Abstract
Gastrointestinal cancers are responsible for more cancer deaths than any other system of the body. This review summarises how Wnt pathway dysregulation contributes to the development of the most common gastrointestinal cancers, with a particular focus on the nature and frequency of upstream pathway aberrations. Tumors with upstream aberrations maintain a dependency on the presence of functional Wnt ligand, and are predicted to be tractable to inhibitors of Porcupine, an enzyme that plays a key role in Wnt secretion. We summarise available pre-clinical efficacy data from Porcupine inhibitors in vitro and in vivo, as well as potential toxicities and the data from early phase clinical trials. We appraise the rationale for biomarker-defined targeted approaches, as well as outlining future opportunities for combination with other therapeutics.
Keywords: Porcupine inhibitor, Wnt, gastrointestinal cancer, RNF43, RSPO, clinical trials
Abbreviations: APC, adenomatous polyposis coli; CAF, cancer associated fibroblast; CMS, consensus molecular subtype; CRC, colorectal cancer; CSC, cancer stem cell; EMT, epithelial-mesenchymal transition; Dkk, Dickkopf; HCC, hepatocellular carcinoma; LGR, leucine-repeat G-coupled receptor; LRP, low-density lipoprotein receptor-related protein; MSI, microsatellite instability; PCP, planar cell polarity; ROR, receptor tyrosine kinase-like orphan receptors; RSPO, R-spondin; SFRP, Secreted frizzled-related protein; TP53, transformation-related protein 53
1. Introduction
Secreted Wnt proteins were originally discovered as key players in development and oncogenesis (Nusse & Varmus, 1982; Nüsslein-Volhard & Wieschaus, 1980; Sharma & Chopra, 1976) and subsequently found to be important in tissue homeostasis, with pivotal roles in stem cell-mediated regeneration of the intestinal epithelium and maintenance of bone integrity (Clevers & Nusse, 2012) Dysregulation of Wnt signaling has been implicated in various cancers, particularly those of the gastrointestinal tract (Jackstadt, Hodder, & Sansom, 2020; Sphyris, Hodder, & Sansom, 2021).
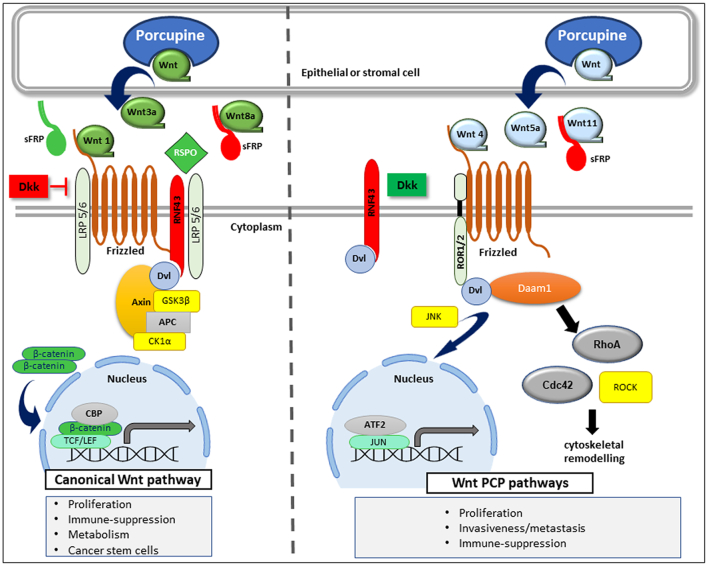
Extracellular Wnts can be divided into canonical or non-canonical according to the cellular pathways they activate (Fig. 1). Canonical Wnts bind Frizzled receptors which, in complex with low-density lipoprotein receptor-related protein (LRP) 5 or 6 and Dishevelled, release β-catenin from a destruction complex of Axin, adenomatous polyposis coli (APC), casein kinase 1α (CK1α) and glycogen synthase kinase 3β (GSK3β). This permits accumulation of β-catenin and its entry into the nucleus to initiate activation of target genes in combination with transcription factor/lymphoid enhancer binding factors (TCF/LEF) (Clevers & Nusse, 2012). The pathway is subject to several checks and balances, particularly in the extracellular space (Niehrs, 2012). The transmembrane E3 ligase RNF43 and its close relative, ZNRF3, negatively regulate canonical Wnt signaling through ubiquitylation of Frizzled receptors, which targets them to lysosomes for destruction (Koo et al., 2012). Soluble Dickkopf (Dkk) proteins compete with Wnt binding to LRP5/6 (Kagey & He, 2017) to block canonical signaling. Wnt activation can be fine-tuned by extracellular R-spondins (RSPOs), which bind to leucine-repeat G-coupled receptors (LGR/5/6) on the cell surface, bringing RSPO into contact with RNF43/ZNRF3. This interaction leads to membrane clearance of RNF43/ZNRF3 with a concomitant increase in membrane Frizzled receptors, thus enhancing Wnt signalling (De Lau, Peng, Gros, & Clevers, 2021). Secreted frizzled-related proteins (SFRPs) can sequester Wnts and prevent them binding Frizzled receptors, although they have also been reported to act as agonists, depending on concentration and/or context (Routledge & Scholpp, 2019; Xavier et al., 2014).
Fig. 1.
Canonical and Non-canonical Wnt Signaling in Cancer
Both canonical and non-canonical Wnt ligands require Porcupine activity for secretion. Canonical Wnts bind Frizzled receptors in complex with LRP5/6 to activate downstream signaling by phosphorylation and stabilization of β-catenin, which enters the nucleus to activate gene transcript via TCF/LEFs. Non-canonical Wnts also act via Frizzled receptors, activating ROR1/2 or recruiting Daam1 to activate the planar cell polarity (PCP) pathways, which can activate gene transcription or promote actin cytoskeleton rearrangements. Both pathways are subject to extracellular regulation, with R-spondins (RSPO) potentiating canonical signaling, and the ubiquitin-ligase RNF43 (or its close relative ZNRF3) and Dkk acting as negative regulators (depicted in red). sFRPs may act as positive or negative regulators of canonical signalling depending on context. sFRPs and RNF43 can also regulate non-canonical signaling, although Dkks have been shown to activate PCP pathways, in one of several examples of cross-talk between different Wnt pathways. LRP: low-density lipoprotein receptor-related protein, APC: adenenomatous polyposis coli, GSK3β: Glycogen synthase kinase 3β, CK1α: casein kinase 1α, Daam1: dishevelled associated activator of morphogenesis 1, Dvl: dishevelled; ROR1/2, receptor-tyrosine kinase-like orphan receptor 1/2, TCF/LEF: transcription factor/lymphoid enhancer binding factors, sFRP: secreted frizzled-related protein, Dkk; Dikkopf.
Pathways not involving β-catenin are termed non-canonical. One of the most well understood is the planar cell polarity (PCP) pathway, in which non-canonical Wnts such as Wnt5a activate Frizzled and co-receptors such as tyrosine kinase-like orphan receptors (RORs) to activate gene expression via various transcription factors through Jun N-terminal kinase (JNK) activation, or promote rearrangements of the cytoskeleton via dishevelled associated activator of morphogenesis 1 (Daam1) which activates small GTPases such as Cdc42 and RhoA (Mattes et al., 2018; Menck, Heinrichs, Baden, & Bleckmann, 2021). Non-canonical Wnts are also subject to direct negative regulation by SFRPs (Satoh, Matsuyama, Takemura, Aizawa, & Shimono, 2008) and RNF43 (Radaszkiewicz et al., 2021; Tsukiyama et al., 2015) but not Dkks, which exclusively and negatively regulate canonical signaling via LRP5/6. Indeed, Dkks have been suggested to activate non-canonical signaling in a non-direct manner, possibly by releasing Frizzled receptors from LRP5/6 complexes, thereby making them available for non-canonical Wnts (Niehrs, 2006). Dkk1 may also function via other receptors, such as cytoskeleton-associated protein 4, an interaction which may be of relevance in pancreatic and lung cancer (H. Kimura et al., 2016). Cross-talk between Wnt pathways is common (Topol et al., 2003), and it has been proposed that Wnt signaling should be viewed as a complex, integrated network, rather than a linear pathway (Amerongen & Nusse, 2009; Menck et al., 2021).
2. Dysregulation of Wnt Pathways in Gastrointestinal Cancers
Elevated β-catenin, indicative of canonical Wnt pathway activation, has been observed in many gastrointestinal tumors. Such activation may be driven by mutations in upstream pathway components such as RNF43 and RSPO fusions and tumors will remain dependent on Wnt ligand, or mutations in downstream Wnt pathway components such as APC or CTNNB1, the gene encoding β-catenin, where the pathway maybe activated independent of Wnt ligand. Deregulation of extracellular Wnt regulators such as Dkks and SFRPs via epigenetic mechanisms may also play a role (for reviews see e.g (Kleeman & Leedham, 2020; White, Chien, & Dawson, 2012)). Activation of the non-canonical pathway in cancers has also been associated with poor prognosis (Chen, Chen, Tang, & Xiao, 2021). Although proliferation and stem cell regeneration have been identified as potential cancer-promoting mechanisms, Wnt pathways also promote tumor evasion of host immunity (Luke, Bao, Sweis, Spranger, & Gajewski, 2019; Oderup, Lajevic, & Butcher, 2021; Valencia et al., 2011; Wang, Kalland, Ke, & Qu, 2018), as well as increasing migration and invasiveness via epithelial-mesenchymal transition (EMT) (Hlubek et al., 2007). These findings have led to a search for therapeutics targeting Wnt pathways. One of several emerging drug candidates is the membrane-bound protein-serine O-palmitoleoyltransferase Porcupine, encoded by the PORCN gene. Porcupine is located in the endoplasmic reticulum of stromal or epithelial cells and palmitoylates Wnt ligands, an enzymatic process essential for their secretion and binding to Frizzled receptors (Liu et al., 2013). Since Porcupine targets both canonical and non-canonical Wnts (Madan et al., 2016), inhibitors have the potential to hit multiple pro-tumorigenic mechanisms. However, they will only block signaling that remains dependent on secreted Wnts. In the next section, we summarise evidence for the involvement of Wnt pathway aberrations in five leading gastrointestinal cancers, with a focus on ligand-dependent mechanisms.
3. Wnt pathway dysregulation in colorectal cancer (CRC)
CRC is a Wnt-pathway driven disease, with 63-93% of patients having mutations in at least one component of the canonical Wnt pathway (Fig. 2) (Cancer Genome Atlas Network, 2012; Kleeman et al., 2020; Yaeger et al., 2018). The majority of mutations occur in downstream elements of the pathway. Most common are loss of function mutations in APC, which account for 60-80% of Wnt pathway aberrations in colorectal cancers (Kinzler & Vogelstein, 1996; Kleeman et al., 2020; Yaeger et al., 2018), and to a lesser extent, aberrations in other downstream components AXIN1, AXIN2, and CTNNB1. In APC-mutated cancer models, restoration of functional APC protein is effective in reversing tumorigenesis (Dow et al., 2015; Faux et al., 2004; Zilberberg, Lahav, & Rosin-Arbesfeld, 2010), but with current technologies, APC restoration is not a practical therapeutic option. In theory, cancers driven by such downstream activation mechanisms are unlikely to respond to Porcupine inhibition, as they do not depend on Wnt ligand, and are therefore termed ligand-independent. However, there are reports of APC-deficient and CTNNB1-mutant colorectal cell lines that remain dependent on Wnt ligands, and whose proliferation was reduced by Porcupine inhibition (J. Li, Zhang, Wang, & Zhang, 2019; Voloshanenko et al., 2013). Whether the dependency of APC- or CTNNB1-mutant CRC cells on Wnt extends beyond in vitro models requires further investigation.
