Abstract
Aims
To analyse if antidiabetic treatment was associated with better COVID-19 outcomes in type 2 diabetic patients, measured by hospital admission and mortality rates as severe outcomes.
Methods
Cohort study including COVID-19 patients registered in the Primary Care electronic records, in March-June 2020, comparing exposed to metformin in monotherapy with exposed to any other antidiabetic. Data source: SIDIAP (Information System for Research in Primary Care), which captures clinical information of 5,8 million people from Catalonia, Spain.
Results
We included 31,006 diabetic patients infected with COVID-19, 43.7% previously exposed to metformin, 45.5% of them in monotherapy. 16.4% were admitted to hospital and 15.1% died. Users of insulin in monotherapy (OR 1.29, 95% CI 1.11–1.50), combined with metformin (OR 1.38, 1.13–1.69) or IDPP4 alone (OR 1.29, 1.03–1.63) had higher risk of severe outcomes than those in metformin monotherapy. Users of any insulin (OR 1.61, 1.32–1.97) or combined with metformin (OR 1.69, 1.30–2.20) had a higher risk of mortality.
Conclusions
Patients receiving metformin monotherapy in our study showed a lower risk of hospitalization and death in comparison to those treated with other frequent antidiabetic agents.
We cannot distinguish if better outcomes are related with the antidiabetic therapy or with other factors, such as metabolic control or interventions applied during the hospital admission.
Abbreviations: ACE2, Angiotensin-2 converting enzyme; ACEI, Angiotensin converting enzyme inhibitors; AQuAS, Catalan Agency of Health Quality and Evaluation; ARB, Angiotensin receptor blockers; ATC, Anatomical, chemical classification system; BMI, Body mass index; CI, Confidence interval; COVID-19, Coronavirus disease 2019; DPP4, Dipeptidyl peptidase 4; ECAP, Electronic health records in Primary Health Care of the Catalan Health Institute; GLP1, Glucagon-like petptide-1; HNI, Hypoglycaemic agents excluding insulins; HR, Hazard ratio; ICD-10, International Classification of Disease-10 codes; iDPP4, Dipeptidyl peptidase 4 inhibitors; IQR, Interquartile range; iSGLT2, Sodium-glucose co-transporter 2 inhibitors; LTCF, Long-term care facilities; NSAID, Non-steroidal anti-inflammatory drugs; OR, Odds ratio; PCR, Polymerase chain reaction; PHC, Primary Health Care; SARS-CoV-2, Severe acute respiratory syndrome-coronavirus-2; SD, Standard deviation; SIDIAP, Information System for Research in Primary Care
Keywords: COVID-19, Electronic health records, Primary health care, Diabetes, Antidiabetic agents
1. Introduction
Coronavirus disease 2019 (COVID-19) is a viral respiratory illness caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) that emerged as a global public health crisis in 2020 [1], [2], [3] and it is significantly associated with worse outcomes in people with chronic conditions, such as diabetes [4].
A series of inflammatory markers have been identified in diabetic patients, pointing out that this disease could be a risk factor for the progression and prognosis of COVID-19 [5]. Different mechanisms could increase the risk and the severity of COVID-19 among diabetic patients, for instance the decrease in T CD4 positive cells, [6] the increase of interleukin-6 expression, or the positive regulation of the expression of angiotensin-2 converting enzyme (ACE2),[5] as SARS-CoV-2 can infect host cells by means of interaction with membrane-bound ACE2 on the respiratory epithelium [7].
As coronaviruses also bind dipeptidyl peptidase 4 (DPP4),[8] the interaction of SARS-CoV-2 and DPP4 may represent a potential factor promoting the virulence of the virus, and thus the inhibition of this interaction might curb the inflammatory storm seen in patients with severe COVID-19 [9], [10]. DPP4 inhibitors (iDPP4) are highly selective hypoglycaemic agents which increase the bioavailability of the glucagon-like petptide-1 (GLP1) and have anti-inflammatory and immunoregulatory effects, therefore, they could be useful to prevent COVID-19 fatal outcomes or improve the course of the disease in diabetic patients [11], [12].
Other antidiabetic drug, metformin, has also anti-inflammatory activity and could be beneficial in treating COVID-19 at multiple levels by improving glucose controls, reducing insulin resistance, or suppressing mitochondrial reactive oxygen species.[13] Significant reduction in mortality has been reported among high-risk diabetic COVID-19 patients treated with metformin, so it may play a role in attenuating COVID-19 complications [14], [15] Other oral antidiabetic agents might be able to bind SARS-CoV-2 receptor (repaglinide, canagliflozin, glipizide, gliquidone, glimepiride and linagliptin), suggesting a possible inhibition of the replication and transcription of the virus [16]. Also GLP1 analogues have been suggested as possible candidates to treat COVID-19 for their anti-inflammatory and anti-obesogenic properties, pulmonary protective effects and benefits on gut microbiome,[17] or insulin for its anti-inflammatory effects [18].
