SUMMARY
A patient with progressive metastatic pancreatic cancer was treated with a single infusion of 16.2×109 autologous T cells that had been genetically engineered to clonally express two allogeneic HLA-C*08:02–restricted T-cell receptors (TCRs) targeting mutant KRAS G12D expressed by the tumors. The patient had regression of visceral metastases (overall partial response of 72% according to the Response Evaluation Criteria in Solid Tumors, version 1.1); the response was ongoing at 6 months. The engineered T cells constituted more than 2% of all the circulating peripheral-blood T cells 6 months after the cell transfer. In this patient, TCR gene therapy targeting the KRAS G12D driver mutation mediated the objective regression of metastatic pancreatic cancer. (Funded by the Providence Portland Medical Foundation.)
Pancreatic ductal adenocarcinoma is resistant to current immunotherapies and remains one of the most lethal cancers in humans. The resistance of pancreatic cancer to immunotherapy could be due in part to a paucity of neoantigen-reactive tumor-infiltrating lymphocytes resulting from the low mutational burden of the disease; such lymphocytes may be critical mediators of immunotherapy responses in solid cancers.1–3 Adoptive cell therapy with the use of T cells that have been engineered to express allogeneic T-cell receptors (TCRs) targeting “hot-spot” mutations commonly found in pancreatic cancer may address this challenge
We previously identified HLA-C*08:02–restricted TCRs targeting KRAS G12D in the tumor-infiltrating lymphocytes of a patient with metastatic colorectal cancer. 4 Treatment of this patient with her autologous KRAS G12D–reactive tumor-infiltrating lymphocytes led to the objective regression of visceral metastases, which suggested that the KRAS G12D–reactive TCRs derived from these tumor-infiltrating lymphocytes may be used in TCR gene therapy to treat other patients whose tumors express HLA-C*08:02 and KRAS G12D. Pancreatic cancers frequently harbor hot-spot mutations in KRAS and thus represent an ideal cancer in which to test the efficacy of TCR gene therapy against mutant KRAS. Here, we describe the case of a patient with metastatic pancreatic cancer who was treated with autologous T cells that had been engineered to clonally express two allogeneic HLA-C*08:02–restricted KRAS G12D–reactive TCRs.
CASE REPORT
A 71-year-old woman (Patient CRI-4483) had received a diagnosis of adenocarcinoma of the head of the pancreas at 67 years of age, after recurrent episodes of pancreatitis and biliary stricture. Levels of CA 19–9 and other tumor markers were not elevated. In 2018, she received four cycles of neoadjuvant FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin), followed by pylorus-preserving open Whipple resection of a poorly differentiated adenocarcinoma (measuring 4.5 cm in the largest dimension) with negative margins and 2 involved lymph nodes of the 21 lymph nodes resected; the disease stage after this treatment was IIB (ypT3N1M0). The patient then received four additional cycles of adjuvant FOLFIRINOX, followed by adjuvant chemoradiotherapy at a dose of 50.4 Gy with concurrent capecitabine therapy. She was disease-free until 2019, when fine-needle aspiration of an enlarging nodule in the right lower lobe of the lung confirmed the presence of lung metastasis. She had subsequent asymptomatic progression of metastases in both lungs in the absence of recurrence in the abdominopelvic cavity.
In 2020, the patient participated in a clinical trial of tumor-infiltrating lymphocyte therapy at the University of Pittsburgh Medical Center (ClinicalTrials.gov number, NCT03935893). She was treated with ex vivo expanded tumor-infiltrating lymphocytes and high-dose interleukin-2, but growth of lung metastases was observed within 6 months. Molecular and genomic studies of her tumor showed a programmed death 1 ligand (PD-L1) tumor proportion score of less than 1%; KRAS c.35G→A (p.G12D); a c.451C→T (p.P151S) variant in TP53, the gene for the tumorsuppressor protein p53; a c.172C→T (p.R58*) variant in CDKN2A, the gene for cyclin-dependent kinase inhibitor 2A; ROS1 c6214C→T (p.R2072W; variant of uncertain significance); a microsatellite-stable tumor; a tumor mutational burden of 8.9 mutations per megabase; and no detectable copy-number variations or gene fusions. HLA typing on peripheral-blood samples at the H. Lee Moffitt Cancer Center indicated expression of HLA-C*08:02.
