Abstract
The aim of the study was to evaluate the potential of autologous bone marrow‐derived nucleated cells to enhance the rate of healing of full‐thickness excisional skin wounds in rabbits. The study was conducted on 20 New Zealand white rabbits of either sex. Two, 2 × 2 cm full‐thickness skin (thoracolumabar region) excisional wounds were created; one on each side of the dorsal midline in each animal. The wounds were randomly assigned to either injection of autologous bone marrow‐derived nucleated cells into the wound margins (BI), or topical application of sterile saline solution (normal saline, NS), which served as control. The wound healing was assessed by evaluation of granulation tissue formation, wound contraction, epithelisation and histopathological and histochemical changes up to 28 days after creation of the wound. Granulation tissue appeared significantly faster in BI‐treated wounds (3.22 ± 0.22 days) than in NS‐treated wounds (4.56 ± 0.47 days). Better epithelisation was seen histologically in BI wounds than in NS‐treated wounds. Wound contraction was significantly more in BI wounds when compared with NS wounds on 21 post‐surgery. Histopathological examination of the healing tissue showed early disappearance of inflammatory reaction, significantly more neovascularisation, and more fibroplasias and early lay down and histological maturation of collagen in BI wounds than in control wounds. It was concluded that injection of autologous bone marrow‐derived nucleated cells in the wound margins induced faster and better quality healing of excisional skin wounds in rabbits when compared with normal saline. The injection of autologous bone marrow‐derived nucleated cells can be used to promote healing of large full‐thickness skin wounds in rabbits.
Keywords: Bone marrow‐derived cells, Rabbit, Skin, Wound healing
INTRODUCTION
There are four stages of the process of wound healing, that is, inflammation, debridement, repair and remodelling, and maturation, which are clearly differentiated from each other but overlap in time (1). Despite appreciable advances in understanding the basic principles of wound healing, problems in wound healing continue to cause significant morbidity and suffering in animals. Bone marrow‐derived stromal cells (BMSCs) exhibit extraordinary degree of plasticity and growth factor repertoire (2). Experimental study in mice suggested that adult bone marrow cells gave rise to epidermal keratinocytes, follicular epithelial cells, sebaceous gland cells, dendritic cells, and endothelial cells after transplantation 3, 4. In addition, treatment with BMSCs increased the expression of growth factors critical to proper repair and regeneration of the damaged tissue 5, 6. Researchers have also noted that bone marrow‐derived mesenchymal stem cells when injected around the wound or applied to the wound bed of in excisional wounds significantly enhanced wound healing in normal and diabetic mice (7). Clinical trials in people suggested that bone marrow‐derived cells (BMDCs) applied directly to non healing chronic wounds of greater than one year's duration led to dermal rebuilding and healing (8).
The purpose of the study reported here was to test the hypothesis that bone marrow‐derived nucleated cells injected into the wound margins in excisional wounds of rabbits can enhance the rate of wound healing.
METHODS
Animals
Twenty clinically healthy New Zealand white rabbits of either sex, weighing from 920 to 2300 g (mean weight 1487 ± 377·54 g) and between 3 and 6 months of age, were used. The Institute Animal Ethics Committee of Indian Veterinary Research Institute Izatnagar, India approved the study. All the animals were procured from Laboratory Animals Research (LAR) section, Animal Genetics Division of Indian Veterinary Research Institute, Izatnagar (UP), India. Animals were housed individually, provided with commercial diet and water ad libitum and maintained under uniform conditions. Animals were acclimatised to approaching and handling for a period of 10–15 days before the start of the study.
Animal preparation
Wound creation and bone marrow aspiration were carried out aseptically under general anaesthesia using xylazine (6 mg/kg intramuscularly) followed, 10 minutes later, by ketamine (60 mg/kg intramuscularly). Anaesthesia was maintained by additional dose of intravenous ketamine, if required. Enrofloxacin (5 mg/kg intravenously) and meloxicam (0·2 mg/kg intravenously) were administered preoperatively in all the animals. The animals were restrained in lateral recumbency for collection of bone marrow aspirate and in sternal recumbency for creation of experimental wounds. For the purpose of bone marrow collection and creation of wounds, the antero‐medial aspect of each proximal tibia and the dorsum (thoracolumbar region), respectively, were prepared for aseptic surgery.
