Abstract
In the real-life setting, the combination of brentuximab vedotin and bendamustine was well tolerated and produced an ORR of 75%, CR 50% and a median PFS of 26 months. A significant proportion of heavily pretreated cHL patients may be cured with this approach.
Background:
Patients with relapsed or refractory classical Hodgkin lymphoma (R/R cHL) have limited opportunities for curative therapy. High-dose therapy followed by autologous stem cell transplantation (HDT-ASCT) produces cure rates of 50% to 60%. Patients relapsing after, or ineligible for HDT-ASCT have limited therapeutic options and long-term remission is uncommon. Furthermore, few patients are candidate to allogeneic stem cell transplantation (AlSCT), a potentially curative approach. The combination of brentuximab vedotin and bendamustine (BVB) is a promising treatment for patients with R/R cHL, regardless of SCT eligibility.
Patients and methods:
We conducted a real-life study of BVB in 41 patients with R/R cHL after failure of ≥ 1 therapy including ASCT, AlSCT, or BV.
Results:
Among 40 patients evaluable for efficacy, the overall response rate and complete response (CR) rate were 75% and 50%, respectively. No significant differences were observed between patients with primary refractory and relapsed disease, previously treated with ≤ 2 and ≥ 3 lines of therapy, or BV-exposed and BV-naïve. After a median follow-up of 38 months, the median progression free survival (PFS) for the entire population is 26 months; PFS is not reached, 10.5 months, and 4 months for patients achieving CR, partial response and no response, respectively (P < .0001). BVB was well tolerated and no grade 4 toxicity or new safety signals were observed. The most common treatment-emergent adverse events were infections.
Conclusion:
Our experience supports the efficacy and tolerability of the BVB combination in R/R cHL as a bridge to SCT, or as a definitive therapy for SCT-ineligible patients. Larger comparative studies testing BVB against standards of care are warranted in both settings.
Keywords: Stem Cell Transplantation, Hodgkin lymphoma, Brentuximab, Bendamustine
Introduction
The majority of patients diagnosed with advanced classical Hodgkin lymphoma (cHL) are cured by ABVD (adriamycin, bleomycin, vinblastine and dacarbazine) or ABVD-like chemotherapy, with or without radiation therapy.1 Patients whose disease does not respond to, or relapses after front-line therapy (relapsed/refractory, R/R) are generally treated with second-line multi-agent chemotherapy followed by high-dose chemotherapy (HDT) with autologous stem cell transplantation (ASCT).2–3 The achievement of a complete response (CR) prior to HDT-ASCT is a strong, independent predictor of long-term favorable outcome.4–7
Established second-line regimens include platinum-based combinations such as ICE (ifosfamide, carboplatin, and etoposide), DHAP (cisplatin, cytarabine, and dexamethasone), or gemcitabine-based therapies like IGEV (ifosfamide, gemcitabine, etoposide, and vinblastine), and BeGEV (bendamustine, gemcitabine, etoposide, and vinorelbine). These therapies have been variably effective (CR rates ranging from 21% to 73%),8–11 some of them require inpatient administration, and all can be associated with significant toxicities.
Treatment options for patients relapsing after, or ineligible for HDT-ASCT are limited and long-term remission is uncommon. Furthermore, allogeneic stem cell transplantation (AlSCT), a potentially curative option, is seldom feasible due to the inability of achieving deep and durable enough responses to subsequent lines of therapy.12
The combination of brentuximab-vedotin and bendamustine (BVB) was recently reported as a promising treatment for patients with R/R cHL, including those who are candidate for HDT-ASCT.21–25 Both BV, a monoclonal anti-CD30 antibody covalently conjugated with the microtubule-disrupting agent monomethyl-auristatin E,13 and bendamustine14 are individually active in patients with R/R cHL, but associated with relatively low incidence of CR.15–20 A number of studies have demonstrated that BVB is well tolerated and can produce high overall and complete response rates, including in patients who were previously exposed to single-agent BV.21 As a result, BVB is recognized by the National Comprehensive Cancer Network (NCCN) as an accepted treatment option for patients with R/R cHL.22–25
Herein, we report the analysis of a real-world experience looking at efficacy, safety and outcomes associated with the use of BVB, with an extended follow-up. In this series, BVB was employed either as definitive therapy in patients not candidate to SCT, or as bridging therapy in those who went on to receive SCT.
