Abstract
High-quality, accurate data on liquid contents and aerosol emissions from electronic nicotine delivery systems (ENDS, e.g., e-cigarettes) are crucial to address potential health concerns as these devices evolve and mature. Metals are an important class of ENDS constituents that merit attention as they have various health implications. Proper sampling, handling and aerosol trapping materials are essential to generate accurate quantitative metal data and to reduce the likelihood of inaccurate results originating from inappropriate collection vessels and materials that contribute to high background levels. Published methods that meet these criteria were applied to the analyses of chromium, nickel, copper, zinc, cadmium, tin and lead in liquid and aerosol from mint/menthol and tobacco flavors of currently popular pod-based devices from three manufacturers. Metal concentrations from pods that had not been used for generating aerosol ranged from below our lowest reportable level to 0.164 μg/g for Cr, 61.3 μg/g for Ni, 927 μg/g for Cu, 14.9 μg/g for Zn, 58.2 μg/g for Sn and 2.56 μg/g for Pb. Cadmium was included in our analyte panel and was not present above detection limits in liquid or aerosol. Aerosol metal concentrations (using a 55-mL puff) ranged from below our lowest reportable level to 29.9 ng/10 puffs for Cr, 373 ng/10 puffs for Ni, 209 ng/10 puffs for Cu, 4,580 ng/10 puffs for Zn, 127 ng/10 puffs for Sn and 463 ng/10 puffs for Pb. Our results showed some metal delivery from all the products examined and highly variable metal levels between manufacturer, brand and package.
Introduction
From 2017 to 2018, the use of electronic nicotine delivery systems (ENDS, e-cigarettes) increased by 77.8% (from 11.7% to 20.8%) for high school students and by 48.5% (from 3.3% to 4.9%) for middle school students in the United States (1). For US students, in 2019, electronic cigarettes continued to be the most used tobacco product, with 27.5% of high school students and 10.5% of middle school students reporting electronic cigarette use in the past 30 days before the survey (2). Pod-based devices are a popular type of ENDS with a user replaceable, disposable (or refillable) sub-unit that contains the heating element for aerosol generation and is usually pre-filled with liquid. The JUUL® brand is an example of these popular pod devices, but other manufacturer devices (myblu™ and Vuse Alto®) have similar design concepts.
Information on metals and their concentrations in liquid and in aerosol deliveries from pod devices obtained using well-validated methodologies, including a standard puff regimen, is needed to help address potential health concerns. Limited metal delivery data are currently available on pod devices. In one study on lead levels in electronic cigarette liquids, two JUUL® flavors purchased in 2017 had lead below reportable levels (3). Analyses of additional toxic metals (chromium, nickel, copper, zinc, cadmium, tin and lead) in ENDS liquid and ENDS aerosol have been reported in our previously published studies (4, 5). In those studies, five flavors of JUUL® pods purchased in 2018 were analyzed for metal content in their liquids and aerosols. Among the metals investigated, only nickel was detected at a concentration above the lowest reportable level in the liquid from three pod flavors. For ENDS with brass components, the most notable results from those previous studies were elevated copper and zinc levels. In contrast to other studies (6–9), we have yet to detect cadmium, a metal of importance in combustible tobacco products, in any liquid or aerosol device above our limit of detection (LOD) (4, 5). In this current report, we examined the metal content in ENDS pod-based systems selected from three manufacturers where there was overlap in the flavors offered (menthol/mint and tobacco). The data reported here provide information on these new, increasingly popular product types and fill key information gaps on the contents and evolution of their designs.
Materials and Methods
Sample preparation and analysis
Quantitative analytical measurements from the pod’s liquid contents and from machine-generated aerosol were made using validated triple quadrupole-inductively coupled plasma-mass spectrometry (ICP-QQQ-MS) under standardized conditions (4, 5). All ENDS products were trademarks of their respective manufacturers and were obtained or ordered online from vendors in the Greater Atlanta, GA, USA, in 2019. Unused disposable pods were used for each replicate analytical run with freshly charged batteries for aerosol collection. Results from triplicate analyses were obtained for liquids and aerosols.
For the ENDS liquid analyses, liquid was removed from the pods after careful deconstruction to ensure no sample contamination with any metals. Aliquots of the liquid content were prepared for analysis by dilution with an acid solution as previously described (4). For the ENDS aerosol method, samples were generated and prepared as separately described (5). Aerosol (50 puffs) was generated using CORESTA Method 81 parameters: 55-mL puff volume, 3-second puff duration, rectangular puff profile and 30-second puff interval (10). High-purity fluoropolymer tubing was used for aerosol collection through condensation (5). The tubing trap was rinsed with an acid solution to remove and collect aerosol condensate. The calibration standards, quality control preparation using a second source of standards and dilution factors used for these analyses were also previously described, as were the instrument parameters used with Agilent 8800 ICP-QQQ-MS (Tokyo, Japan) (4, 5). Method LODs, according to Taylor’s method (11) and lowest calibration standards (LSTDs), were determined and reported previously, but they are included in Tables I and II for convenience (4, 5).
Table I.
