related fatalities: A review and pooled analysis. Metabolites. 2021;11(3):189. doi: 10.3390/metabo11030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. House of Lords Library; 2022. Ambulance response times in England: An emergency? London, UK. [Google Scholar]
- Gomes T., Campbell T.J., Kitchen S.A., Garg R., Bozinoff N., Men S., Tadrous M., Munro C., Antoniou T., Werb D., Wyman J. Association between increased dispensing of opioid agonist therapy take-home doses and opioid overdose and treatment interruption and discontinuation. JAMA The Journal of the American Medical Association. 2022;327(9):846–855. doi: 10.1001/jama.2022.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J., Cerda M., Rhodes T. COVID-19 and the health of people who use drugs: What is and what could be? International Journal of Drug Policy. 2020;83 doi: 10.1016/j.drugpo.2020.102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R., Maskrey V., Swift L., Notley C., Robinson A., Nagar J., Gale T., Kouimtsidis C. Treatment retention, drug use and social functioning outcomes in those receiving 3 months versus 1 month of supervised opioid maintenance treatment. Results from the SuperC randomized controlled trial. Addiction. 2014;109(4):596–607. doi: 10.1111/add.12439. [DOI] [PubMed] [Google Scholar]
- Hughes H.E, Hughes T.C., Morbey R., Challen K., Oliver I., Smith G.E., Elliot AJ. Vol. 37. Emerg Dep use Dur COVID-19 as Descr by Syndr surveillance; 2020. pp. 600–604. (Emergency department use during COVID-19 as described by syndromic surveillance). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SB, Dopp AR, Ober AJ, Uscher-pines L. Clinician perspectives on methadone service delivery and the use of telemedicine during the COVID-19 pandemic: A qualitative study. Journal of Substance Abuse Treatment. 2021;124:108288. doi: 10.1016/j.jsat.2021.108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka BP, Janssen T, Garner BR, Yermash J, Yap KR, Ball EL, et al. Impacts of the COVID-19 pandemic on healthcare access among patients receiving medication for opioid use disorder. Drug and Alcohol Dependence. 2021;221(January) doi: 10.1016/j.drugalcdep.2021.108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Compton WM, Han B, Baldwin G, Volkow ND. Methadone-involved overdose deaths in the US before and after federal policy changes expanding take-home methadone doses from opioid treatment programs. JAMA Psychiatry. 2022;79(9):2021–2023. doi: 10.1001/jamapsychiatry.2022.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joudrey PJ, Adams ZM, Bach P, Van Buren S, Chaiton JA, Ehrenfeld L, et al. Methadone access for opioid use disorder during the COVID-19 pandemic within the United States and Canada. JAMA Network Open. 2021;4(7):1–13. doi: 10.1001/jamanetworkopen.2021.18223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice Committee . UK Parliament; London, UK: 2021. The Coroner Service’s response to covid-19 [Internet]https://publications.parliament.uk/pa/cm5802/cmselect/cmjust/68/6811.htm Available from. [Google Scholar]
- Kesten JM, Holland A, Linton M-J, Family H, Scott J, Horwood J, et al. Living Under Coronavirus and Injecting Drugs in Bristol (LUCID-B): A qualitative study of experiences of COVID-19 among people who inject drugs. International Journal of Drug Policy. 2021;98 doi: 10.1016/j.drugpo.2021.103391. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen S.A., Campbell T.J., Men S., Bozinoff N., Tadrous M., Antoniou T., Wyman J., Werb D., Munro C., Gomes T. Impact of the COVID-19 pandemic on the provision of take-home doses of opioid agonist therapy in Ontario, Canada: A population-based time-series analysis. International Journal of Drug Policy. 2022;103 doi: 10.1016/j.drugpo.2022.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman RA, Nielsen S, Weiss RD. Is daily supervised buprenorphine-naloxone dosing necessary? BMJ. 2022;378 doi: 10.1136/bmj-2022-071467. [DOI] [PubMed] [Google Scholar]
- Krawczyk N, Bunting AM, Frank D, Arshonsky J, Gu Y, Friedman SR, et al. “How will I get my next week’s script?” Reactions of Reddit opioid forum users to changes in treatment access in the early months of the coronavirus pandemic. International Journal of Drug Policy. 2021;93:103140. doi: 10.1016/j.drugpo.2021.103140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmadu A., Batthala S., Correia Gabel A.M., Rosenberg M., Ganguly R., Yedinak J.L., Hallowell B.D., Scagos R.P., Samuels E.A., Cerda M., Paull K., Marshall BD. Comparison of characteristics of deaths from drug overdose before vs during the COVID-19 pandemic in Rhode Island. JAMA Network Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.25538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden J, Stillwell G, Jones H, Cooper A, Eastwood B, Farrell M, et al. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction. 2017;112(8):1408–1418. doi: 10.1111/add.13779. [DOI] [PubMed] [Google Scholar]
- Marteau D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5) doi: 10.1136/bmjopen-2015-007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcauley A, Matheson C, Robertson JR. From the clinic to the street : The changing role of benzodiazepines in the Scottish overdose epidemic. International Journal of Drug Policy. 2022;100 doi: 10.1016/j.drugpo.2021.103512. [Internet]Available from. [DOI] [PubMed] [Google Scholar]
