Abstract
Anaerobic microbial toluene catabolism is initiated by addition of fumarate to the methyl group of toluene, yielding (R)-benzylsuccinate as first intermediate, which is further metabolized via β-oxidation to benzoyl-coenzyme A (CoA) and succinyl-CoA. A specific succinyl-CoA:(R)-benzylsuccinate CoA-transferase activating (R)-benzylsuccinate to the CoA-thioester was purified and characterized from Thauera aromatica. The enzyme is fully reversible and forms exclusively the 2-(R)-benzylsuccinyl-CoA isomer. Only some close chemical analogs of the substrates are accepted by the enzyme: succinate was partially replaced by maleate or methylsuccinate, and (R)-benzylsuccinate was replaced by methylsuccinate, benzylmalonate, or phenylsuccinate. In contrast to all other known CoA-transferases, the enzyme consists of two subunits of similar amino acid sequences and similar sizes (44 and 45 kDa) in an α2β2 conformation. Identity of the subunits with the products of the previously identified toluene-induced bbsEF genes was confirmed by determination of the exact masses via electrospray-mass spectrometry. The deduced amino acid sequences resemble those of only two other characterized CoA-transferases, oxalyl-CoA:formate CoA-transferase and (E)-cinnamoyl-CoA:(R)-phenyllactate CoA-transferase, which represent a new family of CoA-transferases. As suggested by kinetic analysis, the reaction mechanism of enzymes of this family apparently involves formation of a ternary complex between the enzyme and the two substrates.
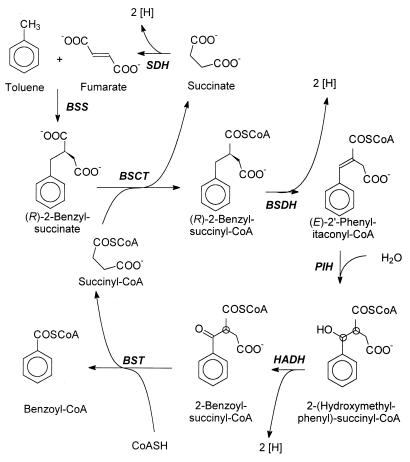
Some bacteria are known to metabolize aromatic hydrocarbons, such as toluene, in the absence of oxygen. The metabolic pathway involved in the anaerobic catabolism of toluene is currently under investigation (for a review, see reference 19). Overall, toluene is oxidized to benzoyl-coenzyme A (CoA), a common intermediate in the anaerobic catabolic pathways of many aromatic compounds (reviewed in reference 20). The first step is an unusual addition of the methyl group of toluene to the double bond of a fumarate cosubstrate (5, 7; Fig. 1). This reaction is catalyzed by the glycyl-radical enzyme (R)-benzylsuccinate synthase and yields exclusively the (R)-(+)-enantiomer of benzylsuccinate (6, 25, 26). Further catabolism of (R)-benzylsuccinate to benzoyl-CoA proceeds via a specific β-oxidation pathway (26; Fig. 1). The enzymes of this pathway are encoded by nine genes, which are arranged in a toluene-induced operon (24). β-Oxidation of benzylsuccinate is initiated by a succinyl-CoA:(R)-benzylsuccinate CoA-transferase producing benzylsuccinyl-CoA (26). Analogous initial reactions as outlined for anaerobic toluene metabolism have recently also been described for anaerobic catabolism of m-xylene (23), m-cresol (28), p-cresol (29), and 2-methylnaphthalene (2), and the corresponding benzylsuccinate analogs of these substrates have been identified (2, 23, 28, 29). However, the subsequent reactions of these pathways are not yet known.
FIG. 1.
Pathway of anaerobic toluene degradation. Enzymes: BSS, (R)-benzylsuccinate synthase; BSCT, succinyl-CoA:(R)-benzylsuccinate CoA-transferase; BSDH, (R)-benzylsuccinyl-CoA dehydrogenase; PIH, (E)-Phenylitaconyl-CoA hydratase; HADH, 3-hydroxyacyl-CoA dehydrogenase; BST, benzoylsuccinyl-CoA thiolase; SDH, succinate dehydrogenase. Chiral C atoms of as-yet-unknown configuration are indicated with circles.
CoA-transferases catalyze the reversible transfer of CoA between organic acids and are known from many bacterial and eukaryotic species. Based on their reaction mechanisms and amino acid sequences, the currently known enzymes can be grouped into three families. (i) CoA-transferases of family I, e.g., for 3-oxoacids (15, 30, 35), acetate, butyrate (4, 33, 36), or glutaconate (9, 22, 27), contain two dissimilar subunits in different aggregation states (α2β2 or α4β4), except for mammalian succinyl-CoA:acetoacetate CoA-transferases, where the subunits are fused to a single polypeptide (15, 30). A conserved amino acid sequence motif in the β-subunit (Prosite entry PS01274) contains an active-site glutamate residue, which is involved in the reaction mechanism (27, 31); another conserved amino acid sequence motif is present in the α-subunit (Prosite entry PS01273). These enzymes operate by a ping-pong mechanism: in the first half of the reaction a covalently bound glutamyl-CoA thioester intermediate of the enzyme is formed by reacting with a suitable CoA-donor compound. The enzyme-CoA intermediate then reacts with a CoA-acceptor compound in the other half of the reaction (27, 31, 35). CoA-transferases of family I are specifically inactivated by treatment with hydroxylamine or borohydride when they are preincubated with a CoA-donor compound. The former inhibitor causes formation of a hydroxamate at the CoA-activated glutamate, and the latter reduces glutamyl-CoA to glutamic alcohol (27, 31, 35). (ii) A second family of CoA-transferases is defined by the transferase subunits of citrate and citramalate lyases. The physiological substrate transferred by these enzymes is an enzyme-associated acyl-carrier protein (ACP) subunit containing a protein-bound CoA moiety. Transfer of free CoA moieties is only observed as a side reaction under in vitro conditions (10, 11, 17). The catalytic mechanisms of these enzymes require ternary-complex formation of the enzyme, donor ACP-thioester (acetyl-ACP), and a thioester-acceptor compound (citrate or citramalate; 10, 11). (iii) Finally, the first members of a third family of CoA-transferases were recently characterized: (E)-cinnamoyl-CoA:(R)-phenyllactate CoA- transferase from Clostridium sporogenes (16) and formyl-CoA:oxalate CoA-transferase from Oxalobacter formigenes (3, 32). These enzymes differ in sequence from the enzymes of families I and II, and a reaction mechanism similar to that of family II enzymes has been suggested for one member (16).
In this communication, we report on the purification and biochemical characterization of succinyl-CoA:(R)-benzylsuccinate CoA-transferase from the denitrifying bacterium Thauera aromatica, the second enzyme of anaerobic toluene metabolism. We provide evidence that this enzyme belongs to the emerging family III of CoA-transferases.