Related with the use of antidiabetic drugs, in studies conducted with diabetic patients with COVID-19, a poor control of blood glucose levels have been associated with higher mortality in comparison with those with optimal metabolic control [19], [20]. Although type 2 diabetes does not increase the susceptibility against SARS-CoV-2 infection, it can worsen the outcomes of the infection, particularly in those people with acute and chronic conditions.[4], [21] Nevertheless, there are no guidelines focused on the treatment of COVID-19 in diabetic patients [22].
In this study we aimed to analyse if antidiabetic agents might be associated with a reduction in COVID-19 complications in diabetic patients, measured by hospital admission and mortality rates as severity outcomes, through a population-based cohort study including type 2 diabetes patients attended in Primary Health Care (PHC) who were infected with COVID-19 in Catalonia, Spain.
2. Methods
2.1. Study design
Cohort study including adult patients with COVID-19 diagnosis registered as confirmed (by polymerase chain reaction, PCR) or as probably (not confirmed by PCR) in the PHC records in Catalonia, Spain; from the pandemics’ onset (March 2020) to June 30th, 2020. We compared type 2 diabetic patients with a diagnosis registered in the PHC records who were exposed to metformin in monotherapy with those exposed to any other antidiabetic drugs alone or in combination or those not receiving any antidiabetic agent.
2.2. Data source
The study data source is the Information System for Research in Primary Care (SIDIAP), [23], [24] which captures clinical information of approximately 5,8 million people from Catalonia, Spain (around 80% of the Catalan population). This information is pseudonymized, originated from different data sources: 1) ECAP (electronic health records in PHC of the Catalan Health Institute); including socio-demographic characteristics, residents in nursing homes/long-term care facilities (LTCF), comorbidities registered as International Classification of Disease (ICD)− 10 codes, [25] specialist referrals, clinical parameters, toxic habits, sickness leave, date of death, laboratory test data, and drug prescriptions issued in PHC, registered as anatomical, chemical classification system (ATC) codes; [26] 2) pharmacy invoice data corresponding to the PHC drug prescriptions; 3) database of diagnoses at hospital discharge [27] and 4) COVID-19 data from the Catalan Agency of Health Quality and Evaluation (AQuAS). [28].
2.3. COVID-19 classification
Subjects were classified according to the following criteria: confirmed cases were those with a confirmed COVID-19 diagnostic record, PCR+ and/or a positive serology test. Those with a non-confirmed diagnosis or test (possible or unclear) along with any individual with a record of hospitalization, pneumonia and/or death related to COVID-19 were considered possible cases. During the first wave of the COVID-19 pandemics in Catalonia, PCR tests were not routinely conducted to all patients with compatible symptoms, due to the unavailability of laboratory kits to do the tests. Thus, we needed to capture those patients with possible diagnosis of COVID-19, such as those admitted to hospital with pneumonia or other COVID-19 symptoms who were not tested. We designed an algorithm to classify patients as “COVID possible” when there was not a test result available. The date of COVID-19 diagnosis was set to be the first record used per patient.
To guarantee that our algorithm is not far from the Catalan population, the resulting cohort was compared to the official COVID-19 cases provided by the AQuAS during the pandemic [28], [29].
2.4. Drug exposure
Patients were classified as exposed to metformin or other antidiabetics when they had at least one prescription issued during the previous 6 months to the COVID-19 diagnosis and with a minimum duration of 30 days. Those diabetic patients not meeting this criterion were considered as untreated. The reference group was composed by those people exposed to metformin in monotherapy.
The rest of the antidiabetic agents were classified as: insulins, sulfonylureas, iDPP4, sodium-glucose co-transporter 2 inhibitors (iSGLT2), GLP1 and other hypoglycaemic agents excluding insulins (HNI), including acarbose, pioglitazone or repaglinide.
2.5. Variables
At baseline, the variables captured were sex, age, body mass index (BMI), residence in LTCF, smoking habit, glycated haemoglobin (HbA1c) measured up to 6 months before COVID-19 infection, years since type 2 diabetes diagnosis, comorbidities (ICD-10 codes), and drug exposure (ATC codes).
Main severity outcomes: rates of hospital admission and mortality. The risk of these events was analysed comparing patients exposed to metformin with those non-exposed to metformin (but with exposure to any other antidiabetic drug).
2.6. Statistical analysis
Quantitative variables were described as the mean and standard deviation or median and interquartile range, whereas categorical variables were described as the proportion over the exposed and non-exposed individuals. Univariate analyses were based on Student’s t-test or Chi-square test depending on the variable type.