A single-patient investigational new drug application was approved by the Food and Drug Administration in May 2021. After review by an institutional review board, the patient was treated in June 2021 with autologous peripheral-blood T cells that had been retrovirally transduced in two separate batches to express two allogeneic, HLA-C*08:02–restricted TCRs targeting mutant KRAS G12D. A preconditioning regimen of tocilizumab (600 mg, administered intravenously) and cyclophosphamide (30 mg per kilogram of body weight per day, administered intravenously for 2 days) was started 5 days before the cell infusion. At 18 hours after the cell infusion, the patient began receiving high-dose interleukin-2 (600,000 IU per kilogram, administered intravenously every 8 hours, for a total of five doses).
METHODS
GENERATION OF THE INFUSION PRODUCT
Autologous peripheral-blood mononuclear cells were stimulated with anti-CD3 antibody (clone OKT3) in the presence of interleukins 2, 7, and 21 and transforming growth factor β (TGF-β), the latter three cytokines being associated with the generation of less-differentiated T cells5–7 and tissue-resident memory T cells.8 Two days later, aliquots of these cells were separately transduced with a Good Manufacturing Practices–grade gammaretroviral vector encoding either an HLA-C*08:02–restricted TCR targeting the mutant KRAS G12D 9 amino acid–long peptide GADGVGKSA (called 9mer TCR) or the mutant KRAS G12D 10 amino acid–long peptide GADGVGKSAL (called 10mer TCR). After a rapid-expansion protocol consisting of irradiated allogeneic peripheral-blood–mononuclear-cell feeder cells, anti-CD3 (OKT3), interleukin-2, interleukin-21, and TGF-β,9 the cells were harvested for infusion. Additional details of the gammaretroviral vector and cell-manufacturing process is provided in Figures S1 and S2A and S2B and the Supplementary Materials and Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org.
INFUSION PRODUCT CHARACTERIZATION AND IMMUNE MONITORING
Flow cytometry was used to phenotype and detect the TCR-transduced T cells in the infusion product and peripheral blood after the infusion. Both the 9mer and 10mer TCRs were engineered to contain the mouse TCRα and TCRβ constant regions, which promoted the pairing of the introduced TCR and allowed for the identification of the TCR-transduced T cells. The 9mer TCRβ chain was TRBV5–6 (also known as Vβ5.2), and thus the T cells coexpressing mouse TCRβ and Vβ5.2 were categorized as the 9mer-transduced T cells. The quantification of soluble cytokines and other proteins within serum samples and media supernatant from coculture assays was evaluated with the use of Luminex and LEGENDplex multiplex cytokine bead assays. Additional details are provided in the Supplementary Materials and Methods section.
RESULTS
NEOANTIGEN TCR GENE THERAPY
Five days before the cell infusion, the patient received a single flat dose of tocilizumab (600 mg, administered intravenously) in order to prevent cytokine release syndrome, which can occur after the infusion of gene-engineered T cells,10 and to potentially mitigate the formation of suppressive myeloid cells during immune reconstitution.11 A reduced-intensity preconditioning regimen was used, which consisted of cyclophosphamide (30 mg per kilogram per day) administered intravenously on day 5 and day 4 before the cell infusion, in light of the prolonged cytopenia that had been observed with previous tumor-infiltrating lymphocyte therapy involving the use of standard-dose preconditioning therapy with cyclophosphamide and fludarabine.