MATERIALS AND METHODS
Materials and bacterial strains.
Chemicals were obtained from Fluka, Merck, Sigma, or Roth; biochemicals were from Boehringer or Gerbu; and the ATP monitoring kit was from Merlin. High-performance liquid chromatography (HPLC) equipment was from Merck or Waters, and fast-performance liquid chromatography (FPLC) equipment was from Pharmacia. [2,3-14C]succinate (specific radioactivity, 2.6 GBq mmol−1) was from American Radiolabeled Compounds. The R and S enantiomers of benzylsuccinate were prepared from the racemate as described previously (26); enantiomer purities were >90%. CoA-thioesters of benzylsuccinate and other dicarboxylic acids were synthesized via the internal anhydrides as described elsewhere (26). T. aromatica strain K172 (1) was isolated by Tschech and Fuchs (34) and has been deposited in Deutsche Sammlung von Mikroorganismen (DSMZ 6984). Azoarcus sp. strains T (DSMZ 9506; 18) and M3 (DSMZ 12184; 21) were obtained from Josef Zeyer, ETH Zürich; Azoarcus sp. strain B5-1 was isolated from an enrichment culture on 3-methylbenzoate in the laboratory of Georg Fuchs, University of Freiburg (unpublished results).
Growth of bacterial cells and preparation of cell extract.
Denitrifying bacteria were grown at 30°C under denitrifying conditions in mineral salt medium, as described previously (7). Cell harvesting and storage were performed as described earlier (7). Cell extract was prepared under aerobic conditions at 4°C. Frozen cells were suspended in one volume of buffer A (10 mM triethanolamine hydrochloride–NaOH [pH 7.5], 10% [vol/vol] glycerol) containing 0.05 mg of DNase I (g of cells)−1. Cells were broken by passage through a French pressure cell at 137 MPa; the lysate was centrifuged at 100,000 × g for 60 min. The supernatant was used immediately or kept frozen at −70°C without detectable loss of activity for up to 3 months.
Enzyme assays.
The forward reaction of succinyl-CoA:(R)-benzylsuccinate CoA-transferase was measured by a photometric assay coupled to the reaction of endogenous succinate dehydrogenase of T. aromatica as described previously (26) or by analysis of benzylsuccinyl-CoA formation via HPLC (reference 26 and as described below). The reverse reaction of the enzyme was measured by a coupled luminometric assay with the endogenous succinate-CoA ligase of T. aromatica and luciferase as auxiliary enzymes. The enzyme was assayed in 100 mM triethanolamine hydrochloride–NaOH (pH 7.5) containing 2.5 mM MgCl2, 5 mM NaH2PO4, 0.1 mM ADP, 1 mM succinate, 2% (vol/vol) ATP-monitoring kit, 0.1 mU of partially purified succinate-CoA ligase (0.2 μg of protein), 10 μl of diluted cell extract or column fraction, and (if necessary) 0.1 mM P1-P5-bis-(adenosine-5′)-pentaphosphate to inhibit myokinase activity. The reaction was started by adding benzylsuccinyl-CoA to a final concentration of 0.1 mM. The reaction was followed continuously in a luminometer 1250 (Pharmacia-LKB) and calibrated with an ATP standard (Merlin). The auxiliary enzyme succinate-CoA ligase was enriched by passing extracts of toluene-grown cells over a DEAE-Sepharose column (Pharmacia; diameter, 2.2 cm; volume, 30 ml). The column was eluted with a linear gradient of 50 to 200 mM NaCl in buffer A over 7 column volumes. Succinate-CoA ligase eluted between 90 and 115 mM NaCl with a recovery of 95% and an enrichment factor of 3.8. The active fractions were pooled; they contained 7.8 mg of protein ml−1 and a specific activity of 0.5 μmol min−1 (mg of protein)−1. No succinyl-CoA:(R)-benzylsuccinate CoA-transferase activity was detectable in these fractions. Steady-state kinetic experiments were performed for the reverse reaction with purified enzyme, using varied concentrations of succinate and chemically synthesized (R)-benzylsuccinyl-CoA that contained both possible regioisomers (2-and 3-benzylsuccinyl-CoA; see below). Concentrations of both substrates varied from 0.25 to 7 times the respective Km values.
Product analysis.
Routine analysis of the CoA-thioesters produced was performed by HPLC at room temperature with UV detection at 260 nm using a C18 reversed-phase column (5 μm; LiChrospher 100 RPC-18; Merck), as described previously (26). However, chemically synthesized benzylsuccinyl-CoA consisted of two isomeric compounds (2- and 3-benzylsuccinyl-CoA), which comigrated under the standard HPLC conditions (26). Partial separation of the two compounds was achieved by a modified HPLC protocol. The column was eluted over 25 min at a flow rate of 1 ml min−1 with a gradient of 1 to 25% (vol/vol) acetonitrile in 50 mM acetate buffer containing 50 mM phosphate (pH 4.5). Chemically synthesized benzylsuccinyl-CoA and enzymatically produced benzylsuccinyl-CoA were analyzed in portions of 1 to 2 nmol per run. Because of the poor peak resolution, comigration tests of the enzymatically produced benzylsuccinyl-CoA regioisomer were confirmed by spiking enzymatic conversion reaction mixtures with different amounts of chemically synthesized benzylsuccinyl-CoA.
Enzyme purification.
All purification steps were performed under aerobic conditions at 6°C with an FPLC System (Pharmacia). Extracts of T. aromatica cells grown on toluene (12.5 ml of a 100,000 × g supernatant) were applied to a DEAE-Sepharose column (Pharmacia; diameter, 2.2 cm; volume, 30 ml) which had been equilibrated with buffer A. The column was washed with buffer A at a flow rate of 1 ml min−1 for 4 column volumes. The column was eluted with steps of 100 and 180 mM NaCl; each step was applied over 4 to 5 column volumes. Fractions of 5 ml were collected. Succinyl-CoA:benzylsuccinate CoA-transferase activity eluted in a volume of 60 ml when the 180 mM NaCl step was applied. Two-thirds of the eluate (40 ml) were loaded on a ceramic hydroxyapatite column (Bio-Rad; diameter, 1.6 cm; volume, 20 ml) equilibrated with buffer A. The column was washed with 60 ml of buffer A, and the CoA-transferase activity was eluted with 22 mM sodium phosphate in buffer A over 2 to 3 column volumes. The active fractions were pooled (final volume, 60 ml). Half of the pool (30 ml) was applied on a Q-Sepharose column (Pharmacia; diameter, 1.1 cm; volume, 10 ml) which was equilibrated with buffer A containing 180 mM NaCl. The column was eluted with steps of 180 and 250 mM NaCl at a flow rate of 1 ml min−1. Each step was applied over 3 to 4 column volumes. The CoA-transferase activity eluted in a volume of 70 ml when 250 mM NaCl was applied. The eluate was concentrated to a volume of 1.5 ml by ultrafiltration with an exclusion limit of 30 kDa (Amicon 8400, with a PM-30 membrane). Concentrated eluate (500 μl) was loaded on a Superdex 200 column (Pharmacia; diameter, 1.6 cm; volume, 120 ml). The column was equilibrated and eluted with buffer A containing 100 mM NaCl at a flow rate of 0.8 ml min−1, and fractions of 1.6 ml were collected. The CoA-transferase eluted in a volume of 8 ml. The fractions were pooled and concentrated 35-fold by ultrafiltration. No loss of activity was recorded during the concentration steps.