Bone marrow aspiration and separation of nucleated cells
Aspiration of bone marrow was done under aseptic condition from the tibial metaphysis. An 18‐gauge biopsy needle was inserted with little force through the skin and muscle of anteriomedial aspect of proximal tibia. Once the needle (with stylet in place) had contacted with the bone, it was advanced further deep by rotating it slowly with steady pressure until the cortical bone was penetrated and the marrow cavity was entered. Usually a sudden change to the penetration force of the needle was felt at this point, which indicated that the needle was in the marrow cavity. The stylet of the biopsy needle was removed and it was connected to a 10‐ml syringe containing 1000 IU of heparin and a negative pressure was applied by forcefully pulling back the plunger. Approximately 2·5 ml bone marrow aspirate was collected from each tibia. Bone marrow‐nucleated cells were collected from the marrow aspirate by volume reduction centrifuge ‘buffy coat' protocol (9). While collecting the buffy coat, it was ascertained that no trace of buffy coat was left in the tube and even a small quantity of plasma with red blood cells (RBCs) was aspirated along with buffy coat. It was recentrifuged for 10 minutes at 313 g to further reduce the volume. The concentrated nucleated cells were mixed with 0·5 ml phosphate buffered saline (PBS) for application to the wounds.
Bone marrow‐nucleated cell count
Bone marrow‐nucleated cell count (BMNC) cells were counted randomly in two samples to have an estimate of the number of cells being transplanted to the site of wound. The buffy coat was collected and diluted with PBS and centrifuged at 1252 g for 10 minutes. The PBS was removed by decanting and aspiration, and the pelleted cells at the bottom with some RBCs were diluted four times and centrifuged at 4000 rpm for 10 minutes. Resuspension of the pelleted cells and counting were as per the method described by earlier researchers (10). The average number of cells counted by this method was 1·4 × 108 cells/ml.
Wound creation
Using a clean transparency sheet template and a permanent marker, the vertices of the experimental wounds of 2 × 2 cm dimensions were outlined on the dorsolumbar region of the rabbits. Two full‐thickness skin wounds including subcutaneous tissue, one on each side of the midline, were excised with a #11 BP blade in each animal. The wounds were created 2 cm away from the midline on the two sides of the dorsum. The wounds were created at the same location on the trunk of each animal regardless of the differences in the body size. Haemorrhage, if any, was controlled by applying pressure with sterile cotton gauze. The two treatments bone marrow‐derived nucleated cells into the wound margins (BI) and normal saline (NS) were assigned to the wounds randomly. In the wounds designated as BI group, bone marrow cells diluted with 0·5 ml of PBS were loaded in a 1‐ml sterile syringe and the contents of the syringe were then injected subcutaneously around the wound edges through a 26 G hypodermic needle. In the wounds designated as NS group, normal saline was applied topically using a piece of sterile cotton gauze. These wounds were taken as control. The antibiotic and an anti‐inflammatory‐analgesic (meloxicam) were administered in all the animals for three consecutive days after the surgery. The animals were observed for restlessness, dullness, loss of appetite, panting, vocalisation and elevated respiratory and heart rates, if any, for 1 week and for wound healing up to 28 days.
Evaluation of wound healing
Granulation tissue
Granulation tissue evaluation was performed daily up to day 7 and then on days 14, 21 and 28 after surgery and categorised with some modifications as: (1) no granulation tissue seen, (2) granulation tissue depressed below the skin edge, (3) granulation tissue proliferated to the level of skin edges, (4) granulation tissue elevated above skin edges and (5) granulation tissue elevated above skin edges, projecting over the advancing border of epithelium (11). A granulation score of 3 was considered better than 4 or 2, which were considered better than 5 or 1.
Time of appearance of granulation tissue was recorded as the first day when the granulation tissue was observed.
Wound contraction
Wound contraction was calculated on 3rd, 7th, 14th, 21st and 28th postoperative days as a percentage reduction in wound area. Progressive decrease in the wound area was monitored periodically by tracing the wound margin on a tracing paper and the area assessed by using graph paper. The total open wound area at each tracing was subtracted from that of the initial tracing to determine the area of contraction and epithelisation during the period since wounding. The area of contraction since wounding was divided by the total wound area of the initial wound tracing and multiplied by 100 to calculate the percentage of wound contraction 12, 13. The wound area, percentage and the mean percentage of wound contraction were calculated in four animals for each interval for both groups of wounds. These four animals were then used for biopsy collection and excluded from the study.
Time of complete healing
Time of complete healing was recorded as the day on which wound healed completely. Healing was considered complete when epithelium covered the entire wound and the area of the remaining granulation tissue was zero (11). The number of days required for each type of wound to heal completely was recorded and mean days to complete healing were calculated for both treatment wounds.
Histomorphological and histochemical evaluation
Full‐thickness skin tissue samples, including about 1‐cm normal skin around the healing wounds from both treatment group, were collected from four animals each on 3rd, 7th, 14th, 21st and 28th postoperative days. After collection of tissues, these wounds were repaired by suturing and the animals were excluded from the study. The tissues were fixed in 10% buffered formalin. After fixation, the tissues were processed by paraffin‐embedding technique to obtain 4–5 µm thick paraffin sections. The sections were stained with haematoxylin and eosin (H&E) stain as per the standard procedure (14). The H&E sections were evaluated microscopically by using histological scoring system with some modifications 15, 16. The histological parameters viz. epithelisation, inflammation, fibroblast were scored 1–4 (1 standing for best similarity to normal skin and 4 for least similarity) and neovascularisation‐score: 1, resembling normal skin, 0–1 new blood vessels; 2, mild, 2–5 blood vessels; 3, moderate, 6–10 blood vessels and 4, severe, greater than 10 new blood vessels.