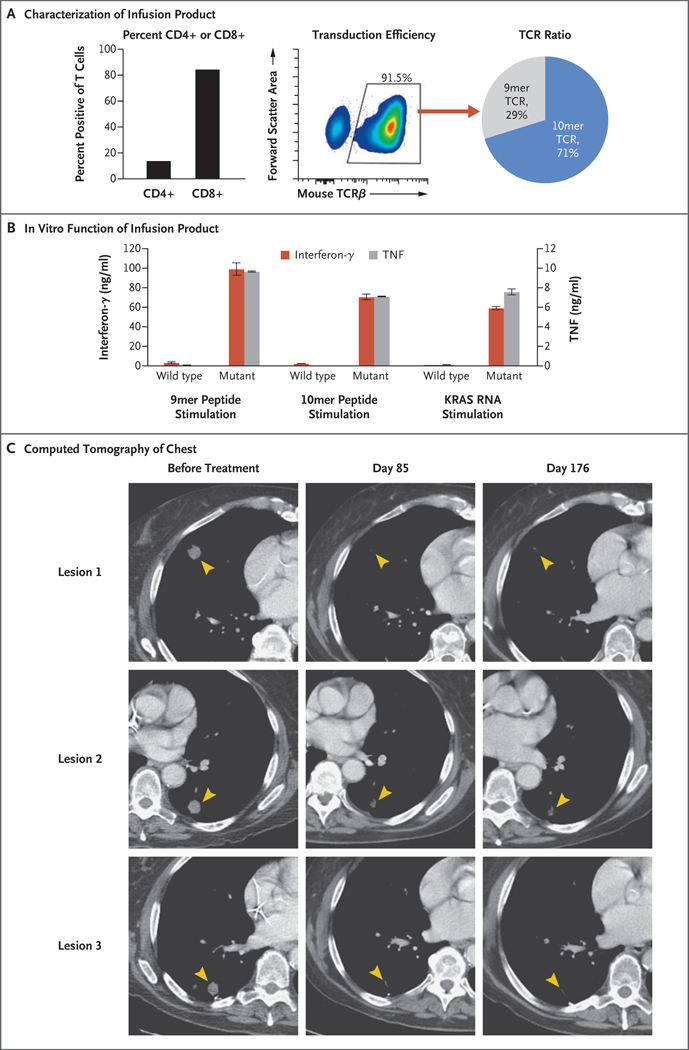
On day 0, the patient received a single infusion of 16.2×109 autologous T cells, which consisted of 85% CD8+ T cells and 15% CD4+ T cells (Fig. 1A, left). Approximately 91.5% of the infusion product (14.8×109 cells) expressed a KRAS G12D–reactive TCR (Fig. 1A, middle). Of the transduced T cells, approximately 29% expressed the 9mer TCR that targets a 9 amino acid–long KRAS G12D peptide and approximately 71% expressed the 10mer TCR that targets a 10 amino acid–long KRAS G12D peptide (Fig. 1A, right). The infusion product was highly specific for mutant KRAS G12D, and the transduced T cells secreted high levels of effector cytokines such as interferon-γ and tumor necrosis factor (TNF) when stimulated in vitro with autologous antigen–presenting cells expressing mutant KRAS G12D peptides or RNA (Figs. 1B and S3B through S3F).
Figure 1. (facing page). Adoptive Immunotherapy with T-Cell Receptor (TCR)–Engineered T Cells Targeting KRAS G12D in a Patient with Pancreatic Cancer.
Panel A shows the frequency of CD4+ and CD8+ T cells (left); the transduction efficiency as measured by the detection of mouse TCRβ constant chain, which was engineered into the TCRs (middle); and the ratio of 9mer and 10mer TCR-transduced T cells (right), as determined on flow cytometry, in the patient’s infusion product. The 9mer TCR and 10mer TCR refer to two different HLA-C*08:02–restricted TCRs that specifically target 9 or 10 amino acid–long KRAS G12D neoepitopes, respectively. Panel B shows the concentrations of the effector cytokines interferon-γ and tumor necrosis factor (TNF) secreted into the supernatant after an overnight coculture of the infusion product (1×105 cells per well) with autologous dendritic cells (0.5×105 cells per well) pulsed with 1 μg per milliliter of the indicated wild-type or mutant KRAS G12D peptide or transfected with 80 μg per milliliter of RNA encoding wild-type or mutant KRAS G12D. I bars represent the standard error. Panel C shows the contrast-enhanced computed tomographic scans of the patient’s chest before infusion and at 85 and 176 days after the infusion of 16.2×109 T cells, approximately 91.5% of which were transduced to express a KRAS G12D–reactive TCR. Arrowheads highlight lesions before and after therapy.
