Introduction
Stigmatizing language used to describe patients and medical conditions is associated with poorer health outcomes. A recent investigation showed that approximately 80% of medical literature focused on alcohol use disorder (AUD) contained stigmatizing terms related to individuals; however, the quantification of stigmatizing terminology for outcomes and processes (STOP) among AUD research is unknown. Thus, our primary objective was to evaluate publications of clinical trials for their inclusion of STOP.
Methods
We performed a systematic search of PubMed for AUD clinical trials between January 1, 2017 and June 30, 2021. Article screening and data extraction were performed in a masked, duplicate manner by 2 investigators. We searched the full text of included manuscripts for STOP. We reported the frequency and percentage of manuscripts with STOP and individual terms. We evaluated associations between STOP usage and several clinical trial characteristics via logistic regression.
Results
Our search returned 1552 articles, which were then randomized and the first 500 were screened for inclusion. Of 147 included articles, 115 (78.2%) included STOP. The most common STOP were “drop out” (38.78%; 57/147), “relapse” (36.05%; 53/ 147), and “adherent, nonadherence” (35.37%; 52/147). No significant associations were found between STOP usage and trial characteristics.
Discussion
STOP was found in a majority of AUD clinical trial publications. As AUD is highly stigmatized, steps should be taken to eliminate usage of STOP in literature pertaining to AUD treatments. Many stigmatizing terms can be replaced by person-centered, more clinically accurate terms to further combat AUD stigma.
Key Words: alcohol use disorder, clinical trials, person centered language, stigma
The use of nonstigmatizing, clinically accurate terminology in the field of addiction medicine is gaining traction in an effort to minimize the negative effects of stigma on patient healthcare outcomes.1 Stigmatizing language is known to negatively alter providers’ perceptions of patients, patients’ self-perceptions, and treatment engagement.2–4 Negative perceptions of patients by providers may impact the quality of care. For example, Kelly et al found that labeling patients as “substance abusers” led to providers pursuingmore punitive measures rather than treatments.2 Stigmatizing language not only impacts provider’s perceptions but also impacts patients’ self-image,5 and recovery capital—the collective personal and institutional resources one has to overcome addiction.5,6 The embedded nature of stigmatizing language in medical culture suggests that reducing the use of stigmatizing language requires intentionality— actively dismantling the presence of stigmatizing language in medical literature, medical terminology, medical education, and patient interactions.2–4 Recent studies have discussed the importance of using nonstigmatizing terminology and patient-centered language and have offered alternative nomenclature that may facilitate positive culture change in medicine, particularly regarding addiction and psychiatric illness.1,7
Person-centered language emphasizes the patient or person above any disease or condition they may have.8 Many studies have highlighted the high prevalence of stigmatizing, nonperson-centered language (labeling, euphemistic language, and emotive terminology) in the medical literature from journals of numerous disciplines and specialties,9–11 including alcohol use disorder (AUD).7 Alcohol use is a major cause of morbidity and mortality and is annually responsible for 3 million deaths worldwide.12 When comparing AUD with other mental health conditions, AUD was less likely to be seen as a chronic medical condition and linked to individual-blame as a component of stigma.13,14 However, it has also been highlighted that the perceived causes of mental health and addiction may have complex stigma effects whereby biomedical attributions may alleviate blame, but may also increase other stigma components such as social distance, and perceived dangerousness or prognostic pessimism.15,16 As such, all efforts seeking to enhance patient care in AUD are important healthcare objectives that warrant careful consideration concerning language and attributional consequences.
Another potential source of pejorative language in medicine is the use of stigmatizing terminology for outcomes and processes (STOP) used in clinical trial reporting.17,18 A study by Ashford et al highlighted the lack of discussion surrounding precise terminology used in treatment outcomes of AUD.3 In clinical trial reporting, many processes and outcomes have been defined with terms that bear a negative connotation,1,17 such as “failing a test” rather than “testing positive,” or “dropping out” of a study rather than “discontinuing participation” or more simply reporting attrition rates. STOP can lead to inaccurate assumptions about the patient receiving care-misidentifying reasons for discontinuity of care or implying that treatment design and clinician opinion supersede a patient’s experience.3 Eliminating the use of STOP in AUD clinical trial reporting may further support the change to nonstigmatizing, more clinically accurate terminology regarding patients with AUD.