Time of epithelisation was recorded as the day when the epithelium was seen histologically for the first time. The duplicate sections of both types of wounds were stained using special staining techniques viz. Masson's Trichrome stain (17) for detection and grading of collagen fibres in the healing wound. The scoring (18) was done as follows: collagen fibre: density ‐score: 1, denser, 2, dense and 3, less dense; thickness ‐score: 1, thicker, 2, thick and 3, thin; arrangement ‐score: 1, best arranged, 2, better, 3, worse and 4, worst arrangement. The mean score of each parameter was calculated for each treatment group and compared.
Statistical analysis
The means of objective parameters were compared by analysis of variance (ANOVA), whereas data from subjective scorings were compared by Kruskal–Wallis one‐way ANOVA (19). For each comparison, differences between groups were considered significant at P < 0·05.
RESULTS
Postoperatively, the animals appeared comfortable as evidenced by normal appetite and behaviour and stable heart and respiratory rates.
Granulation tissue
The time (day) of appearance of granulation tissue was significantly (P < 0·05) less in BI‐treated wounds (3·22 ± 0·22 days) than that in NS‐treated wounds (4·56 ± 0·47 days) wounds. No significant (P > 0·05) difference was seen in the level of granulation tissue in the wounds of the two treatment groups at different intervals.
Epithelium
There was no evidence of epithelisation in NS treatment wounds, whereas regenerating epithelium was detected in one of four wounds in BI wounds on day 3 after surgery. Epithelium was evident histologically in the sections taken from the centre of the wounds in 75% and 100% of the wounds on days 7 and 14 postoperation, respectively, in both BI and NS wounds.
Wound contraction
Although the original wound were created measuring 2 cm × 2 cm dimensions, all the wounds expanded to variable extents possibly because of loose nature of skin of the dorsum in rabbits; thus, after the surgery all the wounds had an area in excess of 4 cm2 (Table 1). However, there was no significant (P > 0·05) difference in the mean of wound area between the groups.
Table 1.
Mean ± SE value of wound area (cm2) and percent wound contraction (in parenthesis) in excisional wounds of rabbits *
| Treatment | Post‐wounding days | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | |
| NS | 5.53 ± 0.28a (0.00) | 4.75 ± 0.27a (14.10) | 3.33 ± 0.30a (39.78) | 1.79 ± 0.37a (67.63) | 0.41 ± 0.17a (92.59) | 0.07 ± 0.04 (98.73) |
| BI | 6.04 ± 0.40a (0.00) | 5.23 ± 0.37a (13.41) | 3.81 ± 0.38a (36.92) | 1.63 ± 0.46a (73.00) | 0.16 ± 0.16b (97.35) | 0.00 ± 0.00 (100) |
BI, bone marrow‐derived nucleated cells into the wound margins; NS, normal saline.
*Values with different superscripts in the same column are significantly different (P < 0.05), and values in the parenthesis indicate percentage of wound contraction.
The wound area decreased gradually in both treatment wounds and the rate of wound although the rate of wound contraction was faster in BI wounds when compared with NS wounds from seventh day onwards. The difference in wound area was significant (P < 0·05) between BI and NS on day 21 postoperation where mean percent contraction of 97·35% and 92·59%, respectively, was recorded. By day 28, difference in the mean wound size and percent contraction between the groups was not significant (P > 0·05), but 100% contraction was recorded in BI‐treated group only.
Time of complete healing
None of the wounds healed completely by 14th day of surgery in two treatment groups. Of the eight animals observed for wound healing, on 21st day after wounding, complete healing was observed in 75% animals in BI treatment group and in 37·5% animals of NS treatment group. On 28th post‐wounding day, the percentage of completely healed wounds treated by BI and NS were 100% and 75%, respectively, of the four animals observed for wound healing at this stage (Table 2).
Table 2.
Number and percentage of wound healed completely in excisional wounds of rabbits*
| Treatments | Between 14 and 21 days (n = 8) | Between 21 and 28 days (n = 4) |
|---|---|---|
| NS | 3/8 (37.50%) | 3/4 (75.00%) |
| BI | 6/8 (75.00%) | 4/4 (100.00%) |
BI, bone marrow‐derived nucleated cells into the wound margins; NS, normal saline.
* n = number of wounds; number in the parenthesis indicates percentage of wound completely healed.
