Abstract
The aim of this study was to explore the effects of supplementing paraformic acid (PFA) to the diet of broiler chickens on intestinal development, inflammation, and microbiota. A total of 378 healthy 1-day-old Arbor Acres broilers with similar birth weight were used in this study, and randomly assigned into two treatment groups. The broiler chickens were received a basal diet or a basal diet supplemented with 1,000 mg/kg PFA. Results showed that PFA supplementation increased (P < 0.05) small intestinal villus height and villus height/crypt depth ratio, elevated intestinal mucosal factors (mucin 2, trefoil factor family, and zonula occludens-1) concentrations, and upregulated mNRA expression of y + L amino acid transporter 1. Moreover, PFA supplementation decreased (P < 0.05) the concentrations of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and interleukin-10), activities of caspase-3 and caspase-8, and mNRA expressions of Toll-like Receptor 4, nuclear factor-kappa B, Bax, and Bax/Bcl-2 ratio in small intestinal mucosa. Dietary PFA supplementation also increased (P < 0.05) alpha diversity of cecal microbiota and relative abundance of Alistipes. The present study demonstrated that supplementation of 1,000 mg/kg PFA showed beneficial effects in improving intestinal development, which might be attributed to the suppression of intestinal inflammation and change of gut microbiota composition in broiler chickens. These findings will aid in our knowledge of the mechanisms through which dietary PFA modulates gut development, as well as support the use of PFA in poultry industry.
Keywords: chickens, inflammation, microbiota, organic acid, small intestine
Introduction
A healthy and well-developed gut is essential for nutrient absorption and serves as a necessary barrier against pathogen invasion (Kogut et al., 2017). However, with the rapid expansion of the scale of intensive farming worldwide, poultry production faces increasing challenges, such as pathogenic bacteria, environmental variables, and feed hygiene, which raise the risks of intestinal diseases in broiler chickens (Qian et al., 2018; Caekebeke et al., 2020). Previous studies have demonstrated that intestinal diseases can limit intestinal development, cause dysfunction of intestinal digestion and absorption, and induce an intestinal flora imbalance, resulting in growth restriction, disease, and even mortality in broiler chickens (Roberts et al., 2015; Shi et al., 2018). For many years, the subtherapeutic use of antibiotic growth promoters (AGP) has been an economically viable means of enhancing animal performance (Bedford, 2000). With the prohibition of AGP due to its significant negative effects on environmental conditions and human health, it is critical to find new feed additives that promote intestinal health and development in poultry production (Castanon, 2007; Muaz et al., 2018).
Acidifiers such as pure organic acids have been used as feed preservatives for decades to prevent microbial and fungal destruction of feedstuffs (Hu et al., 2019; Zhang et al., 2019; Abd El-Hack et al., 2022). Growing studies suggested that organic acids could be used as powerful tool in maintaining gut health by suppressing the proliferation of acid intolerance bacteria, such as E. coli and Clostridium perfringens, leading to improvement of growth performance in poultry (Gharib et al., 2012; Ateya et al., 2019; Hu et al., 2019). It was reported that dietary supplementation with formic acid (FA) benefited to growth performance, immune function, intestinal development, and microbiological characteristics of broilers (Hernández et al., 2006; Garcia et al., 2007; Ragaa and Korany, 2016). However, FA has a strong, pungent odor and corrosiveness to gastrointestinal tract, which limit its usage in animal husbandry (Luise et al., 2020). Recently, FA salts and its derivatives, such as calcium formate, benzoic acid, and potassium diformate, have deserved more and more attention in poultry production due to their little or no corrosive effect and also efficient against harmful microorganisms (Izat et al., 1990; Józefiak et al., 2010; Ragaa and Korany, 2016). Paraformic acid (PFA) is a hyperpolymer formed by dehydration polymerization between two FA molecules. It has not been reported that whether PFA can promote intestinal development of broiler chickens.
Therefore, the aim of this study was to explore the effects of supplementing PFA to the diet of broiler chickens on intestinal development, and provide a reference for the use of PFA in poultry industry.
Materials and methods
Animals and diets
A total of 378 healthy 1-day-old Arbor Acres broilers with similar birth weight (48.47 ± 0.43 g) were used in this study. All broilers were randomly assigned into two treatment groups with seven replicates of 27 broiler chickens each and housed in three-level wired cages placed in a light- and temperature-controlled room with constant illumination in a 42-day study. The treatment groups were as follows: broiler chickens received a basal diet (CON group), or broiler chickens received a basal diet supplemented with 1,000 mg/kg PFA (PFA group). The basal diets (Supplementary Table 1) were formulated based on nutrient requirements of the National Research Council [NAC] (1994), and PFA was provided by Omega Nutrition Group (Spain) & Numega Nutrition Pte. Ltd. The broilers were fed according to a two-phase feeding program (0–21 day and 21–42 day), and had free access to feed and water throughout the experiment. A Newcastle disease vaccine and an inactivated infectious bursal disease vaccine were inoculated on days 7 and 14 of the trial, respectively. The temperature of the room was maintained at 35°C at the first week, and then gradually reduced to 21°C at the rate of 0.5°C daily.
Sample collection
On day 42 of experiment, one broiler from each replicate (7 birds per group) with similar body weight (BW) to the cage average were selected to collect intestinal tissue samples after being narcotized by CO2 asphyxiation. Intestinal segments with a length of 2 cm were cut from the medium of small intestine, and fixed in 4% paraformaldehyde for 24 h after being flushed gently with a 0.9% saline solution. Then mucosal tissue was carefully scraped with a sterile glass slide from the medium of small intestine that had been washed using ice-cold saline solution, and subsequently stored at −80°C after being chilled in liquid nitrogen. Besides, the cecal contents were collected and placed in sterile bags immediately, and stored at -80°C for microbiological analysis.
Measurements of intestinal morphology
The fixed intestinal segments were dehydrated in ethanol and xylene solutions, and embedded according to conventional paraffin-embedding protocol, followed by being cut into 5-μm thin slices using Leica semi-automatic microtome (Leica Co., Wetzlar, Germany). Then the slices were processed by hematoxylin and eosin staining. The intestinal morphology was examined, and villus height (VH), crypt depth (CD) and VH/CD ratio were analyzed according to the method described in Chen et al. (2021).