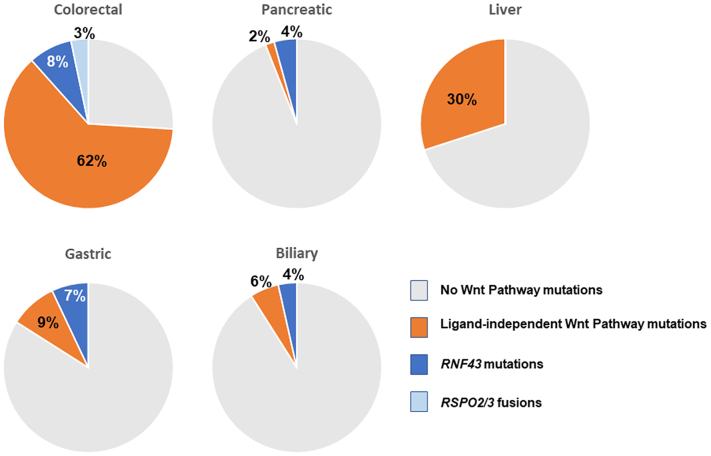
Fig. 2.
Contribution of Genetic Aberrations in Wnt Pathways in Gastrointestinal Cancers
Mutations or fusions in Wnt pathway genes are common in gastrointestinal cancers. Of these, the majority occur in downstream components such as CTNNB1 and APC, which are expected to be largely independent of extracellular Wnt ligands, although ligand-dependent aberrations such as RNF43 mutations or RSPO2/3 fusions are also observed. Mutation data from cBio Portal, (Cerami et al., 2012; Gao et al., 2013), using curated non-overlapping datasets, n = 1564 samples for CRC, n = 922 for pancreatic cancer, n = 1111 for liver cancer, n = 795 for gastric cancer, n = 588 for biliary cancer. CBio Portal accessed 27th Oct 2021. Since RSPO2/3 fusions are not captured in cBio, prevalence is per literature reports (Kleeman et al., 2020).
10-20% of CRC patients have pathway aberrations in which a requirement for Wnt ligand is maintained. The majority of these are in RNF43 or ZNRF3, with RNF43 mutations found in 9-18% of CRC patients, most of which associate with microsatellite instability and are mutually exclusive from genetic aberrations in APC but strongly associated with BRAF mutations (Giannakis et al., 2014; Yaeger et al., 2018). Many RNF43 mutations observed in CRC are truncations and hence loss of function, and therefore predicted to activate both canonical and non-canonical Wnt signaling. Certain truncation mutants have been reported to switch the protein from a tumor suppressor into an activator of downstream β-catenin signaling (Spit et al., 2020). Growth of tumorigenic intestinal organoids that lack functional RNF43 or ZNRF3 remain dependent on local Wnt ligand production (Koo, Van Es, Van Den Born, & Clevers, 2015), and their growth can be blocked by Porcupine inhibitors (Van De Wetering et al., 2015). However, there is debate concerning the most common RNF43 mutation, G659Vfs*41, a frameshift mutation which is almost exclusively found in microsatellite instability (MSI) patients. Initial reports suggested it was associated with cancer cell growth and/or survival, but it has also been reported to generate fully functional protein, although potentially one that is less stable than wild-type and associated with lower RNF43 expression (Giannakis et al., 2014; Li et al., 2020; Tu et al., 2019; Yu et al., 2020). Oncogenic effects of this mutation have been reported to be independent of Wnt signaling (L. Fang et al., 2021). It is therefore unclear whether tumors with this particular frameshift retain Wnt ligand dependency in patients or in fact have enhanced Wnt pathway signalling as a result of the mutation.
RSPO2 and RSPO3 fusions are mutually exclusive and are present in 2-10% of CRC, co-occurring with KRAS or BRAF mutations, but only very rarely with APC and mutually exclusive of CTNNB1 and RNF43 mutations (Giannakis et al., 2014; Seshagiri et al., 2012; Shinmura et al., 2014). The gene encoding RSPO3 fuses with the signal sequence and early section of the PTPRK gene leading to increased expression of RSPO3 and activation of the canonical Wnt pathway. Genetic fusions of RSPO2 have been observed with EIF3E and PIEZO1, and these were associated with increased RSPO2 expression (Hashimoto et al., 2019). Increased stromal RSPO3 expression has also been observed in the absence of gene fusions and this stromal overexpression of RSPO3 is strongly associated with the aggressive mesenchymal consensus molecular subtype 4 (CMS4) CRC phenotype (Kleeman et al., 2020). Targeting RSPO3 overexpression to the murine gut epithelium upregulates Wnt signaling and drives the formation of ectopic niche compartments and intestinal stem cells, leading to adenoma lesions (Hilkens et al., 2017). Inhibition of RSPO3 with function-blocking antibodies in PTPRK-RSPO3-fusion tumor xenografts inhibited growth and promoted epithelial differentiation (Storm et al., 2016).
Excessive Wnt signaling can also arise by non-mutational mechanisms. For example, epigenetic silencing of negative Wnt regulators has been observed during CRC cancer progression and was associated with increased nuclear β-catenin (Caldwell et al., 2004, Silva et al., 2014, Suzuki et al., 2004, Zhang et al., 2008). Epigenetic suppression of Wnt feedback loops, including reduced Axin2 expression, has been observed in CRC and low Axin2 expression has been postulated to be a discriminatory biomarker for ligand-dependent disease (Kleeman et al., 2020). However, the expression of some negative Wnt regulators (Notum, Wif1, Dkk3) is very low/absent in normal murine gut tissue (Flanagan et al., 2021) which may suggest epigenetic silencing takes place prior to tumour formation, possibly confounding the premise of further epigenetic silencing in cancer tissue.
Non-canonical pathways are also dysregulated in colorectal cancer, with high expression of Wnt11a or ROR1 associated with poor prognosis (Nishioka et al., 2013; Zhou et al., 2017). Non-canonical Wnt signaling has been linked to proliferation, invasion, survival and metastasis of cancer stems cells (Katoh, 2017; Nishioka et al., 2013), although it should be noted that the role of non-canonical Wnts is context dependent. In certain situations, non-canonical Wnt5a has shown to be tumor suppressive and promote differentiation, most likely via cross-inhibition of canonical Wnt signaling (Zhou, Kipps, & Zhang, 2017).
4. Wnt pathway dysregulation in pancreatic cancer
RNF43 mutations are the most common Wnt pathway mutations in pancreatic cancer, observed in 5-10% of cases (Fig. 2) (Bailey et al., 2016; Tu et al., 2019; Witkiewicz et al., 2015). Genetic lesions in APC or AXIN1/2 are less common, observed in 1-5% of cases. As expected, RNF43-mutant pancreatic cancer xenografts remained dependent on Wnt ligand. However, a significant proportion of organoids derived from PDAC patients with wild-type RNF43 also remained dependent on Wnt ligands, suggesting opportunities for Porcupine inhibitors and other ligand-dependent therapeutics exist beyond RNF43 mutations in pancreatic cancers (Seino et al., 2018).
At the expression level, upregulation of β-catenin, Wnts and Frizzled receptors was seen in a gene-array analysis of pancreatic ductal adenocarcinomas (Pasca Di Magliano et al., 2007) and elevated nuclear β-catenin is associated with high grade neoplasms and poor prognosis (Al-Aynati, Radulovich, Riddell, & Tsao, 2004; Sano et al., 2016). High epithelial expression of Wnts in pancreatic tumors was also associated with poor prognosis (Seino et al., 2018). Ectopic expression of Wnt1 or β-catenin accelerated tumor growth and decreased survival in a murine pancreatic cancer model (Sano et al., 2016).
Wnt5a is elevated in pancreatic cancer tissue versus surrounding non-cancerous tissues and promotes invasion and proliferation whilst inhibiting apoptosis. Although typically considered a non-canonical Wnt, these effects appear to be via β-catenin signaling (Bo, Gao, Chen, Zhang, & Zhu, 2016; Griesmann et al., 2013; Ripka et al., 2007). The non-canonical pathway receptor ROR1 is overexpressed in circulating tumor cells from pancreatic cancer patients and knockdown of ROR1 reduces invasiveness (Xu, Shen, Xu, Wang, & Ni, 2018). Consistent with non-canonical signaling playing a role in pancreatic cancer, ROR2 expression in tumor epithelium and stroma is associated with poor prognosis in this indication (J. Huang et al., 2015).
5. Wnt pathway dysregulation in gastric cancer
Gastric cancers have one of the highest incidence of Wnt pathway mutations among cancers of the digestive tract (Flanagan, Vincan, & Phesse, 2017). CTNNB1 mutations occur in up to 26% of gastric cancers and are associated with nuclear localization of β-catenin (Clements et al., 2002). Several CTNNB1 single-nucleotide polymorphisms are associated with gastric cancer susceptibility and prognosis (Wang et al., 2012). APC and AXIN1/2 mutations also occur in 5-10% of patients, although prevalence of APC mutations has been reported to be as high as 25% in some studies (Z. Fang et al., 2012; Horii et al., 1992; Pan, Liu, Zhang, You, & Lu, 2008). A significant proportion of gastric cancers contain ligand-dependent aberrations. RNF43 mutations occur in 10% of patients, rising to 54% of MSI tumors, a high proportion of which are the aforementioned G659Vfs*41 frameshift truncations (Wang et al., 2014). RSPO2 fusions have also been reported in gastric tumor xenografts (Li et al., 2018), although prevalence data is not available. Interestingly, patient-derived gastric tumor organoids mimic the variety of Wnt-pathway mutations observed in the clinic and reveal novel mutational and methylation patterns (CpG island methylation phenotype (CIMP+); DNA methylation) to achieve Wnt and/or RSPO independence. Importantly, organoids independent of RSPO (typically RNF43 loss of function mutants) remain sensitive to Wnt inhibition (Nanki et al., 2018).
Beyond mutations, gene expression analysis found 46% of gastric cancers had upregulation of canonical and non-canonical Wnts, or their downstream pathway components (Ooi, Ivanova, Wu, Lee, & Tan, 2009). Conversely, multiple SFRPs and Dkks are downregulated by promoter hypermethylation (Chiurillo, 2015). Of note, Frizzled7 and Wnt5a are highly expressed in gastric cancer and both are associated with poor outcomes (Li et al., 2018; Maeda et al., 2020). Frizzled7 was identified as the predominant receptor responsible for transmitting Wnt signaling in human gastric cancer cells and genetic deletion of Frizzled7 prevented growth of gastric adenomas with or without APC mutations (Flanagan et al., 2019). Inhibiting Wnt5a blocked fibroblast-induced gastric cancer cell proliferation and migration in vitro, and metastasis of gastric cancers in vivo (Hanaki et al., 2012; Kurayoshi et al., 2006; Maeda et al., 2020). Together, these observations suggest that, even in the presence of downstream Wnt mutations, upstream inhibitors including those targeting Porcupine could have utility in gastric cancer.
6. Wnt pathway dysregulation in biliary cancer (Cholangiocarcinoma)
Only a small proportion of bile duct cancers carry genetic mutations in Wnt pathway components. RNF43 mutations were observed in 1-2% of patients (Weinberg et al., 2019), although higher rates (4-9% have been seen in Asian cohorts, particular those associated with liver fluke infection (Chan-On et al., 2013; Ong et al., 2012). However, high expression of stromal Wnt7b and Wnt10a, and upregulation of several Frizzled receptors has been observed (Boulter et al., 2015), with greater than 75% of patient samples in another study showing positive expression of Wnt3a, Wnt5a and Wnt7b (Loilome et al., 2014). In addition Boulter and colleagues demonstrated efficacy in a biliary tract preclinical model with C-59, a Porcupine inhibitor, demonstrating that Wnt ligand is required for proliferation of these tumours (Boulter et al., 2015). Hypermethylation induced silencing of negative pathway regulators such as SFRP2 and DKK2 has also been observed (Goeppert et al., 2014). These data are suggestive of highly activated extracellular Wnt signaling in biliary cancers, which may therefore be suitable targets for inhibitors of Porcupine.
7. Wnt pathway dysregulation in hepatocellular carcinoma (HCC)
CTNNB1 and AXIN1/2 mutations occur in a significant proportion of HCC patients (Miyoshi et al., 1998; Schulze et al., 2015; Taniguchi et al., 2002), and mice with liver-targeted disruption of APC or overexpression of an oncogenic form of β-catenin develop hepatic tumors indicating a role for downstream canonical Wnt pathway activation (Colnot et al., 2004; de La Coste et al., 1998). Another study showed that partial ablation of APC also induced liver tumorigenesis in mice (Buchert, Athineos, Abud, Burke, & Faux, 2010).