Histomorphological and histochemical evaluation
Day 3
Granulation tissues were wider and compact in BI‐treated wounds when compared with NS (Figure 1.). The mean score values of inflammation were significantly (P < 0·05) higher (i.e. more inflammatory reaction) in the NS group than that in the BI group. With regard to neovascularisation, significantly (P < 0·05) higher scores (i.e. more neovascularisation) were recorded in BI‐treated wounds than in NS‐treated wounds. Although statistically insignificant, the mean score values for fibroblasts were higher in BI than in control group (Table 3).
Figure 1.
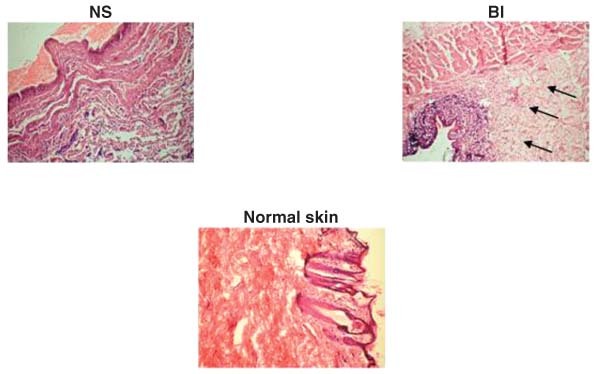
Photomicrographs showing healing pattern on day 3 wounds treated with normal saline (NS), and autologous bone marrow‐derived cells injected to wound margins (BI). Healing tissue in BI‐treated wound was better with compact and wider granulation tissue (arrows) than NS‐treated wound (H&E sections 10×, day 3).
Table 3.
Mean ± SE score values of histopathological and histochemical parameters of excisional wounds of rabbits in various treatment groups at different intervals *
| Parameters | Post‐wounding days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | ||||||
| NS | BI | NS | BI | NS | BI | NS | BI | NS | BI | |
| Epithelialisation † | 4.00 ± 0·00 | 3.75 ± 0·25 | 2.50 ± 0·50 | 2.75 ± 0·48 | 2.50 ± 0·50 | 2.00 ± 0·00 | 3.00 ± 0·00 | 3.00 ± 0·00 | 3.75 ± 0·25a | 1.50 ± 0·29b |
| Inflammation ‡ | 4.00 ± 0·00a | 3.00 ± 0·00b | 2.50 ± 0·29a | 1.25 ± 0·25ab | 2.50 ± 0·29 | 1.00 ± 0·00 | 2.25 ± 0·25 | 1.50 ± 0·29 | 1.50 ± 0·50 | 1.00 ± 0·00 |
| Fibroblast § | 2.33 ± 0·33 | 3.00 ± 0·50 | 2.50 ± 0·29 | 3.50 ± 0·29 | 3.00 ± 0·00 | 4.00 ± 0·00 | 4.00 ± 0·08 | 3.25 ± 0·25 | 2.75 ± 0·48 | 2.75 ± 0·25 |
| Neovascularisation ‖ | 1.00 ± 0·00a | 2.50 ± 0·29b | 2.50 ± 0·29 | 3.75 ± 0·25 | 3.00 ± 0·00 | 3.00 ± 0·00 | 3.00 ± 0·00 | 2.00 ± 0·00 | 3.25 ± 0·25a | 1.50 ± 0·29b |
| Collagen fibre density ¶ | 3.00 ± 0·00 | 3.00 ± 0·00 | 2.75 ± 0·25 | 2.25 ± 0·25 | 2.50 ± 0·29 | 2.50 ± 0·29 | 2.75 ± 0·25 | 2.00 ± 0·00 | 2.75 ± 0·25ab | 1.50 ± 0·29b |
| Collagen fibre thickness ** | 3.00 ± 0·00 | 3.00 ± 0·00 | 3.00 ± 0·00 | 2.75 ± 0·25 | 3.00 ± 0·00 | 2.50 ± 0·29 | 3.00 ± 0·00 | 2.50 ± 0·29 | 2.50 ± 0·29a | 2.00 ± 0·00b |
| Collagen fibre arrangement † , † | 3.00 ± 0·00 | 3.75 ± 0·25 | 2.25 ± 0·25 | 3.00 ± 0·41 | 2.50 ± 0·87 | 1.75 ± 0·48 | 2.50 ± 0·29 | 1.50 ± 0·29 | 1.75 ± 0·25 | 1.00 ± 0·00 |
BI, bone marrow‐derived nucleated cells into the wound margins; NS, normal saline.
*Values of the two groups differ significantly, if possess different superscripts within the same row at the specific day of observation (P < 0·05).
†Epithelialisation graded as: 1, resembling normal skin; 2, slightly thick to normal skin; 3, moderately thick to normal skin; 4, thicker than normal skin.
‡Inflammation: 1, resembling normal skin; 2, mild; 3, moderate; 4, severe.
