Abstract
Purpose:
There are few evidence-based treatments for language deficits in primary progressive aphasia (PPA). PPA treatments are often adopted from the poststroke aphasia literature. The poststroke aphasia literature has shown promising results using Verb Network Strengthening Treatment (VNeST), a behavioral therapy that focuses on improving naming by producing verbs and their arguments in phrases and sentences. Emerging research in poststroke aphasia and PPA has shown promising results pairing behavioral language therapy with transcranial direct current stimulation (tDCS).
Method:
This study used a double-blind, within-subjects, sham-controlled crossover design to study the effect of anodal tDCS applied to left inferior frontal gyrus (IFG) plus VNeST versus VNeST plus sham stimulation in two individuals with nonfluent variant PPA and one individual with logopenic variant PPA. Participants received two phases of treatment, each with 15 1-hr sessions of VNeST. One phase paired VNeST with tDCS stimulation, and one with sham. For each phase, language testing was conducted at baseline, and at 1 week and 8 weeks posttreatment conclusion. For each participant, treatment efficacy was evaluated for each treatment phase by comparing the mean change in accuracy between baseline and the follow-up time points for naming trained verbs (primary outcome measure), untrained verbs, and nouns on the Object and Action Naming Battery. Mean change from baseline was also directly compared between tDCS and sham phases at each time point.
Results:
Results revealed a different pattern of outcomes for each of the participants. A tDCS advantage was not found for trained verbs for any participant. Two participants with nonfluent variant PPA had a tDCS advantage for generalization to naming of untrained verbs, which was apparent at 1 week and 8 weeks posttreatment. One participant with nonfluent variant also showed evidence of generalization to sentence production in the tDCS phase.
Conclusion:
VNeST plus anodal tDCS stimulation of left IFG shows promising results for improving naming in PPA.
Primary progressive aphasia (PPA) is a neurologic syndrome caused by neurodegenerative disease such as frontotemporal lobar degeneration pathology and Alzheimer's disease pathology (Gorno-Tempini et al., 2011). PPA is characterized by the progressive impairment of language functioning. Cognitive skills are typically relatively preserved compared to language skills in the early stages of PPA. PPA often appears in the fifth or sixth decade of life—earlier than other forms of dementia—and has devastating effects on the patient and their family. There are three main variants of PPA, each of which have distinct patterns of language deficits and are often associated with different patterns of atrophy in the brain. Semantic variant (svPPA) is characterized by difficulty understanding single words and impaired naming skills (Gorno-Tempini et al., 2011). SvPPA is typically accompanied by bilateral atrophy in ventrolateral anterior temporal lobes that is greatest in the left hemisphere. Individuals with the nonfluent/agrammatic variant (nfvPPA) experience apraxia of speech (AOS) or agrammatic language production, or both. Many patients with nfvPPA become mute relatively early in the disease progression (Gorno-Tempini et al., 2006). Brain atrophy in individuals with nfvPPA is often greatest in posterior frontal and insular regions of the left hemisphere. The third variant, logopenic variant PPA (lvPPA), is characterized by word retrieval deficits and sentence repetition impairments (Gorno-Tempini et al., 2011). Atrophy is often greatest in left temporoparietal regions in individuals with lvPPA. There are also many patients with PPA who do not fit the specific criteria for any of the three variants (Utianski et al., 2019). Different underlying pathologies can lead to any PPA variant, but svPPA is most often associated with ubiquitin-positive TAR DNA-binding protein 43 pathology; nvPPA is most commonly associated with tau-positive pathology, and lvPPA with Alzheimer's pathology. Word-level comprehension is generally spared in the early stages of nfvPPA and lvPPA, whereas all variants experience difficulty with word retrieval. Anomia is apparent in the early stages of lvPPA and svPPA but often emerges later in the progression of nfvPPA (Hillis et al., 2002).
Behavioral Language Therapy in PPA
Speech-language pathologists overwhelmingly do not feel prepared to treat individuals with PPA due to lack of information about the best treatment options (Taylor et al., 2009). There are few evidence-based treatment options for individuals with PPA. Many studies have focused on treating naming since almost all individuals with PPA regardless of the variant will experience anomia. Several studies have found promising results across all three variants where participants have been able to relearn words that were lost at baseline (Jokel et al., 2006, 2010), maintain words they were able to name at baseline (Meyer et al., 2016), and in some cases maintain these treatment effects for several weeks or months posttreatment (Henry et al., 2019; Jokel et al., 2006, 2010). Given that PPA is neurodegenerative in nature, language therapy that results in maintenance of language skills is considered beneficial. Treatments that induce short-term or even long-lasting improvements in language are even more enticing for targeting deficits in PPA. For example, Henry et al. (2019) investigated the efficacy of a word retrieval treatment in individuals with lvPPA and svPPA at several time points. They compared naming performance in trained and untrained items between a pretreatment baseline, and 3–6 and 12 months posttreatment. Individuals with both subtypes of PPA improved in the naming of trained items for up to 1 year posttreatment and also showed generalization to untrained items up to 6 months posttreatment.
Poststroke aphasia treatment studies far outnumber those conducted in PPA. As poststroke aphasia treatments are readily available and potentially directly applicable to PPA, many PPA treatment studies aim to test the efficacy of poststroke aphasia treatment protocols as a means for increasing treatment options for people with PPA. One treatment that has been found to be particularly effective in improving naming in poststroke aphasia is Verb Network Strengthening Treatment (VNeST; Edmonds, 2016; Edmonds et al., 2009, 2014). VNeST centers on producing sentences around target verbs (e.g., read) and related arguments (e.g., the author [agent], the book [patient]). VNeST is designed to strengthen semantic relationships between verbs and their thematic roles, such as the agent and the patient (Edmonds et al., 2014). The VNeST design was theoretically motivated by past research showing that thematic roles prime or facilitate activation of associated verbs (Edmonds & Mizrahi, 2011; McRae et al., 2005), and the opposite relationship—that verbs prime activation of related agents and patients (Edmonds & Mizrahi, 2011; Ferretti et al., 2001)—is also true. In poststroke aphasia, this treatment has been found to not only improve naming of trained verbs, but also to promote generalization to naming of untrained verbs and nouns, and to promote sentence production (Edmonds, 2016; Edmonds et al., 2009, 2014). Furthermore, for some patients, the benefits of VNeST extend to sentence comprehension (Edmonds, 2016; Edmonds et al., 2014). While VNeST does not explicitly target sentence comprehension, the authors surmise since that VNeST may benefit sentence comprehension because the procedure requires answering many questions, including complex Wh-questions. We are not aware of any former studies that have investigated the utility of treating word retrieval deficits in PPA with VNeST, but its success in poststroke aphasia suggests this approach is worthy of investigation in PPA.