At 18 hours after the cell infusion, the patient began receiving high-dose interleukin-2 (600,000 IU per kilogram, administered intravenously every 8 hours) for five of the six planned doses in order to support the expansion of infused T cells. The sixth dose of interleukin-2 was not given owing to hypotension that led to the use of vasopressor support with phenylephrine (peak intravenous administration, 65 μg per minute). The patient had transient, expected toxic effects that have been associated with cyclophosphamide preconditioning therapy (e.g., nausea and myelosuppression) and with high-dose interleukin-2 therapy (e.g., hypotension, increased aminotransferase levels, rigors, fever, and fatigue). No toxic effects of the engineered T-cell therapy were observed.
The patient was discharged from the hospital on day 11 and received myeloid growth factor and blood-product support as an outpatient (5 single-donor apheresis units of platelets and 1 unit of red cells). She had recovery of the absolute neutrophil count by day 21 after the cell infusion and platelet recovery by day 28. As had occurred with the previous tumor-infiltrating lymphocyte therapy that the patient had received in 2020, orthostatic hypotension developed during the prolonged course of daily filgrastim administration; this effect was managed with intravenous fluid support and a brief course of oral fludrocortisone (0.1 mg daily for 10 days). The clinical treatment scheme and complete description of the clinical course are shown in Figure S2C and the Supplementary Clinical Details section.
CLINICAL EVALUATION
Regression of the patient’s metastatic lung lesions was observed on computed tomography at the first clinical follow-up 1 month after the cell infusion, with an overall objective partial response according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, of 62%. Tumor regression was ongoing at the latest follow-up, 6 months after the cell transfer, with an overall partial response according to RECIST, version 1.1, of 72% (Fig. 1C).
ANALYSIS OF SERUM CYTOKINE LEVELS
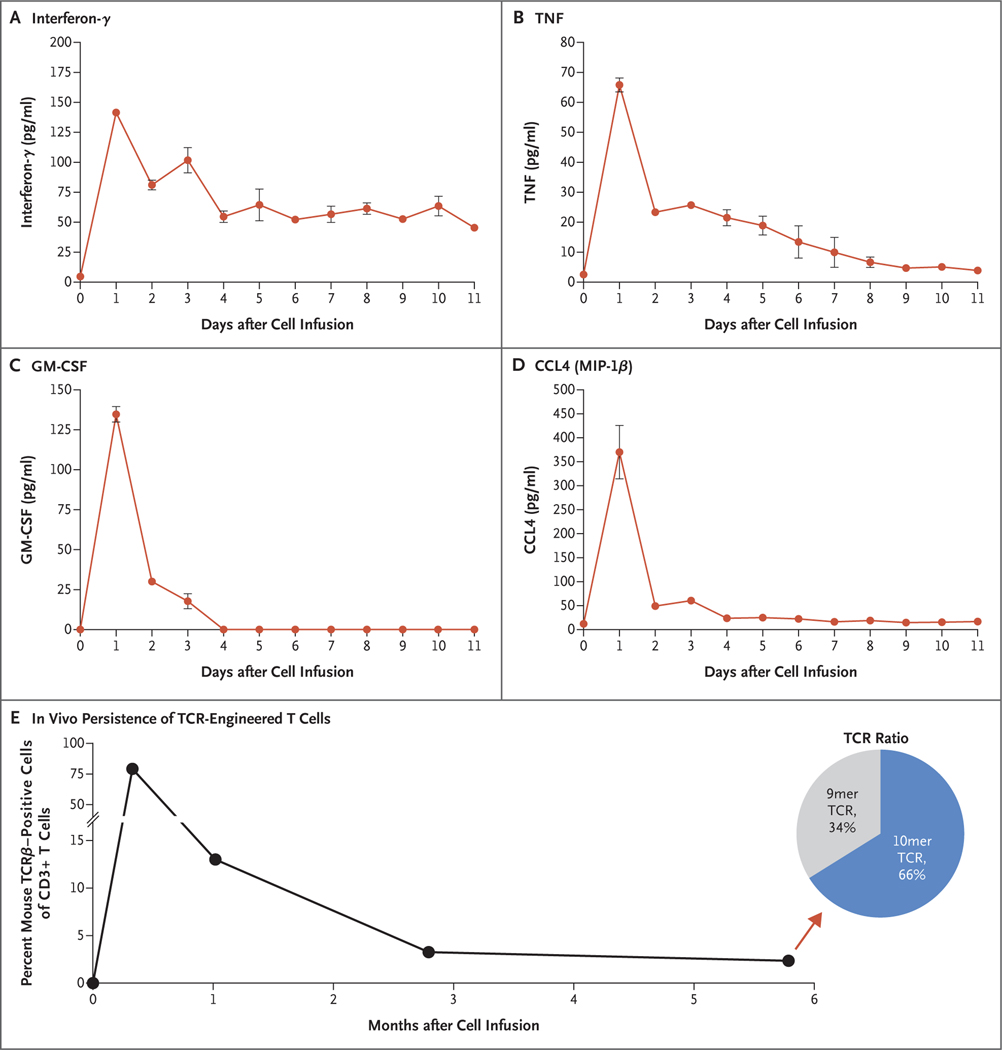
Concentrations of the effector T-cell cytokines interferon-γ, TNF, granulocyte–macrophage colony-stimulating factor (GM-CSF), and CCL4 (macrophage inflammatory protein [MIP]–1β) in the serum were low at baseline but increased to picogram levels and peaked 1 day after the cell infusion (Fig. 2A through 2D). When the patient was discharged 11 days after the cell infusion, the levels of interferon-γ remained elevated as compared with baseline.