ENDS Liquid Metal Concentrations from Triplicate Measurements of Pod Devices, Including LODs and LSTDs (μg/g)
| Cr | Ni | Cu | Zn | Cd | Sn | Pb | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| LOD | 0.031 | 0.032 | 3.15 | 1.27 | 0.108 | 0.099 | 0.066 |
| LSTD | 0.025 | 0.025 | 2.00 | 1.00 | 0.100 | 0.100 | 0.100 |
| JUUL® Mint | <0.025 | <0.025–0.0635 | <2.00 | <1.00–2.40 | <0.100 | <0.099 | <0.066 |
| JUUL® Classic Tobacco | <0.025 | 0.0589–0.0806 | <2.00 | <1.00 | <0.100 | <0.099 | <0.066 |
| myblu™ Intense Mint-sation | 0.131–0.150 | 33.3–61.3 | <2.00 | <1.00 | <0.100 | <0.099 | <0.066 |
| myblu™ Intense Tobacco Chill | 0.093–0.164 | 2.54–9.37 | 573–927 | <1.00–4.63 | <0.100 | 34.2–58.2 | <0.066 |
| Vuse Alto® Menthol | <0.025 | 0.943–1.26 | 10.0–33.1 | 2.19–14.4 | <0.100 | <0.100 | <1.00–2.40 |
| Vuse Alto® Rich Tobacco | <0.025 | 1.09–1.36 | 18.1–24.7 | 9.38–14.9 | <0.100 | <0.100–0.219 | 1.30–2.56 |
Table II.
ENDS Aerosol Metal Concentrations from Triplicate Measurements of Pod Devices, Including LODs and LSTDs (ng/10 Puffs)
| Cr | Ni | Cu | Zn | Cd | Sn | Pb | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| LOD | 0.125 | 0.250 | 0.200 | 5.00 | 0.050 | 0.100 | 0.050 |
| LSTD | 0.500 | 0.500 | 1.00 | 10.0 | 0.200 | 0.200 | 0.500 |
| JUUL® Mint | <0.500a | <0.500–2.09 | <1.00–16.0 | <5.00–17.8 | <0.050 | <0.100–0.603 | 0.602–0.872 |
| JUUL® Classic Tobacco | <0.500a | <0.500–2.87 | <0.200–3.67 | <10.0 | <0.050 | <0.100–0.438 | <0.500 |
| myblu™ Intense Mint-sation | 0.626–0.727 | 30.1–108 | 146–174 | <10.0 | <0.050 | 81.2–127 | <0.500–1.17 |
| myblu™ Intense Tobacco Chill | <0.500a | 0.749–2.30 | 46.1–53.2 | <10.0 | <0.050 | 9.42–51.0 | <0.500–2.88 |
| Vuse Alto® Menthol | 8.90–29.9 | 158–373 | 17.7–209 | 867–4,580 | <0.050 | 9.88–44.1 | 96.5–463 |
| Vuse Alto® Rich Tobacco | <0.125–1.80 | <0.500–97.9 | <1.00–14.6 | <10.0–40.5 | <0.050 | <0.100–0.290 | 0.908–16.3 |
Some results reported as <LSTD may also have replicates <LOD.
The pH analysis was performed using a MANTECH pH meter (ManSci Inc., Orlando, Florida) calibrated at pH 4.01 and 7.00. A 5-mL aliquot of distilled, deionized water was added to a 0.50 g sample of liquid from the pod. Liquid from one pod was used per replicate unless there was insufficient liquid, and a combination of two pods was necessary. The samples were stirred for 1 hour, and pH readings were measured at 5-, 15-, 30- and 60-minute intervals and averaged as previously described (12, 13). Each ENDS flavor was analyzed in quintuplicate.
Microscopy instrumentation and conditions
The analyses of components of ENDS devices were acquired with a Quanta 250 field emission gun scanning electron microscope (SEM) (Thermo-FEI Co., Hillsboro, OR, USA). Images were acquired in high-vacuum mode (20 kV) using the Everhart Thornley detector. The SEM was equipped with an energy-dispersive X-ray (EDS) attachment with an 80 mm2 X-MaxN silicon drift detector (Oxford Instruments, Concord, MA, USA, with Aztec software).
Results
Pod liquid results
For ENDS liquid, ranges from triplicate analysis of the samples were determined along with method LODs, and the concentrations of the LSTDs were expressed in terms of μg/g (Table I).
With the exception of nickel in myblu™ pods and tin in myblu™ Tobacco Chill pods, many analytes were similar to those previously reported liquid metal concentrations (4). Cadmium was <LSTD for all products, as previously reported (4). This is expected as no device components were found to contain cadmium or cadmium alloys, so any measured cadmium would likely be from contamination (14). The JUUL® liquid results from 2018 (4) and 2019 were very similar. Flavored JUUL® liquids purchased in 2018 and in 2019 both had some detectable nickel values, whereas most other analytes were below reportable levels (4). An exception was the low, but detectable, levels of zinc in 2019 JUUL® Mint. In general, JUUL® liquid metal concentrations were overall the lowest of the liquid samples analyzed.
For myblu™ and Vuse Alto®, chromium values were below or at low reportable levels and were comparable to previously published results (4). Previously, we reported 4.04 ± 0.10 μg/g nickel liquid concentrations from a 2-year-old blu™ Classic Tobacco High Nicotine (4). Although variable, the recently purchased myblu™ Tobacco Chill nickel concentrations were comparable to previous blu™ Classic Tobacco results (4). However, myblu™ Mint-sation’s nickel concentrations were elevated compared to other pod samples obtained (Table I). The two Vuse Alto® flavors and the previously reported ENDS nickel concentrations were similar (4).