The primary objective of the current study was to examine the prevalence of STOP among publications of clinical trials of alcohol use interventions, including AUD and heavy or binge drinking, from January 1, 2017 to June 30, 2021. These dates were selected because they follow the publication of Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health19-which specifically called for reducing stigma regarding substance use disorders. Findings from this study may reveal areas of medical literature that can be actively improved to further reduce stigma experienced by patients living with AUD.
METHODS
Journal Selection and Publication Randomization and Reduction
We conducted a systematic search via PubMed for publications of clinical trial results for alcohol interventions, from January 1, 2017 to June 30, 2021, with a search strategy adapted from the Practice Guidelines For The Pharmacological Treatment of Patients with Alcohol Use Disorder20 (Supplement 1, http://links.lww.com/JAM/A326). Search returns were extracted from PubMed as a comma-sorted-value file and imported to Stata (StataCorp LLC, College Station, TX) for randomization (Supplement 2, http://links.lww.com/JAM/A327). Search returns were randomly sorted, and the first 500 articles were selected to screen for inclusion by investigator VL and MHe. Screening was conducted in a masked, duplicated fashion.
Article Eligibility
Publications of primary or secondary results from clinical trials of alcohol use interventions including AUD and heavy or binge drinking were included for this investigation. Binge or heavy drinking studies were included when the publication operationalized and reported these terms. We included trials in any phase assessing feasibility, safety, or efficacy of pharmacologic treatments, devices, behavioral interventions, or other medical treatments. Studies must have been published between January 1, 2017 and June 30, 2021, and be available in English.
Data Extraction
Investigators (VL and Mhe) used a pilot-tested Google Sheet to report assessment of STOP in articles and to extract study characteristics. Before full initiation of extraction, authors VL and MHe extracted data from the first 30 manuscripts and compared results to ensure uniform answers. Data extraction responses were masked until completion, upon which the 2 investigators were unmasked and resolved any discrepancies.
STOP Identification
To explore our primary research question, we systematically searched each article for the presence of the following terms related to trial outcomes which were developed a priori to the study being conducted: “Clean,” “Dirty,” “Resistant,” (as in “treatment-resistant”), “Compliant*” (as in compliant, compliance, or non-), “Adherent*” (as in adherent, adherence, or non-), “Dropout,” “Fail” (as in failed, failure: related to treatment or testing), “Relapse,” “Wagon,” “Recovered.” These terms have been shown to carry negative connotations within patient populations3 or have been identified by experienced alcohol and addiction researchers as having negative bias.17,21,22 The terms “medication-assisted treatment” or “-therapy,” “recurrence,” and “recovery” were not included, as Ashford et al found differing associations among patient populations when compared to terms such as “relapse,” which was frequently associated with negative connotations.3 We evaluated the full text of each article for any occurrence of the STOP listed previously using the Find feature in Adobe Acrobat Reader DC (Acrobat.adobe.com). Articles containing STOP were coded as “including STOP,” and the incidence of each term was recorded.
Other Study Characteristics
During extraction, we also identified the type of intervention involved, the institution type of the first author, the study’s funding source, the journal’s H-index from Scientific Journal Rankings,23 and whether the study mentioned adherence to the CONsolidated Standards Of Reporting Trials (CONSORT) reporting guidelines.
Data Analysis
From the systematic search, we reported (1) the total number of journals and articles returned from the PubMed search, (2) the number of studies that were screened, and (3) the number of studies that were included based on the eligibility criteria from each selected journal. To estimate the presence of STOP within our sample, we calculated the frequency and proportion of articles with stigmatizing terminology in the sample. Further, to evaluate the most common STOP within these articles, we calculated frequencies and percentages for each STOP term overall. To evaluate associations between STOP occurrence and study characteristics, we used bivariate and multivariate regression analysis.