Transcranial Direct Current Stimulation
Transcranial direct current stimulation (tDCS) is a safe, noninvasive, and relatively painless form of neurostimulation that involves applying a weak electrical current to the brain via two electrodes, which are inserted into saline-soaked sponges and placed on the scalp. Together the sponges placed over the two electrodes form the anode and cathode. A low-intensity current is induced that flows from the anode toward the cathode, which results in neuromodulation of areas targeted by the stimulation. Anodal stimulation enhances the likelihood of neuronal firing, whereas cathodal stimulation inhibits neuronal firing (Gomez Palacio Schjetnan et al., 2013). Research suggests the after effects of tDCS are induced through the modulation of sodium- and calcium-dependent channels and N-methyl-D-aspartate-receptor activity (Liebetanz et al., 2002). Enhancing the neuronal firing rate via anodal tDCS (A-tDCS) promotes mechanisms that underlie long-term potentiation, which refers to a persistent strengthening of synapses following stimulation of synaptic activity, and long-term depression, a reduction of synaptic activity. Frequently studies employing tDCS administer tDCS for the first ~20 min of the session as the cerebral excitability produced by tDCS can last over an hour after tDCS has ceased (Nitsche & Paulus, 2001). Another important advantage of tDCS is that it is quite simple to create a sham-controlled condition because most participants typically only feel tingling on their scalp for the first ~30 s of receiving real tDCS. Thus, a sham condition can mimic real tDCS by providing stimulation for the first 30 s and then ramping down to no stimulation over the next ~15 s (Gandiga et al., 2006).
tDCS Paired With Language Therapy in PPA
An exciting area of language treatment research in PPA is pairing tDCS with language therapy. When paired with language therapy, tDCS has shown promising results in poststroke aphasia (Baker et al., 2010; Fridriksson et al., 2011, 2019; Jung et al., 2011; Richardson et al., 2015; Sebastian et al., 2020) and PPA (Cotelli et al., 2014; de Aguiar, Zhao, Faria, et al., 2020; de Aguiar, Zhao, Ficek, et al., 2020; Fenner et al., 2019; Ficek et al., 2018; Gervits et al., 2016; McConathey et al., 2017; Tsapkini et al., 2014). While many studies in PPA often use a small number of patients, a recent meta-analysis determined that noninvasive brain stimulation does offer a benefit that surpasses the benefit of language therapy alone in PPA (Nissim et al., 2020).
Several studies have shown that tDCS can add to the benefit of behavioral naming therapy alone in individuals with PPA. For example, in a sham-controlled study of individuals with nfvPPA who received naming therapy paired with anodal stimulation over left dorsolateral prefrontal cortex, Cotelli et al. (2014) found a significantly greater improvement in naming of trained items at 12 weeks posttherapy conclusion in individuals who received real stimulation compared to a group with sham stimulation. Functional communication also improved in the tDCS group but not the sham-controlled group. Another research group has conducted several sham-controlled studies investigating written naming and spelling therapy paired with anodal tDCS over left inferior frontal gyrus (IFG) in PPA (de Aguiar, Zhao, Faria, et al., 2020; de Aguiar, Zhao, Ficek, et al., 2020; Fenner et al., 2019; Ficek et al., 2018; Tsapkini et al., 2014, 2018). In several studies, they found that tDCS had the advantage of producing longer term treatment gains and enhancing generalization to untrained items compared to sham stimulation (de Aguiar, Zhao, Faria, et al., 2020; de Aguiar, Zhao, Ficek, et al., 2020; Fenner et al., 2019; Tsapkini et al., 2014, 2018).
Most naming treatment studies have focused on noun naming (Tippett et al., 2015), and only one study of which we are aware has investigated treatment of verb naming in individuals with PPA (Fenner et al., 2019). Fenner et al. (2019) investigated oral and written verb naming + spelling therapy in individuals with lvPPA and nfvPPA using a sham-controlled crossover design. In this study, participants were given anodal tDCS to left IFG paired with written verb naming + spelling therapy in one phase, and sham stimulation paired with the same therapy in the other phase. Production of word-level written verb naming improved significantly more in the tDCS compared to the sham condition. Similar to studies of written noun naming (de Aguiar, Zhao, Faria, et al., 2020; de Aguiar, Zhao, Ficek, et al., 2020; Tsapkini et al., 2014, 2018), the effects induced by tDCS generalized to naming of untrained items and were long lasting as they remained significant at 8 weeks posttreatment. Of note, the treatment in Fenner et al. (2019) was at the word level and did not target the role of the verb with its arguments in a sentence. Recently, in a behavioral treatment study, Meyer et al. (2020) found that grammatical ability was a significant predictor of verb naming impairment in a group of individuals with PPA encompassing all three variants and recommended future research investigate treatments that focus on retrieving verbs and their arguments, such as VNeST.
This Study
This study paired tDCS with VNeST, which targets verbs at the phrase- and sentence-level. We used a randomized, double-blind, sham-controlled, within-subject crossover design to investigate the efficacy of VNeST therapy paired with tDCS (VNeST+tDCS) compared to VNeST therapy paired with sham stimulation (VNeST+sham) in one participant with lvPPA and two participants with nfvPPA. Each participant served as their own control and received VNeST therapy augmented with anodal stimulation of left IFG in one phase and VNeST therapy augmented with sham-stimulation in the other phase. Left IFG was chosen as the stimulation site because it is a vital component of the language network that is associated with language production (Amunts et al., 2004; Costafreda et al., 2006; Grande et al., 2012; Grodzinsky, 2000; Hagoort, 2014; Hickok & Poeppel, 2007; Horwitz et al., 2003; Schnur et al., 2009), and is particularly involved in verb naming (Crescentini et al., 2010; den Ouden et al., 2009; Havas et al., 2015; Martin & Cheng, 2006; Thompson-Schill et al., 1998). Furthermore, anodal tDCS stimulation of left IFG has been found to enhance verb learning in neurologically healthy populations (Fiori et al., 2018), increase verb and sentence naming in poststroke aphasia (Marangolo et al., 2013), and improve written verb production in individuals with PPA (Fenner et al., 2019).
This study had several aims: (a) determine whether anodal tDCS stimulation of left IFG paired with VNeST therapy (VNeST+tDCS) will improve verb naming performance of trained verbs above and beyond the effect of VNeST paired with sham stimulation (VNeST+sham) at 1 week posttreatment, (b) evaluate whether naming improvement of trained verbs will last longer after VNeST+tDCS treatment compared to VNeST+sham treatment as measured at 8 weeks posttreatment, (c) assess whether VNeST+tDCS will induce greater generalization to naming of untrained verbs and nouns and to sentence production and comprehension relative to VNeST+sham, and (d) determine whether generalization effects would be longer lasting in the VNeST+tDCS condition. We hypothesized that VNeST+tDCS would result in greater immediate (1 week posttreatment) improvements in trained verbs, as well as generalization to untrained items (nouns, verbs, sentence production, and sentence comprehension) compared to the VNeST+sham condition. We also hypothesized that VNeST+tDCS would result in longer lasting (8 weeks posttreatment) improvements to naming trained verbs and generalization naming of untrained verbs, nouns, sentence production and sentence comprehension.
Method
Participants
In order to be eligible to participate in the study, participants had to be adults with a diagnosis of PPA and presence of naming deficits. Naming deficits were indicated by picture naming ability below 80% accuracy on the Object and Action Naming Battery (OANB; Druks & Masterson, 2000). Participants were required to have at least 10 years of education, be a native English speaker, have normal or corrected-to-normal vision and hearing, and be medically stable. Exclusion criteria included a history of neurological or psychiatric disorder affecting the brain or behavior besides PPA, a history of significant drug or alcohol abuse, a history of seizures in the last 12 months, and history of brain surgery or metal in the head. Participants were also excluded if they were taking medications that substantially lower the seizure threshold (e.g., methylphenidate) or N-methyl-D-aspartate (2) antagonists, which can reduce the effects of tDCS. Participants were also required to pass a screening that consisted of walking the participant through each VNeST step for a sample verb to ensure they were able to successfully engage in VNeST therapy. One potential participant did not pass this screening step and did not enroll in the study. Also, two participants with semantic PPA enrolled in the study but did not complete both phases of treatment. One participant dropped out due to increasing behavioral difficulties that are often a concern in individuals with more severe PPA. The other participant dropped out due to medical concerns unrelated to PPA and increasing difficulty following the treatment protocol due to language decline. The data from both of the participants who did not complete the study are not included in this study.
This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. All participants, or their legally authorized representative, provided informed consent to participate.