Analysis of substrate preference.
The products of the CoA-transferase reaction were analyzed by HPLC as described previously (26). To test the substrate preference of succinyl-CoA:(R)-benzylsuccinate CoA-transferase, succinate was replaced by (R,S)-methylsuccinate, maleinate, fumarate, formate, acetate, propionate, glutarate, malonate, or oxalate; benzylsuccinate was replaced by (R,S)-methylsuccinate, (R,S)-phenylsuccinate, 2- or 3-phenylpropionate, 4-phenylbutyrate, or benzylmalonate. An analogous test was performed with benzoyl-CoA as the potential alternative CoA donor instead of succinyl-CoA and (R,S)-benzylsuccinate as acceptor. The assays (total volume, 100 μl) were performed in 100 mM Tris-HCl [pH 7.5] containing a 2.5 mM concentration of the substrate analog to be tested and 3 μg of purified CoA-transferase after preparative gel filtration. The reactions were started with 0.5 mM of the appropriate CoA-thioester and incubated at 30°C. After 10 min, the assay mixtures were split: one half (50 μl) of each assay mixture was retrieved and acidified to pH 3 with formic acid (10% [vol/vol] final concentration); the remaining half was brought to pH 9 by adding 10 μl of 0.1 M NaOH and heating at 80°C for 15 min to hydrolyze CoA-thioesters. The alkaline-treated samples were then acidified to pH 3 with formic acid (10% [vol/vol] final concentration). Precipitated protein was removed by centrifugation (20,000 × g; 4°C; 10 min), and the samples were analyzed by HPLC (26). If the substrate analogs were converted to CoA-thioesters, new peaks absorbing at 260 nm were observed, which disappeared after alkaline treatment. The correlation of these peaks with the predicted CoA-thioesters was checked by chemical synthesis and HPLC analysis of CoA-thioesters of maleate, methylsuccinate, and phenylsuccinate. Although the chemically synthesized CoA-thioesters were mixtures of the possible positional and conformational isomers, they all contained one compound that comigrated with the enzymatically formed thioester (data not shown).
Isotope exchange experiments.
Assay mixtures containing 100 mM Tris-HCl (pH 7.5), 6.5 μg of purified CoA-transferase, and 125 Bq of [14C]succinate (final concentration, 0.5 μM) were prepared in a total volume of 100 μl. Control assay mixtures also contained 1 mM benzylsuccinate. The reactions were started by adding 1 mM succinyl-CoA and incubating at room temperature. After 5 and 20 min, samples (50 μl) were taken; the reaction was stopped by adding formic acid (10% [vol/vol]) and reaction products were analyzed by HPLC as described before. UV absorption at 260 nm and radioactivity (Ramona detector; Raytest) of the eluate were monitored simultaneously.
Inactivation experiments.
Two types of experiments were performed. (i) The first type was inactivation by sodium borohydride. Enriched CoA-transferase after the Q-Sepharose step (160 μg of protein) was added to 1 ml of a 0.5 M triethanolamine hydrochloride buffer (pH 7.5), which either contained or lacked benzylsuccinyl-CoA (250 μM). The enzyme was treated with 10 μl of a 1 M NaBH4 solution in 1 M NaOH, and 10 μl of a 1 M HCl solution was added immediately afterwards. The mixtures were incubated for 10 min at room temperature and tested for CoA-transferase activity by the coupled luminometric assay. (ii) The second type of experiment involved inactivation by hydroxylamine. The same enzyme batch as used for the above experiments (3.2 μg of protein) was added to 0.1 ml of a 0.1 M Tris-HCl buffer (pH 7.5), which either contained or lacked benzylsuccinyl-CoA (250 μM). The enzyme was treated with 10 mM hydroxylamine for 15 min at room temperature and assayed by the coupled luminometric assay.
Native molecular mass determination.
The molecular mass of the native CoA-transferase was determined by size exclusion chromatography and by Ferguson plot analysis. Concentrated eluate of the preparative gel filtration (100 μl, 1.4 mg ml−1) was applied on a Superdex 200-HR (Pharmacia; diameter, 1.1 cm; volume, 20 ml). The column was equilibrated and eluted with buffer A containing 100 mM NaCl. The following molecular mass standard proteins were used to calibrate the column: ferritin (450 kDa), catalase (240 kDa), alcohol dehydrogenase (150 kDa), bovine serum albumin (67 kDa), and ovalbumin (45 kDa); inclusion and exclusion volumes were determined with blue dextran and vitamin B12, respectively. Additionally, the native mass of the enzyme was confirmed by Ferguson plot. Purified CoA-transferase and standard proteins were separated on native acrylamide gels (5, 6, 6.5, and 8% polyacrylamide [wt/vol]). The following proteins were used as standards: different aggregation forms of bovine serum albumin (monomer, 67 kDa; dimer, 134 kDa; trimer, 201 kDa; tetramer; 268 kDa); ovalbumin (45 kDa); and carboanhydrase (29 kDa). The migration positions of the proteins were used to calculate the molecular mass of the native CoA-transferase after staining the gels with Coomassie blue or by silver staining (12).
Other methods.
Evaluation of kinetic experiments and fitting to equations describing different two-substrate mechanisms was performed by the program Leonora (13), using the original data from duplicated experiments. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8% [wt/vol] polyacrylamide gels) was performed as described previously (12). Proteins were stained with Coomassie blue. Protein concentrations were determined according to the method of Bradford (8) or by a modification of the Lowry method (12), using a bovine serum albumin standard which had been calibrated by its absorption at 280 nm. UV-visible spectra were recorded by a Perkin-Elmer UV/Vis spectrometer Lambda 2S. Electrospray-mass spectrometry of purified protein was performed with a Finnigan TSQ700 mass spectrometer with electrospray interface. The protein was applied on a C4 HPLC column (0.8 by 150 mm; Vydac) directly coupled to the mass spectrometer and eluted by a linear gradient from 10 to 95% acetonitrile in 0.1% trifluoroacetic acid at a flow rate of 20 μl min−1.
RESULTS
Enzyme assays for succinyl-CoA:(R)-benzylsuccinate CoA-transferase.