For each outcome, composite severe event and death related to covid-19 infection, we fitted a logistic regression model to estimate an odds ratio (OR) comparing the prevalence of each outcome among individuals exposed to metformin monotherapy (reference population) to those exposed to other antidiabetic treatments or not being treated. The logistic model was fitted including other covariables such as gender, age, obesity, smoking habits, HbA1c at index date, history of diabetes, comorbidities, and concomitant drugs. Variable selection was performed using the AIC-based stepwise backward procedure and we used the Wald test on the fitted coefficient to determine whether the log-odds was significantly different from zero at a 0.05 level. All analyses were performed in R software (v4.1.0 or above).
3. Results
We included 31,006 people with type 2 diabetes who were infected with SARS-CoV-2 from March to June 2020, 21,131 (68.2%) were receiving treatment with antidiabetic drugs and 13,549 (43.7%) of them were exposed to metformin. There were more diabetic men (15,903, 51.3%) than women, the mean age of all patients included was 71.5 years-old and 50.9% were current smokers. The most frequent comorbidities were hypertension (71.1%), obesity (57.2%) and respiratory diseases (38.2%), and the most prescribed comedications were psychotropic drugs (52.0%), non-steroidal anti-inflammatory drugs (43.9%), and angiotensin converting enzyme inhibitors (30.1%). Table 1 shows the baseline characteristics for all population and for those receiving metformin in monotherapy.
Table 1.
Baseline sociodemographic and clinical characteristics of patients included in the study.
| N (%) | All diabetic patients with COVID-19 N = 31,006 |
Diabetic patients with COVID-19 exposed to metformin in monotherapy N = 6168 (19.9%) |
|---|---|---|
| Gender, female | 15,103 (48.7) | 2979 (48.3) |
| Age, mean (SD) | 71.5 (15.5) | 71.07 (14.44) |
| Smoker | 15,775 (50.9) | 3194 (51.8) |
| LTFC resident | 7148 (23.1) | 1364 (22.1) |
| Hb1Ac, mean (SD) 46.5% missing values |
7.20 (1.43) | 6.75 (1.06) |
| Years since diabetes diagnosis, median (IQR) | 7.00 [3.00, 13.00] | 6.00 [2.00, 10.00] |
| Comorbidities | ||
| Cancer | 5978 (19.3) | 1152 (18.7) |
| Cerebrovascular diseases | 2563 (8.3) | 461 (7.5) |
| Chronic kidney disease | 6980 (22.5) | 839 (13.6) |
| Coronary heart disease | 4288 (13.8) | 658 (10.7) |
| Heart failure | 3740 (12.1) | 493 (8.0) |
| Hypertension | 22,036 (71.1) | 4329 (70.2) |
| Obesity (ICD-10 code and/or BMI ≥30 kg/m2) | 17,748 (57.2) | 3676 (59.6) |
| Respiratory diseases | 11,847 (38.2) | 2313 (37.5) |
| Comedications | ||
| ACEI | 9345 (30.1) | 2157 (35.0) |
| ARB | 6289 (20.3) | 1262 (20.5) |
| Beta blockers | 7545 (24.3) | 1410 (22.9) |
| Calcium channel blockers | 6248 (20.2) | 1203 (19.5) |
| NSAID | 13,610 (43.9) | 2744 (44.5) |
| Oral anticoagulants | 3972 (12.8) | 722 (11.7) |
| Psychotropic drugs | 16,110 (52.0) | 3256 (52.8) |
SD: standard deviation, LTCF: long-term care facilities, IQR: interquartile range, ICD-10: international classification of diseases version 10, BMI: body mass index, ACEI: angiotensin converting enzyme inhibitors, ARB: angiotensin receptor blockers, NSAID: non-steroidal anti-inflammatory drugs.
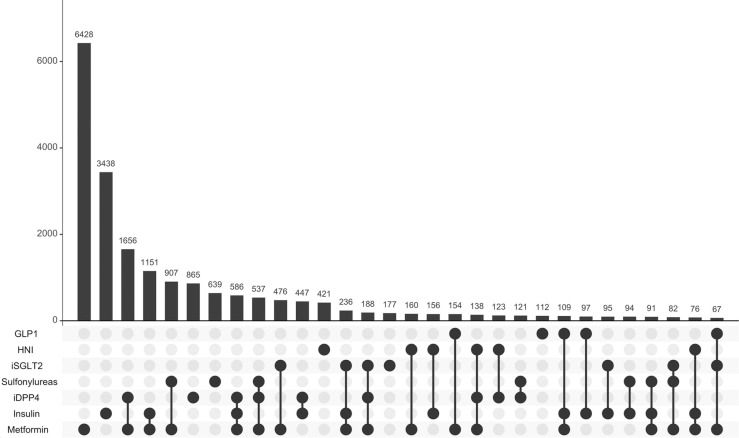
The exposure to antidiabetic drugs is described in Table 2; 56.7% of the antidiabetic drugs’ users were treated in monotherapy. The most used pharmacological groups were metformin (64.1%), insulins (26.4%) and iDPP4 (24.3%). The most frequent drugs in monotherapy and in combination are shown in Fig. 1.