§Fibroblast: 1, resembling normal skin; 2, mild; 3, moderate; 4, severe.
‖Neovascularisation: 1, resembling normal skin, 0–1 new blood vessels; 2, mild, 2–5 new blood vessels; 3, moderate, 6–10 new blood vessel; 4, severe, >10 new blood vessels.
¶Collagen fibre density (1–3): 1, denser; 2, dense; 3, less dense.
**Collagen fibre thickness (1–3): 1, thicker; 2, thick; 3, thin.
††Collagen fibre arrangement (1–4): 1, best arranged; 2, better; 3, worse; 4, worst arrangement.
Day 7
As on day 3 after surgery, there was significantly (P < 0·05) more inflammatory reaction in NS‐treated wounds than the BI‐treated wounds. Higher mean score values for fibroblasts and neovascularisation as well as denser and thicker collagen fibres were recorded in BI‐treated wounds than in NS‐treated wounds, but the difference was not significant (P > 0·05) (Table 3; Figure 2).
Figure 2.
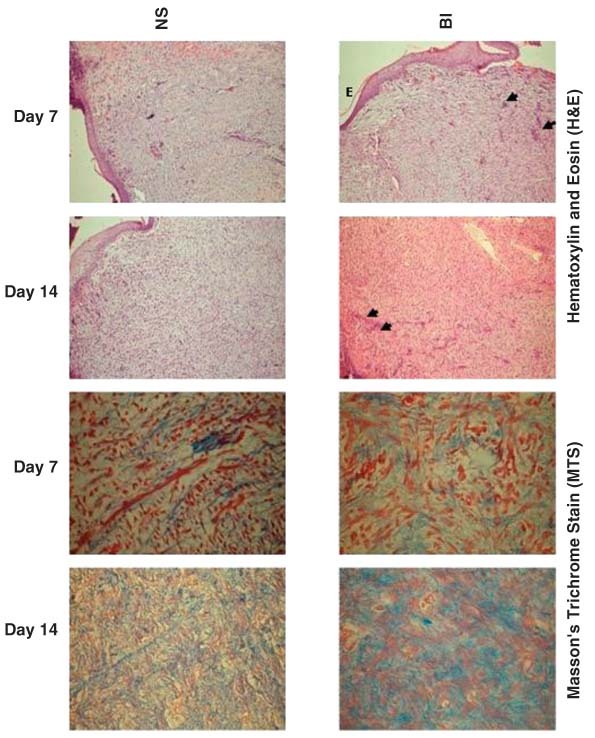
Photomicrographs of granulation tissue of control (normal saline, NS) and bone marrow‐derived nucleated cells into the wound margins (BI) treated animals showing thick epithelium covering wider surface (E), mild to nil inflammatory reaction and more neovascularisation (arrow heads) by days 7 and 14 (shown in H&E) and moderate deposition of collagen fibres which are denser and thicker in the sections from BI group (arrow head) than in NS groups (shown in MTS) (HE 10× and MTS sections, 40×, days 7 and 14).
Day 14
In comparison with NS group, BI‐treated wounds had more dense and thick collagen fibres and showed more fibroblasts of collagen phenotype (Figure 2).
Day 21
The mean scores of inflammation, fibroblast, neovascularisation, collagen fibre density, collagen fibre thickness and collagen fibre arrangement were lower in BI‐treated wounds than in NS‐treated wounds; but no significant (P > 0·05) difference were found in mean scores of any parameter between the treatments (Table 3; Figure 3).
Figure 3.
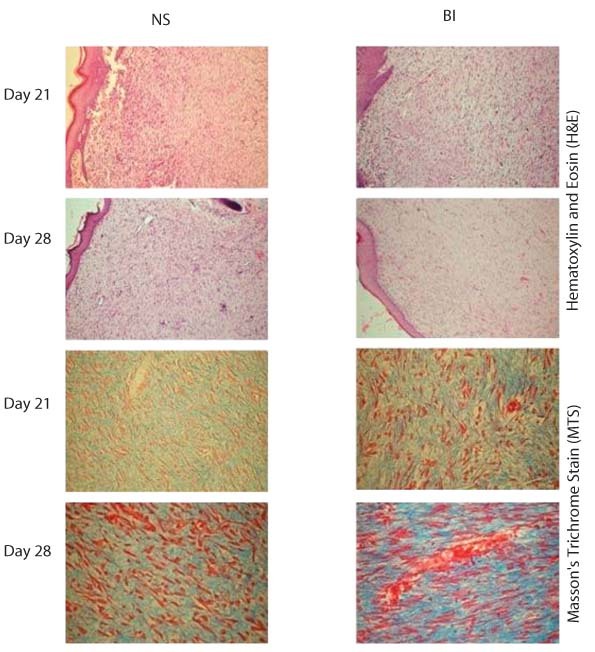
Photomicrographs of granulation tissue from normal saline (NS), and bone marrow‐derived nucleated cells into the wound margins (BI) treated wounds showing histologically more mature granulation tissue of the wounds treated by BI than by NS. Sections of granulation tissue from BI‐treated wounds showed denser and thicker collagen fibres and lesser fibroblasts than that of NS on days 21 and 28 (H&E 10× and MTS 40×, days 21 and 28).