Continuous enzyme assays were developed to measure the forward and reverse reactions of succinyl-CoA:(R)-benzylsuccinate CoA-transferase of toluene-grown cells of T. aromatica. The assay for the forward reaction coupled benzylsuccinate-dependent formation of free succinate from succinyl-CoA to the succinate dehydrogenase reaction. The rate (± standard deviation) measured by this assay in cell extracts was 15 ± 5 nmol min−1 (mg of protein)−1 (26). The reverse reaction, synthesis of succinyl-CoA from benzylsuccinyl-CoA and succinate, was measured by a luminometric coupled enzyme assay with succinate-CoA ligase and firefly luciferase. Partially purified succinate-CoA ligase from T. aromatica was used as auxiliary enzyme. The enzyme preparation was devoid of CoA-transferase activity and had a specific succinate-CoA ligase activity of 510 ± 15 nmol min−1 (mg of protein)−1. The specific activity of succinyl-CoA:(R)-benzylsuccinate CoA-transferase in cell extracts, as measured by this assay, was 25 ± 5 nmol min−1 (mg of protein)−1.
Analysis of reaction products.
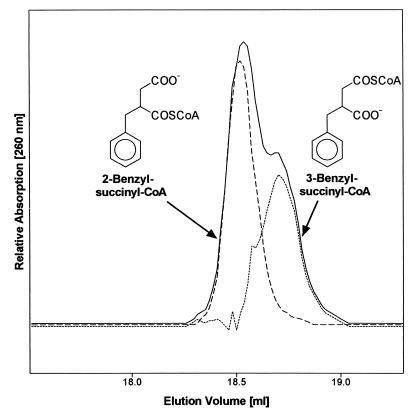
We have previously shown that succinyl-CoA:(R)-benzylsuccinate CoA-transferase specifically forms a CoA-thioester from (R)-benzylsuccinate (26). However, the biologically relevant benzylsuccinyl-CoA isomer has not yet been fully identified, since two different regioisomers can be formed, 2- or 3-benzylsuccinyl-CoA (Fig. 2). Chemically synthesized (racemic) benzylsuccinyl-CoA indeed consisted of 2- and 3-benzylsuccinyl-CoA in a reproducible molar ratio of 2:1, respectively, as evident from the chemical shifts of the thioester-carbonyl groups detected by 13C-nuclear magnetic resonance (NMR) analysis (26; W. Eisenreich, A. Bacher, C. Leutwein, and J. Heider, unpublished data). The two isomers of racemic or enantiomerically pure benzylsuccinyl-CoA comigrated under standard HPLC conditions but were partially separated by a modified HPLC procedure (Fig. 2). The molar ratio of the two isomers was calculated as 2:1 from the integrated peak areas, assuming similar absorption coefficients at 260 nm. Therefore, the earlier eluting peak can be assigned to 2- and the latter to 3-benzylsuccinyl-CoA (Fig. 2). Although enzymatically synthesized benzylsuccinyl-CoA was not obtained in sufficient amounts for 13C-NMR analysis, it eluted as a single compound in HPLC assays and comigrated with 2-benzylsuccinyl-CoA (Fig. 2).
FIG. 2.
HPLC analysis of the enzymatically formed benzylsuccinyl-CoA isomer. Chemically synthesized (solid line) and enzymatically synthesized (broken line) benzylsuccinyl-CoA were separated by HPLC and monitored by their absorption at 260 nm. The dotted line represents the hypothetical elution profile of 3-benzylsuccinyl-CoA; it represented 30 to 40% of the amount of chemically synthesized benzylsuccinyl-CoA. The curve was calculated by subtracting the elution profile of enzymatically synthesized benzylsuccinyl-CoA from that of the chemically synthesized isomer mixture. The structures of the benzylsuccinyl-CoA isomers were inferred from correlation with NMR data as shown.
Purification of succinyl-CoA:(R)-benzylsuccinate CoA-transferase.
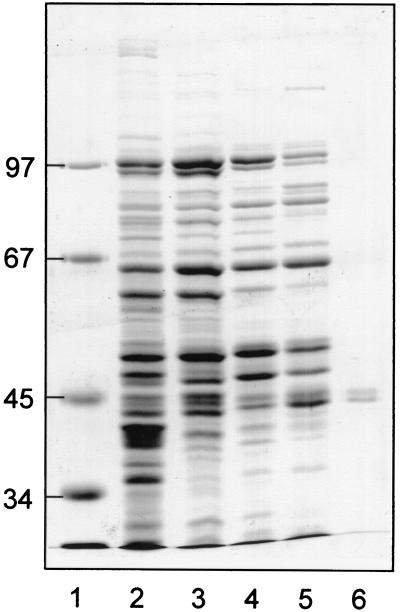
Succinyl-CoA:(R)-benzylsuccinate CoA-transferase was purified from toluene-grown cells of T. aromatica in four chromatographic steps (Table 1). The enzyme was stable in air, and therefore all purification steps were performed under aerobic conditions. In initial experiments, we observed that the enzyme was completely inactivated during passage over DEAE-Sepharose when eluted with linear gradients of NaCl. Therefore, all anion exchange and hydroxyapatite chromatographic steps were performed with step gradients, which were not detrimental for enzyme activity. Monitoring of enzyme activity during purification was done by the luminometric assay for the reverse reaction. Addition of the myokinase inhibitor bis-adenosine pentaphosphate was necessary for luminometric assays with cell extracts and fractions of the first column, but not with the active fractions of the later steps of the purification. Activity eluted from the DEAE-Sepharose column between 100 and 180 mM NaCl. The larger portion of the active fractions was applied to a hydroxyapatite column, and enzyme activity eluted in a step between 0 and 22 mM sodium phosphate. The next purification step was chromatography on Q-Sepharose: half of the active fraction after hydroxyapatite chromatography was applied, and the enzyme eluted in a step between 180 and 250 mM NaCl. The final step of the purification, gel permeation chromatography on Superdex 200 HR, was only applied for small aliquots. Although this step obviously caused severe inactivation of the enzyme, it was necessary for purification. Several other columns, such as Mono-Q, hydrophobic columns, or dye affinity columns, were tested, but they either inactivated the enzyme or did not yield further purification effects. An SDS-polyacrylamide gel of the active fractions during the purification is shown in Fig. 3; the enzyme is essentially pure after the gel filtration step. As calculated from the enrichment factor and yield, the CoA-transferase corresponds to at least 0.6% of the total soluble protein of toluene-grown cells of T. aromatica.
TABLE 1.
Purification of succinyl-CoA:benzylsuccinate CoA-transferase from T. aromaticaa
| Purification step | Volume (ml) | Protein (mg) | Activity (nmol min−1) | Specific activity (nmol min−1 mg−1) | Enrichment factor | Yield (%) |
|---|---|---|---|---|---|---|
| Crude extract | 12.5 | 56.3 | 13.3 | 23.7 | 1 | 100 |
| DEAE-Sepharose | 60 | 17.7 | 12.1 | 68.1 | 2.9 | 91 |
| Hydroxyapatite | 60 | 84 | 7.5 | 89.4 | 3.8 | 73 |
| Q-Sepharose | 70 | 22.5 | 2.3 | 100 | 4.2 | 45 |
| Gel filtration | 8 | 0.5 | 0.14 | 300 | 12.7 | 9 |
Yield values have been corrected for the fractions of active pools which were not used for the next purification steps.