Copper and zinc concentrations were elevated compared to the other analyzed metals in previously reported liquid results for select products (4). Copper concentrations among all the pods were variable but were within previous concentration ranges (4). The zinc values were also variable for 2019 pods, and they were relatively low compared to previously reported results.
Tin concentrations in liquids from ENDS previously examined were less than 1.00 ug/g, with the majority below the reportable level (4). Tin concentrations in most of the 2019 pod liquids measured in this work were also below reportable levels, with the exception of myblu™ Tobacco Chill. The tin concentrations in the myblu™ Tobacco Chill pods were higher than we previously reported for ENDS liquids (4). Lead liquid concentrations were predominantly low or below reportable levels. Lead concentrations in previous generations of Vuse® liquids were below reportable levels (4); however, recently purchased Vuse Alto® pod liquid lead concentrations were detectable but within the concentration ranges from other ENDS devices (4).
Aerosol results
Aerosol results are reported per 10 puffs (55 mL/puff). We selected 10 puffs for reporting results to serve as a basis for comparison, because this is in the intermediate puff range for US cigarettes smoked using World Health Organization intense smoking regimen (15). All aerosol cadmium concentrations were below the method LOD, as was the case for liquid cadmium concentrations. Previously, we reported JUUL® aerosol metal concentrations below the lowest standard or method LOD (5); however, in some JUUL® pods purchased in 2019 (Table II), low but detectable nickel, copper, zinc, tin and lead levels were reported. All of the JUUL® pod metal aerosol deliveries were within ranges previously observed for select ENDS (5).
Chromium concentrations in aerosol from myblu™ and Vuse Alto® pods mostly were low, below the lowest standard, or below the method LOD, with the exception of the Vuse Alto® Menthol pods. The highest chromium aerosol concentration observed from one pod (29.9 ng/10 puffs) was 10 times more than the highest previously reported aerosol chromium concentration (1.85 ± 1.11 ng/10 puffs) (5). myblu™ Tobacco Chill aerosol nickel concentrations were similar to previously reported results (5). Pod-to-pod variabilities were high, but aerosol nickel levels from myblu™ Mint-sation, Vuse Alto® Menthol and Rich Tobacco were higher than we previously reported (Table II) (5).
Detectable copper aerosol concentrations (Table II) were observed, with the highest copper concentration range coming from Vuse Alto® Menthol. Vuse Alto® Menthol aerosols had a higher zinc concentration range than the other products, with notable variation among the three replicates. The highest previously reported ENDS aerosol zinc concentration was 339 ± 90 ng/10 puffs from Flavor Vapes® Blueberry (5).
Previously reported aerosol tin concentrations were typically lower (less than 2.00 ng/10 puffs (5)) than those reported here. Aerosol tin concentrations from myblu™ Mint-sation, Tobacco Chill and Vuse Alto® Menthol pods, however, were highly variable and ranged from <LOD to ~130 ng/10 puffs (Table II). Previously reported aerosol lead concentrations were mostly below reportable levels. The highest concentration we previously reported was 11.4 ± 4.14 ng/10 puffs in a Joyetech™ eGo tank system with My Vapor Store® Gold liquid (5). Lead was detected in the aerosols from all 2019 pod devices, except for JUUL® Classic Tobacco. Aerosol lead concentrations from Vuse Alto® pods were variable, but lead concentrations from one Vuse Alto® Rich Tobacco pod and from all three Vuse Alto® Menthol pods were higher (maximum lead observed was 463 ng/10 puffs in one pod) than any we have previously reported (Table II) (5).
pH results
There is a possibility that metal concentrations in pod liquids might arise from internal corrosion, and this may be correlated to pH of the liquid contents. The pH measurements (Table III) showed that the myblu™ liquids had the lowest pH, JUUL® liquids intermediate pH and Vuse Alto® liquids only slightly acidic.
Table III.
Pod Liquid pH (N = 5)
| Liquid sample | pH |
|---|---|
|
| |
| JUUL® Mint 5% Nicotine | 5.52 ± 0.06 |
| JUUL® Classic Tobacco 5% Nicotine | 5.52 ± 0.01 |
| myblu™ Intense Mint-sation 3.6% Nicotine | 4.02 ± 0.05 |
| myblu™ Intense Tobacco Chill 4% Nicotine | 4.55 ± 0.02 |
| Vuse Alto® Menthol 5% Nicotine | 6.79 ± 0.05 |
| Vuse Alto® Rich Tobacco 5% Nicotine | 6.65 ± 0.05 |
Discussion
Chronic inhalation exposure to metals from any source, including ENDS aerosols, can have various health consequences, including inflammation, sensitization, toxicities and cancer (16). The aerosol metals to which a pod device user might be exposed likely originate from corrosion of the internal metal-containing components of the devices, as previously reported (4, 14), although components among ENDS devices differ somewhat. Alloys used for heating elements, vapor tubes and electrical connectors (14) could corrode and possibly contribute various toxic metals to the ENDS liquids. Stainless steel components and nichrome are possible sources of iron, chromium and nickel. However, we have previously shown using dual-element-single particle-ICP-MS that metal pairing data in the particles did not support nichrome as a major source of metals in aerosol from pod devices (14). Zinc and copper oxides result from corrosion of brass connectors and contacts, but copper in these devices may also result from electrical contacts made from gold-plated alloys that contain copper (14). SEM–EDS did not show any evidence of brass connectors in these pods, contrary to some of the earlier generation devices (4, 14). Tin could have originated from corrosion resistant coatings on some steels or from substrates beneath compromised conductive coatings on electrical connectors.