Table 2.
Exposure to antidiabetic drugs.
| N (%) | Diabetic patients treated N = 21,131 (68.2) |
|---|---|
| Monotherapy | 11,975 (56.7) |
| 2 drugs | 6018 (28.5) |
| ≥ 3 drugs | 3138 (14.9) |
| Exposure to antidiabetic drugs* | |
| Metformin In monotherapy |
13,549 (64.1) 6168 (29.2) |
| Insulins | 8182 (26.4) |
| iDPP4 | 5131 (24.3) |
| Sulfonylureas | 2817 (13.3) |
| iSGLT2 | 1809 (8.6) |
| Other blood glucose lowering drugs | 1401 (6.6) |
| GLP1 | 782 (3.7) |
GLP1; glucagon-like peptide-1 analogues, iDPP4; dipeptidyl peptidase 4 inhibitors, iSGLT2; sodium-glucose co-transporter 2 inhibitors. *Patients may belong to > 1 group.
Fig. 1.
Frequencies of exposure to antidiabetic drugs in monotherapy and in combination This figure shows the 30 most frequent antidiabetic treatments, in monotherapy and in combinations of up to 4 different drugs. The bars are ordered along the X-axis from highest to lowest frequency. GLP1; glucagon-like peptide-1 analogues, iDPP4; dipeptidyl peptidase 4 inhibitors, iSGLT2; sodium-glucose co-transporter 2 inhibitors.
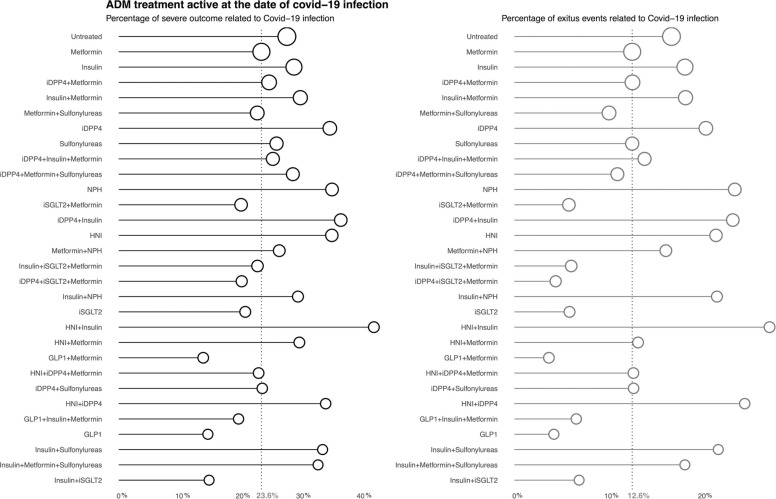
At least one of the main outcomes of the study occurred in 8035 (25.9%) diabetic patients: hospitalization in 5096 (16.4%) and death in 4678 (15.1%). In Fig. 2 we present the frequency of events (in percentage) per antidiabetic treatment. Vertical dotted line shows the frequency of events in those under metformin monotherapy, the reference group. Treatments with the circle on the right of the line, as untreated, treated with insulin monotherapy, with metformin plus iDPP4, or with a combination of insulin and metformin showed higher frequencies of severe events in comparison to the reference drug.
Fig. 2.
Frequency of severe outcomes of the COVID-19 infection per antidiabetic treatment: a) Any of the severe outcomes; hospitalization or mortality. b) Mortality. Fig. 2 shows the frequency of events per antidiabetic treatment. The vertical dotted line corresponds to the frequency of events in those under metformin monotherapy. The treatments showed are the 30 most frequent ones and include: GLP1; glucagon-like peptide-1 analogues, iDPP4; dipeptidyl peptidase 4 inhibitors, iSGLT2; sodium-glucose co-transporter 2 inhibitors, HNI; other hypoglycaemic agents, non-insulin.