FIG. 3.
SDS-PAGE of active fractions during purification of succinyl-CoA:benzylsuccinate CoA-transferase. A Coomassie-stained gel is shown, in which the pooled fractions of the purification were analyzed. Low polyacrylamide concentrations (8% [wt/vol]) were used to separate the two CoA-transferase subunits. The purified enzyme was checked for the absence of contaminating proteins smaller than 30 kDa, which migrated in the front in these gels. Lanes: 1, protein standards; 2, crude extract (16 μg of protein); 3, DEAE-Sepharose fraction (12 μg of protein); 4, hydroxyapatite fraction (12 μg of protein); 5, Q-Sepharose fraction (12 μg of protein); 6, gel filtration fraction (1.5 μg of protein). The masses of standard proteins in kilodaltons are indicated on the left margin.
Molecular properties of succinyl-CoA:(R)-benzylsuccinate CoA-transferase.
Purified succinyl-CoA:(R)-benzylsuccinate CoA-transferase consisted of two polypeptides migrating at apparent masses of 44 and 45 kDa during SDS-PAGE (Fig. 3). Both subunits of the purified enzyme were blocked for N-terminal sequencing. However, exact molecular masses of the subunits were determined by electrospray-mass spectrometry (44.81 and 45.47 kDa). These masses match well with those predicted for two recently identified toluene-induced proteins, BbsF (44.798 kDa) and BbsE (45.482 kDa). These are encoded in a common operon (bbs, for beta-oxidation of benzylsuccinate) together with other enzymes involved in β-oxidation of benzylsuccinate (24). The mass of the native enzyme (mean ± standard deviation) was determined as 215 ± 15 kDa by passage over a calibrated Superdex 200 HR gel filtration column and as 225 ± 26 kDa by a Ferguson plot from native gel electrophoresis. Thus, the subunit composition of succinyl-CoA:(R)-benzylsuccinate CoA-transferase appears to be α2β2. The UV-visible spectrum of purified enzyme did not exhibit unusual features. The absorption coefficient of the enzyme at 280 nm was 304 mM−1 cm−1 per holoenzyme (α2β2), which is in reasonable agreement with the absorption coefficient calculated from the tryptophan and tyrosine content of BbsE and BbsF (ɛ280 = 284 mM−1 cm−1).
Catalytic properties of succinyl-CoA:(R)-benzylsuccinate CoA-transferase.
Purified enzyme was assayed with succinyl-CoA and (R)- or (S)-benzylsuccinate (2 mM final concentration); the products formed after different incubation times were analyzed by HPLC. Only (R)-benzylsuccinate was converted to 2-benzylsuccinyl-CoA at rates of 320 ± 50 nmol min−1 (mg protein)−1, whereas no reaction was observed with the (S) enantiomer. Rates of 2-(R)-benzylsuccinyl-CoA formation were not significantly lowered in assays containing 2 mM (R)-benzylsuccinate plus (S)-benzylsuccinate in up to fivefold excess. Enantiomer specificity of the reverse reaction was tested by the luminometric assay, using chemically prepared (R)-, (S)-, and racemic benzylsuccinyl-CoA. All preparations were mixtures of 2- and 3-benzylsuccinyl-CoA (molar ratio of 2:1, as analyzed by HPLC). Purified enzyme was active with (R)-benzylsuccinyl-CoA, whereas no activity was detected when (S)-benzylsuccinyl-CoA was used as substrate. In contrast to the free acid, (S)-benzylsuccinyl-CoA inhibited CoA-transferase activity, as evident from enzyme assays containing (R)-benzylsuccinyl-CoA (90 μM) and increasing concentrations of (S)-benzylsuccinyl-CoA. Activities dropped to 70% with racemic benzylsuccinyl-CoA, to 40% with a 5-fold excess, and below the detection limit with a 10-fold excess of (S)-benzylsuccinyl-CoA.
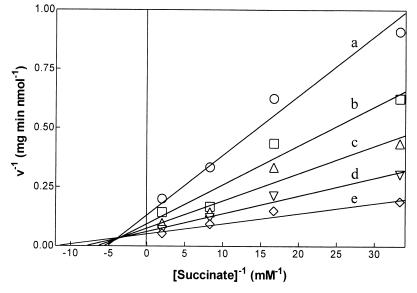
Kinetic parameters of succinyl-CoA:(R)-benzylsuccinate CoA-transferase were only recorded for the reverse reaction because the assays for the forward direction were not sensitive enough to obtain reliable initial activity values at low substrate concentrations. The assays were performed with chemically synthesized (R)-benzylsuccinyl-CoA (2- and 3-benzylsuccinyl-CoA in 2:1 ratio). The indicated concentrations of (R)-benzylsuccinyl-CoA relate to the isomer mixture as applied in the experiments; concentrations of the relevant regioisomer correspond to 67% of these values. The kinetic data were evaluated by curve fitting of the data to the Michaelis-Menten equation and statistical evaluation (13). They were consistent with an assumed ternary-complex mechanism but significantly different from those expected from ping-pong kinetics. This is also evident from double-reciprocal plots of activity versus benzylsuccinyl-CoA or succinate concentrations, which yielded intersecting lines (Fig. 4). Km values of 40 ± 8 μM for 2- plus 3-(R)-benzylsuccinyl-CoA and 160 ± 30 μM for succinate were calculated from the best-fitting ternary complex equation (13, 14).
FIG. 4.
Double-reciprocal plot of succinyl-CoA:benzylsuccinate CoA-transferase activity versus succinate concentration. The assays were performed by the standard luminometric assay in the presence of 5 (a), 15 (b), 30 (c), 60 (d), or 120 (e) μM concentrations of a chemically synthesized regioisomer mixture of 2- and 3-(R)-benzylsuccinyl-CoA (molar ratio, 2:1). Reactions were started with the indicated concentrations of succinate. The symbols represent mean values of two measurements.