Some manufacturers use a variety of metal components that may be in contact with the liquid. For example, the JUUL® website (17) states that pods contain “a stainless steel vapor path, an industry standard silica wick, and nichrome coil heater.” Battery to coil electrical connections are also partially exposed to the liquid. Metallic components from JUUL® pods, including the vapor path, heating element and connectors, are shown in Figure 1. myblu™ components, the vapor tube, heating element and similar connectors, are shown in Figure 2. A Vuse Alto® statement simply describes the pods as containing an “alloy-heater and ceramic-wick assembly” (18). This design does not include the traditional wick and heating coil seen in both the JUUL® and myblu™ brands and instead uses an absorbent ceramic wick. Figure 3 shows this design with the Vuse Alto® gray ceramic wick assembly and the metallic heaters.
Figure 1.
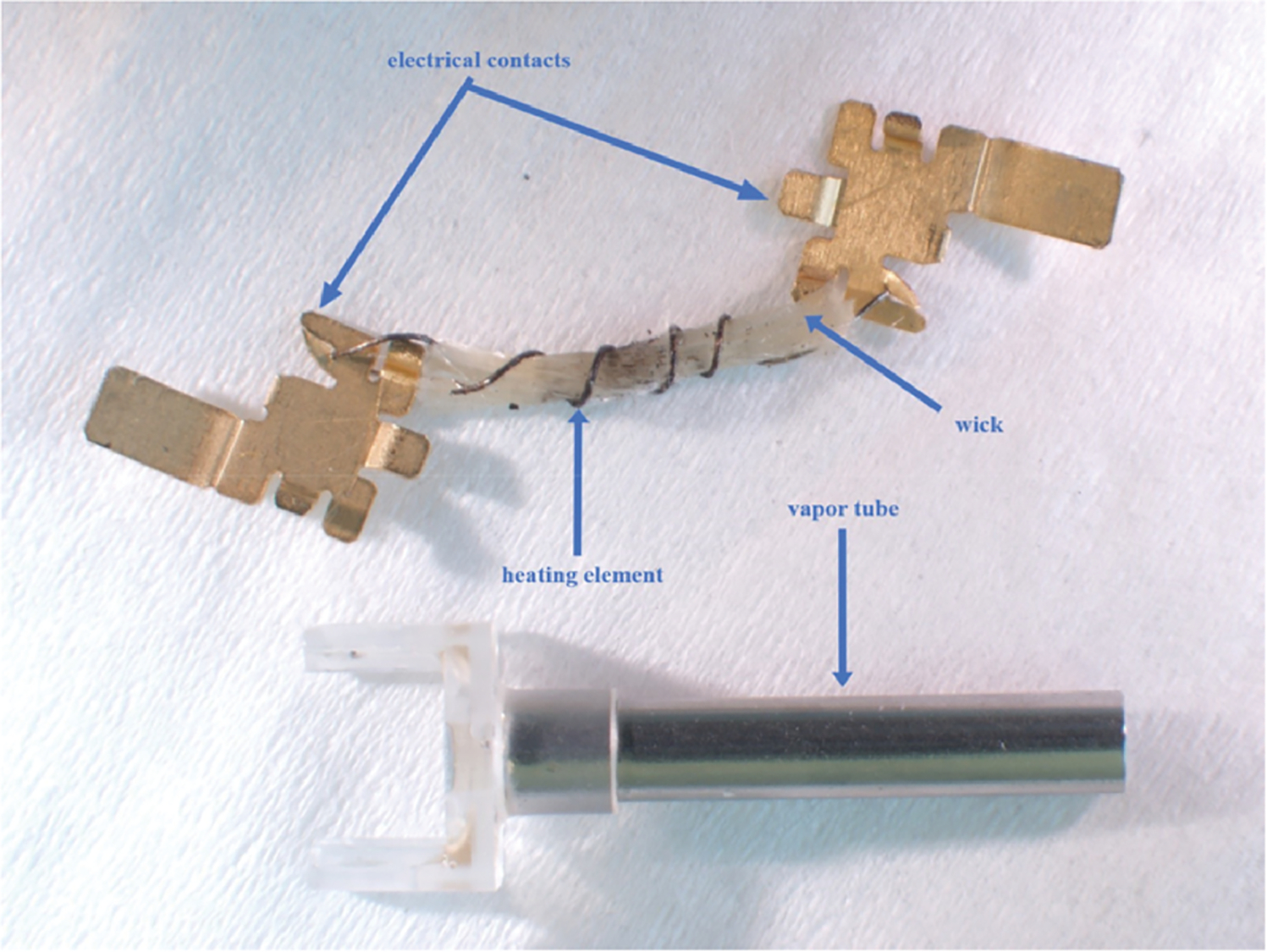
Components within JUUL® pods: vapor tube, electrical contacts, heating element and wick.
Figure 2.