Determination of intestinal mucosal barrier factors and total protein concentrations
Intestinal mucosal barrier factors including mucin 2 (MUC2), trefoil factor family (TFF), transforming growth factor-α (TGF-α), and zonula occludens-1 (ZO-1) were determined with the specific ELISA kits (Jiangsu Meimian Industrial Co., Ltd., Jiangsu, China), following the manufacturer’s instructions strictly. Briefly, the supernatants of intestinal mucosa samples were harvested after being homogenized in ice-cold saline solution (1:9, wt/vol) and centrifugated at 12,000 × g for 15 min. Then 50 μl of diluted standard solutions and supernatants were added to the prepared microplates, respectively, and incubated 30 min at 37°C after being sealed with microplate sealers. The HRP-Conjugate reagent (50 μl) was added to each well after the microplate was washed five times with Wash Buffer, and the microplate was incubated 30 min at 37°C again. After being washed five times again, the wells were added with Chromogenic solution A and B (50 μl each) in order, and the microplate was allowed to stand at 37°C for 10 min under dark condition after gently shaking. Finally, the optical density of each well was determined in 15 min using a microplate reader set at 450 nm, following addition of Stop Solution (50 μl). The concentrations of MUC2, TFF, TGF-α, and ZO-1 were normalized to each sample’s total protein concentration which was quantified with a BCA protein assay (Jiangsu Meimian Industrial Co., Ltd.) (Lang et al., 2022).
Determination of intestinal mucosal caspases activities
Intestinal mucosal activities of caspase-3, caspase-8, and caspase-9 were determined with the specific ELIAS kits purchased from Beyotime Biotech (Shanghai, China) as descried previously (Chen et al., 2021), and normalized to each sample’s total protein concentration.
Determination of intestinal mucosal inflammatory cytokines and secretory immunoglobulin A concentrations
The intestinal mucosal concentrations of tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), interferon-γ (IFN-γ), and secretory immunoglobulin A (SIgA) were examined with ELISA kits (R&D Systems Inc., Minneapolis, MN, United States) according to the detection steps of ELISA operation described in Chen et al. (2021). Inflammatory cytokines and SIgA concentrations in intestinal mucosa were normalized to each sample’s total protein concentration.
Determination of relative mRNA expression in intestinal mucosa
The mRNA expression levels of occludin (OCLN), claudin 2 (CLDN2), claudin 3 (CLDN3), ZO-1, glucose transporter 2 (GLUT2), Na + /glucose cotransporter (SGLT1), y + L amino acid transporter 1 (y + LAT1), fatty acid binding protein (FABP1), cationic amino acid transporter 1 (CAT1), Bax, Bcl-2, Toll-like Receptor 4 (TLR4), and nuclear factor-kappa B (NF-κB) in intestinal mucosa samples were assessed using a CFX-96 real-time PCR detection system (Bio-Rad, Hercules, CA, United States). Primer sequences used for real-time PCR in this study are shown in Supplementary Table 2. The detailed procedure of the relative mRNA expression determination was described in Zhang P. et al. (2021). The β-actin gene was amplified in parallel as the internal control for gene normalization and quantification. The 2–Δ Δ Ct method was used to calculate the relative mRNA abundances of target genes in intestinal mucosa samples. All samples were measured in triplicate, and product sizes and quantities were determined by agarose gel electrophoresis.
Determination of pH values of cecal digesta
The determination of pH values of cecal digesta was conducted as previously described in Li et al. (2020) using the pH meter (PHS-3C PH, Shanghai, China).
Microbial analysis
Total genomic DNA was isolated from cecal digesta by the Omega Bio-tek E.Z.N.A. ™ stool DNA kit (Norcross, GA, United States), followed by DNA concentration and purity examination by agarose gel electrophoresis (Chen et al., 2021). Briefly, the V4 hypervariable region of 16S rDNA was amplified by using 515F and 806R primer (Li et al., 2019). The produced library quality was examined using a Qubit 2.0 Fluorometer (Thermo Fisher Scientific, United Kingdom). Sequencing of the library was carried out on the Illumina HiSeq PE2500 platform at the Novogene Bioinformatics Technology Co., Ltd. (Beijing, China), after which 250 bp paired-end sequences were generated. Paired-end sequences were merged using FLASH (v1.2.7) (Magoè and Salzberg, 2011), and the chimera sequences were removed through comparing with the Silva database using UCHIME algorithm to obtain the effective sequences after quality filtering on the raw tags (Edgar et al., 2011). Sequences were clustered into operational taxonomic units (OTUs) at least 97% sequence similarity using Uparse software (Edgar, 2013), and taxonomic information was annotated using the Silva Database based on Mothur algorithm (Quast et al., 2013). Alpha diversity (including observed species, Shannon index, Simpson index, ACE index, and Chao 1 index) and beta diversity were used to analyze complexity of species diversity for a sample and differences of samples in species complexity, respectively. Principal Coordinate Analysis (PCoA) was performed based on bray-curtis distances to visualize the dissimilarity matrices of OTUs, and the analysis of similarity (ANOSIM) was applied to examine the significant differences among the microbial communities.
Statistical analyses
The individual chicken data were used to access the effects on all variables. Statistical analyses for all data were performed with t-test of SAS (9.4 Inst. Inc., Cary, NC, United States). The Shapiro-Wilk W statistic was applied to check normality of the data, and the data that were not normally distributed were transformed to achieve approximated normality. Spearman’s correlation and linear regression analysis were used to assess the associations between bacterial abundance and intestinal mucosal concentrations of immunological markers, as well as between microorganisms. Values are expressed as mean ± standard error. Statistical significance was set at P < 0.05, and a trend toward significance was considered at 0.05 ≤ P < 0.10.
Results
Intestinal morphology
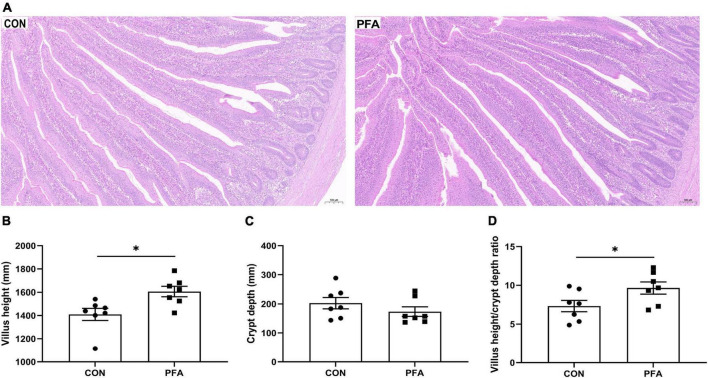
The effects of PFA supplementation on intestinal morphology are presented in Figure 1. Compared with CON group, the villus in PFA group showed thicker and denser (Figure 1A). Broilers fed the diet supplemented with PFA had significantly higher (P < 0.05) intestinal VH (Figure 1B) and VH/CD ratio (Figure 1D) than broilers fed the CON diet. There was no significant difference (P > 0.05) in intestinal CD (Figure 1C) between broilers fed the CON diet and the PFA diet.
FIGURE 1.
Effect of dietary paraformic acid supplementation on intestinal morphology in broiler chickens. (A) Hematoxylin and eosin photomicrographs obtained at 100 × magnification; (B) Villus height; (C) Crypt depth; (D) Villus height/crypt depth ratio. CON, broiler chickens fed basal diet; PFA, broiler chickens fed basal diet supplemented with 1,000 mg/kg paraformic acid. Values are mean ± standard error (n = 7). Differences between treatments were displayed by *P < 0.05.