This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for reporting and guidance from Guidelines for reporting meta-epidemiological methodology research24—as our study followed a research-on-research approach. Analyses were performed with a Type 1 error rate set at 0.05 and performed using STATA 16.1 (StataCorp LLC). This study was determined not to be human subjects research by an institutional review board. A protocol for this study, written before starting our study can be found on Open Science Framework (osf.io/sr65f/).
RESULTS
Search Returns and Study Characteristics
Our systematic search returned 1552 articles from 389 journals. Articles were then randomized and 500 were screened for inclusion—from which 147 met inclusion criteria (Fig. 1). Among the 147 included articles, 73 (49.66%) used behavioral interventions, 45 (30.61%) used pharmacologic treatments, 25 (17.01%) used devices, and 4 (2.72%) were grouped as Other (Table 1). A majority of studies were grant-funded (117/147; 79.59%). Fifty of the studies (34.01%) mentioned adherence to CONSORT reporting guidelines, and 103 were published in journals that required American Medical Assocation or International Committee of Medical Journal Editors (AMA or ICMJE) reporting guidelines. Study characteristics can be found in Table 1.
FIGURE 1.
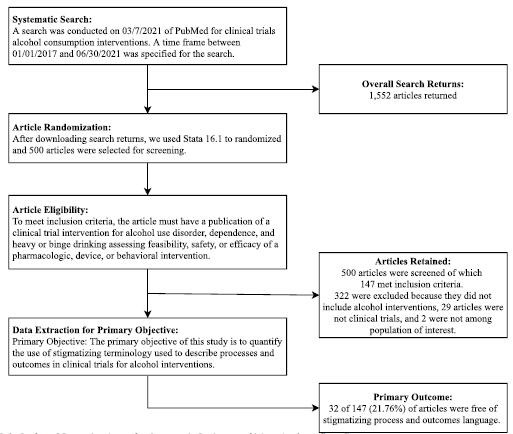
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
TABLE 1.
Clinical Trial Publication Characteristics and Associations Containing Stigmatizing Terminology for Outcomes and Processes (STOP)
| Article Characteristics | Articles With STOP (115) No. (%) | Articles Without STOP (32) No. (%) | Total (147) No. (%) | OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|---|
| Type of intervention | |||||
| Behavioral | 58 (50.43) | 15 (46.88) | 73 (49.66) | 1 (Ref) | 1 (Ref) |
| Device | 18 (15.65) | 7 (21.88) | 25 (17.01) | 1.5 (0.53–4.26) | 2.07 (0.67–6.4) |
| Pharmacologic | 35 (30.43) | 10 (31.25) | 45 (30.61) | 1.1 (0.45–2.73) | 1.19 (0.43–3.29) |
| Other | 4 (3.48) | 0 (0) | 4 (2.72) | 1 - | 1 - |
| Article funding | |||||
| Grant | 94 (81.74) | 23 (71.88) | 117 (79.59) | 1 (Ref) | 1 (Ref) |
| Industry | 3 (2.61) | 1 (3.13) | 4 (2.72) | 1.36 (0.14–13.71) | 1.64 (0.14–18.53) |
| No funding | 2 (1.74) | 0 (0) | 2 (1.36) | — | — |
| No Statement | 12 (10.43) | 5 (15.63) | 17 (11.56) | 1.7 (0.55–5.32) | 1.41 (0.42–4.8) |
| Public | 4 (3.48) | 3 (9.38) | 7 (4.76) | 3.07 (0.64–14.66) | 6.83 (0.97–48.05) |
| Mention of CONSORT | |||||
| No | 74 (64.35) | 23 (71.88) | 97 (65.99) | 1 (Ref) | 1 (Ref) |
| Yes | 41 (35.65) | 9 (28.13) | 50 (34.01) | 0.71 (0.3–1.67) | 0.64 (0.24–1.68) |
| First author employment | |||||
| Government | 14 (12.17) | 4 (12.5) | 18 (12.24) | 1 (Ref) | 1 (Ref) |
| Private | 25 (21.74) | 5 (15.63) | 30 (20.41) | 0.7 (0.16–3.04) | 0.69 (0.14–3.29) |
| Public | 74 (64.35) | 23 (71.88) | 97 (65.99) | 1.07 (0.32–3.58) | 0.97 (0.27–3.5) |
| Journal requires AMA/ICMJE guidelines | |||||
| Government | 32 (27.83) | 12 (37.5) | 44 (29.93) | 1 (Ref) | 1 (Ref) |
| Private | 83 (72.17) | 20 (62.5) | 103 (70.07) | 0.64 (0.28–1.46) | 0.6 (0.25–1.44) |
| Journal H-index* | |||||
| Mean (SD) | 132.42 (64.97) | 123.75 (61.58) | 130.53 (64.14) | 1 (0.99–1) | 1 (0.99–1.01) |
*H-index from Scientific Journal Rankings (https://www.scimagojr.com/journalrank.php) current as of July 21, 2021.