Figure 2. Serum Cytokine Levels and In Vivo T-Cell Persistence.
Panels A through D show concentrations of the effector T-cell cytokines interferon-γ, TNF, granulocyte–macrophage colony-stimulating factor (GM-CSF), and CCL4 (macrophage inflammatory protein [MIP]–1β) in the serum of the patient before cell therapy on day 0 and after cell therapy up to her discharge on day 11. I bars represent the standard error. (I bars for small standard errors may not be visible.) Panel E shows the in vivo persistence of the transferred TCR-engineered T cells in the peripheral blood as determined on flow cytometric analysis of mouse TCRβ. The pie chart (Panel E, inset) shows the ratio of 9mer and 10mer TCRs within the transduced T cells at day 176 after the receipt of cell therapy (arrow). Data were gated on CD3+ lymphocytes.
PERSISTENCE OF TCR-ENGINEERED T CELLS
The transferred T cells constituted approximately 13% of all T cells in circulation at approximately 1 month after the cell transfer, 3.3% of all T cells at 3 months, and 2.4% of all T cells at 6 months; in the transduced T-cell population at 6 months, 34% were 9mer TCR cells and 66% were the 10mer TCR cells (Fig. 2E). The vast majority of the persisting transduced T cells were CD8+ (as in the infusion product), and the ratio of the 9mer to 10mer TCR transduced T cells within CD8+ transduced T cells in the peripheral blood remained similar to that in the infusion product (Fig. S4). TCR-transduced T cells that persisted in the peripheral blood at approximately 3 months after the cell transfer were able to produce the effector cytokines interferon-γ and TNF on in vitro stimulation with mutated KRAS G12D peptides (Fig. S5).
DISCUSSION
We report an ongoing 6-month overall objective partial response according to RECIST, version 1.1, of 72% in a patient with metastatic pancreatic adenocarcinoma that had been refractory to standard therapy and tumor-infiltrating lymphocyte therapy. The patient received a single infusion of autologous T cells that had been engineered to express allogeneic TCRs targeting neoantigen KRAS G12D expressed by the tumors, as well as high-dose interleukin-2 support. The engineered cells represented approximately 2.4% of the total circulating T cells at 6 months. The TCR-transduced cell infusion product consisted of CD8+ and CD4+ T cells, in an approximate 6:1 ratio, expressing a TCR targeting either a 9 or 10 amino acid–long KRAS G12D peptide. These TCR-transduced T-cell populations showed similar persistence in the peripheral blood over time.