Patients and methods
Patient population
This retrospective multicenter study was conducted at 11 Italian academic institutions. Consecutive patients with biopsy-proven, CD30-positive R/R cHL, who were deemed candidate for BVB were included in this report. For the purposes of the analysis, we sought to compare and contrast different groups of patients: primary refractory vs. relapsed disease; previously treated with ≤ 2 vs. ≥3 lines of therapy; BV exposed vs. BV naïve.
Treatment and assessments
Treatment consisted of intravenous (IV) BV (1.8 mg / kg) given on day 1 and IV bendamustine (90 mg / m2) given on days 1 and 2 of a 21-day cycle for up to 8 cycles. The duration of therapy was chosen by the individual physicians according to the patient’s response and tolerance, plan for consolidation strategies, and availability of alternative treatments. Supportive care was left at the discretion of the treating physician. Premedication generally consisted of methylprednisolone (100 mg IV) or hydrocortisone (100 mg IV) and diphenhydramine (50 mg IV) or chlorphenamine (10 mg IV) administered before BV on day 1; antimicrobial prophylaxis included trimethoprim-sulfamethoxazole (800/160 mg orally twice daily two times a week) and acyclovir (800 mg orally daily) throughout. Filgrastim (30 MU) administered subcutaneously was used as primary prophylaxis or treatment of neutropenia. Eligible patients underwent HDT-ASCT or AISCT according to the specific circumstance and center’s institutional policy.
Responses were assessed at the end of the therapy using the 2014 Lugano criteria26 whereby a score of ≤ 3 on the Deauville 5-point scale (DS) was considered indicative of CR.27 Treatment-emergent adverse events (AE) were graded according to CTCAE definitions, v 4.0.28
Treatment was administered under a compassionate use program and according to the principle of the patient’s best interest. As such, it was approved by the ethics committees at each participating center and an informed written consent was obtained from each patient prior to initiating therapy.
Statistical analyses
The primary objectives of the study were to describe the best overall response rate (ORR) and the CR rate (CRR) after treatment with BVB. Secondary objectives included an evaluation of safety, progression free survival (PFS) and overall survival (OS). PFS was defined as the time from treatment initiation to progression of disease, relapse, or death from any cause. If none of these events had occurred, PFS was censored at the date of last follow up. In order to better understand the role of BVB in individuals for whom consolidation strategies were planned, patients undergoing SCT were not censored at the time of consolidation. The OS was calculated from the date of enrollment until death from any cause and it was censored at the date of last follow up for surviving patients.
Descriptive statistics were performed using percentages and frequency tables for categorical variables and mean ± standard deviation (SD) for continuous variables. Categorical variables were evaluated by chi-square analysis or Fisher’s exact test. Non-parametric Mann-Whitney Test was performed to compare continuous variable with no normal distribution. ORR and CRR were evaluated as means of indicator variables with logit-transformed confidence intervals; PFS, and OS were analyzed according to the Kaplan-Meier method and survival curves were compared using the log-rank test. The Cox model was used to estimate hazard ratios and related 95% confidence intervals (CI). A P value of less than .05 was considered to be statistically significant. Statistical analysis was performed with STATA 16.1 (StataCorpLP, Collage Station TX, USA).
Results
Patient characteristics and disposition
Between July 2015 and June 2020, 41 patients with R/R cHL were treated with BVB (Table 1). The median age at the start of therapy was 36 years (range: 16–74) and 28 patients (68%) had stage III or IV disease. Patients had received a median of 2 previous treatments (range: 1–5) and 28 (68%) had primary refractory disease. Twenty-six (63%) had previously received BV monotherapy (median: 4 cycles, range 1–11) and 16 (39%) had failed to achieve a response to it. Seven patients had previously undergone HDT-ASCT and one had had both HDT-ASCT and AlSCT. Six patients (14.6%) received BVB at the time of their first relapse. Nineteen patients received up to 4 cycles of BVB and 21 received 6 or more cycles.
Table 1.
