Abstract
Objective:
This article delineates best practices in the application of the experimental therapeutics framework for evaluating interventions within the context of randomized controlled trials (RCTs), offering a methodological primer and guiding framework for this approach. We illustrate these practices using an ongoing clinical trial conducted within the framework of a National Institute of Mental Health exploratory phased-innovation award for the development of psychosocial therapeutic interventions for mental disorders (R61/R33), describing the implementation of a novel ‘Facial Affect Sensitivity Training’ (FAST) intervention for children with callous-unemotional (CU) traits. CU traits (e.g., lack of guilt or remorse, low empathy, shallow affect) are an established risk factor for persistent and severe youth misconduct, which reflect impairment in identified neurocognitive mechanisms that interfere with child socialization, and predict poor treatment outcomes, even with well-established treatments for disruptive behavior.
Method:
We outline the stages, goals, and best practices for an experimental therapeutics framework. In the FAST trial, we assert that impaired sensitivity for emotional distress cues (fear and/or sadness) is mechanistically linked to CU traits in children, and that by targeting sensitivity to facial affect directly via a computerized automated feedback and incentive system, we can exert downstream effects on CU traits.
Results:
In the context of an open pilot trial, we found preliminary support for feasibility and mechanism engagement using FAST.
Conclusions:
We summarize pilot study limitations and how they are being addressed in the R61/R33 RCTs, as well as challenges and future directions for psychosocial experimental therapeutics.
Keywords: experimental therapeutics, callous-unemotional traits, affect, emotion processing
Recent initiatives within National Institutes of Health, including the National Institute of Mental Health (NIMH), emphasize an experimental therapeutics approach to identification of new treatments (Insel, 2015; NIMH, Support for clinical trials at NIMH). Multiple mechanisms of therapeutic change can be addressed – including biological, behavioral, cognitive, and interpersonal (Gordon, 2017). An empirically grounded, mechanistic, and phased approach involves a series of goals: first, identifying and evaluating change in theoretically based potentially malleable targets (mechanism or mediator); second, evaluating the extent to which the targeted mechanism is adequately engaged in a reliable and measurable way by the intervention; and lastly, testing whether the change in the target impacts clinical outcomes of interest. Herein, we define “adequate engagement” of the target to mean reliable, objectively measurable change (usually based on a pre-specified threshold) in the mechanism, which is demonstrably due to the intervention itself rather than to other influences (e.g., normal development/maturation). Such milestone-driven testing can be best established methodologically via a stringent RCT design to compare target change among those randomized to the intervention versus a control condition in which the target is not strategically engaged.
The experimental therapeutics approach has a long history in medicine and pharmacological interventions in particular, extends traditional tests of efficacy and effectiveness (intervention impact on clinical outcomes under controlled or naturalistic conditions) and can capture underlying mechanisms through which interventions work. It holds significant promise for advancing psychosocial interventions for child psychopathology by explicitly specifying and targeting a priori hypothesized key mechanisms inside the proverbial “black box.” This parsimonious approach stands in contrast to the tradition of first establishing the efficacy of an intervention and only later investigating which aspects improved clinical outcomes and how (Insel et al., 2010).
To facilitate application of experimental therapeutics to psychosocial interventions, the NIMH developed funding opportunity announcements (FOAs) for clinical trials that focus on best practices for an experimental therapeutics approach. Targets may be disease mechanisms or significant risk factors that an intervention is intended to modify in order to ameliorate symptoms or improve behavioral or functional outcomes (NIMH, Support for clinical trials at NIMH). Targets may be identified at various systemic levels that are hypothesized to contribute to the development, exacerbation, or maintenance of symptoms and/or functional impairment. This can include intrapersonal factors (e.g., genetic, molecular, neural, cognitive, emotional, behavioral) and interpersonal, family, community, or higher-level processes that influence risk for disorder and could be strategically altered by an intervention. A conceptual framework is first developed that specifies the intervention target, provides evidence supporting its relevance to the clinical outcome of interest, offers a well-grounded hypothesis on the intervention’s ability to engage the target, and explains how change in the target will be measured in an objective, sensitive, valid, reliable manner. Reliance on self-report/subjective measures of target engagement is discouraged. Multiple targets can be specified, but it must be clear how intervention strategy elements uniquely engage each target. Even if target engagement fails to be associated with symptom reduction or functional change, this null outcome can help inform redirection of effort in subsequent trials. Repeated measures are also expected, as well as consideration of optimal intervention dose (intensity, frequency, duration) in relation to level of target engagement.
While the experimental therapeutic framework applies to a number of grant mechanisms, herein we focus on outlining a Phased “Exploratory Clinical Trial” (R61/R33, “Development of Psychosocial Therapeutic and Preventative Interventions for Mental Disorders” https://grants.nih.gov/grants/guide/pa-files/PAR-21–135.html). This funding mechanism promotes pilot research that develops and rigorously tests innovative psychosocial interventions with novel targets and/or new intervention strategies within an experimental therapeutics framework. Overall, the goal is to support the translation of burgeoning research on psychopathology processes and mechanisms into new interventions or augmentations of efficacious interventions. While such studies may be “high risk” and entail higher failure rates, the framework supports early, objective, efficient investigations that can inform which interventions should be further developed, managing risk from inconclusive or negative results by using a phased innovation (R61/R33) approach.
The primary objective of this article is to summarize best practices within the experimental therapeutics framework for evaluating interventions within the context of randomized clinical trials (RCTs). Using our preliminary work and current trial, we illustrate these practices in order to offer a methodological primer and guiding framework for this approach. While we cite prior research, including some findings from our pilot studies that used smaller samples and an open-trial design, the purpose of referencing this earlier work herein is to exemplify the experimental therapeutics approach, not to demonstrate intervention efficacy.
Stages, Goals, and Best Practices in an Experimental Therapeutics Framework
Table 1 presents our guiding framework for the stages, goals, and best practices of an experimental therapeutics approach for psychosocial intervention development. The three stages include: (1) target identification, (2) target engagement, and (3) linking target engagement to clinical efficacy. We contextualize this staged approach in reference to an ongoing NIMH-funded R61/R33 study. Because this study is currently underway, and because Stage 1 (target identification) establishes the foundation for subsequent stages, we emphasize the target identification stage, in which the primary goal is to specify an objective measure of one or more psychosocial disease mechanisms or risk factors that contribute to the clinical outcome of interest.
Table 1.