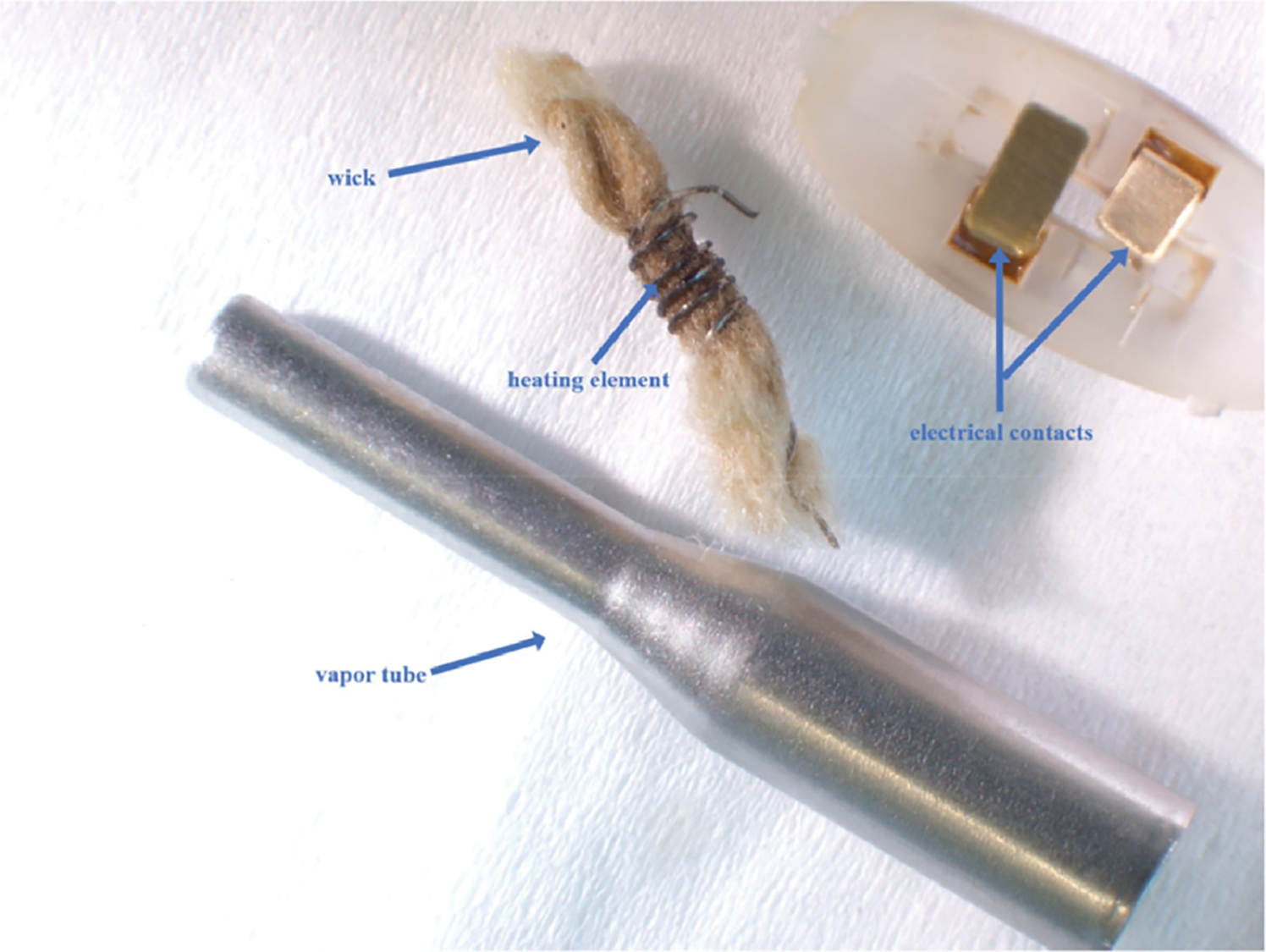
Components within myblu™ pods: vapor tube, electrical contacts, heating element and wick.
Figure 3.