Patient Characteristics (n = 41)
| Characteristic | n. (%) or median [range] |
|---|---|
| Gender | |
| Female | 19 (46) |
| Male | 22 (54) |
| Median age, years | 36 [16–74] |
| Histological subtype | |
| Nodular sclerosis | 28 (68) |
| Mixed cellularity | 6 (15) |
| Lymphocyte-rich | 2 (5) |
| Not classified | 5 (12) |
| ECOG PS | |
| 0–1 | 37 (90) |
| ≥2 | 4 (10) |
| Stage at the time of BVB initiation | |
| II | 13 (32) |
| III | 14 (34) |
| IV | 14 (34) |
| Systemic symptomsa | 15 (37) |
| Previous radiation therapy | 14 (34) |
| Previous HDT-ASCT | 8 (20) |
| Previous AlSCT | 2 (5) |
| N. previous lines of therapy | |
| ≤2 | 28 (68) |
| ≥3 | 13 (32) |
| Median N. previous therapies | 2 [1–5] |
| Primary refractory | 28 (68) |
| Relapsed | 13 (32) |
| Previous BV | 26 (63) |
| BV-refractory | 16 (39) |
Abbreviations: AlSCT: allogenic stem cell transplantation; BV: brentuximab vedotin; BVB: brentuximab vedotin and bendamustine; ECOG PS: Eastern Cooperative Oncology Group Performance Status; HDT-ASCT: high-dose therapy-autologous stem cell transplantation.
Includes: Night sweats, recurrent fevers, and unexplained ≥ 10% weight loss.
Responses
Among 40 evaluable patients (one discontinued treatment after one cycle because of febrile neutropenia and diarrhea), the ORR was 75% (95% CI: 59%−86%) and the CRR was 50% (95% CI: 35%−66%). Results were similar in patients with refractory (ORR: 71%, 95% CI 52–85; CRR 43%, 95% CI 26–62) or relapsed (ORR 83%, 95% CI: 51–96; CRR 67%, 95% CI: 37–87) cHL and in those who had received ≤2 lines of therapy (ORR 73%, 95% CI 46–90; CRR 