Intestinal mucosal barrier functions, apoptosis regulators, and inflammatory factors concentrations
Dietary supplementation with 1,000 mg/kg PFA significantly increased (P < 0.05) MUC2 (Figure 2A), TFF (Figure 2B), and ZO-1 (Figure 2C) concentrations, and significantly decreased (P < 0.05) activities of caspase-3 (Figure 2D) and caspase-8 (Figure 2E) and concentrations of TNF-α (Figure 2F), IL-1β (Figure 2G), IL-6 (Figure 2H), and IL-10 (Figure 2I) in intestinal mucosa of broilers. Besides, PFA supplementation tended to decrease (P < 0.10) intestinal mucosal IFN-γ concentration compared with CON group (Figure 2J). There were no significant differences (P > 0.05) in TGF-α and SIgA concentrations as well as caspase-9 activity in intestinal mucosa of broilers (Supplementary Figure 1).
FIGURE 2.
Effects of dietary paraformic acid supplementation on intestinal mucosal barrier functions, apoptosis regulators, and inflammatory factors concentrations in broiler chickens. (A) Mucin (MUC2); (B) Trefoil factor family (TFF); (C) Zonula occludens-1 (ZO-1); (D) Caspase-3; (E) Caspase-9; (F) Tumor necrosis factor-alpha (TNF-α); (G) Interleukin-1beta (IL-1β); (H) Interleukin-6 (IL-6); (I) Interleukin-10 (IL-10); (J) Interferon-γ (IFN-γ). CON, broiler chickens fed basal diet; PFA, broiler chickens fed basal diet supplemented with 1,000 mg/kg paraformic acid. Values are mean ± standard error (n = 7). Differences between treatments were displayed by #0.05 ≤ P < 0.10, *P < 0.05, and **P < 0.01.
Genes expressions in intestinal mucosa
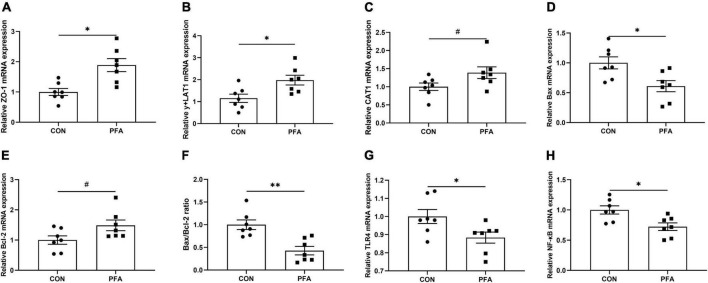
As shown in Figure 4, PFA supplementation in broiler diet significantly increased (P < 0.05) ZO-1 (Figure 3A) and y + LAT1 (Figure 3B) mRNA expressions, and tended to increase (P < 0.05) CAT1 (Figure 3C) and Bcl-2 (Figure 3E) mRNA expression in intestinal mucosa. Besides, PFA group showed significantly lower (P < 0.05) mucosal Bax (Figure 3D), TLR4 (Figure 3G) and NF-κB (Figure 3H) mRNA expressions as well as Bax/Bcl-2 ratio (Figure 3F). There were no significant differences (P > 0.05) in the mRNA expressions of OCLN, CLDN2, CLDN3, GLUT2, SGLT1, and FABP1 in intestinal mucosa (Supplementary Figure 2).
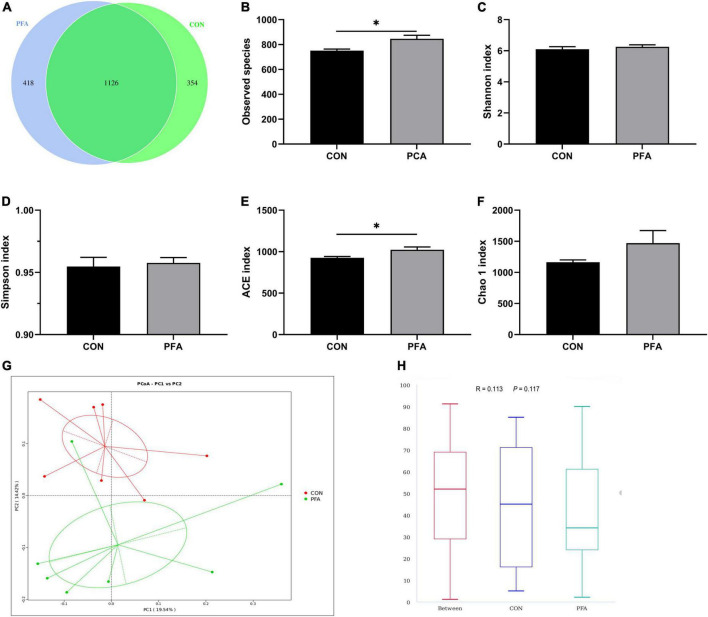
FIGURE 4.
Effect of dietary paraformic acid supplementation on diversity and richness of cecal microbiota in broilers. (A) A Venn diagram generated to display the common and unique operational taxonomic units between two groups; (B) Observed species; (C) Shannon index; (D) Simpson index; (E) ACE index; (F) Chao 1 index; (G) The principal coordinate analysis (PCoA) profile of bray_curtis distance; (H) Analysis of similarity. CON, broiler chickens fed basal diet; PFA, broiler chickens fed basal diet supplemented with 1,000 mg/kg paraformic acid. Values are mean ± standard error (n = 7). Differences between treatments were displayed by *P < 0.05.
FIGURE 3.
Effect of dietary paraformic acid supplementation on relative mRNA expression in intestinal mucosa of broilers. (A) Zonula occludens-1 (ZO-1); (B) y + L amino acid transporter 1 (y + LAT1); (C) Cationic amino acid transporter 1 (CAT1); (D) Bax; (E) Bcl-2; (F) Bax/Bcl-2 ratio; (G) Toll-like Receptor 4 (TLR4); (H) Nuclear factor-kappa B (NF-κB). CON, broiler chickens fed basal diet; PFA, broiler chickens fed basal diet supplemented with 1,000 mg/kg paraformic acid. Values are mean ± standard error (n = 7). Differences between treatments were displayed by #0.05 ≤ P < 0.10, *P < 0.05, and **P < 0.01.
Cecal pH of digesta
Effect of dietary paraformic acid supplementation on pH values of cecal digesta in broiler chickens is shown in Table 1. Broilers in PFA group had significantly lower (P < 0.05) cecal pH values than those in CON group.
TABLE 1.
Effect of dietary paraformic acid supplementation on pH values of cecal digesta in broiler chickens.
| Items, % | Treatment1 |
P-value | |
| CON | PFA | ||
| pH value | 6.70 ± 0.07 | 6.43 ± 0.08 | 0.034 |
Values are mean ± standard error (n = 7). Differences were considered statistically significant when P < 0.05.