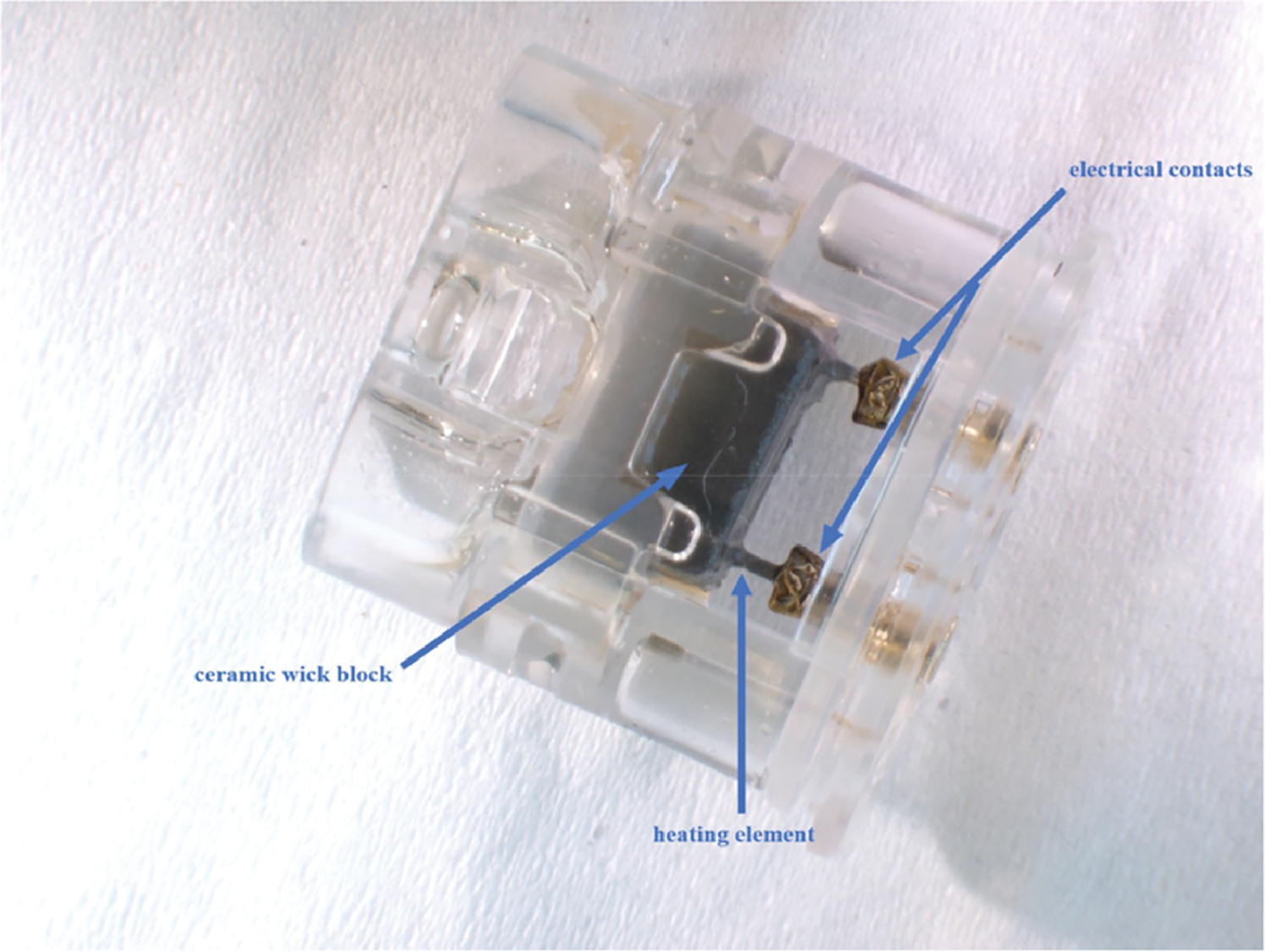
Components within Vuse Alto® pods: electrical contacts, heating element and ceramic wick block.
In contrast to data reported by Zervas et al. (19), the data in this study confirm our previous findings that transfer of metals from the liquid contained within the pods to aerosol emissions from the device is not efficient because of the low heating temperature of ENDS, typically 190–290°C (20). The discrepancies between results reported here and previously (5) and those reported by Zervas et al. (19) may be explained by the fact that our aerosol analyses were performed collecting aerosol from actual ENDS devices using a standard aerosol collection regimen with a high-purity fluoropolymer trap. However, Zervas et al. heated metal materials in liquid placed in a quartz flask, of unknown metals purity, with acid dissolution from a condenser (19).
Metal transfer in conventional, combustible tobacco product smoke is influenced by the temperatures attained by the burning coal of the tobacco product. A traditional cigarette coal temperature can reach up to 950°C, much higher than the heating temperature of the ENDS pod devices (21). We previously analyzed combustible cigarette filler and smoke for 10 toxic metal analytes, which included chromium, nickel, cadmium and lead (15, 22, 23). Chromium concentrations reported here for pod liquids were lower than the lowest (1.3 ± 0.3 μg/g) chromium concentrations reported in cigarette filler tobacco (22). Nickel concentrations in these pod liquids were similar to the lowest concentrations in filler tobacco, except for the myblu™ ENDS flavors (22). The chromium and nickel concentrations in 10 aerosol puffs from the pod devices ranged from comparable deliveries in mainstream smoke to concentrations exceeding cigarette smoke when comparing per stick to 10 puffs (15). Although cadmium and lead concentrations are typically low in filler tobacco (22), they are readily transported in mainstream smoke as these metals are relatively volatile. Cadmium and lead are among the highest concentrations of metals in mainstream cigarette smoke due to volatility above 900°C (15). This differs for cadmium concentrations in ENDS, which were below reportable levels in both liquid and aerosol. Interestingly, detected lead concentrations were comparable between the tested ENDS liquid and traditional cigarette filler tobacco. The lead concentrations in most ENDS aerosols, however, were much lower than in mainstream smoke, with the exception of the Vuse Alto® Menthol aerosols. The typical lower delivery of lead in most ENDS likely is due to the lower temperatures in ENDS heating coils (yielding a lower transport efficiency) compared to a burning cigarette coal temperature during inhalation.
Despite having the more acidic liquids, myblu™ liquids were not among the highest concentrations for zinc and lead. Instead, Vuse Alto® flavors, the least acidic, were the highest for zinc and lead. JUUL® flavors were intermediate in pH levels, but their metal liquid concentrations were the lowest out of the three brands. Chromium, nickel, copper and tin concentrations in some myblu™ liquids were among the highest. Whether pH or length of time between manufacture, purchase and use allowing more time for metals to be leached or corroded from the internal metal components plays a greater role in measured metal concentrations is not known at this time. Therefore, although pod liquid acidity likely plays a role in the oxidation of internal pod metals, contact time of the pod liquids with the metal components may play a more significant role, as none of these liquids are strongly acidic.