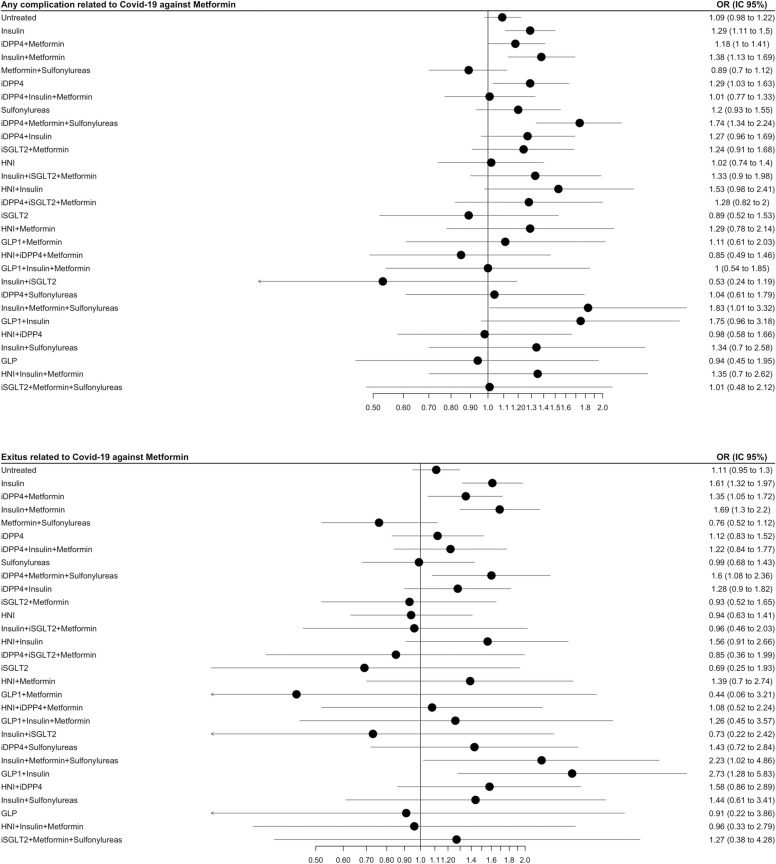
Fig. 3 shows the risk of severe outcomes per antidiabetic treatment. Those patients treated with insulins alone (OR 1.29, 1.11–1.50) or in combination with metformin (OR 1.38, 1.13–1.69), or iDPP4 alone (OR 1.29, 1.03–1.63) had a significant higher risk of experiencing any of the severe outcomes than those in metformin monotherapy. For untreated, the results were not statistically significant (OR 1.09, 95% CI 0.98–1.22). Other less frequent combinations showed significant higher risk of hospitalisation or death than metformin; metformin + sulfonylureas + either iDPP4 or /insulin, or insulin plus other hypoglycaemic drugs.
Fig. 3.
Risk of the severe outcomes per antidiabetic treatment: a) Any of the severe outcomes; hospitalization or mortality. b) Mortality. The treatments showed are the 30 most frequent ones and include: GLP1; glucagon-like peptide-1 analogues, iDPP4; dipeptidyl peptidase 4 inhibitors, iSGLT2; sodium-glucose co-transporter 2 inhibitors, HNI; other hypoglycaemic agents, non-insulin. OR and 95% CI, adjusted.
Those receiving any insulin alone (OR 1.61, 1.32–1.97) or insulins combined with metformin (OR 1.69, 1.30–2.20) had a higher risk of mortality than those in metformin monotherapy. Other less common combinations showing a higher mortality risk were metformin + sulfonylurea + insulin and insulin + GLP1.
4. Discussion
We analysed the outcomes of COVID-19 infection in 31,006 diabetic patients, comparing those chronically treated with metformin with those treated with other antidiabetic drugs. In our population, patients receiving only insulin, insulin combined with metformin or iDPP4 in monotherapy had a higher risk of experiencing hospitalization or death in comparison with people treated with metformin in monotherapy.
A recent study published in Spain analysed the sociodemographic, clinical and analytical variables associated with the need for hospital admission due to COVID-19 complications in a PHC cohort and specifically assessed whether diabetes entailed higher risk, finding that it did not increased risk of hospitalization in this population (OR 1.18, 95% CI 0.80–1.72) [30].
Different antidiabetic drugs have been studied for their anti-inflammatory properties and other effects which could help improving COVID-19 complications in diabetic patients from different settings. Yang W. et al. conducted a meta-analysis of 17 studies including 20,719 COVID-19 diabetic patients and found that metformin could benefit mortality and severity (OR 0.64, 95% CI 0.51–0.79; and OR 0.81, 95% CI 0.66–0.99, respectively) [31]. In our study, we found lower risk of severe outcomes for patients receiving metformin in monotherapy when compared to other antidiabetic treatments, as insulin alone or associated to metformin, or iDPP4, but not comparing with other treatments.