CI indicates confidence interval; CONSORT, CONsolidated Standards Of Reporting Trials; OR. odds ratio.
Inclusion of STOP
We found that 115 of the 147 (78.2%) articles included 1 or more instances of STOP. Among the 147 articles, we found 214 unique instances of STOP (Fig. 2). The most frequent STOP was “drop out”—related to a person(s) leaving treatment or discontinuing a study—found in 38.78% (57/147) of articles (Fig. 2). This was closely followed by “relapse” found in 36.05% (53/147) of articles and “adherent” (or “adherence” or “non-adherence/adherent”) found in 35.37% (52/147) of articles. Further, “compliant” (or “compliance,” or “non-”) was found in 14.97% (22/147) of publications, and “fail” (or “failed” or “failure”) was found in 14.29% (21/147). Instances of STOP present in articles by journal are presented in Supplement 3, http://links.lww.com/JAM/A328.
FIGURE 2.
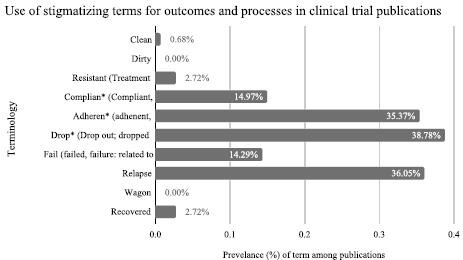
Use of STOP in clinical trial publications. STOP indicates stigmatizing terminology for outcomes and processes.
Associations Between STOP and Study Characteristics
We used bivariate and multivariable regressions to determine associations between the incidence of STOP and (1) the type of intervention, (2) funding source, (3) mention of CONSORT, (4) first author employment, (5) journal requirements for adhering to AMA/ICMJE guidelines, and (6) journal H-index from Scientific Journal Rankings23; however, no statistically significant relationships were found. The multivariable logistic regression model, which included all study characteristics, accounted for 5.4% of the variance among articles including STOP (X2(10)=8.13, P = 0.62; Table 1).
DISCUSSION
Our investigation suggests that stigmatizing terminology for outcomes and processes (STOP) among AUD clinical trials is frequent and pervasive—being present in nearly 4 out of 5 studies. This finding highlights the need for improvement regarding clinically accurate, nonstigmatizing language in AUD research reporting. The use of STOP in reports of AUD clinical trials reinforces the stigma surrounding addiction, prevents positive change in medical culture, and may contribute to negative healthcare outcomes.1,2 To our knowledge, our research is the first to quantify the prevalence of STOP within medical literature; however, as person-centered language and STOP are interrelated, comparisons can be drawn from person-centered language research in AUD and other fields. In previous research from our team, Hartwell et al. found that nearly 80% of medical literature focused on AUD was not adherent to the person-centered language guidelines presented in the American Medical Association’s Manual of Style.7 Similar rates of stigmatizing language have been found in medical literature focused on psoriasis,9 amputations, 25 and heart failure,26—the latter of which prompted the Editor-in-Chief of the Journal for Patient Centered Care to assess the stigmatizing language appearing within the journal.27 Therefore, in the context of these previous studies, our findings further highlight the prevalence of stigmatizing language in medical literature.