The substrate preference of succinyl-CoA:(R)-benzylsuccinate CoA-transferase was tested by HPLC analysis of the CoA-thioesters formed in enzyme assays with chemical analogs of benzylsuccinate and succinate, respectively. Benzylsuccinate analogs were tested with succinyl-CoA, and succinate analogs were tested with (racemic) benzylsuccinyl-CoA as CoA donor (Table 2). From these tests, it became evident that the enzyme is quite specific for the two natural substrates. Detectable CoA-thioester formation was observed with (R, S)-phenylsuccinate and benzylmalonate in lieu of benzylsuccinate, and with maleinate replacing succinate. (R, S)-Methylsuccinate was the only substrate tested that replaced either succinate or benzylsuccinate as a CoA-acceptor compound (Table 2). The CoA-transferase reaction was also analyzed for isotope exchange activity between unlabeled succinyl-CoA and [2,3-14C]succinate. About 50% of 14C-labeled succinate was converted to [14C]succinyl-CoA after 20 min in the absence of benzylsuccinate, suggesting that the enzyme indeed catalyzes CoA transfer between succinyl-CoA and succinate. However, no isotope exchange between [14C]succinate and [14C]succinyl-CoA occurred under the same conditions in the presence of 1 mM benzylsuccinate. Finally, succinyl-CoA could not be replaced as CoA donor for benzylsuccinate by benzoyl-CoA, the second product of β-oxidation of benzylsuccinyl-CoA besides succinyl-CoA (7).
TABLE 2.
Substrate analogs of succinyl-CoA:benzylsuccinate CoA-transferase
| CoA acceptor | CoA donor | Thioester formed (% relative amt) | HPLC retention times of CoA-thioesters (min) |
|---|---|---|---|
| Succinate | Benzylsuccinyl-CoA | 100 | 6.0 |
| Methylsuccinate | Benzylsuccinyl-CoA | 82 | 8.2 |
| Maleinate | Benzylsuccinyl-CoA | 28 | 8.0 |
| Benzylsuccinate | Succinyl-CoA | 100 | 14.3 |
| Methylsuccinate | Succinyl-CoA | 58 | 8.2 |
| Benzylmalonate | Succinyl-CoA | 46 | 10.3 |
| Phenylsuccinate | Succinyl-CoA | 6 | 12.0 |
CoA-thioester formation from the given compounds was determined after 20 min of incubation with benzylsuccinyl-CoA or succinyl-CoA as CoA donors. The 100% values correspond to 96 μM for formation of succinyl-CoA and 84 μM for formation of benzylsuccinyl-CoA. No CoA-thioesters were formed with benzylsuccinyl-CoA as CoA donor and formate, acetate, propionate, oxalate, malonate, glutarate, or fumarate as acceptors, or with succinyl-CoA as CoA donor and 2-phenylpropionate, 3-phenylpropionate, or 4-phenylbutyrate as acceptors.
Inactivation assays of succinyl-CoA:(R)-benzylsuccinate CoA-transferase.
CoA-transferases of family I are specifically inactivated by incubation with NaBH4 or hydroxylamine in the presence of CoA-thioesters (27, 31). Therefore, we tested the effect of these compounds on succinyl-CoA:(R)-benzylsuccinate CoA-transferase. Enzyme was incubated in the absence of CoA-thioesters or in the presence of 0.25 mM benzylsuccinyl-CoA for 10 min with 0.1 to 10 mM NaBH4 and with 10 mM hydroxylamine, respectively, and then assayed by the coupled luminometric test. No inactivation by hydroxylamine was recorded in the tests with and without benzylsuccinyl-CoA, suggesting that no reactive enzyme-CoA intermediate was present. However, the inactivation data with borohydride were somewhat ambiguous. No inactivation was detected with 0.1 mM NaBH4, but a decrease of activity in the presence of benzylsuccinyl-CoA to 25% and further to 3.5% was observed at NaBH4 concentrations of 1 and 10 mM, respectively. The same treatments did not affect the enzyme in the absence of benzylsuccinyl-CoA. The reason for this partial inactivation is not known.
Succinyl-CoA:benzylsuccinate CoA-transferase activity in other denitrifying bacteria.
General occurrence of the benzylsuccinate-activating enzyme was tested by determining succinyl-CoA:benzylsuccinate CoA-transferase activity by the luminometric test in other alkylbenzene-metabolizing denitrifying bacteria; the toluene-degrading Azoarcus sp. strain B5-1 and the Azoarcus sp. strains T and M3, which degrade toluene or m-xylene (18, 21), were chosen for these tests. Enzyme activity was detected in extracts of toluene-grown cells of all these strains, whereas extracts of control cells grown on benzoate did not show detectable activity (Table 3). The observed specific enzyme activities were close to the calculated minimum values which are needed to explain the observed growth rates of cells grown on toluene. Surprisingly, m-xylene-grown cells of Azoarcus sp. strains T and M3 also contained specifically induced CoA-transferase activity for the “wrong” substrate, benzylsuccinyl-CoA, which was more than two times higher than the activity in toluene-grown cells of the same strains (Table 3).
TABLE 3.
Succinyl-CoA:benzylsuccinate CoA-transferase activities in toluene- and m-xylene-degrading denitrifying bacteriaa
| Growth substrate | Toluene | Benzoate | m-Xylene | 3-Methylbenzoate |
|---|---|---|---|---|
| T. aromatica | 29 ± 4 | <0.1 | NA | NA |
| Azoarcus sp. strain B5-1 | 116 ± 8 | <0.1 | NA | NA |
| Azoarcus sp. strain T | 9 ± 1 | <0.1 | 23 ± 10 | <0.1 |
| Azoarcus sp. strain M3 | 25 ± 4 | <0.1 | 49 ± 3 | <0.1 |
Strains B5-1, T, and M3 are all Azoarcus sp. Values are in nanomole of succinyl-CoA formed per minute per milligram of protein, as determined by the luminometric assay NA = not applicable.
DISCUSSION
Succinyl-CoA:benzylsuccinate CoA-transferase of T. aromatica is a strictly toluene-induced enzyme that catalyzes the reversible regio- and enantio-selective synthesis of 2-(R)-benzylsuccinyl-CoA. The detected specific activity in cell extracts is in accordance with the calculated rate of toluene degradation. The enzyme is the first described CoA-transferase consisting of two subunits of very similar amino acid sequences. The subunits were identified as the gene products of the toluene-induced bbsE and bbsF genes. The two subunits of succinyl-CoA:benzylsuccinate CoA-transferase are similar to the sequences of known and putative CoA-transferases of family III, e.g., oxalyl-CoA:formate CoA-transferase from O. formigenes (32), (E)-cinnamoyl-CoA:(R)-phenyllactate CoA-transferase from C. sporogenes, and a probable homologue of the latter derived from the Clastridium difficile genome (16). No sequence similarity was detected with CoA-transferases of families I and II. CoA-transferase activities acting on benzylsuccinate were found in different strains of toluene- or m-xylene-degrading denitrifying bacteria. These and previously reported data (5, 26) suggest that activation of benzylsuccinate [and probably also that of (3-methylbenzyl)-succinate generated from m-xylene] occurs by a CoA-transferase rather than an ATP-dependent CoA-ligase reaction in all known strains.