Participant Case Histories
Participant 1 was a 73-year-old woman with a master's degree who retired from a career as an elementary school teacher prior to symptom onset. Between 5 and 10 years prior to this study, she started noticing word retrieval difficulties, and she was formally diagnosed with lvPPA by a neurologist 6 months prior to enrolling in this study. Her MRI revealed global atrophy that was most pronounced in bilateral medial temporal and parietal lobes. She resided with her husband and was independent for some activities of daily living, including basic hygiene, dressing, and feeding herself. However, she relied on her husband for driving, cooking, medication management, financial management, and managing appointments. Difficulty with activities of daily living was partially due to her language impairments and partially due to the evolution of her dementia to a more generalized dementia typical of Alzheimer's disease. In spite of word finding issues, she was able to express herself effectively to communicate her wants and needs. She demonstrated particular difficulty with word retrieval (6/60 on the Boston Naming Test [BNT]; Kaplan et al., 2001) and sentence repetition (3/5 on the sentence repetition task); see Table 1 for additional baseline testing information. Throughout her participation in the study, she also received concurrent speech language therapy (one session every other week) primarily focused on improving/maintaining memory and implementing strategies to improve activities of daily living. She did not receive VNeST therapy or therapy for word finding while participating in this study.
Table 1.
Participant demographic and baseline testing information.
| Participant | PPA subtype | Sex | Education (years) | Postsymptom onset (years) | Stimulation order | tDCS stimulation dose | Baseline testing |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WAB-R (AQ) | BNT | PPT: short form (%) | KD: short form (%) | SR (no. of correct sentences) | SOAP: canonical (%) | SOAP: noncanonical (%) | ABA-2 (apraxia severity) | |||||||
| Participant 1 | Logopenic | F | 18 | 5–10 | tDCS first | 1 mA | 73 | 6 | 100% | 100% | 3 | 75% | 60% | Not assessed |
| Participant 2 | Nonfluent | F | 16 | 5 | Sham first | 2 mA | 61 | 50 | 100% | 100% | 0 | 95% | 60% | Moderate-to-severe |
| Participant 3 | Nonfluent | F | 14 | 7–9 | Sham first | 1 mA | 43.7 | 39 | 100% | 80% | 0 | 55% | 60% | Severe |
Note. PPA = primary progressive aphasia; tDCS = transcranial direct current stimulation; WAB-R = Western Aphasia Battery–Revised (Kertesz, 2007); AQ = Aphasia Quotient from the WAB-R; BNT = Boston Naming Test (Kaplan et al., 2001); PPT = Pyramids & Palm Trees Test Short Form (Breining et al., 2015; Howard & Patterson, 1992); KD = Kissing & Dancing Test Short Form (Bak & Hodges, 2003); SR = Sentence Repetition test from the National Alzheimer's Coordinating Center's Frontotemporal Dementia Battery, from the National Institute on Aging; SOAP = Subject-Relative, Object-Relative, Active, and Passive test of sentence comprehension (Love & Oster, 2002); ABA-2 = Apraxia Battery for Adults–Second Edition (Dabul, 2000).
Participant 2 was a 56-year-old woman with a bachelor's degree. She first noticed difficulties articulating speech about 5 years prior to enrollment. She was diagnosed with nfvPPA by a neurologist 3.5 years before enrolling in this study and was forced to retire from her career as a financial planner at that time due to difficulties associated with PPA. She resided with her partner and was independent for activities of daily living. Participant 2 used to be an avid reader, but at the time of study participation primarily listened to audio books due to difficulties with reading comprehension. She presented with moderate-to-severe AOS, as confirmed by a speech-language pathologist (E.B.G.) using the Apraxia Battery for Adults–Second Edition (Dabul, 2000), and primarily communicated through writing, e-mailing, or texting with her mobile phone. Note that apraxia was assessed at baseline and at the last testing time point (8 weeks after the conclusion of Phase 2 therapy), and apraxia severity did not change during the course of the study. Her spoken and written output was agrammatic. She had some word finding difficulties for objects (50/60 on the BNT), but had particular difficulty comprehending grammatically complex sentences (60% accuracy comprehending noncanonical sentences versus 95% accuracy for canonical sentences on the Subject-Relative, Object-Relative, Active, and Passive test of sentence comprehension (SOAP; Love & Oster, 2002; see Table 1 for additional baseline testing). Due to severe claustrophobia no clinical or research neuroimaging was available. Participant 2 received speech therapy primarily to treat symptoms of AOS for several months shortly after her PPA diagnosis, but stopped therapy as she did not find it beneficial. She did not receive any speech therapy during the course of this study.
Participant 3 was a 70-year-old woman with an associate's degree, who previously held positions in several fields including financial management and inventory control. She retired prior to experiencing any PPA symptoms. After retirement, she regularly provided child care for her four grandchildren, but due to PPA associated difficulties, she stopped providing care 5 years prior to her enrollment in the study. She started noticing difficulties with speech articulation 7–9 years prior to her study enrollment and was initially diagnosed by a neurologist with suspected primary progressive apraxia of speech (PPAOS) 7 years before the study started. Subsequent language testing by speech-language pathologists in the years after her initial suspected diagnoses of PPAOS documented significant decline in overall language skills, particularly in the areas of word finding, written expressive language, and receptive language, which indicated her disorder encompassed progressive worsening of both AOS and language skills. After completing initial baseline testing as part of this study, a neurologist with expertise in diagnosing PPA (A.E.H.) confirmed a diagnosis of nfvPPA with severe AOS. Apraxia was additionally assessed during baseline testing and during the last testing time point in the current study using the Apraxia Battery for Adult–Second Edition (Dabul, 2000). AOS severity remained severe and did not change during the course of the study. Clinical neuroimaging acquired 3–5 years postsymptom onset revealed greater left than right atrophy apparent in left IFR. Several years prior to study enrollment, she received speech-language therapy focusing on her language deficits and training to use an augmentative and alternative communication device (iPad) to communicate. She did not use the iPad consistently, and she communicated primarily using single-word spoken utterances, with markedly reduce intelligibility, and writing via pen and paper (1–2 words). Written output demonstrated spelling errors such as grapheme substitutions, perseverations, and omissions, which negatively impacted her communication effectiveness. She lived with her husband and also had support from her two grown children. Participant 3 was able to perform some activities of daily living independently or with supervision, such as cooking, caring for personal hygiene, dressing, and medication management; she relied on her husband for activities such as driving, financial management, and maintaining appointments. She required frequent supervision on a day-to-day basis. Participant 3 baseline language testing (see Table 1) for this study revealed significant word finding difficulties (39/60 on the BNT) and auditory sentence comprehension deficits across both canonical (55% on the SOAP test; Love & Oster, 2002) and noncanonical sentences (60% on the SOAP test). She did not participate in speech therapy during the course of this study.
Study Design
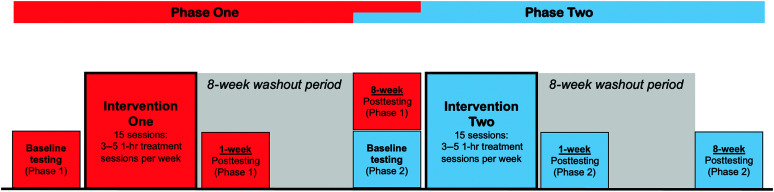
A randomized, double-blind, sham-controlled, within-subject crossover design was used in this study. The crossover design was used to facilitate recruitment, and to reduce individual variability, which is an important consideration as there is inherent heterogeneity in the PPA population. Participants were randomly assigned to receive either the tDCS intervention period followed by the sham intervention period or sham followed by tDCS. Participants received 15 VNeST training sessions within the tDCS intervention period and 15 VNeST sessions within the sham intervention period (see Figure 1). Each session lasted for 1 hr, and participants received 3–5 sessions per week depending on their personal preference and availability. Each treatment phase was followed by an 8-week washout period. The washout period was designed to minimize potential carryover effects from Phase 1 to Phase 2 treatment. Language was evaluated before therapy, 1 week after therapy, and 8 weeks after therapy for each intervention period; the 8-week posttesting assessment for Phase 1 also served as the Phase 2 baseline assessment (see Figure 1).