47%, 95% CI: 23–71) or ≥ 3 lines (ORR 76%, 95% CI: 55–89; CRR 52%, 95% CI: 33–71). Exposure or degree of response to prior BV monotherapy did not affect the likelihood of response to BVB. The ORR and CRR were 80% (95% CI: 58–91) and 47% (95% CI: 23–71) in BV naïve patients, 72% (95% CI: 42–87) and 52% (95% CI: 33–71) in BV exposed patients, and 69%, (95% CI 37–89) and 50%, (95% CI: 27–73) in BV-refractory patients, respectively (Figure 1). There was a trend towards higher CRR in patients who completed at least 6 cycles of BVB (57%, 95% CI: 35–76) as compared to those who received 4 cycles or less (42%, 95% CI: 22–65).
Figure 1.
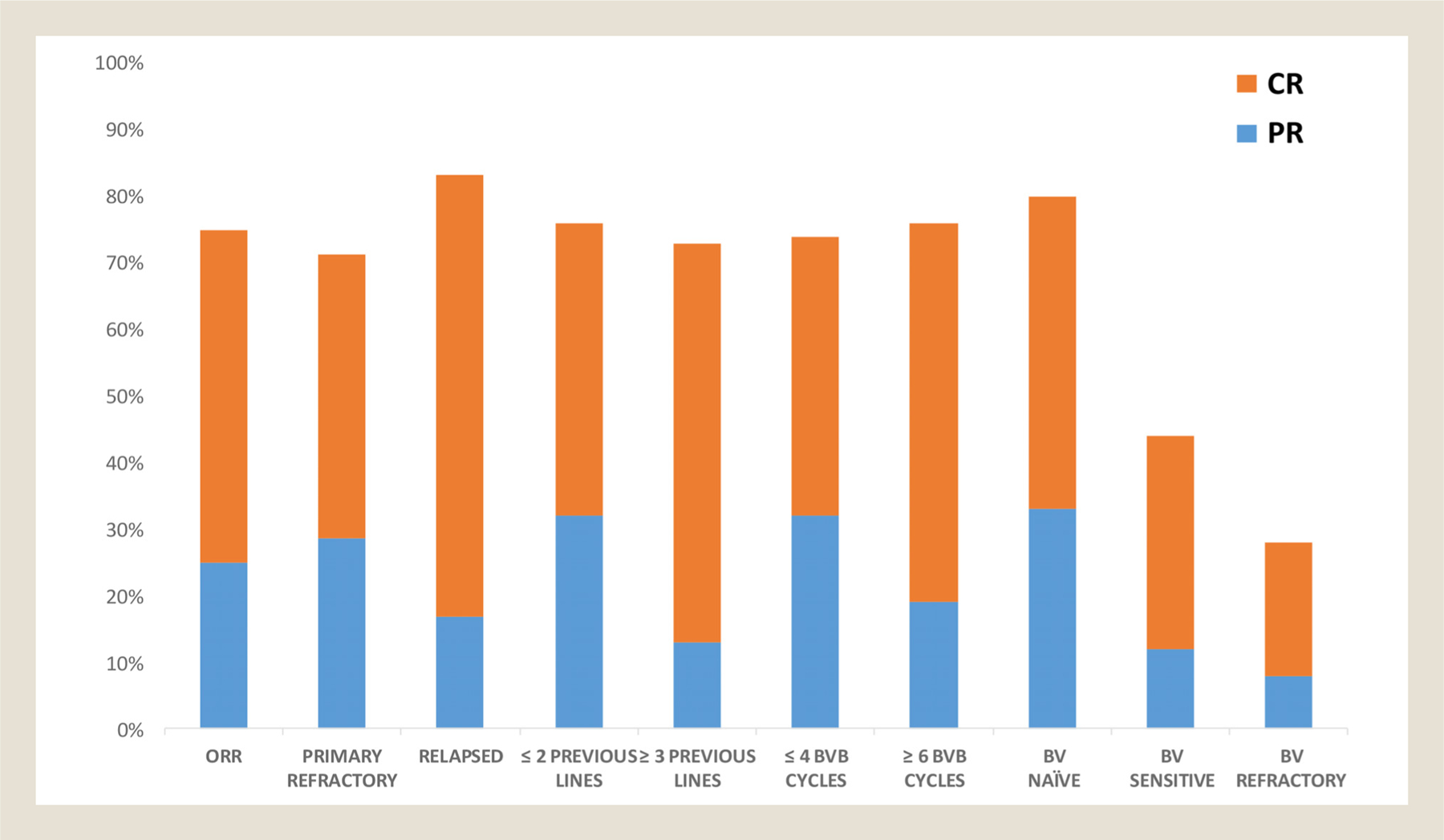
Responses in the entire population and predefined subgroups. ORR = overall response rate; BVB = brentuximab vedotin-bendamustine; BV = brentuximab vedotin; CR = complete response; PR = partial response.
Safety
In the intention-to-treat population, 19 patients (46%) experienced AE of any grade (Table 2). One patient discontinued therapy due to febrile neutropenia and diarrhea after the first cycle and no patient died while on treatment. The most common grade 3 AE were infections (3 patients: one who experienced CMV pneumonia and H1N1 pneumonia, one CMV pericarditis, and one sepsis caused by Klebsiella pneumoniae neutropenic fever), neutropenia (3 patients), and peripheral neuropathy (2 patients). There were no grade 4 toxicities. The most common grade 1–2 AE were gastrointestinal disturbances and skin rash. Overall, peripheral neuropathy was observed in 6 patients and was reversible in all cases.
Table 2.