Chen Y et al. performed a meta-analysis of 35 studies and found that metformin, iDPP4, iSGLT2 and GLP1 (OR 0.74, 0.88, 0.82 and 0.91, respectively) were highly possible to reduce mortality risk and insulin might be related to increased mortality risk (OR 1.8) [32]. We also found a higher mortality risk with insulin.
In another review of 61 studies analysing pre-admission medication, the authors found protective effect against COVID-related death with metformin, GLP1, and iSGLT2 (OR 0.54, 0.50 and 0.60, respectively); and users of iDPP4 and insulin (OR 1.23 and 1.70) were more likely to die during hospitalization [33].
Metformin and sulfonylureas were studied in another meta-analysis including 18 studies with 17,338 patients, which found that both drugs were associated with lower mortality risk in COVID-19 diabetic patients (OR 0.69, 95% CI 0.55–0.86 for metformin and 0.80, 95% CI 0.66–0.96 for sulfonylureas) [34].
With regards to iDPP4, this meta-analysis also found that mortality was not different between users and non-users (OR 0.72, 0.51–1.01) [34]. Yang Y. et al. pooled the results of four articles to investigate if iDPP4 had a protective effect on the progression of COVID-19, and they found an improvement in the mortality risk in iDPP4 users (OR 0.58, 95% CI 0.34–0.99) [35]. Patoulias et al. found that these drugs administered during hospital admission decreased the risk for COVID-19-related death by 50% [36]. We found divergent results for this pharmacological group, with a higher risk of hospital admission or death in comparison with metformin users.
Regarding the insulins, the meta-analysis of Kan et al. mentioned above found that patients treated with insulin exhibited greater mortality (OR 2.20, 1.34–3.60) [34]. Other studies found negative outcomes associated with insulin, as Chen et al. with an OR= 3.58, 95% CI 1.37–9.35 for poor prognosis, [37] or Yu et al. with a significant increase in mortality (27.2% vs 3.5%, HR 5.38, 2.75–10.54) when they compared insulin users with non-users [38]. These results were similar to ours, as we also found a higher risk of severe outcomes for insulin in monotherapy or associated with metformin respect metformin alone. The fact that insulin is the hypoglycaemic agent of choice for managing critically ill patients or that it is a third-line therapy for diabetic patients could have influenced these outcomes, as all of them might have worst prognosis [18], [39].
We found a high percentage of diabetic patients not receiving treatment (31.8%) in our population and we do not know if the results would have been different analysing only antidiabetic users or only adherent patients. We are not able to rule out that the results obtained can be more associated with the level of metabolic control than to the chronic antidiabetic therapy. We have adjusted by years of evolution of the diabetes and by the metabolic control as per HbA1c.
Among other limitations of our study there is the reliability of the COVID-19 diagnoses; we included patients without a confirmed result as during the first wave of the pandemic in our setting PCR test were not always performed. This limitation has been described in other research as during the beginning of the pandemic diagnosis test for COVID-19 were not widely available, and clinical algorithms have been used to assess COVID-19 diagnosis [40]. Another limitation is that our database is a PHC database and it only records the dates of admission and diagnoses and cause of discharge, so it is necessary to consider the lack of hospital information; for instance, we cannot capture ICU admission, ventilation or treatments and interventions administered during the admission, which clearly have influence in the prognosis and outcomes of COVID-19.
Some strengths of our study include the large number of patients included, representativeness for general population, and complete socio-demographic data. We must highlight that our cohort are PHC patients, so we have estimated the risk of death and hospitalization for a different population from the only hospitalized ones that are usually studied.
5. Conclusions
We described hospital admission and mortality rates in a large cohort of diabetic patients infected with SARS-CoV-2.
Patients treated with metformin in monotherapy had lower risk of hospitalization and death in comparison to other frequent antidiabetic treatments in our setting.
With the data available, we cannot distinguish if better outcomes are related with the antidiabetic therapy or with other factors, such as metabolic control or interventions applied during the hospital admission.
AEMPS classification
IDI-VAC-2020–21. Estudio Posautorización con Otros Diseños diferentes al de seguimiento prospectivo, EPA-OD. 15th September 2020.
Ethical considerations
The study protocol was approved by the Research Ethics Committee of IDIAPJGol (June 3rd 2020). This is a database research study which has been conducted according to the guidelines of the Declaration of Helsinki (Fortaleza, Brazil 2013) and does not require consent from the people included to participate or for publication. The need for consent was waived by the Research Ethics Committee of IDIAPJGol as it is deemed unnecessary according to European legislation (Regulation [EU] 2016/679).
Funding
This study was conducted with internal funding from IDIAPJGol (project number 4R20/029).
Conflict of interest
The authors declare no conflict of interest.
Data Availability
The datasets generated and/or analysed during the current study are not publicly available due to patient privacy and data protection concerns, but they are available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article and its supplementary information files.