Owing to rapid tumor regression, we were unable to perform a post-treatment biopsy to assess the extent of tumor infiltration by the transduced T cells. The cytokines that we used for in vitro cell growth during manufacture of the infusion product led to the generation of T cells that phenotypically resembled tissue-resident memory T cells, which express CD103, CD69, and CD49a (Fig. S3A) and are known to traffic to and reside in tumors and correlate with favorable clinical outcomes in a variety of cancers.12
We have treated one additional patient with pancreatic cancer (Patient CRI-3061) at our institute under a separate single-patient investigational new drug application, using autologous T cells that had been engineered to express the two HLA-C*08:02–restricted KRAS G12D–reactive TCRs described in this article. However, the infusion product for this patient was manufactured with the use of different in vitro growth conditions, and additional immune modulation (with tocilizumab) was not part of the preconditioning chemotherapy regimen (Fig. S6).
After the infusion of 29.6×109 T cells, 67.7% of which expressed a single KRAS G12D–reactive TCR (Fig. S7), and the receipt of four doses of high-dose interleukin-2, this other patient had grade 3 cytokine release syndrome and a grade 2 immune-effector cell–associated neurotoxic event. These immune-related clinical symptoms were associated with elevated serum levels of the effector T-cell cytokines interferon-γ, TNF, GM-CSF, and CCL4 (MIP-1β), which peaked approximately 4 days after the cell infusion (Fig. S8A through S8D), and a 1-month transient decrease in the CA 19–9 level.
Radiographic imaging 1 month after the infusion showed modest regression of the patient’s lung metastases and stable liver lesions. However, despite high levels of T-cell persistence in the blood, the patient had progressive disease and died 6 months after receipt of the therapy (Figs. S8E and S9). Next-generation sequencing and bioinformatics analyses of a progressing lesion did not identify common mechanisms of resistance to immunotherapy13 such as loss of HLA, loss of the targeted antigen, mutations in the antigen presentation and processing machinery, and mutations in the interferon-γ signaling pathway (data not shown). The vast majority of tumor and immune cells within a progressing lesion were negative for PD-L1 expression on immunohistochemical staining (data not shown). We continue to investigate the potential mechanisms of therapy failure in this patient.
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 and B-cell maturation antigen (BCMA) has revolutionized the treatment of hematologic cancers.14 However, the transfer of T cells that have been genetically engineered to express CAR against overexpressed pancreatic-cancer antigens such as mesothelin,15,16 CD133,17 and epidermal growth factor receptor18 has been largely ineffective in patients with pancreatic cancer.
TCR gene therapy can target intracellular tumor-specific antigens but is dependent on a patient’s specific HLA genotype. The KRAS G12D–specific TCRs that we used in this study were restricted by HLA-C*08:02, which is expressed by approximately 8% of White persons and 11% of Black persons in the United States and by lower percentages of persons in most other racial and ethnic groups — a situation that limits this therapy to a relatively low percentage of potential patients. However, additional TCRs targeting KRAS G12D and other hot-spot KRAS mutants restricted by different HLA molecules that have been identified19–23 could extend TCR gene therapy against mutant KRAS to a larger number of patients. The immunologic targeting of mutant KRAS with vaccines in patients with pancreatic cancer has also been attempted,24,25 but the clinical usefulness of this approach remains unclear.
Although the durability of the clinical response in our patient remains to be determined, this case report shows that TCR gene therapy targeting the KRAS G12D hot-spot mutation was able to mediate the regression of metastatic pancreatic cancer. Prospective clinical trials are warranted to determine the therapeutic potential of this therapy in pancreatic cancer and other cancers that express KRAS G12D.