Treatment-Emergent Adverse Events (n = 41)
| Adverseevent | Any grade,N. (%) | Grade I, N. (%) | Grade II, N. (%) | Grade III, N. (%) | Grade IV, N. (%) |
|---|---|---|---|---|---|
| Hematological toxicity | 6 (15) | 3 (7) | 0 | 3(7) | 0 |
| Neutropenia | 5 (12) | 2 (5) | 0 | 3 (7) | 0 |
| Thrombocytopenia | 1 (2) | 1(2) | 0 | 0 | 0 |
| Non-hematological toxicity | 25 (61) | 6 (15) | 14 (34) | 5 (12) | 0 |
| Nausea/vomiting | 8 (20) | 4 (10) | 4 (10) | 0 | 0 |
| Peripheral neuropathy | 6 (15) | 2 (5) | 2 (5) | 2 (5) | 0 |
| Skin rash | 4 (10) | 0 | 4 (10) | 0 | 0 |
| Infections | 3 (7) | 0 | 0 | 3 (7) | 0 |
| Diarrea | 2 (5) | 0 | 2 (5) | 0 | 0 |
| Febrile neutropenia | 1 (2) | 0 | 0 | 1 (2) | 0 |
| Fever | 1 (2) | 0 | 1 (2) | 0 | 0 |
| Spine pain | 1 (2) | 0 | 1 (2) | 0 | 0 |
Outcomes
A total of 15 responding patients (36.6%) underwent SCT. Nine patients underwent HDT-ASCT (N. = 7) or AlSCT (N. = 2), after 4 cycles of BVB, 7 in CR and 2 in PR. Eight patients are alive and in CR at the time of this analysis, while 1 was lost to follow-up. Six patients underwent HDT-ASCT (N. = 1) or AlSCT (N. = 5) after ≥ 6 cycles of BVB, 5 in CR and 1 in PR. Three of them are alive in treatment-free remission, 2 relapsed after HDT-ASCT (1) or AlSCT (1) and received, respectively, anti-PD-1 therapy as a bridge to AlSCT and anti-PD-1 therapy followed by BeGEV as palliative therapy. The third patient died due to post-AlSCT infectious complications.
Eight patients achieved CR, but did not undergo SCT. Four are still in remission, while 4 relapsed 5, 6, 16 and 22 months after completing BVB. Among the latter, 3 are currently receiving an anti-PD-1 therapy and one received DHAP chemotherapy followed by HDT-ASCT. Seven patients attained PR and did not undergo HDT-ASCT consolidation. Two of them achieved CR with additional BV or bendamustine monotherapy, 4 received anti-PD-1 therapy, and one died because of CMV pericarditis.
After a median follow-up of 38 months (range 4.2–64, IQR 26.7), the median PFS for the entire study population is 26 months (95% CI: 7.2-not reached (n.r.), Figure 2A). Patients who achieved CR at the end of treatment, had significantly longer PFS (n.r.) compared to those who achieved PR (10.5 months) or did not respond (4 months)(95% CI 22.4-n.r., 4.8-n.r., and 1.38–6.28, respectively, Figure 2B).
Figure 2.
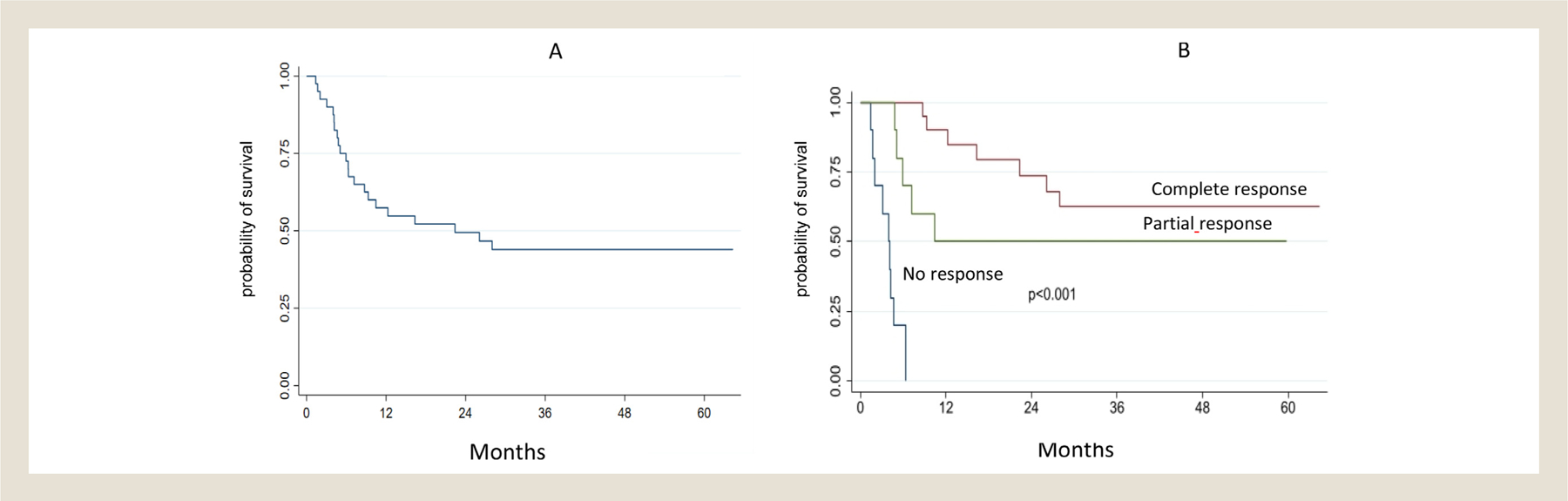
Progression-free survival in the entire population (A) and according to the depth of response to brentuximab vedotin-bendamustine (B).