Figure 1.
Study design. Components of Phase 1 assessment and treatment are depicted in red, and components of Phase 2 assessment and treatment are depicted in blue.
Language Testing
Initial baseline testing included the Western Aphasia Battery–Revised (WAB-R; Kertesz, 2007) to measure overall language impairment severity, and the BNT (Kaplan et al., 2001) to assess object naming. The Pyramids & Palm Trees Test Short Form (Breining et al., 2015; Howard & Patterson, 1992) and a short form of the Kissing and Dancing test (Bak & Hodges, 2003) were administered to assess semantic knowledge of objects and actions, respectively. The SOAP test (Love & Oster, 2002) was given to assess auditory sentence comprehension of active, passive, subject-relative, and object-relative sentences. Baseline testing results for each participant are provided in Table 1. As this was an initial pilot study with limited resources, note that we did not collect multiple baselines to establish a stable baseline. We also did not collect naming probe data as therapy was unfolding during each treatment phase. Future iterations of this study will collect multiple baselines and probes of verb naming.
Testing at each time point was also administered for all primary and secondary outcome measures, and are reported in Table 2. The primary outcome measure in this study was the accuracy of naming 20 trained verbs on the OANB (Druks & Masterson, 2000). The OANB consists of line drawings of 162 objects and 100 actions. We were also interested in investigating generalization to naming of untrained verbs and nouns and generalization to sentence production and comprehension. Therefore, we had several secondary outcome variables. Secondary outcome variables included naming 80 untrained verbs and 162 nouns on the OANB (all untrained items from the OANB). Trained and untrained verbs were matched on number of phonemes, age of acquisition, imageability, and visual complexity (independent samples t tests all p > .05). However, independent samples t tests indicated trained verbs had fewer syllables (p = .014), were more familiar (p = .038), and were more frequent (p = .036) than untrained verbs.
Table 2.
Baseline and two posttreatment scores for naming of trained verbs, untrained verbs, and nouns.
| Assessment | Participant 1 |
Participant 2 |
Participant 3 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tDCS (Phase 1) |
Sham (Phase 2) |
tDCS (Phase 2) |
Sham (Phase 1) |
tDCS (Phase 2) |
Sham (Phase 1) |
||||||||||||||
| Baseline | 1 week post | 8 weeks post | Baseline | 1 week post | 8 weeks post | Baseline | 1 week post | 8 weeks post | Baseline | 1 week post | 8 weeks post | Baseline | 1 week post | 8 weeks post | Baseline | 1 week post | 8 weeks post | ||
| OANB | |||||||||||||||||||
| Trained Verbs (%) (20 items) |
55% | 50% | 60% | 60% | 65% | 50% | 45% | 70% | 55% | 65% | 70% | 45% | 40% | 45% | 20% | 35% | 30% | 40% | |
| Untrained Verbs (%) (80 items) |
41% | 36% | 46% | 46% | 35% | 35% | 40% | 66% | 60% | 64% | 66% | 40% | 25% | 43% | 41% | 35% | 34% | 25% | |
| Nouns (%) (162 items) |
28% | 27% | 27% | 27% | 27% | 21% | 67% | 77% | 44% | 60% | 77% | 67% | 37% | 35% | 32% | 44% | 50% | 37% | |
| NAVS | |||||||||||||||||||
| Sentence Production Priming Test (30 items) |
40% | 37% | 47% | 47% | 40% | 20% | 13% | 37% | 13% | 30% | 17% | 13% | 0% | 7% | 0% | 0% | 7% | 0% | |
| Sentence Comprehension Test (30 items) |
73% | 93% | 90% | 90% | 87% | 87% | 57% | 80% | 70% | 73% | 57% | 57% | 43% | 63% | 70% | 50% | 57% | 43% | |
Note. tDCS = transcranial direct current stimulation; OANB = Object and Action Naming Battery (Druks & Masterson, 2000); NAVS = Northwestern Assessment of Verbs and Sentences (Thompson, 2012).
Secondary outcome variables also included total accuracy producing sentences on the Sentence Priming Production Test and comprehending sentences on the Sentence Comprehension Test using target syntactic forms (i.e., active, passive, subject-relative, object-relative, subject Wh-questions, and object Wh-questions) from the Northwestern Assessment of Verbs and Sentences (NAVS; Thompson, 2012). For the Sentence Priming Production Test, the experimenter shows the participant pairs of pictures and describes the first picture using either an active, passive, subject Wh-question, object Wh-question, subject-relative, or object-relative sentence. Then the experimenter asks the participant to use the same type of sentence to describe the second picture in the pair. There are five items for each sentence-type resulting in 30 total items. The Sentence Comprehension Test also consists of 30 items testing comprehension of active, passive, subject Wh-question, object Wh-question, subject-relative, and object-relative sentences. The experimenter shows the participant a pair of pictures and reads a sentence aloud. The participant is asked to point to the picture that describes the sentence. Scoring of the OANB and the NAVS was completed according to the instructions in each testing manual, but one phonological error per item was acceptable. Two authors, S.M.S. and E.B.G., completed all scoring while blinded to whether participants were completing tDCS or sham phases. Interrater reliability for the OANB and the NAVS Sentence Production Priming Test was determined for the two raters using Cohen's kappa coefficients. Interrater reliability was high as indicated by a Cohen's kappa of 0.873 for the OANB, and a Cohen's kappa of 0.910 for the NAVS Sentence Production Priming Test.
VNeST Language Therapy
A slightly modified version of VNeST was used in this study as described below to target 20 verbs. The 20 verbs were all transitive verbs appropriate for VNeST chosen from verbs that were included on the OANB. Using the same 20 verbs for all participants allowed us to control for item variability. Moreover, in PPA, maintenance of items named correctly at baseline and recovery of items named incorrectly at baseline are both important treatment goals. VNeST follows several steps (see Edmonds, 2016, and Edmonds et al., 2014, for details). All of the materials were presented on a computer monitor positioned in front of the participant, and the clinician typed participants' responses on the screen. First, the participant was asked, “Who can/might (target verb) something?” as they were presented with a screen with Who (target verb) What at the top of the screen. They were asked to produce an agent (the doer of the action) and the patient/theme (the receiver of the action). If they could not provide an appropriate agent, they were presented with three multiple choice options on the screen. Once an agent was produced/selected, they were asked to produce a patient, and if they could not produce an appropriate patient for the agent they selected, they were given three multiple choice options on the screen. Once participants produced three appropriate triads for the target verb, the participant was asked to read each triad aloud. Each participant had the option of adding morphology and inflection (e.g., “The child rides the bike” for the triad: the child–ride–the bike) when reading the triad aloud, but in VNeST accurate syntax is not a requirement for moving to the next treatment step. Next, the participant chose one triad to expand on by answering three Wh-questions (where? [at the park], when? [after school], why? [to get to his friend's house]). If the participant did not understand the Wh-question, the clinician provided clarification regarding the prompt (e.g., Where? What location or places?). Once the expanded sentence was produced, they were next asked to produce the target verb. In the final step, the participant was encouraged to generate five sentences using appropriate agents and patients for the target verb. Throughout each step, the clinician provided support and cueing when appropriate as per the VNeST protocol (Edmonds, 2016; Edmonds et al., 2014). Unlike studies in poststroke aphasia, this modified VNeST protocol did not require participants to decide whether semantic judgment sentences were correct or not (e.g., deciding whether a sentence like “the baby washed the car” has a correct/appropriate agent or patient). We also required participants to generate five sentences in the final step rather than three to four agent–patient pairs. We increased the number of sentences produced in the final step because our goal was to actively combat ongoing neurodegeneration, and increasing the number of productions would result in a greater chance of maintaining, and possibly improving, language skills.
tDCS Parameters
During the tDCS phase, all participants received tDCS using a constant current stimulator (Soterix Medical 1 × 1 clinical trials device) for the first 20 min of the session while VNeST was being simultaneously provided. Stimulation was delivered by the same researcher who also led VNeST therapy in that session (either S.M.S. or E.B.G.). VNeST was initiated as soon as stimulation began and continued to the end of the 1-hr session after stimulation ceased. This ensured the participants benefitted from tDCS throughout the session, as tDCS effects can last over an hour after the conclusion of a 20-min stimulation period (Nitsche & Paulus, 2001). TDCS was delivered using 5 × 5 cm electrodes at 1 mA for Participant 1 and Participant 3, and at 2 mA for Participant 2. Participant 2 received a different stimulation intensity due to a change in study protocol, which was motivated by the promising results obtained by Fenner et al. (2019), who stimulated left IFG with a stimulation intensity of 2 mA in individuals with PPA to improve written verb naming. For all participants, the anode was placed over the left IFG, localized using the F7 coordinate of the international 10–20 system (Homan, 1988), and the cathode on the right shoulder.