Supplementary Material
Acknowledgments
Supported by the Providence Portland Medical Foundation. Dr. Tran’s work is supported by the Society for Immunotherapy of Cancer Steven A. Rosenberg, M.D., Ph.D., Scholars Award. Aldesleukin (Proleukin [interleukin-2]) was provided by Clinigen.
We thank the members of Providence Health and Services and the Earle A. Chiles Research Institute who were involved with patient care and the obtaining of samples, Dr. Brendan Curti for critical review of an earlier version of the manuscript, Ms. Miranda Gilchrist for performing clinical-grade fluorescence-activated cell sorting of T cells from Patient CRI-3061, Dr. Joseph Fass and Mr. Jared Gartner for bioinformatics analyses on samples obtained from Patient CRI-3061, and Dr. Yaping Wu for assistance with the immunohistochemical testing on tumors obtained from Patient CRI-3061.
Footnotes
REFERENCES
- 1.Schumacher TN, Scheper W, Kvistborg P. Cancer neoantigens. Annu Rev Immunol 2019; 37: 173–200. [DOI] [PubMed] [Google Scholar]
- 2.Tran E, Robbins PF, Rosenberg SA. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol 2017; 18:2 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321:1 801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran E, Robbins PF, Lu YC, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 2016; 375:2 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013; 121:5 73–84. [DOI] [PubMed] [Google Scholar]
- 6.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 2008; 111: 5326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahmani A, Janelle V, Carli C, et al. TGFβ programs central memory differentiation in ex vivo-stimulated human T cells. Cancer Immunol Res 2019; 7: 1426–39. [DOI] [PubMed] [Google Scholar]
- 8.Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 2013;3 9: 687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Etto T, Rodríguez-Cruz T, et al. TGF-beta1 induces preferential rapid expansion and persistence of tumor antigen-specific CD8+ T cells for adoptive immunotherapy. J Immunother 2010; 33: 371–81. [DOI] [PubMed] [Google Scholar]
- 10.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124:1 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Innamarato P, Kodumudi K, Asby S, et al. Reactive myelopoiesis triggered by lymphodepleting chemotherapy limits the efficacy of adoptive T cell therapy. Mol Ther 2020; 28: 2252–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okła K, Farber DL, Zou W. Tissue-resident memory T cells in tumor immunity and immunotherapy. J Exp Med 2021; 218(4): e20201605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168: 707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.June CH, Sadelain M. Chimeric anti-gen receptor therapy. N Engl J Med 2018; 379:6 4–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beatty GL, O’Hara MH, Lacey SF, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 2018; 155:2 9–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas AR, Tanyi JL, O’Hara MH, et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther 2019; 27: 1919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Chen M, Wu Z, et al. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Onco-immunology 2018; 7(7): e1440169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Guo Y, Wu Z, et al. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: a phase I clinical trial. Cytotherapy 2020; 22: 573–80. [DOI] [PubMed] [Google Scholar]
- 19.Bear AS, Blanchard T, Cesare J, et al. Biochemical and functional characterization of mutant KRAS epitopes validates this oncoprotein for immunological targeting. Nat Commun 2021;1 2: 4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cafri G, Yossef R, Pasetto A, et al. Memory T cells targeting oncogenic mutations detected in peripheral blood of epithelial cancer patients. Nat Commun 2019; 10: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J, Goulding SP, Conn BP, et al. Systematic discovery and validation of T cell targets directed against oncogenic KRAS mutations. Cell Reports Methods 2021; 1: 100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin N, Paria BC, Vale NR, et al. Identification and validation of T-cell receptors targeting RAS hotspot mutations in human cancers for use in cell-based immunotherapy. Clin Cancer Res 2021; 27: 5084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yossef R, Tran E, Deniger DC, et al. Enhanced detection of neoantigen-reactive T cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight 2018; 3(19): e122467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gjertsen MK, Bakka A, Breivik J, et al. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet 1995; 346:1 399–400. [DOI] [PubMed] [Google Scholar]
- 25.Wedén S, Klemp M, Gladhaug IP, et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer 2011; 128: 1120–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.