There was a non-significant trend towards longer PFS in patients with relapsed disease compared to those with primary refractory disease (n.r. vs. 10.4 months) (Figure 3A), treated with ≤ 2 compared to ≥ 3 lines of therapy (26 vs. 12 months) (Figure 3B) and in individuals who were BV-naïve compared to those who were BV-exposed (n.r. vs. 16.3 months). (Figure 3C) Finally, no statistically significant differences were observed between BVB responsive patients who received HDT-ASCT or AlSCT and those who did not, although there was a trend towards longer PFS in transplanted patients (n.r. vs. 28 months, supplemental material). The median OS has not been reached in the whole population and in various subgroups analyzed. However, a trend towards longer OS was observed in patients achieving CR (HR vs. PR = 3.3 (95% CI: 0.55–19.9), HR vs. NR = 5 (95% CI 0.9–27.6)) approaching statistical significance (P = .06).
Figure 3.
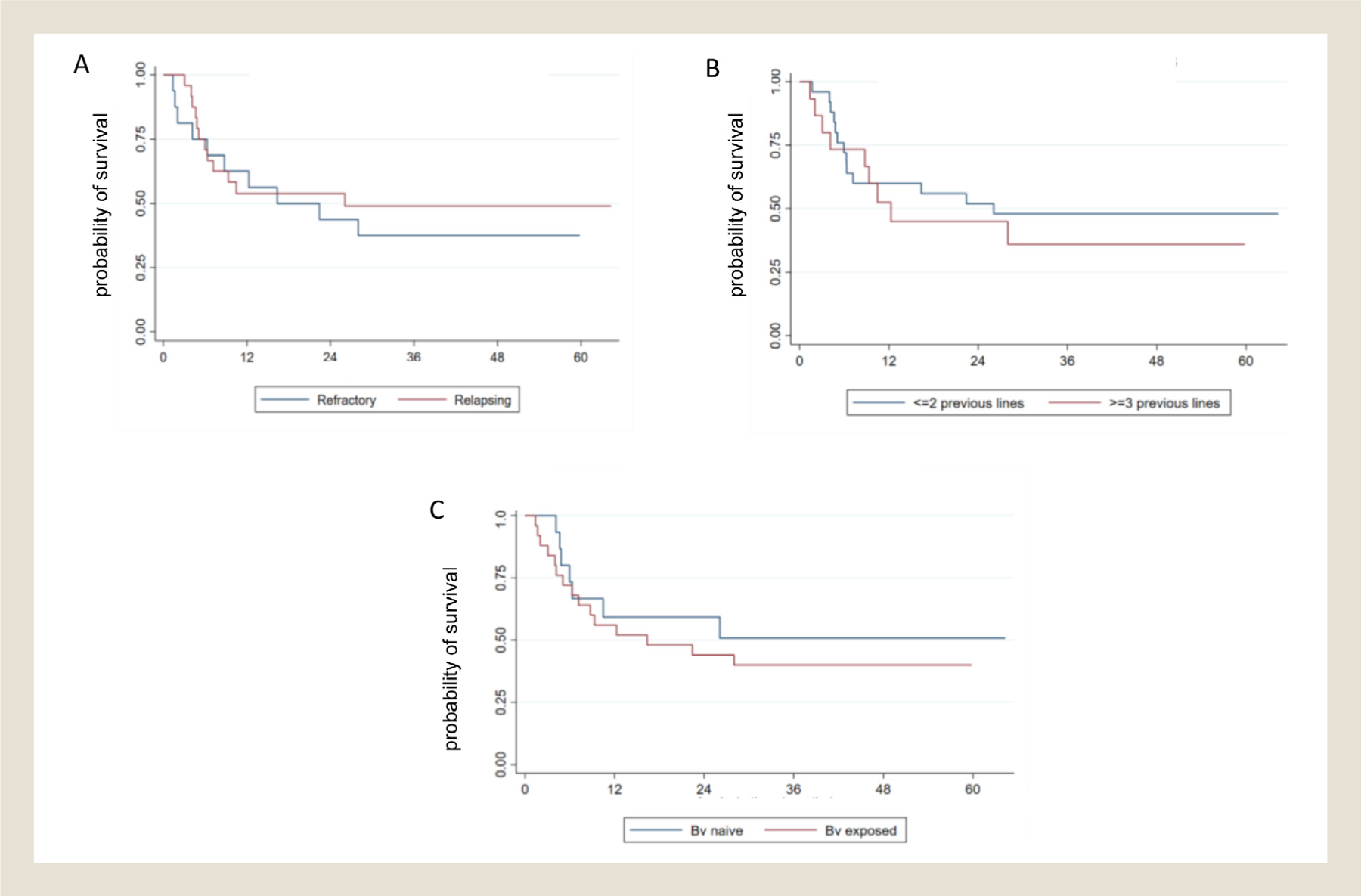
Progression-free survival in predefined patient subgroups. (A) refractory or relapsed after their first therapy; (B) ≤ 2 or ≥ 3 previous lines; (C) brentuximab vedotin naïve or brentuximab vedotin exposed.
Discussion
We reported a real-life experience in R/R cHL patients treated with the BVB combination with a median follow-up of 38 months, the longest ever published in prospective or real-life BVB studies to the best of our knowledge. In this heavily pre-treated cohort, where 39% of patients had already failed to respond to BV monotherapy, the ORR and CRR of 75% and 50%, respectively, appear promising and compare favorably with the results of more intensive and toxic chemotherapy combinations employed in the same setting.8–11 Moreover, our findings are in line with other on-protocol or real-life experiences with BVB.21–25 Although the small sample size and inherent patient heterogeneity prevent detailed cross-study comparisons, the relatively consistent response rates suggest reproducibility of results across centers and geographic regions. The 71% ORR and 43% CRR achieved in the subgroup with primary refractory disease are of particular relevance for patients who have limited therapeutic options, but are still potentially eligible for SCT procedures. Additionally, similar efficacy was observed in patients who received BVB as early