1CON, broilers fed a basal diet; PFA, broilers fed a basal diet supplemented with 1,000 mg/kg paraformic acid.
Microbial diversity in cecal digesta
As shown in Supplementary Table 3, a total of 825,033 total tags, 778,291 taxon tags, 13 unclassified tags, 56,729 unique tags, and 12,147 OTUs were obtained from 14 cecal digesta samples of two treatment groups. Moreover, the species accumulation curves (Supplementary Figure 3) tend to flatten with analyzed sequences number increasing up to 14, demonstrating that our samples were sufficient for OTU testing and prediction of species richness of samples. The bacteria community diversity and richness are shown in Figure 4. The overall OTUs of cecal bacteria differ between groups, and broilers in PFA group showed an increased number of OTUs (Figure 4A). The two groups shared 1,129 common OTUs. Compared with CON group, PFA group showed significantly higher (P < 0.05) observed species (Figure 4B) and ACE index (Figure 4E). No significant differences were observed (P > 0.05) in Shannon index (Figure 4C), Simpson index (Figure 4D), and Chao 1 index (Figure 4F). The PCoA plot (Figure 4G) drawn based on the bray_curtis distances revealed that the PFA samples dispersed far apart with the CON samples, and ANOSIM (Figure 4H) showed the two groups had significantly different bacterial community structures (P < 0.05).
Relative abundance of cecal microbiota
The top 10 phyla in relative abundance of cecal microbiota are shown in shown in Supplementary Table 4. The most predominant phyla in cecal samples of broilers are Bacteroidetes and Firmicutes. Dietary PFA supplementation significantly decreased (P < 0.05) Halobacterota abundance, and tended to decrease (P < 0.10) Campylobacterota abundance.
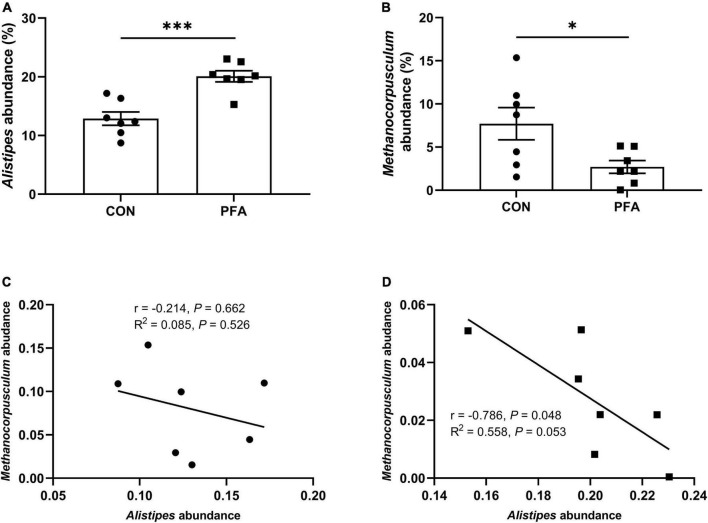
The relative abundance at genus level in broiler cecal microbiota (top 35 genera) in shown in Figure 5 and Supplementary Table 5. The identified most plentiful genera in cecal samples were Alistipes, Bacteroides, and Desulfovibrio. PFA group had significantly higher (P < 0.05) Alistipes abundance and significantly lower (P < 0.05) Methanocorpusculum abundance than CON group. Besides, PFA group tended to had lower (P < 0.10) abundances of Helicobacter, Christensenellaceae_R-7_group, and Ruminococcus in cecal samples than CON group. In PFA group, the Alistipes abundance was significantly negatively (P = 0.048) correlated with Methanocorpusculum abundance, and the Methanocorpusculum abundance tended to (P = 0.053) be linearly decreased with the Alistipes abundance increasing. No significant correlation (P > 0.05) between Alistipes abundance and Methanocorpusculum abundance was observed in cecal digesta of CON group.
FIGURE 5.
Relative abundance of cecal microbiota (at the genus level) that are significantly different between two treatments in broilers. (A,B) Relative abundances of Alistipes and Methanocorpusculum. Differences between treatments were displayed by *P < 0.05 and ***P < 0.001. Values are mean ± standard error. (C,D) The correlation between the Alistipes and Methanocorpusculum abundances for CON group and PFA group based on Spearman’s correlation test and simple linear regression analysis. Statistical significance was set at P < 0.05. CON, broiler chickens fed basal diet; PFA, broiler chickens fed basal diet supplemented with 1,000 mg/kg paraformic acid, n = 7.
Spearman’s correlation analysis between Alistipes and Methanocorpusculum abundances and mucosal immunological markers concentrations
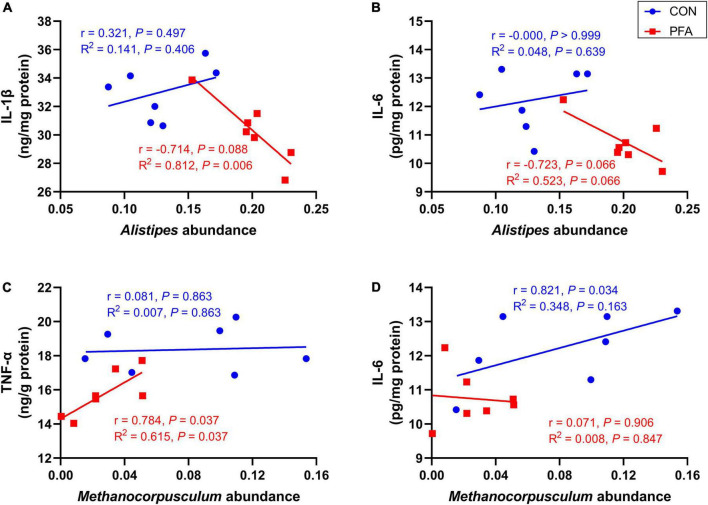
As shown in Figure 6 and Supplementary Figure 4, intestinal mucosal concentrations of IL-1β and IL-6 tended to decrease (P < 0.10) with increasing relative abundance of Alistipes in broilers of PFA group. The relative abundance of Methanocorpusculum was significantly positively (P < 0.05) correlated with mucosal TNF-α concentration of PFA group and IL-6 concentration of CON group, and intestinal mucosal TNF-α concentration increased linearly (P = 0.037) as Methanocorpusculum abundance increased in PFA group.
FIGURE 6.
Correlation analysis between Alistipes and Methanocorpusculum abundances and mucosal immunological markers concentrations. (A,B) Correlation analysis between Alistipes and interleukin-1beta (IL-1β) and interleukin-6 (IL-6) for CON group and PFA group; (C,D) Correlation analysis between Methanocorpusculum and tumor necrosis factor-alpha (TNF-α) and IL-6 for CON group and PFA group. Correlation analysis was based on Spearman’s test and simple linear regression. Statistical significance was set at P < 0.05, n = 7.