Experimental therapeutics framework for psychosocial intervention development
| Stage 1: Target Identification | |
| Goals | Best-Practice Objectives |
| • Specify an objectively measurable, potentially malleable psychosocial disease mechanism or risk factor that contributes to clinical outcome of interest • Provide a strong conceptual basis including both theoretical support and empirical evidence for the target’s relevance to the clinical outcome of interest and its ability to function mechanistically (e.g., temporal precedence; Kendall et al., 2017). • Specify the nature and parameters of the intervention and offer a testable, theory-based hypothesis on how it will engage the target. Propose a strong test of target engagement and clear go/no-go milestones. • Explain how change in the target will be measured in an objective, sensitive, valid, reliable manner. |
• Identify an innovative means of addressing an unmet mental health need or improving outcomes by choosing a novel target and/or intervention approach. • Specify the intervention target based on proposed mechanism of action. Targets may be disease mechanisms or significant risk factors that an intervention is intended to modify in order to ameliorate symptoms or improve behavioral or functional outcomes. • Consider that multiple pathways (equifinality) and thus multiple potentially viable targets may exist at various systemic levels (molecular, cognitive, behavioral, interpersonal, etc.) for the clinical outcome of interest. • Consider prior correlational or cross-sectional research to suggest association between the target mechanism and the clinical outcome. • Select reliable, valid measures of change in the target. Clearly specify key parameters of the intervention that will optimize target engagement. • Consider multimodal indicators of change (e.g., behavioral, neurocognitive, psychophysiological) and avoid exclusive reliance on subject self-report measures of target engagement. • Include repeated measures and examination of optimal intervention dose (intensity, frequency, duration) to maximize target engagement. • Multiple targets can be specified, but explain how intervention strategy elements uniquely engage each target and how this unique engagement will be measured. • Ideally, conduct at least one pilot study to establish preliminary support for feasibility of the intervention and its ability to selectively engage the target |
| Stage 2: Target Engagement | |
| Goals | Best-Practice Objectives |
| • Test whether the intervention results in change in the target | • Consider and minimize threats to internal and external validity in study design (e.g, masking intervention condition from participants and possibly study personnel, using a randomized controlled trial study design to control for potential confounds, selecting inclusion/exclusion criteria that minimize threats and carefully characterizing the sample, ensuring adequate sample size for statistical power and precision, etc.). • Follow Good Clinical Practice guidelines to ensure research participant safety, data integrity, and implementation fidelity. Develop Manual of Procedures following NIMH Clinical Research Toolbox (https://www.nimh.nih.gov/funding/clinical-research/clinical-research-toolbox/nimh-clinical-research-toolbox) guidance documents, templates, sample forms, links to additional resources, and other materials to assist clinical investigators in the development and conduct of high-quality clinical research studies. • Specify the expected timeline and clear “go/no-go” criteria (quantifiable target-engagement milestones) for continuing or not continuing to the next stage. • Determine feasibility of the intervention (e.g., enrollment, attendance, intervention engagement and completion; intervention fidelity, protocol adherence, and consumer satisfaction) • Collect evidence of target engagement and determine whether target-engagement milestones are met to justify continuing to next stage. • Monitor for adverse events. • Identify optimal dose that maximizes target engagement while minimizing cost and subject burden. |
| Stage 3: Link to Clinical Efficacy | |
| Goals | Best-Practice Objectives |
| • Refine the intervention and replicate target engagement • Examine preliminary efficacy of intervention • Determine whether the intervention works through the proposed mechanism • Inform whether the intervention should be further developed in a confirmatory efficacy trial, and if so, how such a trial should be designed |
• Modify the intervention or protocol as needed based on findings from the target-engagement stage regarding feasibility, optimal dosage, etc. • Test whether the change in the target corresponds with clinical symptom or functional outcome improvement, linking target engagement to improvement in clinical outcome. While this stage may not be powered to definitively establish clinical efficacy, it should offer a preliminary estimate the magnitude of relationship between changes in target and clinical outcomes in a clinical or at-risk sample. • Use random assignment again to help control for potential confounding variables and establish the causal link between change in target mechanism and change in clinical outcome. • To further investigate mechanism specificity in relation to clinical outcomes, use an active control condition (equivalent in non-specific factors such as duration) that receives an alternative intervention, rather than no-intervention/waitlist control. • Intervention dosage parameters (e.g., session number, frequency, duration) should be justified in part based on stage 2 findings. • Determine sustained impact of intervention on both target mechanism and clinical outcomes. • Monitor for adverse events. • Further refine and standardize the intervention. • Measure and confirm intervention feasibility, fidelity, and acceptability. |
Stage 1: Target Identification in the Prevention of CU Trait Development
Disruptive behavior problems are among the most prevalent and costly mental health conditions of childhood (Foster et al., 2005; Nock et al., 2006) and the most common antecedent to adult psychiatric disorders (Erskine et al., 2013). A risk factor for particularly severe, chronic, and violent youth misconduct is the presence of CU traits, which reflect reduced concern for others or behavioral consequences, low emotional sensitivity, and impairments in empathy, guilt, and remorse (McMahon et al., 2010; Frick & Ellis, 1999). As shown in prior work, CU traits have unique neurobiological and cognitive correlates (Frick & Ellis, 1999; White & Delk, 2017) and predict elevations in aggression, particularly proactive (instrumental) aggression, which often persists into adulthood (e.g., White et al., 2015). Recognition of CU traits as a key risk factor and treatment barrier has led to a Conduct Disorder specifier, With Limited Prosocial Emotions, in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013). CU traits also correspond to the core affective component of adult psychopathy, a predictor of violence and crime (Rutter, 2012).
In light of these considerations, development of effective early interventions for high-CU youth is a public health priority. Although interventions for disruptive youth are well-established, (Comer et al., 2013; Michelson et al., 2013), and some broad multicomponent interventions designed to target disruptive behavior problems have shown some impact on CU traits, although they were not the target (Kolko & Pardini, 2010; Lochman et al., 2014; Muratori et al., 2017), about 40–50% of such youth do not show substantial or long-lasting benefits (Murrihy et al., 2010; Ollendick et al., 2016). The presence of CU traits predicts poorer outcomes from these multicomponent interventions, even after controlling for pre-treatment conduct problem severity (Frick et al., 2014; Hawes et al., 2014), suggesting more targeted intervention is needed. The vast majority of evidence-based treatments for youth conduct problems focus on coercive exchanges between parents and children with reactive temperaments (e.g., poor impulse and emotion regulation) (Ollendick et al., 2016; Patterson et al., 1989). CU traits represent an alternative developmental pathway to conduct problems (Pardini & Frick, 2013). Given the central role of CU traits in hostile, aggressive, and interpersonally violent behavior, the substantial societal and financial costs associated with such behaviors, and evidence that presence of elevated CU traits predicts poorer response to even our most evidence-based treatments (Frick et al., 2014; Hawes et al., 2014), the development of efficient and effective interventions to modify this risk factor and its sequelae at an early age is critical.
In keeping with a traditional intervention development approach, and a focus on parenting strategies for child disruptive behaviors, Kimonis and colleagues (2019) recently conducted an open trial on feasibility and preliminary efficacy of a promising novel parenting and emotion-training intervention on 23 Australian families with 3–6 year-old children with conduct problems and CU traits. This 21-week intervention, Parent-Child Interaction Therapy-Callous/Unemotional (PCIT-CU), integrates a modified version of PCIT focused on teaching positive parenting strategies with an adjunctive module designed to coach and reward child emotional skills (Kimonis et al., 2019; Kimonis & Fleming, 2019).
The experimental therapeutics approach we have taken, with children slightly older than those in the Kimonis et al. (2019) study and with known FER deficits, is a unique and distinct but complementary one to this more traditional approach to intervention development for children with CU traits. Our focus is, by design, narrow. PCIT-CU involves multiple components, which requires a different approach in order to understand the mechanism of action (e.g., dismantling). Our current investigation, in addition to helping address this unmet clinical/prevention need, involves RCTs with a highly specified target and is designed to be able to clearly support or refute the hypothesized mechanisms of action.