In contrast, ZNRF3 mutations were observed in only 3% of HCC (Schulze et al., 2015) and RSPO fusions are rare, although elevated RSPO2 was seen in a subset of HCC patients. Co-amplification of RSPO2 and MYC has been observed in HCC, although the functional consequence of this is unclear (Sanchez-Vega et al., 2018). Elevated Wnt3/4/5a and suppression of SFRPs have also been observed, an expression pattern that did not correlate with CTNNB1 mutations but was associated with loss of differentiation and cirrhosis, and activation of PCP pathways (Bengochea et al., 2008). Functional overexpression of RSPO2 with combined loss of transformation-related protein 53 (TP53) led to development of liver cancer in mice models (Conboy et al., 2019), implicating a role for extracellular Wnt ligands in liver tumorigenesis.
8. Pre-clinical evidence supporting use of porcupine inhibitors in gastrointestinal cancers
The pivotal role of Wnt signaling in gastrointestinal cancers has led to a search for therapeutics that target this pathway. A major challenge has been finding tractable targets, given the non-enzymatic nature of key intracellular components such as APC, AXIN and β-catenin. The majority of pathway inhibitors explored to date are antibody or biologicals-based approaches such as Frizzled decoy receptors and Frizzled blocking antibodies. Clinical studies of these therapeutics have been limited, hampered by toxicity issues (Table 1). A handful of small molecules have also been developed, such as CWP232291, which degrades β-catenin via an unknown mechanism (Pak et al., 2019), and PRI-724, which has been reported to block β-catenin/CBP interactions. (Table 1). However both compounds require intravenous dosing, and reported effects in the clinic to date are minimal, with no direct evidence of target engagement (El-Khoueiry et al., 2013; K. Kimura et al., 2017; Ko et al., 2016; Lee et al., 2020). Tankyrase inhibitors, which can antagonize Wnt signaling via Axin stabilisation, have failed to advance into the clinic due to lack of a therapeutic window versus gastrointestinal toxicity, as well as non-Wnt related effects (S.-M. A. Huang et al., 2009; Y. Zhong et al., 2016). Antibodies directed against Dkk-1, such as DKN-01 and BHQ880 are also being tested. Although Dkk-1 functions as a naturally occurring negative regulator of Wnt signaling, Dkk-1 expression is often associated with poor prognosis in gastrointestinal cancers, and Dkk-1 blocking antibodies can inhibit tumor growth in pre-clinical animal models (Kagey & He, 2017). Such effects may arise from the complexity of Wnt signaling; for example, Dkk-1 has been linked to promotion of metastasis via activation of the non-canonical PCP-pathway, and also to immune suppression. It is speculated that the role of Dkk1 may be altered in different contexts, including when the canonical Wnt pathway is constitutively activated (Kagey & He, 2017).
Table 1.
Wnt Pathway Inhibitors in Cancer Clinical Trials
| Drug Identifier | Mode of Action | Trial Identifier | Regimen | Cancer Indication | Trial Phase | Study Status or Clinical Results |
|---|---|---|---|---|---|---|
| OMP-54F28 (Ipafricept) | Fzd8-Fc Decoy receptor | NCT01608867 | Monotherapy | Solid tumors | 1 | Dysgeusia and fragility fractures observed. Best response was SD (Jimeno et al., 2017) |
| NCT02092363 | In combination with paclitaxel and carboplatin | ovarian cancer; | 1 | ORR 76%, comparable with historical data. Development in ovarian stopped due to bone toxicity (Moore et al., 2019) | ||
| NCT02069145 | In combination with sorafenib (HCC) | Hepatocellular carcinoma | 1 | Study completed, no results reported | ||
| NCT02050178 | In combination with Gemcitabine and Nab-paclitaxel | 1L pancreatic ductal adenocarcinoma | 1 | 35% PR, 46% SD. Study terminated due to bone toxicity (Dotan et al., 2020) | ||
| OMP18R5 (Vantictumab) | Anti-Fzd7 antibody, also neutralizes binding to Wnts to Fzd 1, 2, 5,8 | NCT01345201 | Monotherapy | Solid tumors | 1 | Study completed, no results reported |
| NCT01957007 | In combination with docetaxel | Previously treated Non-small cell lung cancer; | 1 | Study completed, no results reported | ||
| NCT02005315 | In combination with Nab-Paclitaxel and Gemcitabine | 1L pancreatic ductal adenocarcinoma | 1 | ORR 42%. Study terminated due to bone toxicity (Davis et al., 2020) | ||
| NCT01973309 | In combination with paclitaxel | Locally recurrent/ metastatic breast cancer | 1 | ORR 31%. Wnt pathway gene signature associated with longer PFS/OS. Bone fractures observed (Diamond et al., 2020) | ||
| NCT04176016 | monotherapy | Synovial sarcoma | Recruiting | |||
| OTSA101 | Yttrium90 radiolabelled Anti-Fzd10 antibody | NCT01469975 | monotherapy | Synovial sarcoma | 1 | Most common adverse events were hematologic disorders. SD in 3/8 patients (Giraudet et al., 2018) |
| NCT04176016 | monotherapy | Synovial sarcoma | 1 | Recruiting | ||
| OMP131R10 | Anti-R-spondin3 antibody | NCT02482441 | With or without FOLFIRI | RSPO3 biomarker-positive metastatic colorectal cancer | 1 | Best response was SD in 3/7 patients treated with chemo combination (Bendell et al., 2016) |
| Foxy-5 | Wnt5a mimetic | NCT02655952 | monotherapy | Breast cancer; colorectal cancer; prostate cancer | 1 | Completed. No efficacy data reported |
| NCT02020291 | monotherapy | Breast cancer; colorectal cancer; prostate cancer | 1 | Completed. No efficacy data reported | ||
| NCT03883802 | As Neoadjuvant with surgery+FOLFOX | Wnt5a low colon cancer | 2 | Recruiting | ||
| PRI-724 | Inhibitor of TCF-CBP interaction | NCT01302405 | Monotherapy | Advanced solid cancers | 1 | No responses reported (El-Khoueiry et al., 2013) |
| NCT01606579 | Monotherapy or in combination with dasatinib, or cytarabine | Acute and chronic myelogenous leukaemia | 1/2 | Completed. No results reported | ||
| NCT01764477 | In combination with Gemcitabine | Advanced/metastatic pancreatic adenocarcinoma |
1 | Rest response was SD in 8/20 patients (Ko et al., 2016) | ||
| CWP232291 | Induces degradation of beta catenin. Molecular target not defined | NCT01398462 | Monotherapy | Acute Myeloid Leukaemia | 1 | ORR 3% GI side-effects were most common (Lee et al., 2020) |
| NCT03055286 | In combination with cytabarine | Acute Myeloid Leukaemia | 1/2 | Active, not recruiting | ||
| NCT02426723 | Monotherapy or in combination With Lenalidomide and Dexamethasone | Relapsed/refractory multiple myeloma | 1 | Completed. 4/14 patients with response, 4 had stable disease (Manasanch et al., 2017) | ||
| SM04755 | Small molecule Wnt inhibitor | NCT02191761 | Monotherapy | Advanced CRC, gastric, HCC and pancreatic cancer | 1 | Completed. No results reported |
| Cirmtuzumab | Anti-ROR1 antibody | NCT05156905 | In combination with docetaxel | Metastatic castration resistant prostate cancer | 2 | Not yet recruiting |
Source: Clinicaltrials.gov. Data collected on 10th January 2022. Withdrawn trial or trials terminated early are not included. Dkk blocking antibodies such as DKN01 and BHQ880 were not included due to observed effects on other (non Wnt) pathways.
More recently, several small molecule drug candidates with attractive pharmacokinetic properties have been developed that target Porcupine (Table 2; (Bhamra et al., 2017; B. Chen et al., 2009; J. Jiang et al., 2018; Liu et al., 2013; Madan, Ke, et al., 2016; Proffitt et al., 2013)). Pre-clinical evidence suggests that Porcupine inhibitors exert anti-tumor effects via multiple mechanisms, and data from gastrointestinal models is summarised below.
Table 2.
Porcupine Inhibitors in Cancer Clinical Trials
| Drug Identifier | Trial identifier | Regimen | Cancer Indication | Clinical Phase |
|---|---|---|---|---|
| WNT974 (previously LGK974) | NCT02278133 | In combination with LGX818 and Cetuximab | Metastatic colorectal cancer with BRAFV600 mutations and RSPO fusions or RNF43 mutations | Phase I/II |
| NCT01351103 | Monotherapy OR Combination with PDR001 (Spartalizumab) |
Advanced solid tumors with Wnt pathway activation (monotherapy) Various advanced malignancies naïve or refractory to PD-L1 inhibitors (combination with PDR001) |
Phase I | |
| ETC-159 | NCT02521844 | Monotherapy OR Combination with Pembrolizumab or Denosumab |
Advanced solid tumors | Phase I |
| CGX1321 | NCT02675946 | Monotherapy OR Combination with Pembrolizumab |
Advanced solid tumors (monotherapy) Advanced colorectal cancer (combination) |
Phase I |
| NCT03507998 | Monotherapy | Advanced GI tumors | Phase I | |
| RXC004 | NCT03447470 | Monotherapy and in combination with Nivolumab | Advanced solid tumors | Phase I |
| NCT04907851 | Monotherapy | Advanced solid tumors | Phase II | |
| NCT04907539 | Monotherapy and in combination with Nivolumab | RNF43/RSPO mutant microsatellite stable metastatic colorectal cancer | Phase II | |
| XNW7201 | NCT03901950 | Monotherapy | Advanced solid tumors | Phase I |
Source: Clinicaltrials.gov. Data collected on 10th Jan 2022. Withdrawn trials not included
9. Effects of porcupine inhibitors on tumor growth and proliferation
Porcupine inhibitors have proven effective in inhibiting proliferation of cell lines and organoids derived from gastric, colorectal, pancreatic and biliary cancers. They also show potent inhibition of tumor xenograft growth in mice (Boulter et al., 2015; Li, Cao, et al., 2018; Madan, Ke, et al., 2016; Mo et al., 2013). Effects are limited to models derived from Wnt-dependent tumors, in particular those with RSPO2/3 fusions or RNF43 mutations, consistent with the role of Porcupine in Wnt secretion (X. Jiang et al., 2013; Koo et al., 2015; Woodcock et al., 2019). Inhibition of tumor proliferation was accompanied by downregulation of Wnt targets such as Axin2 and c-Myc, and loss of cell cycle and stem cell markers (Madan et al., 2016). In addition, Porcupine inhibitors reduce glucose uptake by cancer cells in vitro and in vivo (Phillips et al., 2021 and Phillips et al, manuscript submitted for publication) and several studies demonstrate elevated caspase 3/7 expression and induction of apoptosis (Bagheri, Tabatabae Far, Mirzaei, & Ghasemi, 2020; Boulter et al., 2015; Mo et al., 2013), all of which may contribute to inhibition of tumor growth. The multi-modal effects of Porcupine inhibitors may explain why established RSPO2/3-fusion tumours are rapidly cleared following treatment with the porcupine inhibitor LGK974, even though treatment had no effect on the normal growth of intestinal crypts (Han et al., 2017). Effects of Porcupine inhibitors are long-lasting, with no tumor regrowth observed within 6 weeks of treatment withdrawal (Madan, Ke, et al., 2016) and significantly reduced growth was seen when Porcupine-inhibitor treated tumors were reimplanted into naïve, untreated mice (Phillips et al., 2021 and Phillips et al, manuscript submitted for publication).