or late salvage therapy, suggesting that mechanisms of resistance to multiple lines of therapy can be overcome with the use of BVB. Finally, BVB was active in patients who had received, and in many cases were refractory to, BV monotherapy, suggesting possible synergism between BV and bendamustine. Additional pre-clinical and correlative tumor sample analyses are warranted to formally test this hypothesis. Although modest, the increase in CRR observed with more prolonged BVB therapy may imply a correlation between number of treatment cycles and depth of response. This observation is particularly important to maximize the clinical benefit of BVB in those patients who do not have a SCT option available.
The median PFS of 26 months in a population with multiple poor prognostic features is notable. Moreover, the observation that PFS duration was similar in patients with refractory or relapsed disease, and regardless of the number of previous chemotherapy regimens or prior BV exposure suggests that BVB may be considered as a valid therapeutic option for eligible patients with very high-risk cHL at any point in their treatment history. The significantly longer PFS of patients achieving CR to BVB compared to those achieving PR or NR underscores the importance of achieving deeper responses even in the R/R cHL setting. Accordingly, there was a trend towards better PFS between BVB responsive patients who received HDT-ASCT or AlSCT and those who did not.
Conclusion
Our data support the use of BVB as a markedly active, well tolerated combination in patients with R/R cHL, including those who are heavily pretreated. By producing high CRR, BVB allows many patients to proceed onto potentially curative HDT-ASCT or AlSCT. With an extended follow up, we further suggest that a significant proportion of heavily pretreated cHL patients can be cured with this approach. Studies comparing this regimen with traditional chemotherapy, particularly in patients in first relapse, are warranted.
Clinical Practice Points
Treatment options for patients with relapsed or refractory classical Hodgkin lymphoma (cHL) remain limited. The inability to achieve deep response with salvage therapy limits opportunities for cure. Commonly utilized polichemotherapy combinations are associated to severe hematological toxicity.