As an example of how Stage 1 of an experimental therapeutics approach is initiated and implemented, the Facial Affect Sensitivity Training (FAST) intervention was grounded in an established conceptualization of CU traits known as the temperamental fearlessness model (Blair, 1995; Blair, 2006; Lykken, 1957). This framework emphasizes fundamental deficits in affect sensitivity, including reduced attention toward and responsiveness to threat cues, as well as reduced recognition of others’ emotional cues, in particular, signals of distress (e.g., expressions of fear or sadness). This model is supported by evidence that youth with CU traits and psychopathic adults fail to learn from punishment (Lykken, 1957) or acquire fear-conditioned responses (Birbaumer et al., 2005; Flor et al., 2002). There is also substantial correlational evidence across independent research groups of an association between CU traits and deficits in facial emotion recognition (FER), with the largest effect sizes evident for distress-related expressions such as fear and sadness (Billeci et al., 2019; Blair et al., 2001; Dawel et al., 2012; Dadds et al., 2006).
Such deficits are reflected physiologically in fear-potentiated startle response, electrophysiological and event-related potential (ERP) correlates, and underactivity in the amygdala and limbic-prefrontal circuit (Blair, 2008; Brislin et al., 2018; Ledoux, 2000). These patterns are also associated with functional impairment in affective empathy (affective responding to others’ emotional displays) (Blair, 2013). Collectively, such deficits in affect sensitivity are believed to disrupt learning and socialization processes critical to the development of conscience and guilt, and the inhibition of aggressive impulses, predisposing the child to a deleterious developmental cascade culminating in social dysfunction (Akers, 1998; Blair, 2013; Kochanska, 1994).
A variant of the temperamental fearlessness model attributes CU trait-related deficits in distress recognition to a lack of spontaneous orientation of attention to others’ eyes (eye gaze) (Dadds et al., 2006). The eye region is particularly important in signaling distress to others (Adolphs et al., 2005). The amygdala is maximally activated in response to a prototypical of fear expression with wide-open eyes (Morris et al., 2002). Eye region micro-expressions (e.g., sclera showing in the upper eye but not below the iris, upper eyelid elevated, wrinkling between brows) distinguish fear from other expressions (Friesen & Ekman, 1983). Young high-CU males (ages 7 – 18) show less spontaneous gazing at others’ eyes in lab-based eye-tracking studies (Blair, 2013; Dadds et al., 2008) and naturalistic parent-child observations (Dadds et al., 2002; Dadds et al., 2014). Furthermore, this reduced spontaneous eye gaze has been found to contribute to the association between reduced FER for distress expressions (fear or sadness) and CU traits in these youth (Billeci et al., 2019; Dadds et al., 2008). Demetriou and Fanti (2021) recently reported that young (mean-age = 6.35) high-CU children show reduced eye gaze and increased fixation to the mouth area across emotional expressions (fear, sad, happy, angry), which might account for the observed FER deficits across emotions in their sample. A similar eye gaze deficit is seen in amygdala-damaged patients (Adolphs et al., 2005) and adult psychopathic inmates (Richell et al., 2003). Because eye gaze provides critical momentary feedback on others’ emotional states, deficient eye gaze may contribute to the deleterious development in high-CU youth (Dadds et al., 2014; Frick et al., 2014).
Integrating these theoretical models and evidence, we identified reduced sensitivity to facial affect (SFA) as a mechanism underlying the development of CU traits and a viable target for intervention. Herein, we conceptualize the construct of SFA as encompassing sensitivity to and orienting visual attention toward facial affective cues, as well as improved FER, including interpretation of distress signals. Thus, we view SFA as comprising both attention to emotional cues and accurate processing and interpretation of those cues, rapid cognitive processes that potentially overlap in time. Evidence suggests that children high in CU traits do not automatically experience natural incentives that endogenously cue normal eye gaze tendencies that heighten SFA and facilitate FER. Although youth with CU traits show reduced sensitivity both to others’ facial communication signals of fear and to punishment cues (Frick et al., 2014), they do show normal reward signal processing or even a reward-dominant response style (Budhani & Blair, 2005; Byrd et al., 2014; Lykken, 1957). We propose that SFA may be trained in high-CU youth via systematic application of exogenous incentives to improve their sensitivity and orientation to affective stimuli, and more rapid and accurate recognition of others’ distress signals, since their reward systems remain intact or even enhanced. Thus, the target of FAST is to train and automatize SFA via an alternate (reward-based operant conditioning) pathway by cueing eye gaze and incentivizing accurate FER. Specifically, the FAST computerized intervention directly instructs children to “look at the eyes” frequently (cueing visual attention orientation) and provides real-time feedback (auditory tone) and reinforcement (points earned toward prizes) when FER (interpretation of emotion expression) is accurate. On each trial, a fixation cross is followed by an emotional face with eyes directed left, straight ahead, or right (balanced across expressions), followed by a response cue. The child’s task is to indicate the direction the eyes are looking. Stimuli are black and white standardized photographs of men and women models from the Ekman Pictures of Facial Affect (Ekman, 1993) each displaying the 3 gaze directions for 6 emotion expressions (fearful, happy, angry, sad, disgust, and neutral). The child is reminded approximately once per minute (every 15 trials) “Remember, look at the eyes!” to cue eye gaze and instructed to select the emotion displayed in that face from a list of emotions displayed on the monitor. The child receives immediate reinforcement of correct responses via auditory reward tone, which signals points earned toward incrementally “priced” small toys the child can select at the end of each session. The objective of the FAST intervention is to make appropriate eye gaze more automatic during FER (thus improving facial affect sensitivity) by repeatedly pairing correct responses with a reward.
Interestingly, FER for distress expressions is improved by increasing facial stimulus emotional intensity (Blair et al., 2001) or overtly directing the child’s attention by instructing “look at the eyes.” (Dadds et al., 2006) Because psychopathology is typically multiply determined (Rutter, 2012; Lilienfeld et al., 2016), reduced eye gaze is likely an important but not sole manifestation of insensitivity to affect in youth with CU traits (Blair, 2011). Indeed, high-CU youth also show deficient distress recognition across other sensory modalities (e.g., sad vocal tone, crying) (Blair, 1999; Blair et al., 2002). Thus, we see the attention-to-eyes model (Dadds et al., 2006) as one specific but important exemplar of the temperamental fearlessness model, and one with potential intervention implications wherein visual attention to others’ eyes playing a key role in SFA, along with behavioral and neural correlates of FER.
While we use the “CU traits” label, CU tendencies are notably not immutable, at least in young children, but instead show heterogeneous developmental trajectories likely due to environmental influences (e.g., Muratori et al., 2016; Pardini et al., 2007). Thus CU traits are better thought of as malleable behavior patterns (Fontaine et al., 2011; Waller et al., 2016). Whereas intervention efforts for CU traits in early childhood focus primarily or exclusively on parent training (Hawes et al., 2014; Kimonis et al., 2019), the strong evidence for neurocognitive deficits in SFA suggests potential alternative or adjunctive intervention targets. Yet, only three studies to date have attempted to meaningfully alter FER in youth with CU traits. While these studies suggest some promise, methodological limitations limit interpretability of findings, as described below (Dadds et al., 2012; Dadds et al., 2019; Datyner et al., 2016).