Randomization and Blinding
All participants and members of the research team who interacted with participants and scored assessments were blinded to the order in which each participant received tDCS versus sham stimulation. Blinding was achieved by inputting a 6-digit blinded code for initiation of stimulation. As mentioned previously, since most participants only feel the effects of active tDCS on the scalp for the first 30 s of stimulation, an effective sham condition can be created by applying stimulation to the scalp for 30 s and then gradually decreasing stimulation over a period of 15 s (Gandiga et al., 2006). The order that participants received sham versus tDCS stimulation was randomized. Participant 1 received tDCS stimulation in Phase 1 and sham stimulation in Phase 2, whereas Participants 2 and 3 both received sham stimulation in Phase 1 and tDCS in Phase 2 (see Table 1). At the end of each treatment phase, participants, and clinicians were asked to guess whether the participant had received tDCS or sham stimulation during that phase. They were also asked to rate the confidence of their guess on a 5-point Likert scale (5 = extremely confident, 4 = considerably confident, 3 = moderately confident, 2 = slightly confident, 1 = not at all confident). Mean accuracy and confidence ratings were calculated.
Data Analysis
We implemented an analysis approach used in previous tDCS language treatment studies (de Aguiar, Zhao, Faria, et al., 2020; Sebastian et al., 2020; Tsapkini et al., 2018) where we compared change in naming performance between baseline for that phase and each follow-up testing time point. This approach allows us to control for any carry over effects from Phase 1 to Phase 2, and eliminate the possibility that a potential tDCS advantage in Phase 1 would affect the results of Phase 2. Specifically, we compared change in naming performance on trained verbs, untrained verbs, and nouns, which were all measured using the OANB (Druks & Masterson, 2000). We also measured change in sentence production and sentence comprehension using subtests of the NAVS (Thompson, 2012). We compared performance for each phase separately using the Cochran's Q test, which can be used to determine if there are differences on a dichotomous dependent variable across multiple time points. Follow-up pairwise comparisons between the baseline and each follow-up time point (1 week posttreatment and 8 weeks posttreatment) using McNemar χ2 tests, which allows for the investigation of differences on a dichotomous dependent variable between two time points. Additionally, the changes in naming accuracy between the tDCS and the sham phases were compared using Wilcoxon signed-ranks tests. This was accomplished by calculating the change in naming accuracy from baseline to 1 week posttreatment (1 week posttreatment–baseline) and from baseline to 8 weeks posttreatment (8 weeks posttreatment–baseline) and comparing the changes obtained at both the 1 week and 8 weeks posttreatment time points between sham and tDCS. p values were corrected for multiple comparisons using False Discovery Rate correction.
Adverse Effects
Participants were assessed at the end of each treatment session for potential pain and discomfort using the Wong–Baker FACES Pain Rating Scale (Wong & Baker, 1988). We also asked participants if they experienced any discomfort such as tingling or irritation on the scalp at the end of each treatment session. Participants reported no adverse effects.
Results
Integrity of Blinding
To assess whether participants and clinicians were effectively blinded to whether participants were receiving tDCS or sham stimulation in each treatment phase, each participant and clinician was asked to guess the stimulation type at the end of each phase. Each participant and clinician also rated the confidence of their guess on a 5-point Likert scale ranging from 5 (extremely confident) to 1 (not at all confident). Participant guessing accuracy was 66.7% accurate with a mean confidence rating of 2.3. Clinician guessing accuracy was 33.3% accurate with a mean confidence rating of 2.0. These results indicate participants' guessing accuracy was slightly above chance but with a relatively low confidence rating, and clinician accuracy was below chance also with a low confidence rating.
Participant 1 (lvPPA)
Participant 1 was able to successfully engage in all steps of the VNeST protocol during each of the 30 therapy sessions. She enjoyed coming to therapy, and she and her spouse reported her mood was improved while participating in therapy. However, the amount of cueing required remained the same as therapy progressed. Typical cueing required providing semantic/context cues (e.g., “Who might write for their job?”) as well as providing options for agents and patients by presenting the participant with three options with one plausible choice in the set of three. Also, she and her husband did not report that they noticed significant word finding improvement or decline while participating in the study.
Naming
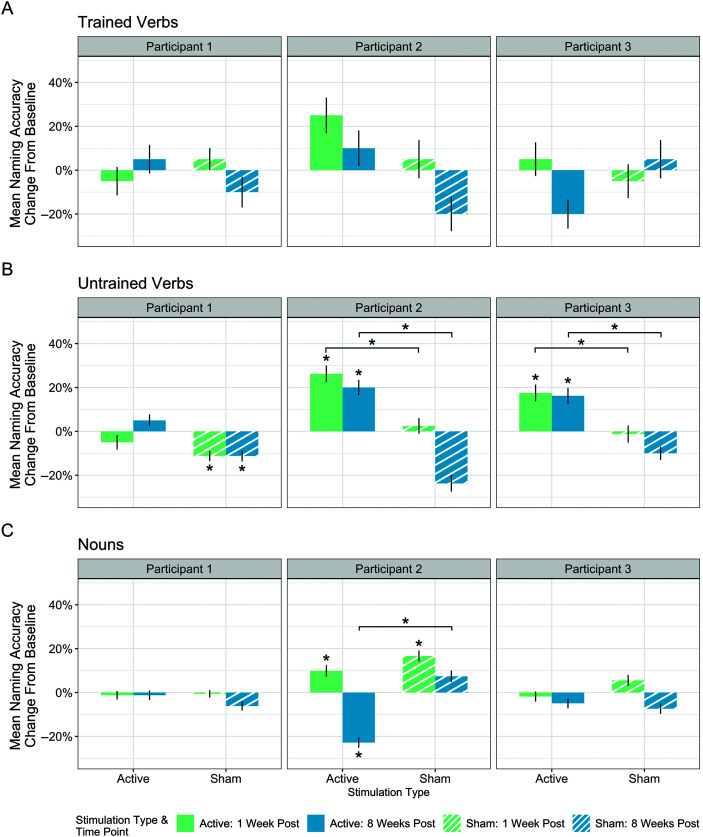
During the tDCS phase (Phase 1), Participant 1's naming of trained verbs (primary outcome measure), untrained verbs, and nouns was maintained without significant change between baseline and either 1 week or 8 weeks posttreatment (see Figure 2A–2C). There were also no significant differences in naming of trained verbs or nouns during the sham phase (Phase 2). However, she showed a significant decline in naming of untrained verbs during the sham phase, χ2(2) = 8.1, p = .017 only (see Figure 2B). Pairwise comparisons revealed a significant decline between baseline and 1 week posttreatment (Z = 2.71, p = .019) and between baseline and 8 weeks posttreatment (Z = 2.50, p = .019) of untrained verbs with sham. Comparisons of the change in naming between the sham and tDCS phase using the Wilcoxon signed-ranks test revealed no significant differences in naming accuracy change for trained verbs, untrained verbs, or nouns between the sham and tDCS phases for either time point.