The most frequently used STOP in our sample were “drop out,” and “relapse.” The term “drop out” is synonymous with leaving high school early—therefore often associated with having inadequate education, poorer health, lower wages, higher rates of incarceration, and inability to achieve life goals.28,29 However, using the term “drop out” to describe individuals who discontinue trial participation is inaccurate and harmful, as participant discontinuation may result from other causes such as lack of access to transportation, changes in housing status, mistrust in the medical community, or stigma surrounding mental health conditions.30 “Relapse” is a term that associates a binary outcome with a person’s engagement in drinking—thereby leading individuals to cross an imaginary line in which all progress is seemingly lost. As Miller points out in a 2015 article, all-or-none progress is antithetical to AUD treatment.21 To portray this fact, Miller relates using the term “relapse” to describe a person with diabetes presenting to the emergency room in a glycemic crisis. An individual with diabetes in the aforementioned setting is not told they have “relapsed”—nor should they be told they have “failed” in their treatment. Although clinical trials often rely on criteria to judge treatment effectiveness, reduced alcohol use or cravings may be best reported on a spectrum as opposed to a dichotomous endpoint. Moreover, individuals with AUD often have varying degrees of success, and if unplanned drinking does resume, it may be due to changes in their treatment needs or because treatment needs are not fully being met. The term “adherence” or “adherent” was also frequently identified and can imply that patients’ outcomes are solely dependent on receiving a full course of treatment which is often imprecise and inaccurate31 It also dichotomizes individuals as adherent or nonadherent which may lead providers to a negative bias towards the latter group32 even though behavior change is not always dependent on treatment engagement31 These terms not only carry a stigma for the person receiving treatment but are also reductive—minimizing the importance of a trial participant’s experience and diminishing the validity of their experiences. Consequently, the continual use of STOP in medical literature may contribute to its persistence in clinical practice27,33 and may perpetuate the negative bias of health care providers toward individuals with substance use disorders.
Translation into Clinical Practice and Society
By implicitly endorsing stigmatizing language, authors of clinical trial publications may be potentially undermining efforts to promote person-centered language and alternatives to STOP. Notably, stigmatizing language has real-world implications for both people with AUD in terms of public stigma, selfchange, and treatment-seeking.14,34,35 Terms such as “relapse” are strongly associated with disease model conceptualizations of alcohol problems which, whilst important in some recovery contexts, can be harmful in others.36 Relapse and other disease model conceptualizations can create a false binary in which people are either viewed as having a “problem” or not. In contrast, models and language promoting more continuum or psychosocially-orientated models of AUD may have important benefits for problem recognition and help-seeking,37,38 potentially mediated by lower stigma.35,39,40 As such, clinical trialists should lead by example, ceasing the use of STOP and replacing it with person-centered and nonstigmatizing language. This change may in turn have important collateral benefits for other AUD discourses including public and policy spheres. For instance, significant efforts to implement alcohol brief interventions programs may have been undermined by binary and stigmatizing conceptualizations of AUD.41 Similarly, underrecognition of the possibility of drinking reduction goals as a valid, self-directed treatment goal has also been associated with the over-application of binary and stigmatizing conceptualizations.34,35 Notwithstanding the right for people to self-label or use terminology that may be common within certain recovery discourses, prioritizing person-centered language is a key strategy for addressing the persistent and damaging effects of addiction stigma.42
Implications for Clinical Trialists
Our findings, in addition to the previously mentioned person-centered language studies, further the need for language reform within the medical research community—especially for those treating stigmatized conditions. The design and implementation of clinical trials guides language within research settings, and it also guides clinical perceptions, patient experiences, and media narratives that report the findings from clinical trials. As such, it is critically important for AUD clinical trialists to consider reporting standards and whether STOP can be avoided in the trial registration, trial documents (including advertisements, consent forms, protocols, etc), and in the reporting of trial results. Considering language in trials and refraining from STOP may not only reduce the stigma of AUD but may also enhance precision and increase the rigor and reproducibility of clinical trial designs. For example, the term “relapse,” used in more than one-third of trials in the current study, does not have a single agreedupon definition. A recent systematic review identified 25 unique definitions of “relapse” used in the alcohol literature, with definitions ranging from any use of alcohol to admission to treatment for acute services.43 Thus, using the term “relapse” in clinical trials is not only stigmatizing, but it also lacks precision. Defining clinical trial processes and outcomes by the observed behaviors is far more likely to enhance rigor and reproducibility.