Most studies of Porcupine inhibitors have focused on tumor models with ligand-dependent Wnt pathway activation, under the assumption that downstream pathway activation such as that seen with mutations in APC and β-catenin would be unaffected by extracellular Wnt levels. Indeed, it has been reported that the Porcupine inhibitor LGK974 accelerates tumorigenesis in APC-deficient mice models by selectively blocking wild-type stem-cell proliferation, and thus favouring growth of APC-mutant cells (Huels et al., 2018). However, it has also been reported that Porcupine inhibitors could inhibit growth of a colon cancer cell line with mutations in CTNNB1 (Voloshanenko et al., 2013). Other colon cancer cells lines, including some with APC mutations also appeared dependent on Wnt/Fzd signaling for growth (Vincan et al., 2007). Small molecule Porcupine inhibition in these cell lines resulted in decreased pathway activation, anti-proliferative effects and reduced cell viability. Taken together these data suggest patients with cancers bearing a subset of downstream mutations in the Wnt pathway, traditionally expected to be insensitive to Porcupine inhibitors, may well in fact receive therapeutic benefit from such treatment. However, further research is needed to determine which APC/CTNNB1 mutation subtypes retain Wnt dependence, and the mechanism(s) involved.
10. Effect of porcupine inhibitors on cancer stem cells
Tumor recurrence following chemotherapy occurs through clonal replacement and reactivation of dormant cancer stem cells (CSCs) (Jahanban-Esfahlan et al., 2019). Canonical Wnt signaling prevents differentiation of CSC and promotes their retention to fuel tumor expansion by direct effects on CSCs, but also via CSC-stromal/immune interactions (Regan et al., 2017; Vermeulen et al., 2010). These findings go some way to reconcile the paradoxical observation that despite all cells within a tumor harbour Wnt-activating mutation, only a subfraction of cancer cells display CSC properties. Non-canonical Wnt signaling also promotes survival and resistance to therapy of CSCs through PI3K-AKT signaling activation (reviewed in Katoh, 2017). The Porcupine inhibitor LGK974 inhibited the ability of breast cancer stem cells to proliferate and form mammospheres (Zhao et al., 2014), and reduced the cancer stem cell compartment of established cutaneous squamous cell carcinomas in mice (Zimmerli et al., 2018). Mutation of RNF43 or ZNRF3 in mice intestines led to adenoma growth from LGR5-expressing stem cells, which was dependent on Wnt3 secreted by Paneth cells, and could be blocked by treatment with a Porcupine inhibitor (Koo et al., 2015). Acute treatment of mice with a high dose of a Porcupine inhibitor (i.e. higher doses than those which have shown anti-tumor effects) induced an initial burst of proliferation of intestinal stem cells as they converted into transit-amplifying cells, with a loss of stem cell self-renewal, supporting the critical function of Wnt signaling to maintain stemness and prevent differentiation in the gut (Kabiri et al., 2018). Consistent with this, Porcupine inhibitors have shown a striking ability to promote epithelial differentiation within the tumor, most notably by upregulation of mucins (X. Jiang et al., 2013; Madan, Ke, et al., 2016).
11. Effect of porcupine inhibitors on invasion and metastasis
Epithelial-mesenchymal transition (EMT) has been linked to invasion and metastasis in intestinal cancers. Reactivation of key developmental pathways, including the Wnt pathway, leads to loss of epithelial architecture and disruption of the basement membrane, which enables cancer cells to invade healthy tissues and migrate into the lymphatic system or bloodstream, leading to metastasis. Canonical and non-canonical PCP Wnt pathways have been implicated in CRC cell migration (Al-Aynati et al., 2004; Katoh, 2017; Y. Zhang et al., 2016; Z. Zhang, Wang, Zhang, Zhong, & Yang, 2018). The Porcupine inhibitor IWP-2 was shown to reduce migration and invasion of a gastric cancer cell line (Mo et al., 2013), and other Porcupine inhibitors reduced invasion of renal cancer cells in vivo (Li et al., 2020 and prolonged metastasis-free survival in an in vivo model of Ewing sarcoma (Hayashi et al., 2017). Effects of Porcupine inhibitors on migration in vivo may be due to direct effects on cancer cells and/or cell-nonautonomous effects related to interactions with neighboring stromal tissues.
12. Effects of porcupine inhibitors on fibrosis
Another corollary of EMT is the induction of fibrosis. Wnt signaling has long been implicated in fibrosis (Akhmetshina et al., 2012) and stromal signaling plays a key role in cancers, with growing evidence that cancer-associated fibroblasts (CAFs) play a role in immunosuppression as well as remodelling (Hilmi, Nicolle, Bousquet, & Neuzillet, 2020). Wnt5a functions as a supportive niche factor in gastric cancer, where it is upregulated by the tumor microenvironment (Hayakawa et al., 2015; Maeda et al., 2020). Wnt5a is also associated with the strong desmoplastic reaction observed in pancreatic cancer (Pilarsky et al., 2008). In colorectal cancers, upregulation of endogenous RSPO2/3 was strongly associated with a defined CAF signature and with the CMS4 (mesenchymal) consensus molecular subtype (Kleeman et al., 2020). Although data on fibrosis is lacking from cancer models, Porcupine inhibitors have demonstrated profound anti-fibrotic effects in models of skin, lung and kidney fibrosis (Bunyard et al., 2019; C.-W. Chen et al., 2017; Madan, Patel, et al., 2016).
13. Reversal of immune evasion by porcupine inhibition
Immune evasion plays a key role in tumor development. The presence of Wnt ligands in the microenvironment in gastrointestinal tumors positions them perfectly to impact the local tumor immune response. There is strong association between upregulation of Wnt signaling and downregulation of immune markers in cancers, including those of the gastrointestinal tract (Luke et al., 2019; Spranger, Bao, & Gajewski, 2015). Tumors may be classed as “hot” (i.e. inflamed), or “cold”, the latter state characterized by a tumor microenvironment lacking infiltrating lymphocytes and with high levels of suppressive regulatory T-cells (Ochoa de Olza, Navarro Rodrigo, Zimmermann, & Coukos, 2020). Cold tumors do not respond to immunotherapy agents. Of note, several canonical and non-canonical Wnt ligands and Frizzled receptors were upregulated in melanoma patients that failed to respond to anti-PD-1 therapy. The Porcupine inhibitor C59 reversed the ability of melanoma cancer cell-conditioned media to induce generation of dendritic cell-mediated regulatory-T cells in vitro (DeVito et al., 2021). In the immune cold B16F10 (C57BL/6 mice) melanoma tumor model, anti-PD-1 therapy was not effective, but treatment with the Porcupine inhibitor RXC004 was able to reduce tumor size both as a monotherapy and in combination with an anti-PD-1 inhibitor. Flow cytometry showed that the RXC004 treatment reduced the myeloid derived suppressor cells in the tumor microenvironment (Phillips et al., 2019, Phillips et al, manuscript submitted for publication). A combination of the Porcupine inhibitor C59 with anti-CTLA4 antibodies was also shown to have synergistic anti-tumor effects in the same model (Holtzhausen et al., 2015). These data suggest that Porcupine inhibitors may be effective in combination with immunotherapy in patients that currently do not respond to such therapies.
Further evidence that Porcupine inhibitors can reverse Wnt pathway-induced immune evasion comes from the immune hot CT26/BALBc colorectal syngeneic model, where RXC004 in combination with anti-PD-1 antibody resulted in a significant increase in the cytotoxic to regulatory T-cell ratio within the tumor. This was not seen in either monotherapy treatment arm (Phillips et al., 2019, Phillips et al, manuscript submitted for publication). The ability to reverse Wnt-ligand induced immune evasion in the wider tumor microenvironment may provide an explanation for the observed anti-tumor effects of Porcupine inhibitors even in cases where Wnt pathway is activated by downstream mutations within the tumor cell.
14. Potential toxicities of porcupine inhibitors identified in vivo
Wnt ligands play a vital homeostatic role in stem cell renewal, which means targeting the pathway has the potential for adverse effects, particularly within the intestinal epithelium. Ectopic expression of the negative Wnt regulator Dkk1 in mice caused loss of intestinal architecture (Kuhnert et al., 2004; Pinto, Gregorieff, Begthel, & Clevers, 2003), and high doses of Porcupine inhibitors have adverse effects on stem cell renewal with associated disruption of villi architecture (Kabiri et al., 2018). However, anti-tumor effects of Porcupine inhibitors have been observed at doses that have no effect on the normal intestine (Koo et al., 2015; J. Liu et al., 2013; Phillips et al, manuscript submitted for publication), suggesting there may be a safe therapeutic window for these compounds. The effects of Dkk1 overexpression on intestinal architecture were reversible, consistent with other studies that indicate the intestine has the ability to regenerate after insult (Kuhnert et al., 2004). A possible explanation for this may be redundancy in the stem cell populations, with HOPX-expressing stem cells, typically located higher in the intestinal crypt, able to compensate for loss of LGR5 expressing stem cells at the base of crypts, and vice-versa (Takeda et al., 2011), although such plasticity may be a relatively short-lived response to injury. Stem cells located at the intestinal crypt base are exposed to the highest levels of Wnt ligand provided by the niche (Paneth cells and stromal cells), and therefore may be more protected from drug-induced reduction of Wnt secretion (Kosinski et al., 2007). Consistent with this, mice with genetic ablation of PORCN in intestinal epithelial cells retained normal intestinal homeostasis due to compensatory Wnt secretion from neighboring stromal cells (Kabiri et al., 2014). Furthermore, pericryptal stromal cells have high expression of drug transporters, protecting the stem cell niche from xenobiotics, including Porcupine inhibitors (Chee et al., 2018).
Other in vivo toxicities observed with Porcupine inhibitors include loss of bone volume and density, most likely due to effects on osteoclasts. However, these effects could be mitigated by prophylactic treatment with bisphosphonates (Madan et al., 2018).
15. Porcupine inhibitors in the clinic
To date, five Porcupine inhibitors have entered phase I/II clinical trials (Table 2) in patients with advanced solid tumors. In NCT02521844, a maximum tolerated dose of 30 mg for ETC-159 (previously known as ETC-1922159) was established, limited by high bone marker turnover and compression fractures. Bone effects at lower concentrations were mitigated by prophylactic treatment with denosumab. Dysgeusia (loss or change in taste) was common, even at lower doses. Target engagement was demonstrated by inhibition of Axin2 expression in hair follicles and increased infiltration of T-cells into the tumor microenvironment. There were no objective responses, although 17% (2/12) patients had stable disease (Tan et al., 2018).
The WNT974 (previously LGK974) monotherapy dose-finding study did not find a maximum tolerated dose. Dysgeusia was the most common side effect, although bone effects were also observed in a subset of patients. Target engagement was demonstrated by reduction of Axin2 expression in skin. There were no objective responses;16% of 98 patients had stable disease (Rodon et al., 2021). In this study, 28 patients were genetically selected for Wnt pathway activation as part of a dose expansion arm, the majority of which had RNF43 mutations. Of these 28 patients, 10 patients had stable disease. This result has led to others concluding that monotherapy Porcupine inhibitor treatment failed even with this enrichment for upstream Wnt pathway aberrations. However, since this dose expansion arm was run in 2015, knowledge of exactly which RNF43 mutations result in loss of function has increased (Spit et al., 2020; Tu et al., 2019; Yu et al., 2020). With our current improved understanding, and excluding patients with co-occuring downstream Wnt pathway mutations, only 8 of these patients would now be considered to have RNF43 loss of function mutations. Of these 8 patients 7 had a best tumor response between -27 and +20%, suggesting a stringent patient selection strategy is likely needed in the clinic for Porcupine inhibitors (Rodon et al., 2021). When WNT974 was paired with the anti-PD-1 antibody spartalizumab, dose-limiting toxicities were reported in 2 patients, including one spinal compression fracture. One patient (4%) with triple-negative breast cancer had a partial response, 11 patients (41%) had stable disease, 13 patients (48%) had progressive disease. 75% of patients experienced a treatment-related adverse events, with dysgeusia and osteopenia among the most common (Janku et al., 2020).
Preliminary data from NCT03447470, a dose finding study for RXC004 in solid tumors, has recently been reported. The most common treatment related adverse events were fatigue, nausea, dysgeusia, vomiting and anorexia. No grade 4/5 adverse events or bone fragility events were reported, with patients receiving prophylactic denosumab. Five patients, all with Wnt pathway activated tumors, had stable disease, including one patient with biliary tract cancer, and two CRC patients with either an RNF43 mutation or a RSPO fusion (Cook et al., 2021).