- Meteliuk A, Galvez SJ, Leon D, Madden LM, Pykalo I, Fomenko T, et al. Rapid transitional response to the COVID-19 pandemic by opioid agonist treatment programs in Ukraine. Journal of Substance Abuse Treatment. 2021;121(January):108164. doi: 10.1016/j.jsat.2020.108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat AC, et al. 4th edition. Pharmaceutical Press; London: 2011. Clarke’s analysis of drugs and poisons. [Google Scholar]
- O'Neal C.L., Poklis A. Postmortem production of ethanol and factors that influence interpretation: A critical review. American Journal of Forensic Medicine and Pathology. 1996;17(1):8–20. doi: 10.1097/00000433-199603000-00002. [DOI] [PubMed] [Google Scholar]
- Office For National Statistics . Office of National Statistics; 2022. Deaths related to drug poisoning in England and Wales: 2021 registrations [Internet]. Released on 3rd August 2022.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2021registrations [cited 2022 Sep 18]. Available from. [Google Scholar]
- Otiashvili D, Mgebrishvili T, Beselia A, Vardanashvili I, Dumchev K, Kiriazova T, et al. The impact of the COVID-19 pandemic on illicit drug supply, drug-related behaviour of people who use drugs and provision of drug related services in Georgia: Results of a mixed methods prospective cohort study. Harm Reduction Journal [Internet] 2022;19(1):1–15. doi: 10.1186/s12954-022-00601-z. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyekan P.J., Gorton H.C., Copeland CS. Are the high safety profiles of antihistamines leading to their unsafe use? British Journal of Clinical Pharmacology. 2021;87(10):3978–3987. doi: 10.1111/bcp.14819. [DOI] [PubMed] [Google Scholar]
- Public Health England . Public Health England; London, UK: 2020. NDTMS. [Google Scholar]
- Public Health England . Public Health England; London, UK: 2020. COVID-19: Guidance for commissioners and providers of services for people who use drugs or alcohol [Internet] [Google Scholar]; Available from https://web.archive.org/web/20200620083630/https:/www.gov.uk/government/publications/covid-19-guidance-for-commissioners-and-providers-of-services-for-people-who-use-drugs-or-alcohol/covid-19-guidance-for-commissioners-and-providers-of-services-for-people
- Roncero C, Vicente-Hernández B, Casado-Espada NM, Aguilar L, Gamonal-Limcaoco S, Garzón MA, et al. The impact of COVID-19 pandemic on the Castile and Leon addiction treatment network: A real-word experience. Front Psychiatry. 2020;11(November):1–8. doi: 10.3389/fpsyt.2020.575755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow I, Bartak M, Bloomfield K, Braddick F, Bye EK, Kilian C, et al. Changes in alcohol consumption during the covid-19 pandemic are dependent on initial consumption level: Findings from eight European countries. International Journal of Environmental Research and Public Health. 2021;18(19):1–12. doi: 10.3390/ijerph181910547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulle R., Vecchi S., Gowing L. Supervised dosing with a long-acting opioid medication in the management of opioid dependence. Cochrane Database of Systematic Reviews (Online) 2017;27(4) doi: 10.1002/14651858.CD011983.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield J., Dumbrell J., Browne T., Bancroft A., Gallip I., Matheson C., Parkes T. University of Stirling; Stirling: 2020. The impacts of COVID-19 on people who use drugs [Internet]https://covid-drugs.stir.ac.uk/ [cited 2022 Sep 17]. Available from. [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Hall W, Hickman M, Bird SM. Impact of supervision of methadone consumption on deaths related to methadone overdose (1993-2008): Analyses using OD4 index in England and Scotland. BMJ. 2010;341(7774):640. doi: 10.1136/bmj.c4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J. Death matters: Understanding heroin/opiate overdose risk and testing potential to prevent deaths. Addiction. 2015;110(S2):27–35. doi: 10.1111/add.12904. [DOI] [PubMed] [Google Scholar]
- Trujols J., Larrabeiti A., Sandchez O., Madrid M., De Andres S., Duran-Sindreu S. Intreased flexibility in methadone take hom scheduling during the COVID-19 pandemic: Should this practice be incorporated into routine clinical care? Substance Abuse Treatment. 2020;119 doi: 10.1016/j.jsat.2020.108154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper C., Stoye G., Zaranko B. The wider impacts of the coronavirus pandemic on the NHS. Fiscal Studies. 2020;41(2):345–356. doi: 10.1111/1475-5890.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield M, Reed H, Webster J, Hope V. The impact of COVID-19 restrictions on needle and syringe programme provision and coverage in England. International Journal of Drug Policy. 2020;83(January) doi: 10.1016/j.drugpo.2020.102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoganathan P, Claridge H, Chester L, Englund A, Kalk N, Copeland C. OSF; 2020. Synthetic cannabinoid-related deaths in England, 2012-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky M.J., Garey L., Rogers A.H., Schmidt N.B., Vujanovic A.A., Storch E.A., Buckner J.D., Paulus D.J., Alfano C., Smits J.A., O'Cleirigh C. Psychological, addictive, and health behavior implications of the COVID-19 pandemic. Behaviour Research and Therapy. 2020;134 doi: 10.1016/j.brat.2020.103715. [DOI] [PMC free article] [PubMed] [Google Scholar]