Succinyl-CoA:(R)-benzylsuccinate CoA-transferase shows a rather strong preference for its natural substrates, succinate and (R)-benzylsuccinate, respectively, and the corresponding CoA-thioesters, and it does not react with the (S)-enantiomer of benzylsuccinate. Surprisingly, the enzyme is inhibited by high concentrations of (S)-benzylsuccinyl-CoA, while it is apparently not affected by free (S)-benzylsuccinate. It may be speculated that the attached CoA moiety affords better binding of the wrong enantiomer to the enzyme. However, since no defined 2-or 3-(S)-benzylsuccinyl-CoA isomers are available, we have not further investigated the mechanism of this inhibition so far. The enzyme is partially active when succinate is replaced by maleate and is inactive with fumarate, suggesting that the two carboxyl groups of succinate bind to the enzyme in syn conformation. The distance of the carboxyl groups of succinate seems to be another important factor, since neither malonate nor glutarate were accepted as substrate analogs. Alternative substrates partially replacing (R)-benzylsuccinate were benzylmalonate and phenylsuccinate, but the enzyme did not accept analogs with only one carboxy group, such as 3-phenylpropionate and 4-phenylbutyrate. Thus, recognition of (R)-benzylsuccinate probably requires both carboxyl groups. The isotope exchange experiment between succinate and succinyl-CoA and the relatively high conversion rates of succinyl-CoA with methylsuccinate indicate that the benzylsuccinate-binding site may also accept small nonaromatic dicarboxylic acids.
The kinetic investigations on succinyl-CoA:(R)-benzylsuccinate CoA-transferase indicate that catalysis proceeds via a ternary complex of enzyme and the two substrates without an enzyme-bound intermediate. In addition to these data, several other observations are consistent with an assumed ternary-complex mechanism of the enzyme. (i) The recently characterized similar family III enzyme (E)-cinnamoyl-CoA:(R)-phenyllactate CoA-transferase was also shown to operate by a ternary-complex mechanism by kinetic analysis (16). (ii) Succinyl-CoA:(R)-benzylsuccinate CoA-transferase preincubated with benzylsuccinyl-CoA was insensitive against hydroxylamine treatment, which would inactivate CoA-transferases of family I. (iii) The observed inhibition of isotope exchange between succinyl-CoA and succinate by added benzylsuccinate may be explained by outcompeting succinate for binding to the benzylsuccinate site, if a ternary-complex mechanism is assumed. The only observation not consistent with this assumption is the inactivation of the enzyme by high concentrations of NaBH4, but the same behavior was also reported for the related enzyme (E)-cinnamoyl-CoA:(R)-phenyllactate CoA-transferase (16). Therefore, we suggest a CoA transfer mechanism between succinyl-CoA and (R)-benzylsuccinate without an enzyme-bound CoA intermediate, similar to the proposed mechanism of (E)-cinnamoyl-CoA:(R)-phenyllactate CoA-transferase (16). It remains to be proven whether this is a general property of CoA-transferases of family III. A ternary-complex mechanism is also used by CoA-transferases of family II; the major difference between the enzymes of families II and III seems to reside in the activated substrates used, as the natural substrates of family II enzymes are thioesters of small enzyme-associated ACPs (10, 11, 17), whereas all known family III enzymes employ diffusible thioesters of CoA.
Apart from their similar amino acid sequences and enzyme kinetics, family III CoA-transferases seem to be surprisingly different. This relates to the relatively strong substrate preference of the respective enzymes, and especially to their subunit compositions. All three characterized enzymes exhibit different oligomeric states: formyl-CoA:oxalate CoA-transferase is monomeric (3); succinyl-CoA:(R)-benzylsuccinate CoA-transferase is an α2β2 heterotetramer of two very similar subunits; and the subunit of (E)-cinnamoyl-CoA:(R)-phenyllactate CoA-transferase is part of a larger phenyllactate dehydratase enzyme complex which also contains the subsequent enzyme of the metabolic pathway, (R)-phenyllactyl-CoA dehydratase (16).
ACKNOWLEDGMENTS
We thank G. Fuchs (Mikrobiologie, Universität Freiburg) for his support and many helpful discussions during this work. P. Gräber and A. Labahn (Physikalische Chemie, Universität Freiburg) are thanked for help in the development of the luminometric assay and for making their luminometer available. W. Eisenreich (Organische Chemie, Technische Universität München) is acknowledged for recording NMR spectra, and P. Hoerth and W. Haehnel (Biochemie der Pflanzen, Universität Freiburg) are acknowledged for electrospray-mass spectrometry analysis.
This work was supported by grants from the Deutsche Forschungsgemeinschaft.
Footnotes
Dedicated to W. Buckel on the occasion of his 60th birthday.
REFERENCES
- 1.Anders A, Kaetzke A, Kämpfer P, Ludwig W, Fuchs G. Taxonomic position of aromatic degrading denitrifying pseudomonad strains K172 and KB740 and their description as new members of the genera Thauera, T. aromatica sp. nov., and Azoarcus, A. evansii sp. nov., respectively, members of the beta subclass of Proteobacteria. Int J Syst Bacteriol. 1995;45:327–333. doi: 10.1099/00207713-45-2-327. [DOI] [PubMed] [Google Scholar]
- 2.Annweiler E, Materna A, Safinowski M, Kappler A, Richnow H H, Michaelis W, Meckenstock R U. Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture. Appl Environ Microbiol. 2000;66:5329–5333. doi: 10.1128/aem.66.12.5329-5333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baetz A L, Allison M J. Purification and characterization of formyl-coenzyme A transferase from Oxalobacter formigenes. J Bacteriol. 1990;172:3537–3540. doi: 10.1128/jb.172.7.3537-3540.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker H A, Jeng I M, Neff N, Robertson J M, Tam F K, Hosaka S. Butyryl-CoA:acetoacetate CoA-transferase from a lysine-fermenting Clostridium. J Biol Chem. 1978;253:1219–1225. [PubMed] [Google Scholar]
- 5.Beller H R, Spormann A M. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J Bacteriol. 1997;179:670–676. doi: 10.1128/jb.179.3.670-676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beller H R, Spormann A M. Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product. J Bacteriol. 1998;180:5454–5457. doi: 10.1128/jb.180.20.5454-5457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biegert T, Fuchs G, Heider J. Evidence that oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. doi: 10.1111/j.1432-1033.1996.0661w.x. [DOI] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Buckel W, Dorn U, Semmler R. Glutaconate CoA-transferase from Acidaminococcus fermentans. Eur J Biochem. 1981;118:315–321. doi: 10.1111/j.1432-1033.1981.tb06404.x. [DOI] [PubMed] [Google Scholar]