Discussion
Intestinal morphology and barrier integrity are two crucial indicators of intestinal development (Li et al., 2020; Beumer and Clevers, 2021). In the present study, PFA supplementation in the diet increased VH and VH/CD ratio of small intestine. Previous study also demonstrated that broilers fed the diets supplemented with FA or potassium diformate had higher intestinal VH than those fed a basal diet (Abd El-Hack et al., 2022). The small intestine is the principal organ in charge of nutrient absorption, and the crypt-villus structure is responsible for efficient nutrition intake (Beumer and Clevers, 2021). The VH and VH/CD are related to the digestion and absorption capacity of the intestine (Chen et al., 2021). Consistently, compared with CON group, PFA group had higher intestinal expressions of y + LAT1 and CAT1 which were responsible for amino acids transport (Closs et al., 2006; del Amo et al., 2008). Garcia et al. (2007) reported that dietary FA supplementation significantly increased the digestibility of crude protein. It might suggest that PFA supplementation was helped to enhance the digestion and absorption capacity of the intestine in this study. Besides, we also found that PFA supplementation increased ZO-1, MUC2, and TFF levels in small intestinal mucosa in the current study. As a regulator of paracellular permeability in epithelia and endothelia, the major component of tight junction protein (TJP) ZO-1 plays an important role in maintaining intestinal barrier function and defensing systemic inflammatory diseases through interacting with the gap, actin cytoskeleton, and adherens junction proteins (Dai et al., 2020; Lai et al., 2021). Trefoil factor family and mucins are typical exocrine products of mucous epithelia. Mucin2, predominantly produced by the goblet cells, is the principal mucin in the small intestine and located throughout the surface of the intestinal epithelium (Liu et al., 2020). Recent researches demonstrated that MUC2 is involved in intestinal barrier protection, microbiome homeostasis regulation, and diseases prevention (Kim and Khan, 2013; Liu et al., 2020). Trefoil factor family peptides are typically co-secreted together with mucins, and play vital roles in mucosal innate immune defense, mucosal repair, and prevention of the infiltration of microorganisms (Hoffmann, 2020). It was also reported that organic acids (mainly containing FA, formate ammonia, propionate, acetate, and lactate) supplementation enhanced duodenal TJPs (including ZO-1 and CLDN2) in broilers (Ma et al., 2021). A compound acidifier blend of calcium formate, calcium citrate and calcium lactate with 7:2:1 ratio could increase the expressions of MUC2, OCLN, and CLDN3 in jejunal mucosa of broilers (He et al., 2020). Above all, dietary PFA supplementation could promote small intestinal development through improving intestinal morphology and barrier integrity.
Excessive apoptosis of epithelial cells is an important cause of intestinal mucosal barrier damage (Chen et al., 2021). Caspases are an evolutionary conserved family of cysteine proteases which are centrally involved in inflammation responses and cell death (Abd El-Hack et al., 2022). In the present study, we found that supplementation of 1,000 mg/kg PFA decreased activities of caspase-3 and caspase-8 in small intestinal mucosa. Caspase-8, serving as an initiator caspase, is important player in activation of extrinsic apoptotic pathway (Weng et al., 2014). Caspase-3, an executioner caspase, can be activated by caspase-8 and initiate the process of apoptosis (Van Opdenbosch and Lamkanfi, 2019). Pro-apoptotic Bax and anti-apoptotic Bcl-2 are two important proteins in the Bax family, and upregulation of Bax/Bcl-2 ratio has been proven to increase the activation of caspase-3 and caspase-8 cascade (Du et al., 2014). Consistently, dietary PFA supplementation decreased the expressions of Bax, Bcl-2, and Bax/Bcl-2 ratio in current study. Therefore, our results of this study suggested that PFA supplementation in the diet could improve intestinal morphology and barrier integrity through suppressing the activation of extrinsic apoptotic pathway in small intestinal mucosa.
Inflammatory response is usually associated with excessive cell apoptosis, leading to the intestinal mucosal barrier damage and gastrointestinal disorders (Van Opdenbosch and Lamkanfi, 2019). It was reported that inflammatory cytokines such as TNF-α, IL-1β, IFN-γ, and IL-6 could induce cell apoptosis (Idriss and Naismith, 2000; Kuo et al., 2021; Ren et al., 2021). In the present study, dietary PFA supplementation decreased concentrations of TNF-α, IL-1β, IL-6, IL-10, and IFN-γ in small intestinal mucosa of broilers. Tumor necrosis factor-alpha, IL-1β, and IFN-γ are three major pro-inflammatory cytokines implicated in the pathogenesis of many inflammatory-associated diseases (Idriss and Naismith, 2000; Jorgovanovic et al., 2020; Kuo et al., 2021). Moreover, TNF-α, IL-1β, and IFN-γ can also activate the generation of pro-inflammatory IL-6 which contributes to the pathogenesis of various diseases, such as inflammation, autoimmunity, and cancers (Suzuki et al., 2000; Raeburn et al., 2002; Ishimaru et al., 2013; Yao et al., 2014). Interleukin-10 is a cytokine with anti-inflammatory properties. Under inflammatory condition, increased IL-6 concentration often accompanied an increased IL-10 concentration (Chun et al., 2007). Therefore, the results of our study indicated that dietary PFA supplementation decreased inflammatory response in small intestinal mucosa of broilers. To further explore the mechanisms underlying the inhibition of intestinal inflammatory response by PFA in broiler chickens, TLR4/NF-κB signaling pathway-related genes expressions were examined in this study. Toll-like receptor 4 is a crucial regulator of inflammatory reactions, whose activation triggers its downstream effector NF-κB, which translocates to the nucleus and upregulates the expressions of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IFN-γ (Wei et al., 2015; Ye et al., 2021). In the present study, we found that PFA supplementation inhibited the expressions of TLR4 and NF-κB in small intestinal mucosa of broilers. Previous study has demonstrated that encapsulated essential oils and organic acids mixture (containing 4% carvacrol, 4% thyme, 0.5% hexanoic, 3.5% benzoic, and 0.5% butyric acid) supplementation can inhibit necrotic enteritis-induced increase in genes expressions of TLR4, IL-1β, and IFN-γ in the jejunum (Pham et al., 2022). Overall, dietary PFA supplementation could suppress the inflammation response in small intestinal mucosa partially via inhibiting TLR4/NF-κB signaling pathway.