Day 28
Significant (P < 0·05) difference in the mean scores values of neovascularisation, collagen fibre density and thickness was recorded during this period (Table 3). Better epithelisation was seen in BI‐treated wounds than in NS‐treated wounds. BI‐treated wounds had significantly less neovascularisation than the control (NS). Collagen fibres were significantly (P < 0·05) thicker in BI‐treated wounds than in NS‐treated wounds (Figure 3).
DISCUSSION
Bone marrow cells show the broadest differentiation potential among adult somatic cell populations and are considered as one of the most promising cell sources for clinical applications 3, 4, 20. Bone marrow also contains mesenchymal stem cells (BMSCs) that secrete a large number of growth factors and cytokines, which are critical to proper repair and regeneration of damaged (5).
Considering the reported capacity of bone marrow cells to secrete growth factors and cytokines and the ability to trans‐differentiate it to the healing tissue components, we hypothesised that there would be quantitatively and qualitatively better healing in the wounds‐treated bone marrow cells compared with the control. It was assumed that excisional wounds treated with BI would heal faster with more granulation tissue formation, epithelisation, rapid wound contraction, early histological maturation and complete healing than control group.
The injection of bone marrow cells in BI wounds might have caused some trauma and caused inflammation leading to release of cytokines, which may affect wound healing. However, the injection of bone marrow cells was made slowly using a fine hypodermic needle to minimise the trauma. Furthermore, the injection trauma was considered negligible compared with the surgical trauma inflicted in creation of wounds and thus may be of little significance.
Grossly, the granulation tissue appeared significantly faster in wounds treated with BI than in control group. These findings were also supported by the histopathological findings of early deposition of relatively wider granulation tissues with more cellularity in BI‐treated wounds than in control wounds. This might be attributable to the role of BMNCs in BI group as studies have shown that BMNCs accelerate granulation tissue genesis 9, 21.
Full‐thickness skin wound healing occurs by granulation tissue formation, contraction and epithelisation 22, 23, 24. Epithelisation occurs by migration of undamaged epidermal cells from the wound margins across the granulation bed 1, 25. Recently, there is evidence that adult bone marrow cells transplanted in skin defect had differentiated into epidermal keratinocytes, sebaceous gland cells, follicular epithelial cells, dendritic cells, and endothelial cells and fully differentiated skin with hair was reconstituted within 3 weeks (26). Many researchers also suggested that bone marrow stem cells can produce new skin cells 4, 21. In the present study, early and better epithelisation observed histologically in sections from BI‐treated wounds when compared with control wounds could also be attributed to differentiation of bone marrow cells into epidermal cells 4, 21, 26 or enhanced proliferation of resident epidermal cells in the presence of epidermal growth factor, possibly produced by bone marrow cells (5). Wound contraction plays very important role in the healing of excisional skin wounds. There are two theories of wound contraction that have been proposed in the past. The ‘picture frame’ theory of wound contraction states that myofibroblasts located in the wound margins of an open wound are responsible for the centripetal forces that lead to wound contraction 24, 27. On the other hand, the ‘pull theory’ suggests that fibroblasts distributed throughout the granulation tissue generate the forces responsible for contraction (28). More recently, it has been suggested that wound contraction occurs through a combination of these two processes (29). It was suggested that circulating fibrocytes in the blood are not major sources of wound myofibroblasts, rather an in vivo experiment showed that bone marrow cells differentiated into wound myofibroblasts after they entered the microenvironment of the wound 30, 31. Another study showed that adult, BMDCs participated in wound repair by differentiating into wound fibroblasts (32). In the present study, many of the BMNCs, which were injected into the wound margins, might have differentiated into myofibroblasts and fibroblasts or recruited more fibroblasts from the surrounding tissues through chemotaxis (2) that resulted in significantly early and fast wound contraction and early closure of full‐thickness wounds in BI group than control group.
It was suggested that histopathological assessment of mode and rate of healing in open wounds allows more precision than clinical examination (33). Inflammation is necessary for healing as it plays a role in combating infection and inducing the proliferation phase, but healing proceeds only after inflammation is controlled 34, 35. Thus early disappearance of inflammation in BI wounds in the present study might have facilitated the progress to the next phase of wound healing. Because the activity of fibroblasts and epithelial cells requires oxygen, angiogenesis is imperative for other stages in wound healing, such as epidermal and fibroblast migration. Neovascularisation occurs concurrently with fibroblast proliferation when endothelial cells, originating from parts of uninjured blood vessels, migrate to the area of the wound (36). Bone marrow stem cells are reported to release factors that recruit macrophages and endothelial lineage cells into wound (37). In another study, it was suggested that bone marrow cells enhance neovascularisation and promote wound healing through differentiation and release of pro‐angiogenic factors (8). This might be the main reason for the significantly higher neovascularisation in early phases of wound healing in BI wounds than control wounds.