- 10.Buckel W, Buschmeier V, Eggerer H. The action mechanism of citrate lyase from Klebsiella aerogenes. Hoppe-Seyler's Z Physiol Chem. 1971;352:1195–1205. [PubMed] [Google Scholar]
- 11.Buckel W, Bobi A. The enzyme complex citramalate lyase from Clostridium tetanomorphum. Eur J Biochem. 1975;64:255–262. doi: 10.1111/j.1432-1033.1976.tb10295.x. [DOI] [PubMed] [Google Scholar]
- 12.Coligan J E, Dunn B M, Ploegh H L, Speicher D W, Wingfield P T. Current protocols in protein science. New York, N.Y: John Wiley & Sons Inc.; 1995. [Google Scholar]
- 13.Cornish-Bowden A. Analysis of enzyme kinetic data. Oxford, United Kingdom: Oxford University Press; 1995. [Google Scholar]
- 14.Cornish-Bowden A. Fundamentals of enzyme kinetics. Oxford, United Kingdom: Oxford University Press; 1995. [Google Scholar]
- 15.Corthésy-Theulaz I E, Bergonzelli G E, Henry H, Bachmann D, Schorderet D F, Blum A L, Ornston N. Cloning and characterization of Helicobacter pylori succinyl-CoA:acetoacetate CoA-transferase, a novel prokaryotic member of the CoA-transferase family. J Biol Chem. 1997;272:25659–25667. doi: 10.1074/jbc.272.41.25659. [DOI] [PubMed] [Google Scholar]
- 16.Dickert S, Pierik A J, Linder D, Buckel W. The involvement of coenzyme A esters in the dehydration of (R)-phenyllactate to (E)-cinnamate by Clostridium sporogenes. Eur J Biochem. 2000;267:3874–3884. doi: 10.1046/j.1432-1327.2000.01427.x. [DOI] [PubMed] [Google Scholar]
- 17.Dimroth P, Eggerer H. Isolation of subunits of citrate lyase and characterization of their function in the enzyme complex. Proc Natl Acad Sci USA. 1975;72:3458–3462. doi: 10.1073/pnas.72.9.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolfing J, Zeyer J, Binder-Eicher P, Schwarzenbach R P. Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch Microbiol. 1990;154:336–341. doi: 10.1007/BF00276528. [DOI] [PubMed] [Google Scholar]
- 19.Heider J, Spormann A M, Beller H R, Widdel F. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol Rev. 1999;22:459–473. [Google Scholar]
- 20.Heider J, Fuchs G. Microbial anaerobic aromatic metabolism. Anaerobe. 1997;3:1–22. doi: 10.1006/anae.1997.0073. [DOI] [PubMed] [Google Scholar]
- 21.Hess A, Zarda B, Hahn D, Häner A, Stax D, Höhener P, Zeyer J. In situ analysis of denitrifying toluene- and m-xylene-degrading bacteria in a diesel fuel-contaminated laboratory aquifer column. Appl Environ Microbiol. 1997;63:2136–2141. doi: 10.1128/aem.63.6.2136-2141.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob U, Mack M, Clausen T, Huber R, Buckel W, Messerschmidt A. Glutaconate CoA-transferase from Acidaminococcus fermentans: the crystal structure reveals homology with other CoA-transferases. Structure. 1997;5:415–426. doi: 10.1016/s0969-2126(97)00198-6. [DOI] [PubMed] [Google Scholar]
- 23.Krieger C J, Beller H R, Reinhard M, Spormann A M. Initial reactions in anaerobic oxidation of m-xylene by the denitrifying bacterium Azoarcus sp. strain T. J Bacteriol. 1999;181:6403–6410. doi: 10.1128/jb.181.20.6403-6410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leuthner B, Heider J. Anaerobic toluene catabolism of Thauera aromatica: the bbs operon codes for the enzymes of β-oxidation of the intermediate benzylsuccinate. J Bacteriol. 2000;182:272–277. doi: 10.1128/jb.182.2.272-277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuthner B, Leutwein C, Schulz H, Hörth P, Haehnel W, Schiltz E, Schägger H, Heider J. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalyzing the first step in anaerobic toluene metabolism. Mol Microbiol. 1998;28:615–628. doi: 10.1046/j.1365-2958.1998.00826.x. [DOI] [PubMed] [Google Scholar]
- 26.Leutwein C, Heider J. Anaerobic toluene catabolic pathway in denitrifying Thauera aromatica: activation and β-oxidation of the first intermediate, (R)-(+)-benzylsuccinate. Microbiology. 1999;145:3265–3271. doi: 10.1099/00221287-145-11-3265. [DOI] [PubMed] [Google Scholar]
- 27.Mack M, Buckel W. Identification of glutamate beta 54 as the covalent-catalytic residue in the active site of glutaconate CoA-transferase from Acidaminococcus fermentans. FEBS Lett. 1995;357:145–148. doi: 10.1016/0014-5793(94)01351-z. [DOI] [PubMed] [Google Scholar]
- 28.Müller J A, Galushko A S, Kappler A, Schink B. Anaerobic degradation of m-cresol by Desulfobacterium cetonicum is initiated by formation of 3-hydroxybenzylsuccinate. Arch Microbiol. 1999;172:287–294. doi: 10.1007/s002030050782. [DOI] [PubMed] [Google Scholar]
- 29.Müller J A, Galushko A S, Kappler A, Schink B. Initiation of anaerobic degradation of p-cresol by formation of 4-hydroxybenzylsuccinate in Desulfobacterium cetonicum. J Bacteriol. 2001;183:752–757. doi: 10.1128/JB.183.2.752-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parales R E, Harwood C S. Characterization of the genes encoding β-ketoadipate succinyl-coenzyme A transferase in Pseudomonas putida. J Bacteriol. 1992;174:4657–4666. doi: 10.1128/jb.174.14.4657-4666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selmer T, Buckel W. Oxygen exchange between acetate and the catalytic glutamate residue in glutaconate CoA-transferase from Acidaminococcus fermentans: implications for the mechanism of CoA-ester hydrolysis. J Biol Chem. 1999;274:20772–20778. doi: 10.1074/jbc.274.30.20772. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu H, Ogden S D, Lung H-Y, Luttge B G, Baetz A L, Peck A B. DNA sequencing and expression of the formyl-coenzyme A transferase gene, frc, from Oxalobacter formigenes. J Bacteriol. 1997;179:3378–3381. doi: 10.1128/jb.179.10.3378-3381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sramek S J, Frerman F E. Purification and properties of Escherichia coli coenzyme A-transferase. Arch Biochem Biophys. 1975;171:14–26. doi: 10.1016/0003-9861(75)90002-8. [DOI] [PubMed] [Google Scholar]
- 34.Tschech A, Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987;148:213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]
- 35.White H, Jencks W P. Properties of succinyl-CoA:3-ketoacid coenzyme A transferase. J Biol Chem. 1976;251:1708–1711. [PubMed] [Google Scholar]
- 36.Wiesenborn D P, Rudolph F B, Papoutsakis E T. Coenzyme A-transferase from Clostridium acetobutylicum ATCC 824 and its role in the uptake of acids. Appl Environ Microbiol. 1989;55:317–322. doi: 10.1128/aem.55.2.323-329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]