Given the importance of the eye region in particular to distress signaling, we proposed that strategically increasing high-CU youths’ attention and sensitivity to facial emotional information, particularly information provided by others’ eyes, can improve FER for distress signals. However, the intervention was carefully designed to consider temperamental patterns common to youth with CU traits. Importantly, we posited that while eye gaze is temporarily corrected via direct instruction (Dadds et al., 2006), these changes may not be durable beyond a matter of seconds, and there is no evidence that they create appropriate eye contact in the youth’s social world or influence CU traits. In other words, the simple directive to “look at the eyes” alone would be insufficient. Thus, we proposed that difficulties identifying distress facial emotions in these children would be more pronounced and sustained when the sensitivity to social affective stimuli is increased via operant conditioning of attentional orientation toward and accurate processing of emotional expressions.
The fundamental premise of our conceptual mechanistic model is that impaired sensitivity for emotional distress cues (fear and/or sadness) is mechanistically linked to CU traits in children, and that by targeting SFA directly via a computerized automated feedback and incentive system, we can exert downstream effects on CU traits (Figure 1). Whereas youth with CU traits are insensitive to others’ distress signals and to punishment cues (Frick et al., 2014), they have intact reward processing or even a reward-dominant response style (Budhani & Blair, 2005; Byrd et al., 2014; Lykken, 1957). We thus hypothesize that children high in CU traits do not automatically experience natural incentives that endogenously cue normal eye gaze tendencies, but they may nevertheless be trained via exogenous incentives to become more sensitive and oriented to affective stimuli and better able to recognize others’ distress signals. Such training, implemented early in childhood, may interrupt the developmental cascade toward antisocial outcomes.
Figure 1.

Conceptual model of Facial Affect Sensitivity Training on SFA in Youth with CU Traits
To summarize, our central hypothesis is that impaired SFA, specifically to distress cues (fear and/or sadness), is mechanistically linked to CU traits in children, and that, by targeting affect sensitivity directly via a computerized real-time automated feedback and incentive system, we can exert downstream effects on CU traits. This project will directly target SFA in high-CU youth. Per the NIMH Strategic Plan (Objective 3.1), we proposed an experimental therapeutics approach to develop a novel neurocognitive intervention for CU traits with SFA as a clearly identified target that is engaged and assessed via primary (distress FER accuracy, heightened eye gaze) and secondary (ERP) neurocognitive and behavioral processes. Our long-term goal is to apply this targeted intervention to the wider range of problems associated with CU traits.
Stage 2: Target Engagement
The primary goal of the second stage, Target Engagement, is to test whether the intervention results in change in the target. Because prior research shows that intentional training of eye gaze facilitates FER, FAST training is expected to improve, via use of structured practice with exogenous contingent reinforcement, both attention to the eye region and FER for novel face stimuli, processes we will assess separately. For sake of establishing a clear go/no-go criterion, we use two dimensions of SFA as our primary targets: (1) FER (behavioral performance; accurate interpretation of distress expressions) and (2) eye-gaze indices to measure attentional orientation and allocation. As secondary indicators of SFA, using electroencephalography (EEG) we also measure ERP signals as neural markers of sensitivity to, encoding, and processing of facial expressions. This approach allows us to quantify how FER improvement corresponds to changes in eye gaze and ERPs, informing our SFA/FAST-intervention conceptual model.
We defined specific aims for the target engagement (R61) stage of our FAST clinical trial. We will first demonstrate, in a preliminary RCT (N=84), that a new neurocognitive intervention (FAST) can improve SFA [target engagement] in children with elevated CU traits. SFA will be measured primarily by FER accuracy for distress expressions and/or heightened attention to the eye region (eye gaze), and secondarily by neural activity (specifically, N170 and P200 ERP components, based on prior findings with high-CU adults and youth; Brislin et al., 2018; Hoyniak et al., 2018). We have several objectives within this stage: 1) Establish that distress FER accuracy and/or eye gaze can be altered in a reliable manner among young children with elevated CU traits; 2) Determine whether FAST improves secondary neural indices of SFA (brain activity during processing of emotional faces); 3) Refine FAST for subsequent evaluation by determining optimal dose parameters with regard to number of sessions for FER and/or eye gaze improvement via a nonlinear mixed model for small samples (e.g., timing of local bump or decay, amount of change, when maximal change occurs), and participant satisfaction with session frequency, length, and number; and 4) Deliver a computerized training program (FAST) capable of providing real-time automated feedback and reinforcement of accurate FER performance.
We also established the following milestones (Go/No-Go Criteria): The FAST intervention will engage the target (SFA), indexed by enhancing distress FER accuracy and/or eye gaze in high-CU youth. We will examine individual growth rates and test slope differences between conditions (FAST v control). Target engagement will be defined as medium effect size (d = .50; Cohen, 1988) in the comparison of FAST vs. no-treatment control on the primary SFA indices (distress FER and/or eye gaze).
Stage 3: Linking Target Engagement to Clinical Efficacy
The objectives of the third stage, linking target engagement to clinical efficacy, include replicating target engagement, examining preliminary efficacy of intervention, determining whether the intervention works through the proposed mechanism, and informing whether the intervention should be further developed in a confirmatory efficacy trial, and if so, how such a trial should be designed.
Returning to our illustrative example of the FAST clinical trial, should we find support during the second stage (R61 phase) for FAST intervention feasibility and target engagement, in keeping with our open-trial pilot findings, our specific aims for this final stage (R33) of our FAST clinical trial are to replicate target engagement with a new, larger high CU sample and evaluate feasibility and preliminary efficacy of FAST, in the context of an RCT (N = 84) in which FAST is compared to an active control condition (ACC; implicit eye gaze training). In addition, we plan to validate the functional role of SFA by examining downstream change in CU as a result of FAST. FAST will produce reliable increases in FER accuracy for distress cues in others. Furthermore, FAST completers will show greater improvement in CU/empathic behaviors than ACC completers. Our two main objectives for this stage are thus: 1) Replicate target engagement of SFA; 2) Determine if improved SFA leads to reduction in CU.
Preliminary Support for Each Stage of the SFA/FAST Model
We briefly summarize some pilot investigations in order to illustrate the last best-practice objective in Stage 1 in Table 1 (i.e., conduct at least one pilot study to establish preliminary support for feasibility of the intervention and its ability to selectively engage the target). Several sets of pilot data from our lab support the conceptual basis for SFA deficits in children with CU traits as well as intervention feasibility and mechanism malleability. First, we have unpublished cross-sectional data (Delk & White, 2018) from a sample of 86 children ages 6–9 showing elevated CU traits are associated with reduced fear FER for boys (r = −.41, p = .010), though interestingly not for girls (r = .03, p = .838). In this sample, ability to track eyes consistently was high (82% of trials). For boys, eye gaze duration was associated with fear FER (r = .47, p = .003) and inversely with CU traits (r = −.34, p = .036), but for girls, eye gaze duration was not associated with fear FER (r = .10, p = .487) or with CU traits (r = −.18, p = .202). Because this was a cross-sectional study, the question of whether eye gaze serves as a mechanistic pathway awaits further investigation with a longitudinal, multi-wave design study that accounts for the temporal ordering of these processes. The n=26 6-year-olds included in this sample demonstrated that young children can complete a full session protocol and provide valid eye-tracking data (100%).