Gut microbiota plays an important role in host gut health by improving gastrointestinal development, enhancing the immune function, and competitively suppressing pathogens (Ley et al., 2008; Camara-Lemarroy et al., 2018; Zhang S. et al., 2021). It has been proven that FA is efficient against pathogenic bacteria through reducing intestinal pH, leading to improvement of gut health in poultry (Hernández et al., 2006; Gharib et al., 2012; Ateya et al., 2019). In the present study, we also found that supplementation of 1,000 mg/kg PFA decreased pH value in cecal digesta of broilers. Besides, PFA supplementation increased the observed species and ACE index of cecal microbiota in broilers. Observed species and ACE index are two important indicators of alpha diversity, and used to calculate unique OTUs and estimate community richness, respectively (Li et al., 2020; Chen et al., 2021). Gut dysbiosis and gastrointestinal inflammatory disease are usually characterized by reduced bacterial richness (Clemente et al., 2018). The results in Song et al. (2022) showed that necrotic enteritis challenge increased intestinal inflammatory cytokine gene expression levels, inhibited intestinal development, and caused intestinal damage, as well as reduced alpha diversity of microbiota in ileum of broilers. Bacteroidetes and Firmicutes were the most predominant phyla in cecal samples of broilers in this study, which was in accord with the results of previous study (Cui et al., 2021). Importantly, dietary PFA supplementation increased the abundance of Alistipes that was the dominate genus, and decreased Methanocorpusculum abundance in cecal digesta of broilers. Zhang et al. (2022) also found that Alistipes was the most plentiful genera in cecum of broilers. Alistipes is classified as Gram-negative anaerobic rods, and found primarily in the gut of healthy humans (Shkoporov et al., 2015). Previous study showed that Alistipes finegoldii supplementation could decrease the severity of the colitis in mice (Dziarski et al., 2016). Besides, Zhang et al. (2022) reported that dietary rhamnolipids addition could enhance the immunity, improve intestinal barrier function, and increase the cecal abundance of Alistipes in broilers. The results suggested that Alistipes genus might have a protective role in inflammatory bowel disease. The correlation analysis in this study also showed that the relative abundance of Alistipes was negatively correlated with intestinal mucosal concentrations of pro-inflammatory IL-1β and IL-6 which were significantly decreased in PFA group. Previous studies suggesting that Alistipes was a short-chain fatty acids (SCFAs) producer (Oliphant and Allen-Vercoe, 2019), which might be contributed to the decreased pH in cecal digesta in this study. Short-chain fatty acids are identified as a principal energy source for intestinal epithelial cells and are known to strengthen the gut barrier function (Parada Venegas et al., 2019). It was reported that SCFAs could lower inflammation and oxidative stress through reducing intestinal permeability and circulating endotoxins (Kim et al., 2018). Besides, SCFAs may signal through cell surface G-protein coupled receptors to activate signaling cascades that regulate immune functions and production of cytokines (Vinolo et al., 2011). Methanocorpusculum was the predominant methanogen (Duan et al., 2014), and Methanocorpusculum abundance was positively correlated with intestinal mucosal pro-inflammatory TNF-α and IL-6 concentration. The decrease of Methanocorpusculum in PFA group might suggested that PFA supplementation benefited to the mitigation of methane emissions in poultry production and the decrease of inflammatory response in intestine of broilers. Therefore, the increased microbial diversity and beneficial bacteria might be another reason for the improved intestinal development in PFA broilers.
Conclusion
In conclusion, the present study demonstrated that supplementation of 1,000 mg/kg PFA showed beneficial effects in improving intestinal development and function including intestinal morphology, barrier integrity, and nutrients transformation. It might be attributed to the suppression of apoptosis though inhibiting intestinal inflammation partially via inactivating TLR4/NF-κB signaling pathway and change of gut microbiota composition in broiler chickens. These findings will aid in our knowledge of the mechanisms through which dietary PFA modulates gut development, as well as support the use of PFA in poultry industry.
Data availability statement
The data presented in this study are deposited in the NCBI Sequence Read Archive repository, accession number: PRJNA847797.
Ethics statement
This animal study was reviewed and approved by the Care and Use Committee of Shandong Agricultural University (protocol code SDAUA-2021-019).
Author contributions
NJ, WY, and YLi: conceptualization. JL, YLiu, and JN: data curation and project administration. YLiu and CJ: formal analysis. WY and YLi: funding acquisition, supervision, and writing – review and editing. CJ, NJ, LY, and YLi: investigation. YLiu, JN, NJ, LH, SJ, and YLi: methodology. JN, LH, and LY: resources. JL and YLiu: software and writing – original draft. SJ and YLi: validation. CJ and WY: visualization. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Shandong Province Pig Industry Technology System (SDAIT-08-04) and Postdoctoral Science Foundation of Shandong Agricultural University (040/76598).
Conflict of interest
JL was employed by Qingdao Huaxin Feed Co., Ltd. CJ was employed by Shandong Chinwhiz Co., Ltd. LY was employed by Shandong New Hope Liuhe Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.975056/full#supplementary-material
References
- Abd El-Hack M. E., El-Saadony M. T., Salem H. M., El-Tahan A. M., Soliman M. M., Youssef G. B. A., et al. (2022). Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird’s health and production. Poult. Sci. 101:101696. 10.1016/j.psj.2022.101696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateya A. I., Arafat N., Saleh R. M., Ghanem H. M., Naguib D., Radwan H. A., et al. (2019). Intestinal gene expressions in broiler chickens infected with Escherichia coli and dietary supplemented with probiotic, acidifier and synbiotic. Vet. Res. Commun. 43 131–142. 10.1007/s11259-019-09753-z [DOI] [PubMed] [Google Scholar]
- Bedford M. (2000). Removal of antibiotic growth promoters from poultry diets: implications and strategies to minimise subsequent problems. World’s Poult. Sci. J. 56 347–365. 10.1079/WPS20000024 36007395 [DOI] [Google Scholar]
- Beumer J., Clevers H. (2021). Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 22 39–53. 10.1038/s41580-020-0278-0 [DOI] [PubMed] [Google Scholar]
- Caekebeke N., Ringenier M., De Meyer F., Ducatelle R., Ongena N., Van Immerseel F., et al. (2020). A study on risk factors for macroscopic gut abnormalities in intensively reared broiler chickens. Avian. Pathol. 49 193–201. 10.1080/03079457.2019.1711019 [DOI] [PubMed] [Google Scholar]