These early clinical studies have identified bone loss and dysgeusia as class effects of Porcupine inhibitors. The latter likely arises because Wnt signaling has been shown to be vital for proliferation of taste-progenitors and formation of taste buds (Gaillard et al., 2017; Prochazkova et al., 2017). Prophylactic treatment with bisphosphonates or denosumab has been shown to mitigate increases in bone turnover markers (Cook et al., 2021; Tan et al., 2018). Encouragingly, gastrointestinal side effects appear to be limited and manageable. Furthermore, the limited efficacy observed to date may be attributable to the studies being performed in heavily-pretreated patients with poor prognosis and lack of appropriate patient selection.
16. Conclusions and future perspectives
Porcupine inhibitors have the potential for multiple potentially beneficial effects on tumor biology (Fig. 3). There is substantial preclinical data supporting the use of Porcupine inhibitors in genetically-defined populations of patients with gastrointestinal cancers. Functionally relevant mutations are most common in CRC and pancreatic cancers. Patients with RNF43 or ZNRF3 truncations may be amenable to treatment and mutations in RNF43 are particularly common in MSI high CRC and pancreatic cancers, although it should be noted that the majority of RNF43 mutations found in MSI disease are the hotspot G659Vfs*41 frameshift, which has been reported to encode functional protein and may be simply a passenger mutation in this context (Tu et al., 2019). Tumors with RSPO2/3 fusion and RSPO3 stromal overexpression are also likely to be susceptible to Porcupine inhibition, an intriguing prospect for CMS4 CRC subtype patients, where RSPO3 overexpression is common. There is also data to suggest Porcupine inhibitors may be effective in patients where Wnt signaling is activated by non-genetic/epigenetic mechanisms, and in certain patients with downstream Wnt pathway aberrations. However further work is needed to understand the processes involved, and to develop biomarkers to robustly identify such patients.
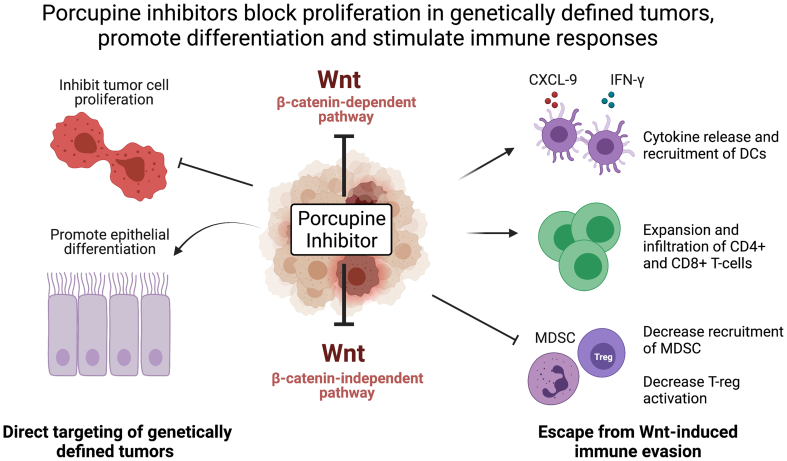
Fig. 3.
Porcupine Inhibitors Have Multiple Effects on Tumor Biology
DC: Dendritic cells, MDSC: myeloid-derived suppressor cells. T-reg: regulatory T-cells. Image created with BioRender.com.
The effects of Porcupine inhibitors in reversing immune evasion provide a strong rationale for their use in combination with checkpoint inhibitors, particularly in patients previously unresponsive to anti-PD-1/PD-L1 therapy. MSI high CRC patients are currently indicated for anti-PD-1 therapy and exploration of anti-PD-(L)1/Porcupine inhibitor combinations in these patients may also be warranted. Similarly, Porcupine and BRAF inhibitors may be an attractive combination in CRC patients with both BRAF and RNF43 mutations, particularly in light of the recent data from the BEACON study which demonstrated benefits of combining the BRAF inhibitor encorafenib with cetuximab in this setting (Tabernero et al., 2021). Additional combination opportunities are being explored pre-clinically, with synergy observed between ETC-159 and PI3K inhibitors or ETC-159 and PARP inhibition in Wnt-driven cancer models (Kaur et al., 2021; Z. Zhong et al., 2019).
Given the role of Wnt pathway in CSC maintenance, combination of Porcupine inhibitors with chemotherapy in selected patients may provide an interesting avenue of research, based on the rationale that Porcupine inhibitors may overcome chemoresistance.
Initial clinical data is promising in that, despite the homeostatic role of Wnt in the digestive tract, gastrointestinal side-effects at doses that engage the target are limited, indicating the presence of a therapeutic window. Effects on bone turnover can be mitigated with Receptor activator of nuclear factor-κB ligand (RANKL) inhibitors or bisphosphonate treatment, although the additional impact on taste may need to be managed in order to minimize associated weight loss. Since future strategies may include combining Porcupine inhibitors with other therapeutics, the potential for combined toxicities will also need to be managed. However initial data from NCT01351103, where WNT974 was used in combination with anti-PD-1 therapy showed the combination to be well-tolerated (Janku et al., 2020).
Conclusive clinical efficacy data on Porcupine inhibitors is currently lacking, with studies to date limited to small numbers of late stage, previously-treated patients with relevant somatic mutations. Key questions remain concerning optimal patient selection strategies for Porcupine inhibitors. Different approaches are being explored, including prospectively targeting patients with RSPO2/3 fusions and RNF43/ZNRF3 mutations with monotherapy, but also broader populations. WNT974 is under investigation in combination with anti-PD-1 in patients refractory to prior anti-PD-1 therapy, as well as other targeted populations. It is also interesting to note differences in dosing strategies between various Porcupine inhibitors; In NCT01351103, WNT974 was given as a priming dose when used in combination with anti- PD-1, RXC004 is dosed one daily, and ETC-159 demonstrated interpatient variability in pharmacokinetics and was dosed every other day. The impact of distinct dosing regimens and patient selection strategies is yet to be determined. Large scale, randomized, controlled clinical trials with robust biomarker assessments will be needed to elucidate whether Porcupine inhibitors will prove useful in gastrointestinal cancers, and to identify which patients are most likely to benefit from such treatments.
Declaration of Competing Interest
S.W, C.E and CP are current employees of Redx Pharma PLC and hold shares or share options in Redx Pharma PLC. D.J.F. and O.J.S have no conflict of interest to declare.
Acknowledgements
O.J. S acknowledges core grant A17196 and A31287 from Cancer Research UK: Medical writing assistance was provided by Marianne Ratcliffe, PhD, of EmWrite Ltd, and was funded by Redx Pharma PLC
Editor: S.J. Enna
References
- Akhmetshina A., Palumbo K., Dees C., Bergmann C., Venalis P., Zerr P.…Distler J.H.W. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nature Communications. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aynati M.M., Radulovich N., Riddell R.H., Tsao M.S. Epithelial-cadherin and β-catenin expression changes in pancreatic intraepithelial neoplasia. Clinical Cancer Research. 2004;10(4):1235–1240. doi: 10.1158/1078-0432.ccr-03-0087. [DOI] [PubMed] [Google Scholar]
- Amerongen R., Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Bagheri M., Tabatabae Far M.A., Mirzaei H., Ghasemi F. Evaluation of antitumor effects of aspirin and LGK974 drugs on cellular signaling pathways, cell cycle and apoptosis in colorectal cancer cell lines compared to oxaliplatin drug. Fundamental & Clinical Pharmacology. 2020;34(1):51–64. doi: 10.1111/fcp.12492. [DOI] [PubMed] [Google Scholar]
- Bailey P., Chang D.K., Nones K., Johns A.L., Patch A.M., Gingras M.C.…Grimmond S.M. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- Bendell J., Eckhardt G.S., Hochster H.S., Morris V.K., Strickler J., Kapoun A.M.…Munster P. Initial results from a phase 1a/b study of OMP-131R10, a first-in-class anti-RSPO3 antibody, in advanced solid tumors and previously treated metastatic colorectal cancer (CRC) European Journal of Cancer. 2016;69:S29–S30. [Google Scholar]
- Bengochea A, de Souza M, Lefrançois L, Le Roux E, Galy O, Chemin I.…Merle P, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. British journal of cancer. 2008;99(1):143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhamra I., Adams N., Armer R., Bingham M., McKeever H., Phillips C., Thompson B., Woodcock S. Novel porcupine (PORCN) inhibitor RXC004: Evaluation in models of RNF43 loss of function cancers. Journal of Clinical Oncology. 2017;35(15_suppl):e14094. [Google Scholar]
- Bo H., Gao L., Chen Y., Zhang J., Zhu M. Upregulation of the expression of Wnt5a promotes the proliferation of pancreatic cancer cells in vitro and in a nude mouse model. Molecular Medicine Reports. 2016;13(2):1163–1171. doi: 10.3892/mmr.2015.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter L., Guest R.V., Kendall T.J., Wilson D.H., Wojtacha D., Robson A.J.…Forbes S.J. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. Journal of Clinical Investigation. 2015;125(3):1269–1285. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert M., Athineos D., Abud H.E., Burke Z.D., Faux M.C. Genetic dissection of differential signaling threshold requirements for the wnt/b-catenin pathway in vivo. PLoS Genetics. 2010;6(1):1000816. doi: 10.1371/journal.pgen.1000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunyard P., Bhamra I., Eckersley K., Chaplin C., Guisot N., Jones C.…Armer R. Preclinical data using a novel porcupine inhibitor demonstrates that inhibition of porcupine is a promising target for the treatment of Idiopathic Pulmonary Fibrosis. European Respiratory Journal. 2019;54(Suppl. 63):PA589. [Google Scholar]
- Caldwell G.M., Jones C., Gensberg K., Jan S., Hardy R.G., Byrd P.…Morton D.G. The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Research. 2004;64(3):883–888. doi: 10.1158/0008-5472.can-03-1346. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network, T Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A.…Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discovery. 2012;2(5) doi: 10.1158/2159-8290.CD-12-0095. 401 LP – 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-On W., Nairismägi M.L., Ong C.K., Lim W.K., Dima S., Pairojkul C.…Teh B.T. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nature Genetics. 2013;45(12):1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- Chee Y.C., Pahnke J., Bunte R., Adsool V.A., Madan B., Virshup D.M. Intrinsic xenobiotic resistance of the intestinal stem cell Niche. Developmental Cell. 2018;46(6):681–695.e5. doi: 10.1016/j.devcel.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Chen B., Dodge M.E., Tang W., Lu J., Ma Z., Fan C.-W.…Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chemical Biology. 2009;5(2):100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-W., Beyer C., Liu J., Maier C., Li C., Trinh-Minh T.…Distler J.H.W. Pharmacological inhibition of porcupine induces regression of experimental skin fibrosis by targeting Wnt signalling. Annals of the Rheumatic Diseases. 2017;76(4):773–778. doi: 10.1136/annrheumdis-2016-210294. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen Z., Tang Y., Xiao Q. The involvement of noncanonical Wnt signaling in cancers. Biomedicine and Pharmacotherapy. 2021;133 doi: 10.1016/j.biopha.2020.110946. [DOI] [PubMed] [Google Scholar]
- Chiurillo M.A. Role of the Wnt/b-catenin pathway in gastric cancer: An in-depth literature review. World Journal of Experimental Medicine. 2015;5(2):84–102. doi: 10.5493/wjem.v5.i2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements W.M., Wang J., Sarnaik A., Kim O.J., MacDonald J., Fenoglio-Preiser C.…Lowy A.M. β-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Research. 2002;62(12):3503–3506. [PubMed] [Google Scholar]
- Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Colnot S., Decaens T., Niwa-Kawakita M., Godard C., Hamard G., Kahn A., Giovannini M., Perret C. Liver-targeted disruption of Apc in mice activates-catenin signaling and leads to hepatocellular carcinomas. Proceedings of the National Academy of Sciences. 2004;101(49):17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy C.B., Vélez-Reyes G.L., Tschida B.R., Hu H., Kaufmann G., Koes N.…Largaespada D.A. R-spondin 2 drives liver tumor development in a yes-associated protein-dependent manner. Hepatology Communications. 2019;3(11):1496. doi: 10.1002/hep4.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N., Blagden S., Lopez J., Sarker D., Greystoke A., Harris N.…P. R. Phase I study of the porcupine (PORCN) inhibitor RXC004 in patients with advanced solid tumours. Annals of Oncology. 2021;32(Suppl. 5):S583–S620. [Google Scholar]