Our study demonstrates that the combination of brentuximab vedotin and bendamustine (BVB) is highly effective, producing an ORR of 75%, CRR 50% and a median PFS of 26 months, and it allows successful stem cell transplantation in eligible patients. With an extended follow up, we further suggest that a significant proportion of heavily pretreated cHL patients can be cured with this approach. Finally, the combination was generally safe, with most AE being mild and/or readily mangeable.
Supplementary Material
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clml.2021.09.018.
References
- 1.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 2013;31:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet 1993;341:1051–1054. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomized trial. Lancet 2002;359:2065–2071. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood 2010;116:4934–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood 2012;119:1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devillier R, Coso D, Castagna L, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin’s lymphoma responding to prior salvage therapy. Haematologica 2012;97:1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentzler RD, Evens AM, Rademaker AW, et al. F-18 FDG-PET predicts outcomes for patients receiving total lymphoid irradiation and autologous blood stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Br. J. Haematol 2014;165:793–800. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high–dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood 2001;97:616–623. [DOI] [PubMed] [Google Scholar]
- 9.Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. AnnOncol 2002;13:1628–1635. [DOI] [PubMed] [Google Scholar]
- 10.Santoro A, Magagnoli M, Spina M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin’s lymphoma. Haematologica 2007;92(1):35–41. [DOI] [PubMed] [Google Scholar]
- 11.Santoro A, Mazza R, Pulsoni A, et al. Bendamustine in Combination With Gemcitabine and Vinorelbine Is an Effective Regimen As Induction Chemotherapy Before Autologous Stem-Cell Transplantation for Relapsed or Refractory Hodgkin Lymphoma: Final Results of a Multicenter Phase II Study. J of Clin Oncol 2016;34:3293–3299. [DOI] [PubMed] [Google Scholar]
- 12.Mehta-Shah N, Bartlett NL. Management of relapsed/refractory classical Hodgkin lymphoma in transplant-ineligible patients. Blood 2018;131:1698–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van de Donk N, Dhimolea E. Brentuximab vedotin Landes Bioscience; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metabolism Gandhi V. and mechanisms of action of bendamustine: Rationales for combination therapies. Seminars in oncology 2002;29(4, suppl. 13):4–11. [DOI] [PubMed] [Google Scholar]
- 15.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J ClinOncol 2012;30:2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016;128:1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopal AK, Chen R, Smith SE, et al. Durable remissions in a pivotal phase II study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood 2015;125:1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R, Palmer JM, Martin P, et al. Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin Lymphoma. Biol Blood Marrow Transplant 2015;21:2136–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskowitz AJ, Hamlin PA Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J ClinOncol 2013;31:456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anastasia A, Stella CC, Corradini P, et al. Bendamustine for Hodgkin lymphoma patients failing autologous or autologous and allogeneic stem cell transplantation: a retrospective study of the Fondazione Italiana Linfomi. British Journal of Haematology 2014;166:140–153. [DOI] [PubMed] [Google Scholar]
- 21.Sawas A, Connors JM, Kuruvilla JG, et al. The Combination of Brentuximab Vedotin (Bv) and Bendamustine (B) Demonstrates Marked Activity in Heavily Treated Patients with Relapsed or Refractory Hodgkin Lymphoma (HL) and Anaplastic Large T-Cell Lymphoma (ALCL): Results of an International Multi-Center Phase I/II Experience. Blood 2015;126:586. [Google Scholar]
- 22.O’Connor OA, Lue JK, Sawas A, et al. Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin’s lymphoma: an international, multicentre, single-arm, phase I-II trial. Lancet Oncol 2018;19:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaCasce AS, Bociek RG, Sawas A, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood 2018;132:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picardi M, Della Pepa R, Giordano C, et al. Brentuximab vedotin followed by bendamustine supercharge for refractory or relapsed Hodgkin lymphoma. Blood Adv 2019;3:1546–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broccoli A, Argnani L, Botto B. First salvage treatment with bendamustine and brentuximab vedotin in Hodgkin lymphoma: a phase II study of the Fondazione Italiana Linfomi. Blood Cancer J 2019;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J ClinOncol 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sally F, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging 2017;44(1):97–110 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Common Terminology Criteria for Adverse Events (CTCAE) U.S.DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute. Version 4.0 Published: 2009 (v4.03: June 14, 2010)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