Recommendations
Within addiction treatment, long-term care for AUD is multifaceted and does not often hinge on pass-or-fail criteria. Completing treatment does not necessarily mean there will not be recurrences of symptoms. A novel approach to overcome these potential recurrences may be to consider that a person’s treatment needs have changed and are no longer fully being met. Additionally, understanding that, in the process of change and recovery, progress should be expected—but not perfection. Changing the language used to describe the outcomes and processes of clinical trials will likely lead to conceptual changes regarding the addiction and recovery process and vice versa. In turn, this change may lead to reduced stigma and an improved, scientifically-driven understanding of addiction treatment. Given the potentially detrimental effects of STOP on patient outcomes, steps should be taken to eliminate STOP in AUD research. We recommend implementing training on STOP in future research to reduce the harm of STOP and using new terms to describe processes and outcomes of clinical trials. Firstly, “recurrence of use,” which describes the non-judgemental return of behaviors or symptoms as used with other disease processes, could replace “relapse” terms, also noting that the word “abuse” is avoided due to its stigmatizing association. Secondly, “concordance with treatment/protocol,” emphasizes the shared responsibility of both the person receiving the treatment and the providers or researchers administering the treatment in following mutually agreed-upon treatment plans or protocols. Lastly, “discontinuation” or “trial attrition rate,” both of which describe the morally neutral event of a participant who is unable to continue with the study or treatment, could replace “drop out” terms. In a similar fashion to how we screened these articles—using the Find feature within the document viewer, we encourage authors to search for STOP and make adjustments to their manuscripts before submission, and we encourage journal editors to become more vigilant in their review, acceptance, and publication of articles in which STOP and nonperson-centered language are included.
Strengths and Limitations
A major strength of our paper is the use of previously published methodology7,26 adapted to systematically search for STOP within articles, and in this case, publications of clinical trials. Further, to enhance transparency and reproducibility, our protocol is publicly available on OSF.io. The limitations of our study include the subjective nature of the terminology assessed within articles; however, this risk was mitigated by identifying our list of search terms a priori and using the Find feature to systematically search each article. Another limitation is that we may not have included all STOP that pertains to AUD, thereby possibly underestimating the frequency in which it seems in the literature. Future research may expand this examination to include systematic reviews of clinical trials, as they are at the apex of the evidence hierarchy, and among other medical topics.
CONCLUSIONS
The current study identified the inclusion of STOP within nearly 80% of clinical trial publications for alcohol interventions—a finding consistent with the rates of nonperson-centered language found in other studies. Our study demonstrates a pervasive use of stigmatizing language within medical literature for AUD, which may lead to poorer health outcomes, lower problem recognition and treatment engagement, and fewer people seeking treatment options. Thus, we, as medical researchers in the field of addiction, must ensure that we use appropriate medical and technical language that incorporates the nature of patient-centered care when reporting outcomes in clinical trial publications.
Footnotes
This study was submitted to ethics review through the Oklahoma State University Center for Health Sciences Institutional Review Board and was determined to be of exempt status. This study adhered to STROBE reporting guidelines.