The fibroblast is the connective tissue cell responsible for collagen deposition that is needed to repair the tissue injury (1). In the present study, also there were more fibroblasts up to day 14 post‐surgery, better collagen formation throughout the observation period were recorded in the BI‐treated wounds than in the NS‐treated wounds. These findings are in agreement with the earlier findings (38) where BMDCs were able to contract a collagen matrix and transcribe both collagen types I and III. In addition, BMSCs secrete several growth factors critical to repair of damaged tissues (5).
Compared with NS, BI‐treated wounds showed more compact and thicker granulation tissue with less fibroblasts and better‐arranged fibrocytes, and denser and thicker collagen fibres by day 21, which indicated maturation phase of the wound healing. By day 28 post‐wounding, there were significantly denser and thicker collagen fibres in the BI wounds than in the control wounds. Epithelisation was also more comparable with the normal skin in the BI wounds than the control wounds. All these findings indicate early histological maturation of test wounds than the controls, which conform to the observations of earlier researchers, who reported that the wounds treated with BMSCs were found to be histologically mature earlier when compared with the untreated wounds (39). Early deposition and maturation of collagen in BI wound may also be attributable to the reported interaction of BMSCs with fibroblasts, which results in promotion of growth and chemotaxis of fibroblasts (2).
It was concluded that injection of bone marrow‐derived nucleated cells to the wound margins facilitated early and better healing than the normal saline. Histological and histochemical observations showed that the bone marrow cells augmented wound healing activity significantly by increasing cellular proliferation, formation of granulation tissue, neovascularisation, synthesis of collagen, epithelisation and early histological maturation in excisional wounds. Bone marrow‐nucleated cells can be used clinically in the management of large skin wounds in rabbits.
REFERENCES
- 1. Fossum TW, Hedlund CS, Johnson AL, Schulz KS, Seim HB, Willard MD, Baer A, Carroll GL. Manual of small animal surgery. St Louis, MO: Mosby, 2007. [Google Scholar]
- 2. Dulchavsky D, Gao X, Liu YB, Deeb D, Arbab AS, McIntosh K, Dulchavsky SA, Gautam SC. Bone marrow‐derived stromal cells (BMSCs) interact with fibroblasts in accelerating wound healing. J Invest Surg 2008;21:270–9. [DOI] [PubMed] [Google Scholar]
- 3. Goodell MA, Jackson KA, Majka SM, Mi T, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK. Stem cell plasticity in muscle and bone marrow. Ann N Y Acad Sci 2001;938:208–18. [DOI] [PubMed] [Google Scholar]
- 4. Krause DS, Theise ND, Collector MI, Hengariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi‐organ, multi‐lineage engraftment by a single bone marrow–derived stem cell. Cell 2001;105:369–77. [DOI] [PubMed] [Google Scholar]
- 5. Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, Bansal M, Chopp M, McIntosh K, Arbab AS, Dulchavsky SA, Gautam SC. Treatment with bone marrow‐derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 2008;3:453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salvolini E, Lucarini G, Zizzi A, Orciani M, Di Benedetto G, Di Primio R. Human skin‐derived mesenchymal stem cells as a source of VEGF and nitric oxide. Arch Dermatol Res 2009; December 25 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Wu Y, Chen L, Scott PG, Tredget EE. Translational and clinical research: mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–59. [DOI] [PubMed] [Google Scholar]
- 8. Badiavas FV, Falanga V. Treatment of chronic wounds with bone marrow–derived cells. Arch Dermatol 2003;139:510–6. [DOI] [PubMed] [Google Scholar]
- 9. Kasten P, Luginbuhl R, Feebuer K, Suda AJ, Egermann M, Gasser B, Beyen I. Instant mesenchymal stem cell therapy: how can we do it? Characterization and concentration of mesenchymal stem cells in vitro. Orthop Surg Euro Cells Mater Suppl 2007;14:39. [DOI] [PubMed] [Google Scholar]
- 10. Barker PE, Knoblock KF. Bovine costimulator. II. Generation and maintenance of bovine costimulator‐dependent bovine lymphoblastoid cell lines. Vet Immunol Immunopathol 1982;3:381–92. [DOI] [PubMed] [Google Scholar]
- 11. Bigbie RB, Schumacker J, Swaim SF, Purohit KC, Wright JC. Effect of amnion and yeast cell derivate on second intention healing in horses. Am J Vet Res 1991;52:1376. [PubMed] [Google Scholar]