- Camara-Lemarroy C. R., Metz L. M., Yong V. W. (2018). Focus on the gut-brain axis: Multiple sclerosis, the intestinal barrier and the microbiome. World J. Gastroenterol. 24 4217–4223. 10.3748/wjg.v24.i37.4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon J. I. (2007). History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 86 2466–2471. 10.3382/ps.2007-00249 [DOI] [PubMed] [Google Scholar]
- Chen J., Li F., Yang W., Jiang S., Li Y. (2021). Supplementation with exogenous catalase from Penicillium notatum in the diet ameliorates lipopolysaccharide-induced intestinal oxidative damage through affecting intestinal antioxidant capacity and microbiota in weaned pigs. Microbiol. Spectr. 9:e0065421. 10.1128/Spectrum.00654-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H. Y., Chung J. W., Kim H. A., Yun J. M., Jeon J. Y., Ye Y. M., et al. (2007). Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J. Clin. Immunol. 27 461–466. 10.1007/s10875-007-9104-0 [DOI] [PubMed] [Google Scholar]
- Clemente J. C., Manasson J., Scher J. U. (2018). The role of the gut microbiome in systemic inflammatory disease. Bmj 360:j5145. 10.1136/bmj.j5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs E. I., Boissel J. P., Habermeier A., Rotmann A. (2006). Structure and function of cationic amino acid transporters (CATs). J. Membr. Biol. 213 67–77. 10.1007/s00232-006-0875-7 [DOI] [PubMed] [Google Scholar]
- Cui L., Zhang X., Cheng R., Ansari A. R., Elokil A. A., Hu Y., et al. (2021). Sex differences in growth performance are related to cecal microbiota in chicken. Microb. Pathog. 150:104710. 10.1016/j.micpath.2020.104710 [DOI] [PubMed] [Google Scholar]
- Dai W., Nadadur R. D., Brennan J. A., Smith H. L., Shen K. M., Gadek M., et al. (2020). ZO-1 regulates intercalated disc composition and Atrioventricular node conduction. Circ. Res. 127 e28–e43. 10.1161/circresaha.119.316415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Amo E. M., Urtti A., Yliperttula M. (2008). Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur. J. Pharm. Sci. 35 161–174. 10.1016/j.ejps.2008.06.015 [DOI] [PubMed] [Google Scholar]
- Du L., Mei H. F., Yin X., Xing Y. Q. (2014). Delayed growth of glioma by a polysaccharide from Aster tataricus involve upregulation of Bax/Bcl-2 ratio, activation of caspase-3/8/9, and downregulation of the Akt. Tumour. Biol. 35 1819–1825. 10.1007/s13277-013-1243-8 [DOI] [PubMed] [Google Scholar]
- Duan Y. F., Al-Soud W. A., Brejnrod A., Sørensen S. J., Elsgaard L., Petersen S. O., et al. (2014). Methanotrophs, methanogens and microbial community structure in livestock slurry surface crusts. J. Appl. Microbiol. 117 1066–1078. 10.1111/jam.12584 [DOI] [PubMed] [Google Scholar]
- Dziarski R., Park S. Y., Kashyap D. R., Dowd S. E., Gupta D. (2016). Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS One 11:e0146162. 10.1371/journal.pone.0146162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10 996–998. 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V., Catala-Gregori P., Hernandez F., Megias M., Madrid J. (2007). Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poult. Res. 16 555–562. 10.3382/japr.2006-00116 [DOI] [Google Scholar]
- Gharib N. K., Rahimi S., Khaki P. (2012). Comparison of the effects of probiotic, organic acid and medicinal plant on Campylobacter jejuni challenged broiler chickens. J. Agric. Sci. Technol. 14 1485–1496. [Google Scholar]
- He J., Ma L., Qiu J., Lu X., Hou C., Liu B., et al. (2020). Effects of compound organic acid calcium on growth performance, hepatic antioxidation and intestinal barrier of male broilers under heat stress. Asian-Australas J. Anim. Sci. 33 1156–1166. 10.5713/ajas.19.0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández F., García V., Madrid J., Orengo J., Catalá P., Megías M. D. (2006). Effect of formic acid on performance, digestibility, intestinal histomorphology and plasma metabolite levels of broiler chickens. Br. Poult. Sci. 47 50–56. 10.1080/00071660500475574 [DOI] [PubMed] [Google Scholar]
- Hoffmann W. (2020). Trefoil Factor Family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: changing the paradigm. Int. J. Mol. Sci. 21:4535. 10.3390/ijms21124535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Wang L., Shao D., Wang Q., Wu Y., Han Y., et al. (2019). Selectived and reshaped early dominant microbial community in the cecum with similar proportions and better homogenization and species diversity due to organic acids as agp alternatives mediate their effects on broilers growth. Front. Microbiol. 10:2948. 10.3389/fmicb.2019.02948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss H. T., Naismith J. H. (2000). TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc. Res. Tech. 50 184–195. [DOI] [PubMed] [Google Scholar]
- Ishimaru M., Tsukimoto M., Harada H., Kojima S. (2013). Involvement of P2Y11 receptor in IFN-γ-induced IL-6 production in human keratinocytes. Eur. J. Pharmacol. 703 67–73. 10.1016/j.ejphar.2013.02.020 [DOI] [PubMed] [Google Scholar]
- Izat A. L., Adams M. H., Cabel M. C., Colberg M., Reiber M. A., Skinner J. T., et al. (1990). Effects of formic acid or calcium formate in feed on performance and microbiological characteristics of broilers. Poult. Sci. 69 1876–1882. 10.3382/ps.0691876 [DOI] [PubMed] [Google Scholar]
- Jorgovanovic D., Song M., Wang L., Zhang Y. (2020). Roles of IFN-γ in tumor progression and regression: a review. Biomark Res. 8:49. 10.1186/s40364-020-00228-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefiak D., Kaczmarek S., Rutkowski A. (2010). The effects of benzoic acid supplementation on the performance of broiler chickens. J. Anim. Physiol. Anim. Nutr. 94 29–34. 10.1111/j.1439-0396.2008.00875.x [DOI] [PubMed] [Google Scholar]
- Kim J. J., Khan W. I. (2013). Goblet cells and mucins: role in innate defense in enteric infections. Pathogens 2 55–70. 10.3390/pathogens2010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. A., Keogh J. B., Clifton P. M. (2018). Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 31 35–51. 10.1017/S095442241700018X [DOI] [PubMed] [Google Scholar]
- Kogut M. H., Yin X., Yuan J., Broom L. (2017). Gut health in poultry. CAB Rev. 12 1–7. [Google Scholar]
- Kuo W. C., Lee C. C., Chang Y. W., Pang W., Chen H. S., Hou S. C., et al. (2021). Structure-based Development of Human Interleukin-1β-Specific Antibody That Simultaneously Inhibits Binding to Both IL-1RI and IL-1RAcP. J. Mol. Biol. 433:166766. 10.1016/j.jmb.2020.166766 [DOI] [PubMed] [Google Scholar]
- Lai W. T., Lee H. C., Huang Y. H., Lo M. H., Kuo H. C. (2021). Tight junction protein ZO-1 in Kawasaki disease. BMC Pediatr. 21:157. 10.1186/s12887-021-02622-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang W., Cheng M., Zheng X., Zhao Y., Qu Y., Jia Z., et al. (2022). Forsythiaside A alleviates methotrexate-induced intestinal mucositis in rats by modulating the NLRP3 signaling pathways. Int. Immunopharmacol. 103:108466. 10.1016/j.intimp.2021.108466 [DOI] [PubMed] [Google Scholar]