- Davis S.L., Cardin D.B., Shahda S., Lenz H.-J., Dotan E., O’Neil B.H.…Cohen S.J. A phase 1b dose escalation study of Wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Investigational New Drugs. 2020;38:821–830. doi: 10.1007/s10637-019-00824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lau W., Peng W.C., Gros P., Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes and Development. 2021;28:305–316. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito N.C., Sturdivant M., Thievanthiran B., Xiao C., Plebanek M.P., Salama A.K.…Hanks B.A. Pharmacological Wnt ligand inhibition overcomes key tumor-mediated resistance pathways to anti-PD-1 immunotherapy. Cell Reports. 2021;35 doi: 10.1016/j.celrep.2021.109071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J.R., Becerra C., Richards D., Mita A., Osborne C., O’Shaughnessy J.…Mita M. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Research and Treatment. 2020;184(1):53–62. doi: 10.1007/s10549-020-05817-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotan E., Cardin D.B., Lenz H.-J., Messersmith W., O’Neil B., Cohen S.J.…Weekes C. Phase Ib Study of Wnt inhibitor ipafricept with gemcitabine and nab-paclitaxel in patients with previously untreated stage IV pancreatic cancer. Clinical Cancer Research. 2020;26:5348–5357. doi: 10.1158/1078-0432.CCR-20-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow L.E., O’Rourke K.P., Simon J., Tschaharganeh D.F., Van Es J.H., Clevers H., Lowe S.W. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell. 2015;161(7):1539–1552. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khoueiry A., Ning Y., Yang D., Cole S., Kahn M., Zoghbi M., Berg J., Fujimori M., Inada T., Kouji H., Lenz H.-J. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. Journal of Clinical Oncology. 2013;31:2501. [Google Scholar]
- Fang L., Ford-Roshon D., Russo M., Brien C., Gurjao C., Grandclaudon M.…Giannakis M. Abstract 960: RNF43 G659fs is an oncogenic mutation in colorectal cancer and sensitizes tumor cells to PI3K/mTOR inhibition. Cancer Research. 2021;81(13 Supplement) 960 LP – 960. [Google Scholar]
- Fang Z., Xiong Y., Li J., Liu L., Zhang W., Zhang C., Wan J. APC gene deletions in gastric adenocarcinomas in a Chinese population: a correlation with tumour progression. Clinical and Translational Oncology. 2012;14(1):60–65. doi: 10.1007/s12094-012-0762-x. [DOI] [PubMed] [Google Scholar]
- Faux M., Ross J.L., Meeker C., Johns T., Ji H., Simpson R.J.…Burgess A. Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. Journal of Cell Science. 2004;117(3):427–439. doi: 10.1242/jcs.00862. [DOI] [PubMed] [Google Scholar]
- Flanagan D.J., Barker N., Di Costanzo N.S., Mason E.A., Gurney A., Meniel V.S.…Vincan E. Frizzled-7 is required for Wnt signaling in gastric tumors with and without Apc mutations. Cancer Research. 2019;79(5):970–981. doi: 10.1158/0008-5472.CAN-18-2095. [DOI] [PubMed] [Google Scholar]
- Flanagan D.J., Pentinmikko N., Luopajärvi K., Willis N.J., Gilroy K., Raven A.P.…Sansom O.J. NOTUM from Apc-mutant cells biases clonal competition to initiate cancer. Nature. 2021;(March 2020) doi: 10.1038/s41586-021-03525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan D.J., Vincan E., Phesse T.J. Winding back Wnt signalling: potential therapeutic targets for treating gastric cancers. British Journal of Pharmacology. 2017;174(24):4666–4683. doi: 10.1111/bph.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D., Bowles S.G., Salcedo E., Xu M., Millar S.E., Barlow L.A. Βeta-catenin is required for taste bud cell renewal and behavioral taste perception in adult mice. PLoS Genetics. 2017;13(8):1–31. doi: 10.1371/journal.pgen.1006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O.…Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science Signaling. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakis M., Hodis E., Jasmine M., Yamauchi M., Rosenbluh J., Cibulskis K.…Garraway L.A. RNF43 is frequently mutated in colorectal and endometrial cancers. Nature Genetics. 2014;46(12):1264–1266. doi: 10.1038/ng.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudet A.-L., Alexandre Cassier P., Iwao-Fukukawa C., Garin G., Badel J.-N., Kryza D., Chabaud S., Gilles-Afchain L., Clapisson G., Desuzinges C., Sarrut D., Halty A., Italiano A., Mori M., Tsunoda T., Katagiri T., Nakamura Y., Alberti L., Cropet C.…Blay J.-Y. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer. 2018;18:646. doi: 10.1186/s12885-018-4544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeppert B., Konermann C., Schmidt C.R., Bogatyrova O., Geiselhart L., Ernst C.…Weichenhan D. Global alterations of DNA methylation in cholangiocarcinoma target the Wnt signaling pathway. Hepatology. 2014;59(2):544–554. doi: 10.1002/hep.26721. [DOI] [PubMed] [Google Scholar]
- Griesmann H., Ripka S., Pralle M., Ellenrieder V., Baumgart S., Buchholz M.…Michl P. WNT5A-NFAT signaling mediates resistance to apoptosis in pancreatic cancer. Neoplasia (United States) 2013;15(1):11–22. doi: 10.1593/neo.121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T., Schatoff E.M., Murphy C., Paz Zafra M., Wilkinson J.E., Elemento O.…Program S. R-Spondin chromosome rearrangements drive Wnt-dependent tumour initiation and maintenance in the intestine. Nature Communications. 2017;8:15945. doi: 10.1038/ncomms15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaki H., Yamamoto H., Sakane H., Matsumoto S., Ohdan H., Sato A., Kikuchi A. An Anti-Wnt5a Antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Molecular Cancer Therapeutics. 2012;11(2):298–307. doi: 10.1158/1535-7163.MCT-11-0682. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Ogawa R., Yoshida H., Taniguchi H., Kojima M., Saito Y., Sekine S. EIF3E–RSPO2 and PIEZO1–RSPO2 fusions in colorectal traditional serrated adenoma. Histopathology. 2019;75(2):266–273. doi: 10.1111/his.13867. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y., Ariyama H., Stancikova J., Sakitani K., Asfaha S., Renz B.W.…Wang T.C. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28(6):800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Baker A., Goldstein S.D., Albert C.M., Jackson K.W., Mccarty G.…Loeb D.M. Inhibition of porcupine prolongs metastasis free survival in a mouse xenograft model of Ewing sarcoma. Oncotarget. 2017;8(45):78265–78276. doi: 10.18632/oncotarget.19432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens J., Timmer N.C., Boer M., Ikink G.J., Schewe M., Sacchetti A.…Bakker E.R.M. RSPO3 expands intestinal stem cell and niche compartments and drives tumorigenesis. Gut. 2017;66:1095–1105. doi: 10.1136/gutjnl-2016-311606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmi M., Nicolle R., Bousquet C., Neuzillet C. Cancer-associated fibroblasts: accomplices in the tumor immune evasion. Cancers. 2020;12:2969. doi: 10.3390/cancers12102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlubek F., Spaderna S., Schmalhofer O., Jung A., Kirchner T., Brabletz T. Wnt/FZD signaling and colorectal cancer morphogenesis. Frontiers in Bioscience : A Journal and Virtual Library. 2007;12:458–470. doi: 10.2741/2075. [DOI] [PubMed] [Google Scholar]
- Holtzhausen A., Zhao F., Evans K.S., Tsutsui M., Orabona C., Tyler D.S., Hanks B.A. Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunology Research. 2015;3(9):1082–1095. doi: 10.1158/2326-6066.CIR-14-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Miyoshi Y., Ichii S., Nagase H., Kato Y., Yanagisawa A., Nakamura Y. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Research. 1992;52(11):3231–3233. [PubMed] [Google Scholar]
- Huang J., Fan X., Wang X., Lu Y., Zhu H., Wang W., Zhang S., Wang Z. High ROR2 expression in tumor cells and stroma is correlated with poor prognosis in pancreatic ductal adenocarcinoma. Scientific Reports. 2015;5(June):1–9. doi: 10.1038/srep12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.-M.A., Mishina Y.M., Liu S., Cheung A., Stegmeier F., Michaud G.A.…Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Huels D.J., Bruens L., Hodder M.C., Cammareri P., Campbell A.D., Ridgway R.A.…Sansom O.J. Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nature Communications. 2018;9(1):1132. doi: 10.1038/s41467-018-03426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackstadt R., Hodder M.C., Sansom O.J. WNT and β-Catenin in Cancer: Genes and Therapy. The Annual Review of Cancer Biology. 2020;4:177–196. [Google Scholar]
- Jahanban-Esfahlan R., Seidi K., Manjili M.H., Jahanban-Esfahlan A., Javaheri T., Zare P. Tumor cell dormancy: threat or opportunity in the fight against cancer. Cancers. 2019;11:1207. doi: 10.3390/cancers11081207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F., de Vos F., de Miguel M., Forde P., Ribas A., Nagasaka M.…De Jonge M. Abstract CT034: Phase I study of WNT974 + spartalizumab in patients (pts) with advanced solid tumors. Cancer Research. 2020;80(16 Supplement):CT034. [Google Scholar]
- Jiang J., Lan C., Li L., Yang D., Xia X., Liao Q.…Zeng C. A novel porcupine inhibitor blocks WNT pathways and attenuates cardiac hypertrophy. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2018;1864(10):3459–3467. doi: 10.1016/j.bbadis.2018.07.035. [DOI] [PubMed] [Google Scholar]
- Jiang X., Hao H.X., Growney J.D., Woolfenden S., Bottiglio C., Ng N.…Cong F. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(31):12649–12654. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A., Gordon M., Chugh R., Messersmith W., Mendelson D., Dupont J.…Smith D.C. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for wnt ligands, in patients with advanced solid tumors. Clinical Cancer Research. 2017;23(24):7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- Kabiri Z., Greicius G., Madan B., Biechele S., Zhong Z., Zaribafzadeh H.…Virshup D.M. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development (Cambridge) 2014;141(11):2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- Kabiri Z., Greicius G., Zaribafzadeh H., Hemmerich A., Counter C.M., Virshup D.M. Wnt signaling suppresses MAPK-driven proliferation of intestinal stem cells. Journal of Clinical Investigation. 2018;128(9):3806–3812. doi: 10.1172/JCI99325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey M.H., He X. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. British Journal of Pharmacology. 2017;174(24):4637–4650. doi: 10.1111/bph.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review) International Journal of Oncology. 2017;51(5):1357–1369. doi: 10.3892/ijo.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A., Lim J.Y.S., Sepramaniam S., Patnaik S., Harmston N., Lee M.A.…Madan B. WNT inhibition creates a BRCA-like state in Wnt-addicted cancer. EMBO Molecular Medicine. 2021;13(4) doi: 10.15252/emmm.202013349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Katsumi F., Shojima K., Nojima S., Osugi Y., Tomihara H., Eguchi H., Shintani Y., Endo H., Inoue M., Doki Y., Okumura M., Morii E., Kikuchi A. CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. Journal of Clinical Investigation. 2016;126(7):2689–2705. doi: 10.1172/JCI84658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Ikoma A., Shibakawa M., Shimoda S., Harada K., Saio M., Imamura J., Osawa Y., Kimura M., Nishikawa K., Okusaka T., Morita S., Inoue K., Kanto T., Todaka K., Nakanishi Y., Kohara M., Mizokami M. Safety, Tolerability, and Preliminary Efficacy of the Anti-Fibrotic Small Molecule PRI-724, a CBP/β-Catenin Inhibitor, in Patients with Hepatitis C Virus-related Cirrhosis: A Single-Center, Open-Label, Dose Escalation Phase 1 Trial. EBioMedicine. 2017;23:79–87. doi: 10.1016/j.ebiom.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K.W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kleeman S.O., Koelzer V.H., Jones H.J.S., Vazquez E.G., Davis H., East J.E.…Leedham S.J. Exploiting differential Wnt target gene expression to generate a molecular biomarker for colorectal cancer stratification. Gut. 2020;69(6):1092–1103. doi: 10.1136/gutjnl-2019-319126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeman S.O., Leedham S.J. Not all wnt activation is equal: Ligand-dependent versus ligand-independent wnt activation in colorectal cancer. Cancers. 2020;12(11):1–16. doi: 10.3390/cancers12113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko A.H., Chiorean E.G., Kwak E.L., Lenz H.-J., Nadler P.I., Wood D.L.…McWilliams R.R. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. Journal of Clinical Oncology. 2016;34(15_suppl):e15721. [Google Scholar]