We also collected pilot data on n = 12 children ages 6–10 with elevated parent-reported CU traits who completed the FAST intervention (White et al., 2019). Separately, we piloted n = 6 children in the same age range with elevated parent-reported CU traits on the active control condition (ACC). The ACC comprised an implicit gaze-training task we developed, in which participants are implicitly tasked with attending to others’ eyes (Dadds et al., 2006) using a computerized gaze training program. Like the FAST intervention program, ACC task uses the same stimuli and trial format. But in contrast to the FAST intervention, in the ACC, the child is simply told to “look at the face,” rather than being explicitly directed to “look at the eyes.” In an effort to implicitly train eye gaze, the child is instructed to identify the gaze direction of each emotional face (left, right, or center). Unlike the FAST intervention, no performance feedback or incentives are provided.
Regarding primary target engagement (FER behavioral performance and/or eye gaze), our pilot data on the 18 youth who either received FAST (n=12) or ACC (n=6), showed promising results such that only children receiving FAST show improvement in FER accuracy. The time*treatment interaction (FAST vs. ACC) was significant for both fear (p <0.01) and sadness (p <0.01). Using a Tobii T60XL eye tracker, we also observed significant effects on eye gaze, such that children who received the FAST intervention exhibited an upward trend in eye gaze over time, while those in the active control showed a downward trend (Figure 2). The time*treatment interactions showed nonsignificant trends for eye gaze primacy (first fixation on the eyes; p =.09) and duration of eye gaze (p =.08).
Figure 2.
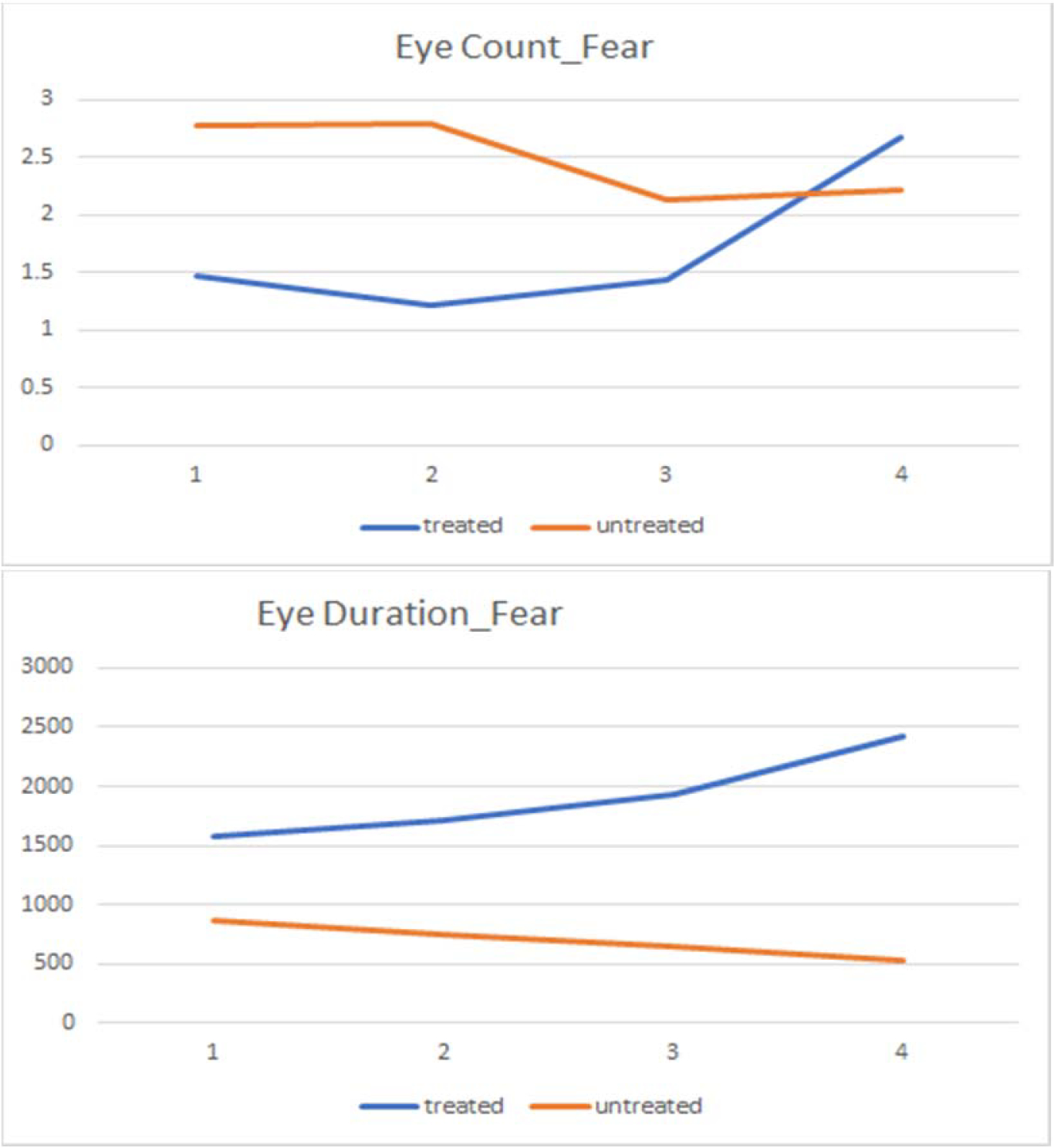
Trends in eye-gaze primacy (“eye count”) and eye-gaze fixation time (“eye duration” in msec) for fear faces in FAST intervention pilot across sessions (x-axis).
Regarding our secondary index of SFA engagement, ERP results for children in the FAST condition revealed that the P200 component demonstrated increased amplitude discrimination (i.e., effect differences between fear and neutral face conditions) from the first session (M = 2.47 μv) to the last session (M = 9.49 μv). This finding suggests that the FAST intervention enhanced early attention processing to negative facial expressions.
Lastly, our pilot data were promising in terms of FAST intervention impact on clinical outcomes. Specifically, we observed a significant effect of FAST intervention relative to the active control with respect to reduction in parent-reported callousness (p =. 01) on the Inventory of Callous Unemotional Traits (Frick, 2004).
DISCUSSION
The primary objective of this review was to summarize best practices within the experimental therapeutics framework for treatment of CU traits in children. We illustrated these practices using the clinical trial we are conducting within the NIMH exploratory phased-innovation award framework for the development of psychosocial therapeutic interventions for mental disorders. We presented open-trial pilot data suggesting the FAST intervention is feasible for children with elevated CU traits and engages the target mechanism, SFA, as measured primarily by FER accuracy for distress expressions and/or heightened attention to others’ eyes (eye gaze), and secondarily by neural activity. We described how the ongoing target engagement demonstration (R61) phase will assess optimal dose parameters and feasibility (e.g., protocol fidelity, consumer satisfaction). We discussed the need to replicate (in the R33 phase) target engagement with a new sample, and to evaluate feasibility and preliminary efficacy of FAST relative to an active control condition (implicit eye gaze training) in producing downstream change in CU traits. If the FAST intervention improves FER and/or eye gaze and reduces CU traits, such training in early childhood could help interrupt the developmental cascade toward antisocial outcomes.
There are several important caveats to interpreting the pilot study findings. Foremost, this was an open trial on a less intensively screened small sample, and the ACC dosage was not equivalent to the FAST intervention (i.e., fewer sessions). Change was not seen in all outcome measures in our pilot data, likely owing at least in part to the high level of variability in initial CU traits in this sample, with some cases below the standard CU cutoffs. Nevertheless, our open-trial pilot data supported our ability to implement our study protocol with high intervention adherence and no adverse events, to produce viable data, and provided a very preliminary indication that the FAST intervention can engage the target and potentially improve clinical outcomes. We are currently completing Stage 2 (R61 phase) of our NIMH-sponsored investigation to determine whether pilot findings replicate in a new, larger sample under strict experimental controls.