MHa receives research support through the National Institutes for Justice unrelated to the present work.KWis a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which during the past 3 years was supported by AbbVie, Alkermes, Dicerna, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor Pharmaceuticals, and Amygdala Neurosciences, Inc.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.journaladdictionmedicine.com).
REFERENCES
- 1.Saitz R Miller SC Fiellin DA, et al. Recommended use of terminology in addiction medicine. J Addict Med. 2021;15(1):3–7. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JF, Dow SJ, Westerhoff C. Does our choice of substance-related terms influence perceptions of treatment need? An empirical investigation with two commonly used terms. J Drug Issues. 2010;40(4):805–818. [Google Scholar]
- 3.Ashford RD, Brown AM, Curtis B. Substance use, recovery, and linguistics: the impact of word choice on explicit and implicit bias. Drug Alcohol Depend. 2018;189:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.P Goddu A O’Conor KJ Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashford RD Brown AM Canode B, et al. A mixed-methods exploration of the role and impact of stigma and advocacy on substance use disorder recovery. Alcohol Treat Q. 2019;37(4):462–480. [Google Scholar]
- 6.Granfield R, Cloud W. Coming Clean: Overcoming Addiction Without Treatment. New York: NYU Press; 1999. [Google Scholar]
- 7.Hartwell M Naberhaus B Arnhart C, et al. The use of person-centered language in scientific research articles focusing on alcohol use disorder. Drug Alcohol Depend. 2020;216:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Committee AMof S, AMA Manual of Style Committee. AMA Manual of Style. Published online 2020. doi: 10.1093/jama/9780190246556.001.0001. [DOI]
- 9.Ottwell R Heigle B Reddy AK, et al. The use of person-centered language in medical research journals focusing on psoriasis: crosssectional analysis. JMIR Dermatology. 2021;4(1):e28415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanford FC, Kyle TK. Respectful language and care in childhood obesity. JAMA Pediatr. 2018;172(11):1001–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson JK Guzman SJ Maryniuk MD, et al. The use of language in diabetes care and education. Diabetes Educ. 2017;43(6):551–564. [DOI] [PubMed] [Google Scholar]
- 12.Alcohol. World Health Organization . Available at: https://www.who.int/health-topics/alcohol. Accessed July 1, 2021.
- 13.Schomerus G Lucht M Holzinger A, et al. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol. 2011;46(2):105–112. [DOI] [PubMed] [Google Scholar]
- 14.Kilian C Manthey J Carr S, et al. Stigmatization of people with alcohol use disorders: an updated systematic review of population studies. Alcohol Clin Exp Res. 2021;45(5):899–911. [DOI] [PubMed] [Google Scholar]
- 15.Loughman A, Haslam N. Neuroscientific explanations and the stigma of mental disorder: a meta-analytic study. Cogn Res Princ Implic. 2018;3(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslam N, Kvaale EP. Biogenetic explanations of mental disorder: the mixed-blessings model. Curr Dir Psychol Sci. 2015;24(5):399–404. [Google Scholar]
- 17.Pfund RA Peter SC Swift JK, et al. Nonstigmatizing and precise terminology to describe processes and outcomes in addiction medicine. J Addict Med. 2021. doi: 10.1097/ADM.0000000000000885. Published online June 14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saitz R. Valid, reproducible, clinically useful, nonstigmatizing terminology for the disease and its treatment: addiction, substance use disorder, and medication. J Addict Med. 2017;11(4):246–247. [DOI] [PubMed] [Google Scholar]
- 19.Substance Abuse and Mental Health Services Administration (US); Office of the Surgeon General (US) . Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health [Internet]. Washington (DC): US Department of Health and Human Services; 2016: Nov. PMID: 28252892. [PubMed] [Google Scholar]
- 20.Reus VI Fochtmann LJ Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86–90. [DOI] [PubMed] [Google Scholar]
- 21.Miller WR. Retire the concept of “relapse”. Subst Use Misuse. 2015;50(8–9):976–977. [DOI] [PubMed] [Google Scholar]
- 22.Broyles LM Binswanger IA Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scimago Lab. Scimago Journal Rankings . Available at: https://www.scimagojr.com/journalrank.php. Accessed August 2, 2021.