Figure 2.
Mean change in naming accuracy between baseline and two follow-up time points for active and sham stimulation for each participant. Results shown for (A) trained verbs, (B) untrained verbs, and (C) nouns. Change above 0% indicates improvement from baseline and change below 0% indicates decline from baseline. A line at 0% change indicates maintenance between baseline at that follow-up time point. Asterisks immediately above bars indicate significant change from baseline at that time point. Brackets indicate a significant difference between sham and baseline at that time point. Vertical error bars indicate standard error.
Sentence Production and Comprehension
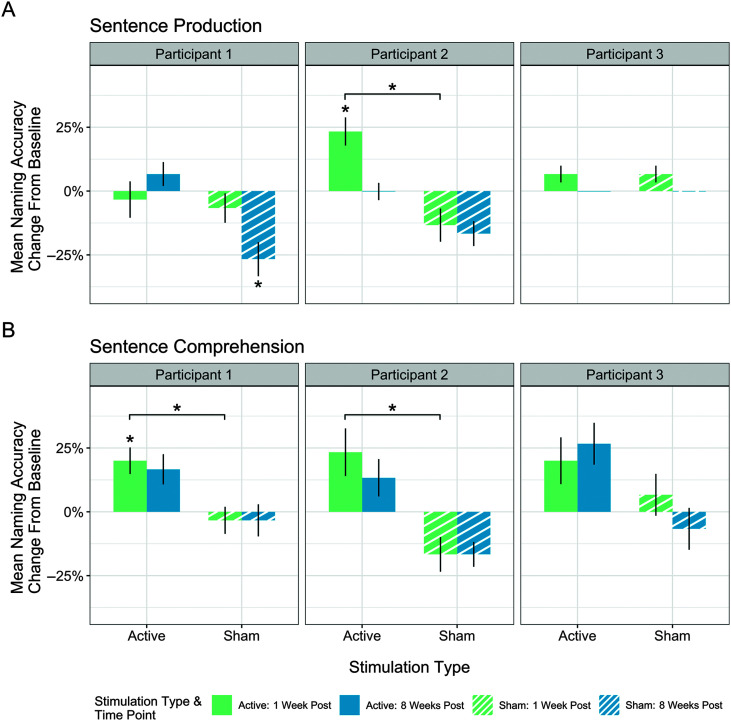
In the tDCS phase, sentence production was maintained between baseline and both follow-up time points, with no significant decline or improvement (see Figure 3A). Sentence comprehension however changed, χ2(2) = 6.89, p = .032. Pairwise comparisons revealed a significant improvement in sentence comprehension between baseline and 1 week follow-up testing (Z = −2.45, p = .043), but not between baseline and 8 weeks posttreatment (see Figure 3B).
Figure 3.
Mean change in sentence production and comprehension accuracy between baseline and two follow-up time points for active and sham stimulation for each participant. Results shown for (A) sentence comprehension and (B) sentence production. Change above 0% indicates improvement from baseline and change below 0% indicates decline from baseline. A line at 0% change indicates maintenance between baseline at that follow-up time point. Asterisks immediately above bars indicate significant change from baseline at that time point. Brackets indicate a significant difference between sham and baseline at that time point. Vertical error bars indicate standard error.
During the sham phase, Participant 1's sentence production significantly declined, χ2(2) = 8.67, p = .013, between baseline and 8 weeks posttreatment (Z = 2.53, p = .034; see Figure 3A). Sentence comprehension was maintained across both follow-up testing time points during the sham phase (see Figure 3B).
Comparisons of sentence production and comprehension accuracy between the tDCS and sham phases revealed no significant differences for sentence production at either follow-up time point. A significant tDCS advantage was revealed for sentence comprehension between baseline and the 1 week posttreatment time point (Z = −2.27, p = .047).
Participant 2 (nvPPA)
Participant 2 was also able to successfully engage in all VNeST steps throughout the study. Modeling and cueing required during therapy sessions reduced as therapy progressed and response diversity improved during each phase. She reported word finding improvements and reported her written sentences were more grammatically accurate in her daily life outside of therapy sessions. She enjoyed participating in therapy, and her partner reported that her mood improved while participating in the study.
Naming
Participant 2's results from the tDCS phase (Phase 2) revealed that naming of trained verbs (primary outcome measure) was maintained with no significant improvement or decline. A significant improvement in naming of untrained verbs, χ2(2) = 17.61, p < .001, was found between baseline and both follow-up time points (1 week: Z = −3.66, p < .001; 8 weeks: Z = −3.14, p = .003; see Figure 2B). Results also revealed a significant difference in noun naming across time points, χ2(2) = 48.20, p < .001; see Figure 2C. Specifically, there was a significant improvement in naming nouns between baseline and 1 week posttreatment (Z = −2.18, p = .03), but a significant decline between baseline and 8 weeks posttreatment (Z = 5.08, p < .001).
The results from the sham phase (Phase 1) revealed no significant changes for the naming of trained verbs (see Figure 2A); however, there was a decline in naming of untrained verbs, χ2(2) = 19.66, p < .001; see Figure 2B). Pairwise comparisons of untrained verb naming demonstrated no decline between baseline and 1 week posttreatment, and a significant decline between baseline and 8 weeks posttreatment (Z = 3.41, p < .001). The only improvement Participant 2 experienced during the sham phase was improved noun naming, χ2(2) = 14.44, p < .001, which was significant from baseline to 1 week posttreatment (Z = −3.71, p = .001) but not between baseline and 8 weeks posttreatment (see Figure 2C).
Analyses comparing change in naming accuracy in the sham versus tDCS phases revealed no significant differences at either time point for trained verbs. There was a tDCS advantage for change in naming of untrained verbs both at 1 week posttreatment (Z = − 2.50, p = .012) and 8 weeks posttreatment (Z = −3.75, p < .001). Results also revealed a significant decrease in naming of nouns in the tDCS versus sham phases at the 8-week posttreatment time point (Z = −3.94, p < .001).
Sentence Production and Comprehension
For the tDCS phase, results revealed a significant difference in sentence production accuracy between time points, χ2(2) = 12.25, p = .002, and pairwise comparisons indicated a significant improvement between baseline and 1 week posttreatment (Z = −2.65, p = .012; see Figure 3A). Sentence comprehension skills were maintained during the tDCS phase with no significant improvement or decline (see Figure 3B). In the sham phase, Participant 2's sentence production and sentence comprehension skills were maintained across baseline and follow-up time points.
The comparison of mean change in sentence production and comprehension accuracy between tDCS and sham phases revealed a significant tDCS advantage for sentence production at 1 week posttreatment (Z = −2.53, p = .023), with no tDCS advantage at the 8-week follow-up time point (see Figure 3A). A tDCS advantage was also revealed for sentence comprehension at 1 week follow-up testing (Z = −2.33, p = .039; see Figure 3B).
Participant 3 (nvPPA)
Similar to Participants 1 and 2, Participant 3 was also able to successfully complete all VNeST steps during all 30 therapy sessions. The amount of cueing required did not lessen during the course of the study. Response diversity did not noticeably improve during each phase as she often recycled the same agent–verb–patient combinations. However, she became familiar with VNeST steps after several sessions and was able to anticipate upcoming steps in the protocol. Her husband reported she was happier and more engaged with her family while participating in the study. He also believed participating in the study helped stave off decline as she declined quite rapidly approximately 6 months after the final testing time point.
Naming
In the tDCS phase (Phase 2), Participant 3's naming of trained verbs (primary outcome measure) and nouns was maintained without significant decline or improvement. However, her naming of untrained verbs improved, χ2(2) = 8.51, p = .014), between baseline and both follow-up time points (1 week: Z = −2.56, p = .019; 8 weeks: Z = −2.50, p = .019).