The pod devices were all purchased and analyzed within 4 months. Unfortunately, none of the products provided the date of manufacture or expiration dates. Presumably, even with shorter corrosion times than for some devices previously reported (4, 5), there were several elevated metal concentrations in the liquids and aerosols. Elevated metal concentrations that were observed in the liquids were typically observed in the aerosols. However, there were exceptions, where only detectable aerosol metal concentrations were observed. Vuse Alto® Menthol chromium levels in liquid, for example, were below the reportable level. However, this product had the highest chromium concentration in aerosol. The Vuse Alto® Rich Tobacco was also below the reportable level in the tested liquid samples, whereas one analytical replicate had low but detectable chromium level in aerosol. Correspondingly, the highest aerosol nickel concentrations were for Vuse Alto® flavors, although they were not elevated in the liquids. In the case of myblu™ Mint-sation copper concentrations, the copper levels in the liquid did not appear to correlate directly with elevated copper in the aerosol. These differences between elevated metal concentrations among pod liquids and aerosols are likely explained by pod-to-pod variability or are impacted by heating of internal components.
The average liquid mass lost from pods before and after 50 aerosol puffs were 0.420 ± 0.040 g for myblu™, 0.259 ± 0.042 g for Vuse Alto® and 0.182 ± 0.023 g for JUUL® (the pods were visually monitored to ensure bubbles did not prevent a full puff). JUUL® had the lowest mass transferred to aerosol per pod compared to the other brands, which may also contribute to the lower metal aerosol deliveries.
Overall, for both liquid and aerosol, the two JUUL® flavors had lower metal concentrations than the other brands in this study. Even taking the lower aerosol mass deliveries into account, the analytes were among the lowest for JUUL® flavors. Zhao et al. (24) have reported analytical results for some metal concentrations in aerosol samples for the JUUL® device, as we have (4, 5, 14), though Zhao et al. have not reported results of analyses of aerosol from myblu™ or Vuse Alto® pod devices. It would be difficult, however, to compare our JUUL results with the results of Zhao et al., as they did not describe LOD calculations in detail, did not described the use of a puff profile with a standardized puff regimen using a standard vaping machine and reported the results in terms of microgram metal per kilogram recovered aerosol, whereas we report the results in terms of nanograms per 10 puffs. The Vuse Alto® Menthol devices consistently produced aerosol with elevated metal concentrations relative to pods tested from the other two brands. Compared to our previously reported results, we observed higher nickel and tin results for newer 2019 pods than from those measured previously (4, 5). A more detailed and extensive set of analyses of pods, from various lots, would be necessary to more fully explore and characterize delivery and variability trends among these devices.
Conclusions
Our previously validated ENDS liquid and aerosol methods were used to analyze chromium, nickel, copper, zinc, cadmium, tin and lead in two common flavor varieties from three different manufacturers (JUUL® Mint and Classic Tobacco, myblu™ Mint-sation and Tobacco Chill, and Vuse Alto® Menthol and Rich Tobacco). Triplicate result ranges were reported because of the inherent high pod-to-pod metal variability. In this work, many results were consistent with previously reported ENDS (4, 5), although there were exceptions. Chromium concentrations were consistently low in liquid and aerosol, except for Vuse Alto® Menthol aerosol. Nickel was detectable in all brands, including some higher concentrations than previously reported (4, 5). In some liquid and aerosol, copper and zinc concentrations were detected, although most were within the previously reported ranges (4, 5), with the exception of Vuse Alto® Menthol zinc (867–4,580 ng/10 puffs). Tin and lead concentrations were low in our previous ENDS results as well as in most pod liquid samples used for this study. One exception for tin was observed in myblu™ Tobacco Chill liquid where the concentration was greater than the other pod liquid concentrations. In aerosol, there were detectable tin and lead values among the pods. The highest tin aerosol concentration ranged from 81.2 to 127 ng/10 puffs, and the highest aerosol lead concentration ranged from 96.5 to 463 ng/10 puffs.
Metal corrosion from pod device components could elevate the exposure of toxic metals to users as we previously reported (4, 5). We specifically reported that the principal forms of the metals in ENDS aerosol were insoluble metal oxide particles (14). Using dual-element-single particle-ICP-MS, we showed that element pairing among particles was consistent with materials in device components (14). Those data were consistent with the total metal data reported here in that chromium concentrations, for example, were generally low, and low relative to nickel, indicating components other than nichrome heating elements as the sources (14). Measurements of metal concentration in the liquids provide some indication of exposures that could be expected in aerosols. Although it is possible that the aerosol metal deliveries could be increased from repeated coil heating and related corrosion chemistry in some devices, pod heating elements are replaced with each new pod.