Some Challenges of the Experimental Therapeutics Paradigm & Future Directions
While the experimental therapeutics approach offers some clear advantages over traditional efficacy trials (e.g., identifying key mechanisms, informative failures, high risk/reward innovation), we conclude by highlighting some of the challenges and future directions to this approach. First, negative results, while potentially more informative from experimental therapeutic designs, can leave lingering questions regarding interpretation and next steps. Failure to move the target mechanism at Stage 2 or clinical outcome at Stage 3 might reflect problems with the sensitivity of measures of change in target mechanism or clinical outcome, intervention design, dosage, or fit with the targeted population. Considering equifinality, potential psychosocial targets for a given condition are often multiple and can be challenging to accurately identify or disambiguate from one another (Lewandowski et al., 2018), and they may interact in complex ways. While the implications of positive results are clear, the implications of negative results may thus be less straightforward. Ensuring a strong study design, conceptual and empirical basis for target selection, and otherwise following best practices outlined in Table 1 (e.g., selecting reliable, valid measures of change and utilizing multimodal indicators of target engagement) can help mitigate such challenges. For instance, to reduce likelihood and range of alternative explanations for negative findings in the FAST trial, we have taken steps to ensure a strong conceptual and empirical basis for target selection, a rigorous design and adequate statistical power, stringent inclusion criteria to ensure target presence (pre-existing FER deficits), and multimodal assessment to carefully and thoroughly characterize the sample and intervention impacts using measures previously shown to be sensitive to change.
Second, improving interventions for youth with CU traits does not end with demonstrating a specified target can be manipulated to impact clinical outcomes via a novel intervention. It is likely that any intervention will not be equally effective for all individuals with a clinical condition since individual factors may moderate intervention efficacy. For instance, the FAST intervention may be more effective for certain high-CU youth (e.g., primary versus secondary variants; Dadds et al., 2018) because such youth have diminished SFA. Thus, diminished SFA may not be the central mechanism for all youth with CU traits, even if it is a common mechanism for many. Heterogeneity of treatment response can complicate identification of relevant psychosocial intervention targets (Lewandowski et al., 2018). Personalized interventions, exemplified by the emerging field of precision medicine, calls for unique approaches to clinical trials (e.g., Lenze et al., 2020). The Introduction to this special issue speaks to the intersection of personalized medicine and experimental therapeutics. As a preliminary step in the FAST trial, we are considering potential moderators such as child age, gender, and CU variant subtypes identified in prior research (e.g., Dadds et al., 2018).
Finally, for interventions supported by experimental therapeutics, challenges may remain regarding their dissemination and implementation (Insel, 2015). If found effective, as a computerized intervention, FAST is highly transportable and requires minimal resources or training, which could facilitate large-scale dissemination and implementation. However, translating research into routine practice requires careful planning and investigation (e.g., Shelton et al., 2020, offer an introduction to D&I methods, frameworks, and opportunities). Thus, it is important to keep in mind both the benefits and limitations of experimental therapeutics, and to consider as a field whether the goals of this approach are being realized to reducing the mental health burden of youth and families. We anticipate that an emphasis on best practices in experimental therapeutics (Table 1) will help inform future work.
Acknowledgements:
Special thanks to Kirby Deater Deckard, Robin Panneton, and Thomas Ollendick for their invaluable input and support during the early stages of development of the FAST proposal.
Funding:
Pilot data collection was supported through grants to B. White from the Virginia Tech Institute for Society, Culture and Environment and the Center for Peace Studies and Violence Prevention. The R61/R33 clinical trial described herein is supported by the National Institute of Mental Health (grant number 5R61MH117192–02).
Footnotes
The authors have no conflicts of interest to report.
Ethics approval: This study was approved by the Institutional Review Board of The University of Alabama IRB# 19–10-2933/
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR (2005) A mechanism for impaired fear recognition after amygdala damage. Nature, 433(7021), 68–72. 10.1038/nature03086 [DOI] [PubMed] [Google Scholar]
- Akers RL (1998). Social learning and social structure: A general theory of crime and deviance. Boston, MA: Northeastern University Press. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Billeci L, Muratori P, Calderoni S, Chericoni N, Levantini V, Milone A, Nocentini A, Papini M, Ruglioni L, & Dadds M (2019). Emotional processing deficits in Italian children with disruptive behavior disorder: the role of callous unemotional traits. Behaviour Research and Therapy, 113, 32–38. 10.1016/j.brat.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, & Flor H (2005). Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry, 62(7), 799–805. 10.1001/archpsyc.62.7.799 [DOI] [PubMed] [Google Scholar]
- Blair RJR (1995). A cognitive developmental approach to morality: Investigating the psychopath. Cognition, 57(1), 1–29. 10.1016/0010-0277(95)00676-p [DOI] [PubMed] [Google Scholar]
- Blair RJR (1999). Responsiveness to distress cues in the child with psychopathic tendencies. Personality and Individual Differences, 27(1), 135–145. 10.1016/S0191-8869(98)00231-1 [DOI] [Google Scholar]
- Blair RJR (2006). The emergence of psychopathy: Implications for the neuropsychological approach to developmental disorders. Cognition, 101(2), 414–442. 10.1016/j.cognition.2006.04.005 [DOI] [PubMed] [Google Scholar]
- Blair RJR (2008). The amygdala and ventromedial prefrontal cortex: Functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1503), 2557–2565. 10.1098/rstb.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2011). Commentary: Are callous unemotional traits all in the eyes? Examining eye contact in youth with conduct problems and callous unemotional traits–reflections on Dadds et al.(2011). Journal of Child Psychology and Psychiatry, 52(3), 246–247. [DOI] [PubMed] [Google Scholar]
- Blair RJR (2013). The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience, 14(11), 786–799. 10.1111/j.1469-7610.2010.02364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Colledge E, Murray L, & Mitchell DGV (2001). A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology, 29(6), 491–498. 10.1023/a:1012225108281 [DOI] [PubMed] [Google Scholar]
- Blair RJR, Mitchell DG, Richell RA, Kelly S, Leonard A, Newman C, & Scott SK (2002). Turning a deaf ear to fear: Impaired recognition of vocal affect in psychopathic individuals. Journal of Abnormal Psychology, 111(4), 682. 10.1037//0021-843x.111.4.682 [DOI] [PubMed] [Google Scholar]