- Koo B.K., Spit M., Jordens I., Low T.Y., Stange D.E., Van De Wetering M.…Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- Koo B.K., Van Es J.H., Van Den Born M., Clevers H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(24):7548–7550. doi: 10.1073/pnas.1508113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski C., Li V.S., Chan Y., Zhang J., Ho C., Yin Tsui W.…Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F., Davis C.R., Wang H.-T., Chu P., Lee M., Yuan J.…Kuo C.J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurayoshi M., Oue N., Yamamoto H., Kishida M., Inoue A., Asahara T., Yasui W., Kikuchi A. Expression of Wnt-5a Is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Research. 2006;66(21):10439–10487. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- de La Coste A., Romagnolo B., Billuart P., Renard C.-A., Buendia M.-A., Soubrane O., Fabre M., Chelly J., Beldjord C., Kahn A., Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Faderl S., Pagel J.M., Won Jung C., Yoon S.-S., Pardanani A.D.…Cortes J.E. Phase 1 study of CWP232291 in patients with relapsed or refractory acute myeloid leukemia and myelodysplastic syndrome. Blood Advances. 2020;4:2032–2043. doi: 10.1182/bloodadvances.2019000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Cao J., Zhang N., Tu M., Xu F., Wei S., Chen X., Xu Y. Identification of RSPO2 fusion mutations and target therapy using a porcupine inhibitor. Scientific Reports. 2018;8:14244. doi: 10.1038/s41598-018-32652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Su Q., Liu H., Wang D., Zhang W., Lu Z., Chen Y., Huang X., Li W., Zhang C., He Y., Fu L., Bi J. Frizzled7 Promotes Epithelial-to-mesenchymal Transition and Stemness Via Activating Canonical Wnt/β-catenin Pathway in Gastric Cancer. International Journal of Biological Sciences. 2018;14(3):280–293. doi: 10.7150/ijbs.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wu G., Xu Y., Li J., Ruan N., Chen Y.…Xia Q. Porcupine Inhibitor LGK974 Downregulates the Wnt Signaling Pathway and Inhibits Clear Cell Renal Cell Carcinoma. BioMed Research International. 2020;2020 doi: 10.1155/2020/2527643. Article ID 2527643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang Z., Wang L., Zhang Y. The oncogenic role of wnt10a in colorectal cancer through activation of canonical Wnt/β-catenin signaling. Oncology Letters. 2019;17(4):3657–3664. doi: 10.3892/ol.2019.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Lavrijsen M., Bakker A., Magierowski M., Magierowska K., Liu P.…Smits R. Commonly observed RNF43 mutations retain functionality in attenuating Wnt/β-catenin signaling and unlikely confer Wnt-dependency onto colorectal cancers. Oncogene. 2020;39(17):3458–3472. doi: 10.1038/s41388-020-1232-5. [DOI] [PubMed] [Google Scholar]
- Liu J., Pan S., Hsieh M.H., Ng N., Sun F., Wang T.…Harris J.L. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(50):20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loilome W., Bungkanjana P., Techasen A., Namwat N., Yongvanit P., Puapairoj A.…Riggins G.J. Activated macrophages promote Wnt/β-catenin signaling in cholangiocarcinoma cells. Tumour Biology. 2014;35:5357–5367. doi: 10.1007/s13277-014-1698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke J.J., Bao R., Sweis R.F., Spranger S., Gajewski T.F. WNT/b-catenin pathway activation correlates with immune exclusion across human cancers. Clinical Cancer Research. 2019;25(10):3074–3083. doi: 10.1158/1078-0432.CCR-18-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan B., Ke Z., Harmston N., Ho S.Y., Frois A.O., Alam J.…Virshup D.M. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35:2197–2207. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan B., McDonald M.J., Foxa G.E., Diegel C.R., Williams B.O., Virshup D.M. Bone loss from Wnt inhibition mitigated by concurrent alendronate therapy. Bone Research. 2018;6(1):17. doi: 10.1038/s41413-018-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan B., Patel M.B., Zhang J., Bunte R.M., Rudemiller N.P., Griffiths R.…Crowley S.D. Experimental inhibition of porcupine-mediated Wnt O-acylation attenuates kidney fibrosis. Kidney International. 2016;89(5):1062–1074. doi: 10.1016/j.kint.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M., Takeshima H., Iida N., Hattori N., Yamashita S., Moro H., Yasukawa Y., Nishiyama K., Hashimoto T., Sekine S., Ishii G., Ochiai A., Fukagawa T., Katai H., Sakai Y., Ushijima T. Cancer cell niche factors secreted from cancer-associated fibroblast by loss of H3K27me3. Gut. 2020;69(2):243–251. doi: 10.1136/gutjnl-2018-317645. [DOI] [PubMed] [Google Scholar]
- Manasanch E.E., Yoon S.-S., Min C.-K., Kim J.S., Shain K.H., Hauptschein R.…Lee K.-J. Interim results from the phase 1a/1b Dose-Finding Study of CWP232291 (CWP291) in Relapsed or Refractory Myeloma (RRMM) Alone or in Combination with Lenalidomide and Dexamethasone. Blood. 2017;130(Supplement 1):3091. [Google Scholar]
- Mattes B., Dang Y., Greicius G., Tamara Kaufmann L., Prunsche B., Rosenbauer J.…Scholpp S. Wnt/PCP controls spreading of Wnt/b-catenin signals by cytonemes in vertebrates. ELIFE. 2018;7 doi: 10.7554/eLife.36953. e36953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menck K., Heinrichs S., Baden C., Bleckmann A. Cells The WNT/ROR pathway in cancer: From signaling to therapeutic intervention. Cells. 2021;10:142. doi: 10.3390/cells10010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y., Iwao K., Nagasawa Y., Aihara T., Sasaki Y., Imaoka S., Murata M., Shimano T., Nakamura Y. Activation of the β-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Research. 1998;58(12):2524–2527. [PubMed] [Google Scholar]
- Mo M.-L., Li M.-R., Chen Z., Liu Z., Sheng Q., Zhou H.-M. Inhibition of the Wnt palmitoyltransferase porcupine suppresses cell growth and downregulates the Wnt/β-catenin pathway in gastric cancer. Oncology Letters. 2013;5:1719–1723. doi: 10.3892/ol.2013.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.N., Gunderson C.C., Sabbatini P., McMeekin D.S., Mantia-Smaldone G., Burger R.A.…O’Cearbhaill R.E. A phase 1b dose escalation study of ipafricept (OMP—54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecologic Oncology. 2019;154(2):294–301. doi: 10.1016/j.ygyno.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanki K., Toshimitsu K., Takano A., Fujii M., Shimokawa M., Ohta Y., Matano M., Seino T., Nishikori S., Ishikawa K., Kawasaki K., Togasaki K., Takahashi S., Sukawa Y., Ishida H., Sugimoto S., Kawakubo H., Kim J., Kitagawa Y.…Sato T. Divergent Routes toward Wnt and R-spondin Niche Independency during Human Gastric Carcinogenesis. Cell. 2018;174(4):856–869.e17. doi: 10.1016/j.cell.2018.07.027. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7481–7569. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Niehrs C. The complex world of WNT receptor signalling. Nature Reviews Molecular Cell Biology. 2012;13(12):767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- Nishioka M., Ueno K., Hazama S., Okada T., Sakai K., Suehiro Y., Okayama N., Hirata H., Oka M., Imai K., Dahiya R., Hinoda Y. Possible involvement of Wnt11 in colorectal cancer progression. Molecular Carcinogenesis. 2013;52(3):207–217. doi: 10.1002/mc.21845. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Ochoa de Olza M., Navarro Rodrigo B., Zimmermann S., Coukos G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. The Lancet Oncology. 2020;21(9):e419–e430. doi: 10.1016/S1470-2045(20)30234-5. [DOI] [PubMed] [Google Scholar]
- Oderup C., Lajevic M., Butcher E.C. Tolerance program dendritic cell responses for canonical and noncanonical wnt proteins. Journal of Immunology. 2021;190:6126–6134. doi: 10.4049/jimmunol.1203002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C.K., Subimerb C., Pairojkul C., Wongkham S., Cutcutache I., Yu W.…Teh B.T. Exome sequencing of liver flukeg-associated cholangiocarcinoma. Nature Genetics. 2012;44(6):690–693. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- Ooi C.H., Ivanova T., Wu J., Lee M., Tan I.B. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genetics. 2009;5(10):1000676. doi: 10.1371/journal.pgen.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak S., Park S., Kim Y., Park J.-H., Park C.-H., Lee K.-J., Kim C.-S., Ahn H. The small molecule WNT/β-catenin inhibitor CWP232291 blocks the growth of castration-resistant prostate cancer by activating the endoplasmic reticulum stress pathway. Journal of Experimental & Clinical Cancer Research. 2019;38:342. doi: 10.1186/s13046-019-1342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan K.-F., Liu W.-G., Zhang L., You W.-C., Lu Y.-Y. Mutations in components of the Wnt signaling pathway in gastric cancer. World Journal of Gastroenterology. 2008;14(10):1570–1574. doi: 10.3748/wjg.14.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca Di Magliano M., Biankin A.V., Heiser P.W., Cano D.A., Gutierrez P.J.A., Deramaudt T.…Hebrok M. Common activation of canonical wnt signaling in pancreatic adenocarcinoma. PLoS One. 2007;2(11) doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C., Bhamra I., Eagle C., Cook A.E., Jones C., Woodcock S. Abstract 506: Wnt/â-Catenin pathway inhibitor RXC004 enhances the immunity of pre-clinical models of cancer. Cancer Research. 2019;79(13_Supplement):506. [Google Scholar]
- Phillips C., Woodcock S., Eagle C., Saunders A., Tilston C., Bhamra I., Armer R. Abstract 998: Mechanism of action of RXC004, a Wnt pathway inhibitor, in genetically-defined models of cancer. Cancer Research. 2021;81(13_Supplement):998. [Google Scholar]
- Pilarsky C., Ammerpohl O., Sipos B., Dahl E., Hartmann A., Wellmann A.…Grützmann R. Activation of Wnt signalling in stroma from pancreatic cancer identified by gene expression profiling. Journal of Cellular and Molecular Medicine. 2008;12(6B):2823–2835. doi: 10.1111/j.1582-4934.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes and Development. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazkova M., Häkkinen T.J., Prochazka J., Spoutil F., Jheon A.H., Ahn Y.…Klein O.D. FGF signaling refines Wnt gradients to regulate the patterning of taste papillae. Development (Cambridge) 2017;144(12):2212–2221. doi: 10.1242/dev.148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffitt K.D., Madan B., Ke Z., Pendharkar V., Ding L., Lee M.A.…Virshup D.M. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Research. 2013;73(2):502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- Radaszkiewicz T., Nosková M., Gömöryová K., Blanářová O.V., Radaszkiewicz K.A., Picková M.…Bryja V. Rnf43 inhibits wnt5a-driven signalling and suppresses melanoma invasion and resistance to the targeted therapy. ELife. 2021;10:1–41. doi: 10.7554/eLife.65759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J.L., Schumacher D., Staudte S., Steffen A., Haybaeck J., Keilholz U.…Lange M. Non-canonical Hedgehog signaling is a positive regulator of the WNT pathway and is required for the survival of colon cancer stem cells. Cell Reports. 2017;21(10):2813–2828. doi: 10.1016/j.celrep.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Ripka S., König A., Buchholz M., Wagner M., Sipos B., Klöppel G.…Michl P. WNT5A - Target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28(6):1178–1187. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- Rodon J., Argilés G., Connolly R.M., Vaishampayan U., De Jonge M., Garralda E., Giannakis M. Phase 1 study of single-agent WNT974, a first-in-class Porcupine inhibitor, in patients with advanced solid tumours. British Journal of Cancer. 2021;125:28–37. doi: 10.1038/s41416-021-01389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge D., Scholpp S. Mechanisms of intercellular Wnt transport. Development. 2019;146(10):dev176073. doi: 10.1242/dev.176073. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vega F., Mina M., Armenia J., Chatila W.K., Luna A., La K.C.…Schultz N. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173(2):321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M., Driscoll D.R., DeJesus-Monge W.E., Quattrochi B., Appleman V.A., Ou J.…Lewis B.C. Activation of WNT/β-catenin signaling enhances pancreatic cancer development and the malignant potential Via Up-regulation of Cyr61. Neoplasia (United States) 2016;18(12):785–794. doi: 10.1016/j.neo.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh W., Matsuyama M., Takemura H., Aizawa S., Shimono A. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/β-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis. 2008;46(2):92–103. doi: 10.1002/dvg.20369. [DOI] [PubMed] [Google Scholar]