During the sham phase (Phase 1) naming of trained verbs, untrained verbs, and nouns was maintained. There was no significant improvement or decline in naming.
Analyses comparing change in naming at 1 week and 8 weeks posttreatment in the tDCS versus sham phases revealed no differences for trained verbs or nouns. However, the tDCS phase yielded significantly more positive change at both time points (1 week: Z = −2.09, p = .037; 8 weeks: Z = −2.68, p = .015) for naming of untrained verbs compared to the sham phase.
Sentence Production and Comprehension
Analyses of the tDCS phase did not reveal any significant difference between baseline testing and follow-up testing time points for sentence production or comprehension (see Figure 3). Participant 3 also did not have any significant decline or improvement of sentence production or comprehension during the sham phase. The comparison of tDCS and sham phases at each follow-up time point did not reveal any significant differences between the tDCS and sham phases for sentence production or comprehension (see Figure 3).
Discussion
In this case series treatment study, we investigated treatment efficacy of VNeST to treat naming paired with tDCS versus sham stimulation in three individuals with PPA. Unlike in the case of poststroke aphasia, in PPA, maintenance of baseline performance can indicate an encouraging outcome since it is a neurodegenerative condition, and naming is expected to decline over time. Any gains in performance are especially encouraging as it indicates positive change in the face of neurodegeneration. Recall this study had several aims. For our first aim, we hypothesized that the addition of tDCS would induce greater improvement in naming of trained verbs. For our second aim, we hypothesized any improvement to trained verbs would last longer in the tDCS phase. However, we found no evidence of a tDCS advantage for trained verbs at any time point. All three of our participants were at least 5 years postsymptom onset and were no longer in the early stages of PPA. It is possible we would have seen improvement in trained verbs in individuals in the earlier stages of PPA, as patients in the later stages of PPA are likely to have more atrophy. Atrophy may ultimately reach a point where the neural network is so severely degraded it cannot be enhanced by tDCS, so the benefits of tDCS may decline over time in PPA. Future studies will investigate the effect of disease progression on treatment effects.
For our third aim, we predicted tDCS would induce greater generalization to naming of untrained items and production and comprehension of sentences not targeted in therapy. We found a clear tDCS advantage to untrained verb naming where naming improved in Participants 2 and 3. There was also a slight tDCS advantage for untrained verb naming for Participant 3 as naming was maintained in the tDCS phase, but declined in the sham phase.
For our fourth aim, we predicted we would find evidence of longer lasting generalization in the tDCS condition as measured at the 8-week posttreatment follow time point. We did find that generalization effects were still present at the final time point for untrained verb naming in all three participants. The pattern of results varied between each patient. First, for Participant 1 with logopenic PPA, VNeST+sham was accompanied by decline of naming untrained verbs at both follow-up time points, whereas VNeST+tDCS resulted in maintenance of untrained verbs. There was no evidence of a significant difference between tDCS and sham phases with regards to mean change of naming accuracy, but she only experienced decline in the sham phase, which indicates a slight tDCS advantage. Her results also revealed a slight tDCS advantage for generalization of VNeST treatment to sentence production and comprehension. Similar to untrained verb naming, the VNeST+sham phase yielded significant decline of sentence production at the 8-week follow-up time point, while VNeST+tDCS resulted in maintenance. Comparisons of accuracy change between sham and tDCS phases; however, it did not reveal significant differences. Thus, for Participant 1, one of the primary benefits of tDCS was the maintenance of untrained verb naming and sentence production that were lost in the sham phase. A clear tDCS advantage was found for sentence comprehension at the 1 week follow-up time point, where the VNeST+tDCS showed improved comprehension and the VNeST+sham showed decline.
Of the three participants, Participant 2 experienced the most evident tDCS advantage. First, untrained verb naming improved between baseline and both follow-up time points during the VNeST+tDCS phase, whereas naming declined during the VNeST+sham phase at the 8-week time point. She also had improved sentence production at the 1 week follow-up time point during VNeST+tDCS, whereas sentence production was simply maintained in VNeST+sham. Finally, sentence comprehension showed significantly greater improvement in the VNeST+tDCS versus VNeST+sham phases at 1-week posttreatment. Collectively, Participant 2's results show that treatment effects generalized to untrained verbs, sentence production, and sentence comprehension. The generalization to untrained verbs was particularly long lasting as it was still evident 2 months after treatment concluded. However, the tDCS advantage did not extend to noun naming; rather noun naming was significantly worse in the VNeST+tDCS relative to the VNeST+sham phase at 8 weeks posttreatment. It should be mentioned that the tDCS phase for Participant 2 occurred during Phase 2 of the study, which is notable because the baseline testing for Phase 1 occurred approximately three months prior to baseline testing for Phase 2. The testing results presented in Table 2 demonstrate that Participant 2's baseline testing during Phase 2 for the tDCS phase revealed poorer performance overall compared to the baseline testing for the sham phase. It is possible that some decline related to neurodegeneration occurred over the course of 3 months that may explain the subsequent decline in noun naming experienced during the tDCS phase.
Similar to Participant 2, Participant 3 also experienced improvement in naming untrained verbs at both 1 week and 8 weeks posttreatment in the VNeST+tDCS. Thus, tDCS produced greater generalization effects for untrained verb naming, which were apparent immediately after, and two months after treatment concluded. There were no other significant differences between VNeST+tDCS and VNeST+sham.
There are several possibilities that could explain why Participant 2 experienced the greatest tDCS advantage of the three participants. First, when comparing Participant 2 and Participant 3, both participants have nfvPPA, yet differed in several respects that may account for these differences in treatment outcomes. First, Participant 3 had more severe overall language deficits at baseline, as indicated by her lower WAB-R Aphasia Quotient (AQ) score of 43.7 compared to Participant 2's AQ of 61 (see Table 1). It is possible that disease severity affects treatment outcomes when language therapy is augmented with tDCS (Nissim et al., 2020), but we need larger randomized clinical trials to effectively determine its exact effect. It is possible that Participant 3's more severe language deficits, or related neurodegeneration, affected how well she responded to both behavioral therapy and tDCS. Additionally, Participant 2 received stronger tDCS stimulation intensity of 2 mA compared to Participant 3's 1 mA. The stronger current may have induced stronger treatment effects in Participant 2. Both Participant 2 and Participant 3 received tDCS in phase 2 of treatment, which could result in a disadvantage during that phase due to neurodegenerative effects sustained over the course of the study. However, the results are quite promising given that a tDCS advantage for the generalization of treatment effects to untrained verbs, and long-term maintenance of these treatment gains, was found in both participants who received tDCS even in the second phase of treatment.
Participant 2 also experienced improvement in areas that Participant 1 only experienced maintenance, such as naming untrained verbs and sentence production. Meyer et al. (2020) found that difficulties with grammatical ability, as measured by production of subject- and object-extracted sentences, were a significant predictor of impaired verb naming in individuals with PPA. They speculated that verb naming impairment could partially be due to difficulties accessing verb argument structure. All three participants in this study struggled with sentence production (see Table 2). Participant 1 primarily used active or subject-relative sentences to describe most pictures even when the task required different sentence structures such as passives, object relatives, and subject and object Wh-questions. Participant 2's output was agrammatic and she struggled most with noncanonical sentences. Participant 3 had difficulty correctly producing any sentence type. Even though each participant struggled with the sentence production task, it is possible Participant 2 achieved the most positive change during treatment because her verb naming deficits may be rooted in impaired access of argument structure, which was directly treated with VNeST. Also, verb argument structure deficits are typically associated with nfvPPA and not lvPPA (Thompson et al., 2012), so this may also explain why both individuals with nfvPPA benefitted more from the behavioral VNeST therapy. Participant 2 also received the strongest stimulation intensity, which may have contributed to the tDCS advantage. Finally, in the comparison of Participant 1 to Participant 3, it should be noted that Participant 3's clinical neuroimaging revealed atrophy in left IFG, whereas Participant 1 had global atrophy particularly affecting bilateral medial temporal and parietal lobes. It is notable that Participant 3 experienced a better tDCS advantage than Participant 1 with stimulation of left IFG, even though she had atrophy in this region. Future research will need to determine whether there is an atrophy threshold after which targeting that area is no longer beneficial.