- 12. Bohling MW, Henderson RA, Swaim SF, Kincaid SV, Wright JC. Cutaneous wound healing in the cat: a macroscopic description and comparison with cutaneous wound healing in the dog. Vet Surg 2004;33:579–87. [DOI] [PubMed] [Google Scholar]
- 13. Majeske C. Reliability of wound surface area measurements. Phys Ther 1992;72:138–41. [DOI] [PubMed] [Google Scholar]
- 14. Bancroft JD, Cook HC. Manual of histological techniques, 2nd edn. Edinburg: Churchill Livingston, 1984. [Google Scholar]
- 15. Parameshwaraiah S, Shivakumar HG. Evaluation of topical formulations of aqueous extracts of Centella asiatica on open wound in rats. Indian J Exp Biol 1998;36:569–72. [PubMed] [Google Scholar]
- 16. Smith MJ, Haltom JD, Gainor BJ. Effects of anticoagulation on wound healing using a tensile test. Orthopedics 2008;31:373. [DOI] [PubMed] [Google Scholar]
- 17. Masson PJ. Some histological methods: trichrome stainings and their preliminary techniques. J Tech Methods 1929;12:75–90. [Google Scholar]
- 18. Ghamsari SM, Acorda JA, Taguchi K, Abe N, Ymada H. Evaluation of wound healing of the teat with and without low level laser therapy in dairy cattle by laser Doppler flowmetry in comparison with histopathology, tensiometry and hydroxyproline analysis. Br Vet J 1996;152:583–91. [DOI] [PubMed] [Google Scholar]
- 19. Snedecor GW, Cochran WG. Statistical methods, 8th edn. New Delhi: Oxford and IBH Publishing Company, 1989. [Google Scholar]
- 20. Poulsom R, Alison MR, Forbes SJ, Wright NA. Adult stem cell plasticity. J Pathol 2002;197: 441–56. [DOI] [PubMed] [Google Scholar]
- 21. Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 2003;196:245–50. [DOI] [PubMed] [Google Scholar]
- 22. Johnston DE. Skin and subcutaneous tissue. In: Bojrab MJ, editor. Pathophysiology of small animal surgery. Philadelphia: Lea and Febiger, 1981:405–24. [Google Scholar]
- 23. Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994;130:489. [PubMed] [Google Scholar]
- 24. Waldron DR, Zimmerman‐Pope N. Superficial skin wounds. In: Slatter DH, editor. Textbook of small animals surgery, 3rd edn, Vol. 2. Philadelphia: Saunders, 2003:259–92. [Google Scholar]
- 25. Deodhar AK, Rana DA. Surgical physiology of wound healing: a review. J Postgrad Med 1997;43:52–6. [PubMed] [Google Scholar]
- 26. Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, Huh N. Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol 2003;163:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grillo HC, Watts GT, Gross J. Studies in wound healing: I. Contraction and the wound contents. Ann Surg 1958;148:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abercrombie M, Flint MH, James DW. Wound contraction in relation to collagen formation in scorbutic Guinea‐pigs. J Embryol Exp Morphol 1956;4:167–75. [Google Scholar]
- 29. Swaim SF, Hinkle SH, Bradley DM. Wound contraction: basic and clinical factors. Compend Contin Educ Prac Vet 2001;23:20–34. [Google Scholar]
- 30. Abe R, Donnelly SC, Peng T, Bucula R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–62. [DOI] [PubMed] [Google Scholar]
- 31. Yamaguchi Y, Kubo T, Murakami T, Takhashi M, Hakamata Y, Kobayashi E, Yoshda S, Hosokowa K, Yoshikawa K, Etami S. Bone marrow cells differentiate into wound myofibroblasts and accelerate the healing of wound with occlusive dressing. Br J Dermatol 2005;152:616–22. [DOI] [PubMed] [Google Scholar]
- 32. Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow‐derived cells during wound repair. FASEB J 2005;19:1561–3. [DOI] [PubMed] [Google Scholar]
- 33. Abramo F, Mantis P, Lloyd DH, Auxilia ST, Noli C, Leotta R, Pfeiffer D. An evaluation of methods for the assessment of healing of open wounds in the dog. Vet Dermatol 2004;15:13. 14989700 [Google Scholar]
- 34. Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 2004;36:1031–7. [DOI] [PubMed] [Google Scholar]
- 35. Diegelmann RF, Evans NC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 36. Kuwahara RT, Rasberry R. Chemical peels. URL http://www.Emedicine.com [accessed on 15 September 2007].
- 37. Chen L, Tredget EE, Wu PYG, WY. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008;3: e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow–derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22: 812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Y, Dulchavsky DS, Xiaohua GD, Kwon D, Chopp M, Dulchavsky S, Gautam SC. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res 2006;136:336–41. [DOI] [PubMed] [Google Scholar]