- 24.Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22(4):139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Headley S Potter I Ottwell R, et al. Adherence rates of person-centered language in amputation research: a cross-sectional analysis. Disabil Health J. 2021;12(1):101172. [DOI] [PubMed] [Google Scholar]
- 26.Pham V Greiner B Ottwell R, et al. Cross-sectional analysis of patient centered language use in journals publishing research focused on heart failure. J Patient Cent Res Rev. 2021;8(3):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgardner DJ. The weight of a word. J Patient Cent Res Rev. 2021;8(3):229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stark P, Noel AM. Trends in high school dropout and completion rates in the United States: 1972-2012. Compendium report. NCES 2015-015. National Center for Education Statistics. Published online June 2015. Available at: http://files.eric.ed.gov/fulltext/ED557576.pdf. Accessed August 6, 2021.
- 29.Belfield CR, Levin HM. The Price We Pay: Economic and Social Consequences of Inadequate Education. Washington D.C.: Brookings Institution Press; 2007. [Google Scholar]
- 30.Woodall A Morgan C Sloan C, et al. Barriers to participation in mental health research: are there specific gender, ethnicity and age related barriers? BMC Psychiatry. 2010;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen E Feinn R Arias A, et al. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86(2–3):214–221. [DOI] [PubMed] [Google Scholar]
- 32.Moyers TB, Miller WR. Is low therapist empathy toxic? Psychol Addict Behav. 2013;27(3):878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Boekel LC Brouwers EPM van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1–2):23–35. [DOI] [PubMed] [Google Scholar]
- 34.Witkiewitz K Wilson AD Pearson MR, et al. A bridge to nowhere: resistance to the possibility of some heavy drinking during recovery and the potential public health implications. JAddict Med. 2021;15(4):352–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris J Moss AC Albery IP, et al. The “alcoholic other”: harmful drinkers resist problem recognition to manage identity threat. Addict Behav. 2021;124:1–7. [DOI] [PubMed] [Google Scholar]
- 36.Walters GD. Twelve reasons why we need to find alternatives to alcoholics anonymous. Addict Disord Their Treat. 2002;1(2):53–59. [Google Scholar]
- 37.Burnette JL Forsyth RB Desmarais SL, et al. Mindsets of addiction: implications for treatment intentions. J Soc Clin Psychol. 2019;38(5):367–394. [Google Scholar]
- 38.Morris J Albery IP Heather N, et al. Continuum beliefs are associated with higher problem recognition than binary beliefs among harmful drinkers without addiction experience. Addict Behav. 2020;105:1–7. [DOI] [PubMed] [Google Scholar]
- 39.Rundle SM, Cunningham JA, Hendershot CS. Implications of addiction diagnosis and addiction beliefs for public stigma: a cross-national experimental study. Drug Alcohol Rev. 2021;40(5):842–846. [DOI] [PubMed] [Google Scholar]
- 40.Violeau L Valery K-M Fournier T, et al. How continuum beliefs can reduce stigma of schizophrenia: the role of perceived similarities. Schizophr Res. 2020;220:46–53. [DOI] [PubMed] [Google Scholar]
- 41.Aira M Kauhanen J Larivaara P, et al. Factors influencing inquiry about patients’ alcohol consumption by primary health care physicians: qualitative semi-structured interview study. Fam Pract. 2003;20(3):270–275. [DOI] [PubMed] [Google Scholar]
- 42.McGinty EE, Barry CL. Stigma reduction to combat the addiction crisis -developing an evidence base. N Engl J Med. 2020;382(14):1291–1292. [DOI] [PubMed] [Google Scholar]
- 43.Maisto SA Witkiewitz K Moskal D, et al. Is the construct of relapse heuristic, and does it advance alcohol use disorder clinical practice? J Stud Alcohol Drugs. 2016;77(6):849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]