- Brislin SJ, Yancey JR, Perkins ER, Palumbo IM, Drislane LE, Salekin RT, Fanti KA, Kimonis ER, Frick PJ, Blair RJR, & Patrick CJ (2018). Callousness and affective face processing in adults: Behavioral and brain-potential indicators. Personality Disorders: Theory, Research, and Treatment, 9(2), 122. 10.1037/per0000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhani S, & Blair RJR (2005). Response reversal and children with psychopathic tendencies: Success is a function of salience of contingency change. Journal of Child Psychology and Psychiatry, 46(9), 972–981. 10.1111/j.1469-7610.2004.00398.x [DOI] [PubMed] [Google Scholar]
- Byrd AL, Loeber R, & Pardini DA (2014). Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clinical Child and Family Psychology Review, 17(2), 125–156. 10.1007/s10567-013-0159-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences, Second Edition. Lawrence Erlbaum Associates Inc. Hillsdale, New Jersey. [Google Scholar]
- Comer JS, Chow C, Chan PT, Cooper-Vince C, & Wilson LA (2013). Psychosocial treatment efficacy for disruptive behavior problems in very young children: A meta-analytic examination. Journal of the American Academy of Child & Adolescent Psychiatry, 52(1), 26–36. 10.1016/j.jaac.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, Allen JL, McGregor K, Woolgar M, Viding E, & Scott S (2014). Callous unemotional traits in children and mechanisms of impaired eye contact during expressions of love: A treatment target? Journal of Child Psychology and Psychiatry, 55(7), 771–780. 10.1111/jcpp.12155 [DOI] [PubMed] [Google Scholar]
- Dadds MR, Cauchi AJ, Wimalaweera S, Hawes DJ, & Brennan J (2012). Outcomes, moderators, and mediators of empathic-emotion recognition training for complex conduct problems in childhood. Psychiatry Research, 199(3), 201–207. 10.1016/j.psychres.2012.04.033 [DOI] [PubMed] [Google Scholar]
- Dadds MR, El Masry Y, Wimalaweera S, & Guastella AJ (2008). Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. Journal of the American Academy of Child & Adolescent Psychiatry, 47(4), 455–463. 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- Dadds MR, English T, Wimalaweera S, Schollar-Root O, & Hawes DJ (2019). Can reciprocated parent–child eye gaze and emotional engagement enhance treatment for children with conduct problems and callous-unemotional traits: a proof-of-concept trial. Journal of Child Psychology and Psychiatry, 60(6), 676–685. 10.1111/jcpp.13023. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Hawes D, & Merz S (2004). The UNSW facial emotion task. Sydney: Department of Psychology, University of New South Wales. [Google Scholar]
- Dadds MR, Kimonis ER, Schollar-Root O, Moul C, & Hawes DJ (2018). Are impairments in emotion recognition a core feature of callous–unemotional traits? Testing the primary versus secondary variants model in children. Development and Psychopathology, 30(1), 67–77. 10.1017/S0954579417000475 [DOI] [PubMed] [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, Solak E, & Abeygunawardane AI (2006). Attention to the eyes and fear-recognition deficits in child psychopathy. The British Journal of Psychiatry, 189(3), 280–281. 10.1192/bjp.bp.105.018150 [DOI] [PubMed] [Google Scholar]
- Datyner A, Kimonis ER, Hunt E, & Armstrong K (2016). Using a novel emotional skills module to enhance empathic responding for a child with conduct disorder with limited prosocial emotions. Clinical Case Studies, 15(1), 35–52. 10.1177/1534650115588978 [DOI] [Google Scholar]
- Dawel A, O’Kearney R, McKone E, & Palermo R (2012). Not just fear and sadness: Meta analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neuroscience & Biobehavioral Reviews, 36(10), 2288–2304. 10.1016/j.neubiorev.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Delk L, & White BA (2018, November 15–18) Eye Tracking: Unveiling the Mechanisms between Callous-Unemotional Behaviors and Emotion Recognition in Young Children. [Poster presentation]. Association for Behavioral and Cognitive Therapies 52nd Annual Convention, Washington, D.C. United States. https://conventionarchives.abct.org/conv2018/ [Google Scholar]
- Demetriou CA, & Fanti KA (2021). Are children high on callous-unemotional traits emotionally blind? Testing eye-gaze differences. Child Psychiatry & Human Development, 1–12. 10.1007/s10578-021-01152-3 [DOI] [PubMed] [Google Scholar]
- Ekman P (1993). Facial expression and emotion. American Psychologist, 48(4), 384. 10.1037/0003-066X.48.4.384 [DOI] [PubMed] [Google Scholar]
- Erskine HE, Ferrari AJ, Nelson P, Polanczyk GV, Flaxman AD, Vos T, Whiteford AH, & Scott JG (2013). Research Review: Epidemiological modelling of attention-deficit/hyperactivity disorder and conduct disorder for the Global Burden of Disease Study 2010. Journal of Child Psychology and Psychiatry, 54(12), 1263–1274. 10.1111/jcpp.12144. [DOI] [PubMed] [Google Scholar]
- Fontaine NM, McCrory EJ, Boivin M, Moffitt TE, & Viding E (2011). Predictors and outcomes of joint trajectories of callous–unemotional traits and conduct problems in childhood. Journal of Abnormal Psychology, 120(3), 730. 10.1037/a0022620. [DOI] [PubMed] [Google Scholar]
- Foster EM, Jones DE, & Conduct Problems Prevention Research Group. (2005). The high costs of aggression: Public expenditures resulting from conduct disorder. American Journal of Public Health, 95(10), 1767–1772. 10.2105/AJPH.2004.061424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, & Patrick CJ (2002). Aversive Pavlovian conditioning in psychopaths: Peripheral and central correlates. Psychophysiology, 39(4), 505–518. https://doi.org/10.1017.s0048577202394046 [DOI] [PubMed] [Google Scholar]
- Frick PJ (2004). The inventory of callous-unemotional traits. Unpublished rating scale. [Google Scholar]
- Frick PJ, & Ellis M (1999). Callous-unemotional traits and subtypes of conduct disorder. Clinical Child and Family Psychology Review, 2(3), 149–168. 10.1023/a:1021803005547 [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, & Kahn RE (2014). Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin, 140(1), 1. 10.1037/a0033076 [DOI] [PubMed] [Google Scholar]
- Friesen WV, & Ekman P (1983). EMFACS-7: Emotional facial action coding system. Unpublished manuscript, University of California at San Francisco, 2(36), 1. [Google Scholar]
- Gordon J (2017, March 20). An experimental therapeutic approach to psychosocial interventions. National Institute of Mental Health. https://www.nimh.nih.gov/about/director/messages/2017/an-experimental-therapeutic-approach-topsychosocial-Interventions.shtml [Google Scholar]
- Hawes DJ, Price MJ, & Dadds MR (2014). Callous-unemotional traits and the treatment of conduct problems in childhood and adolescence: A comprehensive review. Clinical Child and Family Psychology Review, 17(3), 248–267. [DOI] [PubMed] [Google Scholar]
- Hoyniak CP, Bates JE, Petersen IT, Yang CL, Darcy I, & Fontaine NM (2018). Diminished neural responses to emotionally valenced facial stimuli: A potential biomarker for unemotional traits in early childhood. Child Psychiatry & Human Development, 50(1), 72–82. 10.1007/s10578-018-0821-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T (2015). The NIMH experimental medicine initiative. World Psychiatry, 14,151–153 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4471962/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, & Wang P (2010). Research domain criteria (RDoC): Towarda new classification framework for research on mental disorders. The American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Kendall PC, Olino TM, Carper M, & Makover H (2017). On the importance of temporal precedence in mediational analyses. Journal of Consulting and Clinical Psychology, 85(1), 80–82. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, & Fleming GE (2019). Disruptive and conduct disorders, delinquency. In Ollendick TH, White SW, & White BA (Eds.), The Oxford handbook of clinical child and adolescent psychology (pp. 1–32). Oxford: Oxford University Press. [Google Scholar]