- Schulze K., Imbeaud S., Letouzé E., Alexandrov L.B., Calderaro J., Rebouissou S.…Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nature Genetics. 2015;47(5):505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino T., Kawasaki S., Shimokawa M., Tamagawa H., Toshimitsu K., Fujii M., Ohta Y., Matano M., Nanki K., Kawasaki K., Takahashi S., Sugimoto S., Iwasaki E., Takagi J., Itoi T., Kitago M., Kitagawa Y., Kanai T., Sato T. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell. 2018;22(3):454–467.e6. doi: 10.1016/j.stem.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Seshagiri S., Stawiski E.W., Durinck S., Modrusan Z., Storm E.E., Conboy C.B.…De Sauvage F.J. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R.P., Chopra V.L. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Developmental Biology. 1976;48(2):461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Shinmura K., Kahyo T., Kato H., Igarashi H., Matsuura S., Nakamura S., Kurachi K., Nakamura T., Ogawa H., Funai K., Tanahashi M., Niwa H., Sugimura H. RSPO fusion transcripts in colorectal cancer in Japanese population. Molecular Biology Reports. 2014;41(8):5375–5384. doi: 10.1007/s11033-014-3409-x. [DOI] [PubMed] [Google Scholar]
- Silva A.-L., Dawson S.N., Arends M.J., Guttula K., Hall N., Cameron E.A.…Ibrahim A.E.K. Boosting Wnt activity during colorectal cancer progression through selective hypermethylation of Wnt signaling antagonists. BMC Cancer. 2014;14(1):891. doi: 10.1186/1471-2407-14-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sphyris N., Hodder M.C., Sansom O.J. Subversion of niche-signalling pathways in colorectal cancer: What makes and breaks the intestinal stem cell. Cancers. 2021;13:1000. doi: 10.3390/cancers13051000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spit M., Fenderico N., Jordens I., Radaszkiewicz T., Lindeboom R.G., Bugter J.M.…Maurice M.M. RNF 43 truncations trap CK 1 to drive niche-independent self-renewal in cancer. The EMBO Journal. 2020;39(18):1–15. doi: 10.15252/embj.2019103932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S., Bao R., Gajewski T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- Storm E.E., Durinck S., De Sousa E., Melo F., Tremayne J., Kljavin N.…De Sauvage F.J. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature. 2016;529:97–100. doi: 10.1038/nature16466. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Watkins D.N., Jair K.-W., Schuebel K.E., Markowitz S.D., Dong Chen W.…Baylin S.B. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nature Genetics. 2004;36(4):417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- Tabernero J., Grothey A., Van Cutsem E., Yaeger R., Wasan H., Yoshino T.…Kopetz S. Encorafenib plus cetuximab as a new standard of care for previously Treated BRAF V600E–mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. Journal of Clinical Oncology. 2021;39(4):273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N., Jain R., LeBoeuf M.R., Wang Q., Lu M.M., Epstein J.A. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334(6061):1420 1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D., Ng M., Subbiah V., Messersmith W., Teneggi V., Diermayr V.…Matter A. Phase I extension study of ETC-159 an oral PORCN inhibitor administered with bone protective treatment, in patients with advanced solid tumours. Annals of Oncology. 2018;29(Supplement) ix23–ix24. [Google Scholar]
- Taniguchi K., Roberts L.R., Aderca I.N., Dong X., Qian C., Murphy L.M.…Liu W. Mutational spectrum of b-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863–4871. doi: 10.1038/sj.onc.1205591. [DOI] [PubMed] [Google Scholar]
- Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P.J., Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent-catenin degradation. The Journal of Cell Biology. 2003;162(5):899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Fukui A., Terai S., Fujioka Y., Shinada K., Takahashi H.…Hatakeyama S. Molecular role of RNF43 in canonical and noncanonical Wnt signaling. Molecular and Cellular Biology. 2015;35(11):2007–2023. doi: 10.1128/MCB.00159-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J., Park S., Yu W., Zhang S., Wu L., Carmon K., Liu Q.J. The most common RNF43 mutant G659Vfs*41 is fully functional in inhibiting Wnt signaling and unlikely to play a role in tumorigenesis. Scientific Reports. 2019;9:18557. doi: 10.1038/s41598-019-54931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia J., Hernández-López C., Martínez V.G., Hidalgo L., Zapata A.G., Vicente Á.…Sacedón R. Wnt5a Skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. The Journal of Immunology. 2011;187(8):4129–4139. doi: 10.4049/jimmunol.1101243. [DOI] [PubMed] [Google Scholar]
- Van De Wetering M., Francies H.E., Francis J.M., Bounova G., Iorio F., Pronk A.…Clevers H. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L., De Sousa E., Melo F., Van Der Heijden M., Cameron K., De Jong J.H.…Medema J.P. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biology. 2010;12(5):468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Vincan E., Darcy P.K., Farrelly C.A., Faux M.C., Brabletz T., Ramsay R.G. Frizzled-7 dictates three-dimensional organization of colorectal cancer cell carcinoids. Oncogene. 2007;26:2340–2352. doi: 10.1038/sj.onc.1210026. [DOI] [PubMed] [Google Scholar]
- Voloshanenko O., Erdmann G., Dubash T.D., Augustin I., Metzig M., Moffa G.…Boutros M. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nature Communications. 2013;4:2610. doi: 10.1038/ncomms3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Yuen S.T., Xu J., Lee S.P., Yan H.H.N., Shi S.T.…Leung S.Y. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nature Genetics. 2014;46(6):573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- Wang S., Tian Y., Wu D., Zhu H., Luo D., Gong W., Zhou Y., Zhou J., Zhang Z. Genetic variation of CTNNB1 gene is associated with susceptibility and prognosis of gastric cancer in a Chinese population Shizhi. Mutagenesis. 2012;27(6):623–630. doi: 10.1093/mutage/ges027. [DOI] [PubMed] [Google Scholar]
- Wang T., Kalland K.-H., Ke X., Qu Y. Targeting Wnt/β-Catenin signaling for cancer immunotherapy. Trends in Pharmacological Sciences. 2018;39(7):648–658. doi: 10.1016/j.tips.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Weinberg B.A., Xiu J., Lindberg M.R., Shields A.F., Hwang J.J., Poorman K.…Morse M.A. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. Journal of Gastrointestinal Oncology. 2019;10(4):652–662. doi: 10.21037/jgo.2018.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B.D., Chien A.J., Dawson D.W. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142(2):219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewicz A.K., McMillan E.A., Balaji U., Baek G.H., Lin W.C., Mansour J.…Knudsen E.S. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nature Communications. 2015;6:1–11. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock S., Bhamra I., Jones C., Cook A.E., Eagle C., Phillips C. Abstract 3874: Efficacy of the Wnt/Beta-Catenin pathway inhibitor RXC004 in genetically-defined models of cancer. Cancer Research. 2019;79(13_Supplement):3874. [Google Scholar]
- Xavier C.P., Melikova M., Chuman Y., Üren A., Baljinnyam B., Rubin J.S. Secreted Frizzled-related protein potentiation versus inhibition of Wnt3a/β-catenin signaling. Cellular Signalling. 2014;26(1):94–101. doi: 10.1016/j.cellsig.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.L., Shen J., Xu Y.H., Wang W.S., Ni C. ROR1 is highly expressed in circulating tumor cells and promotes invasion of pancreatic cancer (GUI-LIle) Molecular Medicine Reports. 2018;18:5087–5094. doi: 10.3892/mmr.2018.9500. [DOI] [PubMed] [Google Scholar]
- Yaeger R., Chatila W.K., Lipsyc M.D., Hechtman J.F., Cercek A., Sanchez-Vega F.…Schultz N. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 2018;33(1):125–136. doi: 10.1016/j.ccell.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Mohamed Yusoff P.A., Woutersen D.T.J., Goh P., Harmston N., Smits R.…Madan B. The functional landscape of patient-derived RNF43 mutations predicts sensitivity to Wnt inhibition. Cancer Research. 2020;80(24):5619–5632. doi: 10.1158/0008-5472.CAN-20-0957. [DOI] [PubMed] [Google Scholar]
- Zhang W., Glöckner S.C., Guo M., Ota Machida E., Wang D.H., Easwaran H.…Baylin S.B. Epigenetic Inactivation of the Canonical Wnt Antagonist SRY-Box Containing Gene 17 in Colorectal Cancer. Cancer Research. 2008;68(5):2764–2772. doi: 10.1158/0008-5472.CAN-07-6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lin L., Jin Y., Lin Y., Cao Y., Zheng C. Overexpression of WNT5B promotes COLO 205 cell migration and invasion through the JNK signaling pathway. Oncology Reports. 2016;36(1):23–30. doi: 10.3892/or.2016.4772. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang Y., Zhang J., Zhong J., Yang R. COL1A1 promotes metastasis in colorectal cancer by regulating the WNT/PCP pathway. Molecular Medicine Reports. 2018;17:5037–5042. doi: 10.3892/mmr.2018.8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Lu P., Zhang H., Xu H., Gao N., Li M., Liu C. Nestin positively regulates the Wnt/β-catenin pathway and the proliferation, survival and invasiveness of breast cancer stem cells. Breast Cancer Research. 2014;16(4):1–12. doi: 10.1186/s13058-014-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Katavolos P., Nguyen T., Lau T., Boggs J., Sambrone A., Kan D., Merchant M., Harstad E., Diaz D., Costa M., Schutten M. Tankyrase Inhibition Causes Reversible Intestinal Toxicity in Mice with a Therapeutic Index < 1. Toxicologic Pathology. 2016;44(2):267–278. doi: 10.1177/0192623315621192. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Sepramaniam S., Chew X.H., Wood K., Lee M.A., Madan B., Virshup D.M. PORCN inhibition synergizes with PI3K/mTOR inhibition in Wnt-addicted cancers. Oncogene. 2019;38(40):6662–6677. doi: 10.1038/s41388-019-0908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.-K., Zheng Y.-Z., Liu X.-S., Gou Q., Ma R., Guo C.-L.…Peng Y. ROR1 expression as a biomarker for predicting prognosis in patients with colorectal cancer. Oncotarget. 2017;8(20):32864–32872. doi: 10.18632/oncotarget.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Kipps T.J., Zhang S. Wnt5a signaling in normal and cancer stem cells. Stem Cells International. 2017;2017:1–6. doi: 10.1155/2017/5295286. Article ID 5295286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg A., Lahav L., Rosin-Arbesfeld R. Restoration of APC gene function in colorectal cancer cells by aminoglycoside- and macrolide-induced read-through of premature termination codons. Gut. 2010;59(4):496–507. doi: 10.1136/gut.2008.169805. [DOI] [PubMed] [Google Scholar]
- Zimmerli D., Cecconi V., Valenta T., Hausmann G., Cantù C., Restivo G.…Van Den Broek M. WNT ligands control initiation and progression of human papillomavirus-driven squamous cell carcinoma. Oncogene. 2018;37:3753–3762. doi: 10.1038/s41388-018-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]