- Ley R. E., Lozupone C. A., Hamady M., Knight R., Gordon J. I. (2008). Worlds within worlds: evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 6 776–788. 10.1038/nrmicro1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu H., Zhang L., Yang Y., Lin Y., Zhuo Y., et al. (2019). Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int. J. Mol. Sci. 21:31. 10.3390/ijms21010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao X., Zhang L., Zhan X., Liu Z., Zhuo Y., et al. (2020). Effects of a diet supplemented with exogenous catalase from Penicillium notatum on intestinal development and microbiota in weaned piglets. Microorganisms 8:391. 10.3390/microorganisms8030391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yu X., Zhao J., Zhang H., Zhai Q., Chen W. (2020). The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 164 884–891. 10.1016/j.ijbiomac.2020.07.191 [DOI] [PubMed] [Google Scholar]
- Luise D., Correa F., Bosi P., Trevisi P. (2020). A review of the effect of formic acid and its salts on the gastrointestinal microbiota and performance of pigs. Animals 10:887. 10.3390/ani10050887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Mahfuz S., Wang J., Piao X. (2021). Effect of dietary supplementation with mixed organic acids on immune function, antioxidative characteristics, digestive enzymes activity, and intestinal health in broiler chickens. Front. Nutr. 8:673316. 10.3389/fnut.2021.673316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoè T., Salzberg S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27 2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muaz K., Riaz M., Akhtar S., Park S., Ismail A. (2018). Antibiotic residues in chicken meat: global prevalence. threats, and decontamination strategies: a review. J. Food Prot. 81 619–627. 10.4315/0362-028x.Jfp-17-086 [DOI] [PubMed] [Google Scholar]
- National Research Council [NAC] (1994). Nutrient requirements of poultry, 9th edn. Washington, DC: National Academy Press. [Google Scholar]
- Oliphant K., Allen-Vercoe E. (2019). Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7:91. 10.1186/s40168-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M. K., Landskron G., González M. J., Quera R., Dijkstra G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10:277. 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V. H., Abbas W., Huang J., He Q., Zhen W., Guo Y., et al. (2022). Effect of blending encapsulated essential oils and organic acids as an antibiotic growth promoter alternative on growth performance and intestinal health in broilers with necrotic enteritis. Poult. Sci. 101:101563. 10.1016/j.psj.2021.101563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Song K., Hu T., Ying T. (2018). Environmental status of livestock and poultry sectors in China under current transformation stage. Sci. Total Environ. 1 702–709. 10.1016/j.scitotenv.2017.12.045 [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 D590–D596. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeburn C. D., Sheppard F., Barsness K. A., Arya J., Harken A. H. (2002). Cytokines for surgeons. Am. J. Surg. 183 268–273. 10.1016/s0002-9610(02)00781-x [DOI] [PubMed] [Google Scholar]
- Ragaa N. M., Korany R. M. S. (2016). Studying the effect of formic acid and potassium diformate on performance, immunity and gut health of broiler chickens. Anim. Nutr. 2 296–302. 10.1016/j.aninu.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Yan Y., Zhen L., Cao C., Wang Q., Zhang Y., et al. (2021). Zhike Pingchuan Granule suppresses interleukin (IL)-6 or the medium of M2 macrophages induced apoptosis in human bronchial epithelial cells. Bioengineered 12 7694–7703. 10.1080/21655979.2021.1982309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T., Wilson J., Guthrie A., Cookson K., Vancraeynest D., Schaeffer J., et al. (2015). New issues and science in broiler chicken intestinal health: Emerging technology and alternative interventions. J. Appl. Poult. Res. 24 257–266. 10.3382/japr/pfv023 [DOI] [Google Scholar]
- Shi S., Wu S., Shen Y., Zhang S., Xiao Y., He X., et al. (2018). Iron oxide nanozyme suppresses intracellular Salmonella Enteritidis growth and alleviates infection in vivo. Theranostics 8 6149–6162. 10.7150/thno.29303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkoporov A. N., Chaplin A. V., Khokhlova E. V., Shcherbakova V. A., Motuzova O. V., Bozhenko V. K., et al. (2015). Alistipes inops sp. nov. and Coprobacter secundus sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 65 4580–4588. 10.1099/ijsem.0.000617 [DOI] [PubMed] [Google Scholar]
- Song B., Li P., Yan S., Liu Y., Gao M., Lv H., et al. (2022). Effects of dietary astragalus polysaccharide supplementation on the Th17/Treg balance and the gut microbiota of broiler chickens challenged with necrotic enteritis. Front. Immunol. 13:781934. 10.3389/fimmu.2022.781934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Tetsuka T., Yoshida S., Watanabe N., Kobayashi M., Matsui N., et al. (2000). The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-alpha- or IL-1beta-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 465 23–27. 10.1016/s0014-5793(99)01717-2 [DOI] [PubMed] [Google Scholar]
- Van Opdenbosch N., Lamkanfi M. (2019). Caspases in cell death, inflammation, and disease. Immunity 50 1352–1364. 10.1016/j.immuni.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo M. A., Rodrigues H. G., Nachbar R. T., Curi R. (2011). Regulation of inflammation by short chain fatty acids. Nutrients 3 858–876. 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Dejie L., Xiaojing S., Tiancheng W., Yongguo C., Zhengtao Y., et al. (2015). Magnolol inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. Inflammation 38 16–26. 10.1007/s10753-014-0003-2 [DOI] [PubMed] [Google Scholar]
- Weng D., Marty-Roix R., Ganesan S., Proulx M. K., Vladimer G. I., Kaiser W. J., et al. (2014). Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl. Acad. Sci. U.S.A. 111 7391–7396. 10.1073/pnas.1403477111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Huang J., Zhong H., Shen N., Faggioni R., Fung M., et al. (2014). Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 141 125–139. 10.1016/j.pharmthera.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Ye Y., Wang P., Zhou F. (2021). miR-489-3p inhibits TLR4/NF-κB signaling to prevent inflammation in psoriasis. Exp. Ther. Med. 22:744. 10.3892/etm.2021.10176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Jing C., Liang M., Jiang S., Huang L., Jiao N., et al. (2021). Zearalenone exposure triggered cecal physical barrier injury through the TGF-β1/Smads signaling pathway in weaned piglets. Toxins 13:902. 10.3390/toxins13120902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Zhang H., Liu J., Zeng X., Wu Y., Yang C. (2022). Rhamnolipids enhance growth performance by improving the immunity, intestinal barrier function, and metabolome composition in broilers. J. Sci. Food Agric. 102 908–919. 10.1002/jsfa.11423 [DOI] [PubMed] [Google Scholar]
- Zhang S., Shen Y. R., Wu S., Xiao Y. Q., He Q., Shi S. R. (2019). The dietary combination of essential oils and organic acids reduces Salmonella enteritidis in challenged chicks. Poult. Sci. 98 6349–6355. 10.3382/ps/pez457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zhong G., Shao D., Wang Q., Hu Y., Wu T., et al. (2021). Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 100:100935. 10.1016/j.psj.2020.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are deposited in the NCBI Sequence Read Archive repository, accession number: PRJNA847797.