- Kimonis ER, Fleming G, Briggs N, Brouwer-French L, Frick PJ, Hawes DJ, ... & Dadds M (2019). Parent-child interaction therapy adapted for preschoolers with callous-unemotional traits: An open trial pilot study. Journal of Clinical Child & Adolescent Psychology, 48(sup1), S347–S361. [DOI] [PubMed] [Google Scholar]
- Kochanska G (1994). Beyond cognition: Expanding the search for the early roots of internalization and conscience. Developmental Psychology, 30(1)20–22. 10.1037/0012-1649.30.1.20 [DOI] [Google Scholar]
- Kolko DJ, & Pardini DA (2010). ODD dimensions, ADHD, and callous–unemotional traits as predictors of treatment response in children with disruptive behavior disorders. Journal of Abnormal Psychology, 119(4), 713. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23(1), 155 184. 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Rodebaugh TL, & Nicol GE (2020). A framework for advancing precision medicine in clinical trials for mental disorders. JAMA Psychiatry, 77(7), 663–664. 10.1001/jamapsychiatry.2020.0114. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Ongur D, & Keshavan MS (2018). Development of novel behavioral interventions in an experimental therapeutics world: Challenges, and directions for the future. Schizophrenia Research, 192, 6–8. 10.1016/j.schres.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO, Smith SF, Sauvigné KC, Patrick CJ, Drislane LE, Latzman RD, & Krueger RF (2016). Is boldness relevant to psychopathic personality? Meta-analytic relations with non-Psychopathy Checklist-based measures of psychopathy. Psychological Assessment, 28(10), 1172–1185. 10.1037/pas0000244 [DOI] [PubMed] [Google Scholar]
- Lochman JE, Baden RE, Boxmeyer CL, Powell NP, Qu L, Salekin KL, & Windle M (2014). Does a booster intervention augment the preventive effects of an abbreviated version of the coping power program for aggressive children? Journal of Abnormal Child Psychology, 42(3), 367–381. 10.1007/s10802-013-9727-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT (1957). A study of anxiety in the sociopathic personality. Journal of Abnormal and Social Psychology, 55(1), 6–10. 10.1037/h0047232 [DOI] [PubMed] [Google Scholar]
- McMahon RJ, Witkiewitz K, & Kotler JS (2010). Predictive validity of callous unemotional traits measured in early adolescence with respect to multiple antisocial outcomes. Journal of Abnormal Psychology, 119(4), 752–763. 10.1037/a0020796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson D, Davenport C, Dretzke J, Barlow J, & Day C (2013). Do evidence-based interventions work when tested in the “real world?” A systematic review and meta-analysis of parent management training for the treatment of child disruptive behavior. Clinical Child and Family Psychology Review, 16(1), 18–34. 10.1007/s10567-013-0128-0 [DOI] [PubMed] [Google Scholar]
- Morris JS, deBonis M, & Dolan RJ (2002). Human amygdala responses to fearful eyes. Neuroimage, 17(1), 214–222. 10.1006/nimg.2002.1220 [DOI] [PubMed] [Google Scholar]
- Muratori P, Lochman JE, Lai E, Milone A, Nocentini A, Pisano S, Righini E, Masi G (2016). Which dimension of parenting predicts the change of callous unemotional in children with disruptive behavior disorder? Comprehensive Psychiatry, 69, 202–210. 10.1016/j.comppsych.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Muratori P, Milone A, Manfredi A, Polidori L, Ruglioni L, Lambruschi F, Masi G, & Lochman JE (2017). Evaluation of improvement in externalizing behaviors and callous-unemotional traits in children with Disruptive Behavior Disorder: A 1-year follow up clinic-based study. Administration and Policy in Mental Health and Mental Health Services Research, 44, 452–462. [DOI] [PubMed] [Google Scholar]
- Murrihy RC, Kidman AD, & Ollendick TH (Eds.). (2010). Clinical handbook of assessing and treating conduct problems in youth. Springer Science & Business Media. [Google Scholar]
- Nock MK, Kazdin AE, Hiripi EVA, & Kessler RC (2006). Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychological Medicine, 36(5), 699–710. 10.1017/S0033291706007082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollendick TH, Greene RW, Austin KE, Fraire MG, Halldorsdottir T, Allen KB, ... & Wolff JC (2016). Parent management training and collaborative & proactive solutions: A randomized control trial for oppositional youth. Journal of Clinical Child & Adolescent Psychology, 45(5), 591–604. 10.1080/15374416.2015.1004681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini D, & Frick PJ (2013). Multiple developmental pathways to conduct disorder: Current conceptualizations and clinical implications. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 22(1), 20–25. [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Lochman JE, & Powell N (2007). The development of callous-unemotional traits and antisocial behavior in children: Are there shared and/or unique predictors? Journal of Clinical Child and Adolescent Psychology, 36(3), 319–333. 10.1080/15374410701444215 [DOI] [PubMed] [Google Scholar]
- Patterson GR, DeBaryshe BD, & Ramsey E (1989). A developmental perspective on antisocial behavior. American Psychologist, 44(2), 329–335. 10.1037/0003-066X.44.2.329 [DOI] [PubMed] [Google Scholar]
- Richell RA, Mitchell DG, Newman C, Leonard A, Baron-Cohen S, & Blair RJR (2003). Theory of mind and psychopathy: can psychopathic individuals read the ‘language of the eyes’? Neuropsychologia, 41(5), 523–526. 10.1016/s0028-3932(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Rutter M (2012). Psychopathy in childhood: is it a meaningful diagnosis? The British Journal of Psychiatry, 200(3), 175–176. 10.1192/bjp.bp.111.092072 [DOI] [PubMed] [Google Scholar]
- Shelton RC, Lee M, Brotzman LE, Wolfenden L, Nathan N, & Wainberg ML (2020). What is dissemination and implementation science?: an introduction and opportunities to advance behavioral medicine and public health globally. International Journal of Behavioral medicine, 27(1), 3–20. [DOI] [PubMed] [Google Scholar]
- Strategic Plan for Research. Bethesda, MD, National Institute of Mental Health, May 2020. https://www.nimh.nih.gov/about/strategic-planning-reports/goal-3-strive-for-prevention-and-cures) [Google Scholar]
- U.S. Department of Health and Human Services. (n.d.). Support for clinical trials at NIMH. National Institute of Mental Health. https://www.nimh.nih.gov/funding/opportunities-announcements/clinical-trials-foas/#Q3. [Google Scholar]
- Waller R, Dishion TJ, Shaw DS, Gardner F, Wilson MN, & Hyde LW (2016). Does early childhood callous-unemotional behavior uniquely predict behavior problems or callous-unemotional behavior in late childhood? Developmental Psychology, 52(11), 1805–1819. 10.1037/dev0000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BA, Dede B, Heilman M, Delk L, White S, Bui CA (2019, November 21–24). Preliminary Investigation of Facial Affect Sensitivity Training for young children with CU traits. [Poster presentation]. 53rd Annual Convention of the Association for Behavioral and Cognitive Therapies, Atlanta, GA, USA. https://conventionarchives.abct.org/conv2019/ [Google Scholar]
- White BA, & Delk LA (2017). Uncaring young adults show reduced vigilance for others’ fearful expressions. Personality and Individual Differences, 106, 77–80. [Google Scholar]
- White BA, Gordon H, & Guerra RC (2015). Callous–unemotional traits and empathy in proactive and reactive relational aggression in young women. Personality and Individual Differences, 75, 185–189. 10.1016/j.paid.2014.11.031 [DOI] [Google Scholar]