Results reported here revealed that the new generation of pod designs and formulations of the popular pod liquids do not necessarily provide a decrease in toxic metal concentrations in aerosols compared to previous designs. All the aerosol metals detected may represent health risks for ENDS users; however, there is limited evidence for specific dose/response disease relations. Further studies on the specific compositions of the metal concentrations in ENDS liquids and aerosols are necessary to determine the full extent of product aging and related metal exposure to users and specific health impacts. Although more research is needed to fully characterize the deliveries and variabilities of these pod-based designs, and other ENDS, it would be extremely helpful if the manufacturers include the date of manufacture and expiration dates on all these consumer products.
Acknowledgments
We gratefully acknowledge Tameka Lawler for providing pH measurements.
Funding
Our work is supported by congressionally appropriated funds to the CDC.
Footnotes
Conflicts of Interest
The authors affirm no conflicts of interest in the publication of this manuscript.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Use of trade names and commercial sources is for identification only and does not constitute endorsement by the US Department of Health and Human Services, or the US Centers for Disease Control and Prevention.
References
- 1.Gentzke AS, Creamer M, Cullen KA, Ambrose BK, Willis G, Jamal A, et al. (2019) Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. Morbidity and Mortality Weekly Report, 68, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TW, Gentzke AS, Creamer MR, Cullen KA, Holder-Hayes E, Sawdey MD, et al. (2019) Tobacco product use and associated factors among middle and high school students—United States, 2019. Morbidity and Mortality Weekly Report, 68, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunbar ZR, Das A, O’Connor RJ, Goniewicz ML, Wei B, Travers MJ (2018) Brief report: lead levels in selected electronic cigarettes from Canada and the United States. International Journal of Environmental Research and Public Health, 15, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray N, Halstead M, Gonzalez-Jimenez N, Valentin-Blasini L, Watson C, Pappas RS (2019) Analysis of toxic metals in liquid from electronic cigarettes. International Journal of Environmental Research and Public Health, 16, 4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead M, Gray N, Gonzalez-Jimenez N, Fresquez M, Valentin-Blasini L, Watson C, et al. (2020) Analysis of toxic metals in electronic cigarette aerosols using a novel trap design. Journal of Analytical Toxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess CA, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, Rule AM (2017) E-cigarettes as a source of toxic and potentially carcinogenic metals. Environmental Research, 152, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul S, Aherrera A, et al. (2018) Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environmental Health Perspectives, 126(2), 027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. (2014) Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control, 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, et al. (2018) Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Network Open, 1(8), e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CORESTA. Method Number 81. Routine Analytical Machine for E-cigarette Aerosol Generation and Collection – Definitions and Standard Conditions; Paris, France, 2015; pp. 1–6. https://www.coresta.org/routine-analytical-machine-e-cigarette-aerosol-generation-and-collection-definitions-and-standard [Google Scholar]
- 11.Taylor JK Quality Asssurance of Chemical Measurements; Lewis Publishers: Boca Raton, LA, 1987; pp. 79–81. [Google Scholar]
- 12.Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH (2015) Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine & Tobacco Research, 17, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Department of Health and Human Services. (1999) Notice regarding requirement for annual submission of the quantity of nicotine contained in smokeless tobacco products manufactured, imported, or packaged in the United States. Federal Register, 64, 14086–14096. [PubMed] [Google Scholar]
- 14.Pappas RS, Gray N, Halstead M, Valentin-Blasini L, Watson C (2020) Toxic metal-containing particles in aerosols from pod-type electronic cigarettes. Journal of Analytical Toxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappas RS, Fresquez MR, Martone N, Watson CH (2014) Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. Journal of Analytical Toxicology, 38, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pappas RS (2011) Toxic elements in tobacco and in cigarette smoke: inflammation and sensitization. Metallomics, 3, 1181–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.JUUL USA Quality and Manufacturing. What Materials Makeup JUUL and the JUUL Pods? https://support.juul.com/hc/en-us/articles/360023319634-What-materials-makeup-JUUL-and-the-JUULpods (accessed Feb 7, 2020).
- 18.WIRED Brand Lab for Vuse. Vuse Takes Vaping to the Next Level with the Alto. https://www.wired.com/brandlab/2018/11/vuse-alto-new-heights-d/index-ads.html?intcid=polar&utm_medium=nativetile&utm_source=polar (accessed Feb 7, 2020).
- 19.Zervas E, Matsouki N, Kyriakopoulos G, Poulopoulos S, Ioannides T, Katsaounou P (2020) Transfer of metals in the liquids of electronic cigarettes. Inhalation Toxicology, 32, 240–248. [DOI] [PubMed] [Google Scholar]
- 20.Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, et al. (2017) Benzene formation in electronic cigarettes. PLoS One, 12, e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker RR (1981) Product formation mechanisms inside a burning cigarette. Progress in Energy and Combustion Science, 7, 135–153. [Google Scholar]
- 22.Fresquez MR, Pappas RS, Watson CH (2013) Establishment of toxic metal reference range in tobacco from US cigarettes. Journal of Analytical Toxicology, 37, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fresquez MR, Gonzalez-Jimenez N, Gray N, Valentin-Blasini L, Watson CH, Pappas RS (2017) Electrothermal vaporization-QQQ-ICP-MS for determination of chromium in mainstream cigarette smoke particulate. Journal of Analytical Toxicology, 41, 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao D, Navas-Acien A, Ilievski V, Slavkovich V, Olmedo P, Adria-Mora B, et al. (2019) Metal concentrations in electronic cigarette aerosol: effect of open-system and closed-system devices and power settings. Environmental Research, 174, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]