References
- 1.European Centre for Disease Prevention and Control (ECDC), COVID-19 situation updates, 2022.
- 2.World health organization (WHO), Coronavirus disease 2019, 2022.
- 3.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19 — studies needed. N. Engl. J. Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 4.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.I., Skyler J.S., DeVries J.H., Renard E., Eckel R.H., Zimmet P., Alberti K.G., Vidal J., Geloneze B., Chan J.C., Ji L., Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsiki N., Ferrannini E. Anti-inflammatory properties of antidiabetic drugs: a “promised land” in the COVID-19 era? J. Diabetes Its Complicat. 2020;34 doi: 10.1016/j.jdiacomp.2020.107723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts J., Pritchard A.L., Treweeke A.T., Rossi A.G., Brace N., Cahill P., MacRury S.M., Wei J., Megson I.L. Why Is COVID-19 more severe in patients with diabetes? the role of angiotensin-converting enzyme 2, endothelial dysfunction and the immunoinflammatory system. Front. Cardiovasc. Med. 2021;7:1–23. doi: 10.3389/fcvm.2020.629933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y., Ogedegbe G., Hochman J.S. Renin–Angiotensin–Aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Zhang Z., Yang L., Lian X., Xie Y., Li S., Xin S., Cao P., Lu J. The MERS-CoV Receptor DPP4 as a Candidate Binding Target of the SARS-CoV-2 Spike. IScience. 2020;23 doi: 10.1016/J.ISCI.2020.101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal R., Banerjee M., Mukherjee S., Bhogal R.S., Kaur A., Bhadada S.K. Dipeptidyl peptidase-4 inhibitor use and mortality in COVID-19 patients with diabetes mellitus: an updated systematic review and meta-analysis. Ther. Adv. Endocrinol. Metab. 2021;12 doi: 10.1177/2042018821996482. 204201882199648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieffer T.J., Mc Intosh C.H.S., Pederson R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/ENDO.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 12.Solerte S.B., Antonio D.Sabatino, Galli Massimo, Fiorina P. Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol. 2020;57:779–783. doi: 10.1007/s00592-020-01539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheen A.J. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab. 2020;46:423–426. doi: 10.1016/j.diabet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel S.M., Varghese E., Büsselberg D. Therapeutic potential of metformin in COVID-19: reasoning for its protective role. Trends Microbiol. 2021;29:894–907. doi: 10.1016/j.tim.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo P., Qiu L., Liu Y., Liu X.L., Zheng J.L., Xue H.Y., Liu W.H., Liu D., Li J. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 2020;103:69–72. doi: 10.4269/AJTMH.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu H., Zheng Y., Wang Y., Li H., Liu X., Xiong X., Zhang L., Gu J., Yang G., Zhu Z., Zheng H., Ouyang Q. The potential effects of clinical antidiabetic agents on SARS‐CoV‐2. J. Diabetes. 2021;13:243–252. doi: 10.1111/1753-0407.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belančić A., Kresović A., Troskot Dijan M. Glucagon‐like peptide‐1 receptor agonists in the era of COVID ‐19: Friend or foe? Clin. Obes. 2021;11:1–6. doi: 10.1111/cob.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drucker D.J. Coronavirus infections and type 2 diabetes—shared pathways with therapeutic implications. Endocr. Rev. 2020;41:1–13. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landstra C.P., de Koning E.J.P. COVID-19 and diabetes: understanding the interrelationship and risks for a severe course. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.649525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/BMJDRC-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Investig. 2020;43:867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COVID-19 Treatment Guidelines Panel. National Institutes of Health, Coronavirus Disease 2019 (COVID-19) Treatment Guidelines., 2022.
- 23.Recalde M., et al. Data resource profile: the information system for research in primary care (SIDIAP) Int. J. Epidemiol. 2022 doi: 10.1093/ije/dyac068. .http://www.sidiap.org/index.php/en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recalde M., Rodríguez C., Burn E., Far M., García D., Carrere-Molina J., Benítez M., Moleras A., Pistillo A., Bolíbar B., Aragón M., Duarte-Salles T. Data resource profile: the information system for research in primary care (SIDIAP. Int. J. Epidemiol. 2022:1–13. doi: 10.1093/ije/dyac068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO, ICD-10 Version: 2019 Int. Stat. Classif. Dis. Relat. Health Probl. 10th Revis. 2019 https://icd.who.int/browse10/2019/en [Google Scholar]
- 26.WHO Collaborating Centre for Drug Statistics Methodology, ATC/DDD Index 2022, (2022). https://www.whocc.no/atc_ddd_index/.
- 27.CatSalut. Servei Català de la Salut, Conjunt mínim bàsic de dades (CMBD), (2022). http://catsalut.gencat.cat/ca/proveidors-professionals/registres-catalegs/registres/cmbd/.
- 28.Catalan Agency for Health Quality and Evaluation (AQuAS), Updated SARS-CoV-2 data, (2022). https://aquas.gencat.cat/ca/actualitat/ultimes-dades-coronavirus/index.html#googtrans(ca%7Cen).
- 29.Departament de Salut Generalitat de Catalunya, COVID Data, (2022). https://dadescovid.cat/?lang=eng.
- 30.Orozco-Beltrán D., Merino-Torres J.F., Pérez A., Cebrián-Cuenca A.M., Párraga-Martínez I., Ávila-Lachica L., Rojo-Martínez G., Pomares-Gómez F.J., Álvarez-Guisasola F., Sánchez-Molla M., Gutiérrez F., Ortega F.J., Mata-Cases M., Carretero-Anibarro E., Vilaseca J.M., Quesada J.A. Diabetes does not increase the risk of hospitalization due to COVID-19 in patients aged 50 years or older in primary care—APHOSDIAB—COVID-19 multicenter study. J. Clin. Med. 2022;11 doi: 10.3390/jcm11082092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W., Sun X., Zhang J., Zhang K. The effect of metformin on mortality and severity in COVID-19 patients with diabetes mellitus. Diabetes Res. Clin. Pract. 2021;178 doi: 10.1016/j.diabres.2021.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Lv X., Lin S., Arshad M., Dai M. The association between antidiabetic agents and clinical outcomes of COVID-19 patients with diabetes: a bayesian network meta-analysis. Front. Endocrinol. 2022;0:926. doi: 10.3389/FENDO.2022.895458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen N.N., Ho D.S., Nguyen H.S., Ho D.K.N., Li H.Y., Lin C.Y., Chiu H.Y., Chen Y.C. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: a meta-analysis. Metab.: Clin. Exp. 2022;131 doi: 10.1016/J.METABOL.2022.155196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kan C., Zhang Y., Han F., Xu Q., Ye T., Hou N., Sun X. Mortality risk of antidiabetic agents for type 2 diabetes with COVID-19: a systematic review and meta-analysis. Front. Endocrinol. 2021;12:1158. doi: 10.3389/FENDO.2021.708494/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Cai Z., ZhangID J. DPP-4 inhibitors may improve the mortality of coronavirus disease 2019: a meta-analysis. PLoS One. 2021 doi: 10.1371/journal.pone.0251916. https://doi.org/10.1371/journal.pone.0251916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patoulias D., Doumas M. Dipeptidyl peptidase-4 inhibitors and COVID-19-related deaths among patients with type 2 diabetes mellitus: a meta-analysis of observational studies. Endocrinol. Metab. 2021;36:904–908. doi: 10.3803/ENM.2021.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., Liu C., Xiong M., Deng A., Zhang Y., Zheng L., Huang K. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/DC20-0660. [DOI] [PubMed] [Google Scholar]
- 38.Yu B., Li C., Sun Y., Wang D.W. Insulin treatment is associated with increased mortality in patients with COVID-19 and Type 2 diabetes. Cell Metab. 2021;33 doi: 10.1016/J.CMET.2020.11.014. 65-77.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mcgurnaghan S.J., Robertson C., Murray Mscph J., Mcmenamin Mbchb J., Phd S.-P., Frcp M., Blackbourn L.A.K., Mrcp C., Jeyam A., O’reilly J.E., Höhn A., Colombo M., Mellor J., Wild S.H., Mcgurnaghan S.J., Weir A., Bishop J., Kennedy S., Blackbourn L.A.K., Mcallister D.A., Hutchinson S., Caparrotta T.M., Mellor J., Jeyam A., O’reilly J.E., Wild S.H., Hatam S., Höhn A., Colombo M., Robertson C., Lone N., Murray J., Butterly E., Petrie J., Kennon B., Mccrimmon R., Lindsay R., Pearson E., Sattar N., Mcknight J., Philip S., Collier A., Mcmenamin J., Smith-Palmer A., Goldberg D., Mckeigue P.M., Colhoun H.M. Articles Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Www. Thelancet. Com. /Diabetes-Endocrinol. 2021;9:82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almalki Y.E., Qayyum A., Irfan M., Haider N., Glowacz A., Alshehri F.M., Alduraibi S.K., Alshamrani K., Alkhalik Basha M.A., Alduraibi A., Saeed M.K., Rahman S. A novel method for COVID-19 diagnosis using artificial intelligence in chest X-ray images. Healthc. (Basel) 2021;9:1–23. doi: 10.3390/healthcare9050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to patient privacy and data protection concerns, but they are available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article and its supplementary information files.