One of the fundamental aims of VNeST therapy is generalization beyond trained verb networks (Edmonds et al., 2014). We only found VNeST+sham generalization effects for noun naming in Participant 2 but found no other evidence of generalization during the sham phase. However, the greatest benefit of adding tDCS to VNeST treatment was the significant generalization effect, particularly to untrained verbs. In stroke aphasia, VNeST promotes generalization to untrained structures, including naming untrained verbs, naming nouns, and producing and comprehending untrained sentences (Edmonds, 2016; Edmonds et al., 2009, 2014). Perhaps in PPA, supplementing VNeST with tDCS is needed to see generalization effects that are often seen in stroke aphasia with VNeST alone. While we did not observe widespread generalization of treatment effects in the sham phase, the fact that VNeST+sham promoted maintenance of language skills over time is promising. Many patients do not have access to language therapy plus neuromodulation, so it is important to note that VNeST may be an effective therapy for maintenance of language in PPA.
Our results showing tDCS induced generalization are similar to Fenner et al.'s study (2019) of written verb naming and spelling therapy using a similar design to our study. Recall they compared anodal stimulation of left IFG to sham stimulation in 11 participants with either logopenic or nonfluent variant PPA. Unlike our study, they found a significant tDCS advantage for written naming of trained verbs, but similar to our study, they also found evidence of tDCS induced generalization to written naming of untrained verbs that lasted up to 2 months posttreatment. Also, similar to our study, they did not find a significant improvement for oral naming of trained verbs, but they did find an improvement on oral naming of untrained verbs. In Fenner et al.'s study (2019), the number of treated verbs varied by participant (12–35 verbs), with more severe participants focusing on fewer verbs during treatment. In our study, participants all received treatment for 20 verbs. It is possible we did not find a tDCS advantage for trained verbs because a participant with less severe naming deficits who had a baseline naming accuracy greater than 50% would only be able to improve on fewer than 10 trained verbs. Thus, even a relatively meaningful change such as the 25% improvement in naming accuracy that Participant 2 experienced between baseline and 1 week posttreatment in the tDCS phase did not reach statistical significance. Additionally, Fenner et al. (2019) did not assess whether treatment effects extended to the sentence level. While we found evidence of generalization to sentence production in one participant and evidence of generalization to sentence comprehension in two participants, a larger study will be required to determine if more participants would also experience this benefit.
Note that we did not find any changes over the course of the study to apraxia of speech severity in either Participant 2 or 3. Anodal tDCS induces a weak polarization of cortical neurons in the area of stimulation, but it is not strong enough to generate action potentials (Nitsche & Paulus, 2001; Nitsche et al., 2008). Rather, the weak polarization makes it more likely for action potentials to occur when a participant engages in a behavioral task, which results in increased excitability (Bindman et al., 1964; Fridriksson & Hillis, 2021). Therefore, tDCS induced excitability is specific to the task-related network. While we did ask participants to produce phrases and sentences as part of the VNeST protocol in this study, we did not specifically treat apraxia of speech. We did not anticipate seeing changes to apraxia of speech, and unsurprisingly we did not find any changes to apraxia of speech over the course of the study in Participant 2 or 3.
Our study had several limitations. First, only three participants were included in this study. While our results are promising, we cannot make firm conclusions about the added advantage of tDCS above and beyond VNeST alone until a larger group of participants is investigated. Studying the benefits of VNeST+tDCS in a larger group of participants will also allow for the examination of how factors such as PPA severity and PPA subtype influence treatment efficacy. There is a great deal of heterogeneity among individuals with PPA, and rates of decline also vary across variant (Rogalski et al., 2011; Sebastian et al., 2018). Thus, we cannot assume that time post symptom onset is a direct marker of PPA severity. Another limitation is that we did not include anyone with semantic PPA in this study. In a large randomized clinical trial, Tsapkini et al.'s (2018) study of written naming and spelling therapy found a tDCS advantage for individuals with nfvPPA and lvPPA, but not svPPA. Future studies investigating VNeST+tDCS will need to include individuals with svPPA to determine whether they experience similar tDCS induced generalization advantages to the three individuals in this study. We also only had recent imaging for one of the three participants. As tDCS success requires sufficient neurons to stimulate, it is vital for future research to consider how atrophy severity influences tDCS induced treatment outcomes. Additionally, while we did investigate generalization of VNeST treatment effects to untrained words and to sentence-level tasks, we did not investigate generalization to discourse measures or to functional language outcomes. Future research should investigate whether the tDCS induced improvements seen here extend to real world functioning, as this would provide the greatest benefit to patients with PPA. We did not establish stable baselines as we only collected each assessment once during each baseline phase, rather than collecting multiple baselines to establish stability. Future research should ideally collect at least three baseline measures to establish stable baseline measures for comparison of pre- and posttreatment naming. Furthermore, we did not collect naming probe data throughout treatment and, therefore, cannot comment on progress made as each treatment phase progressed.
Conclusions
Anodal tDCS applied to left IFG during VNeST resulted in generalization of treatment effects to untrained verbs that lasted up to 8 weeks posttreatment in two of three individuals with PPA. However, a tDCS advantage was not found for trained verbs. The participant who benefitted the most from tDCS was one of the two participants with nfvPPA. In addition to showing generalization of treatment to untrained verb naming, she also had a tDCS advantage for production and comprehension of untrained sentences at 1 week posttreatment. She may have experienced the most benefits of tDCS due to receiving stronger stimulation intensity at 2 mA versus 1 mA in the other two participants. It is also possible that her verb production deficits were rooted in impaired access to verb argument structure, which was directly treated by VNeST. Furthermore, VNeST+sham appears to be an effective therapy for maintenance of language, particularly naming of trained verbs in all three participants and nouns in two participants. The results show promise for pairing A-tDCS to left IFG with VNeST therapy for treatment of verb naming deficits in PPA. Future work will include individuals with svPPA, and will investigate generalization of effects to discourse and functional language skills.
Acknowledgments
The authors of this article were supported by National Institute on Deafness and Other Communication Disorders, through Grants R01 DC05375, R01 DC015466, P50 DC014664, R01 DC011317 awarded to Argye E. Hillis, and R00 DC015554 awarded to Rajani Sebastian.
Funding Statement
The authors of this article were supported by National Institute on Deafness and Other Communication Disorders, through Grants R01 DC05375, R01 DC015466, P50 DC014664, R01 DC011317 awarded to Argye E. Hillis, and R00 DC015554 awarded to Rajani Sebastian.
References
- Amunts, K. , Weiss, P. H. , Mohlberg, H. , Pieperhoff, P. , Eickhoff, S. , Gurd, J. M. , Marshall, J. C. , Shah, N. J. , Fink, G. R. , & Zilles, K. (2004). Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—The roles of Brodmann areas 44 and 45. NeuroImage, 22(1), 42–56. https://doi.org/10.1016/j.neuroimage.2003.12.031 [DOI] [PubMed] [Google Scholar]
- Bak, T. H. , & Hodges, J. R. (2003). Kissing and dancing—A test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. Journal of Neurolinguistics, 16(2–3), 169–181. https://doi.org/10.1016/S0911-6044(02)00011-8 [Google Scholar]
- Baker, J. M. , Rorden, C. , & Fridriksson, J. (2010). Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke, 41(6), 1229–1236. https://doi.org/10.1161/STROKEAHA.109.576785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman, L. J. , Lippold, O. C. J. , & Redfearn, J. W. T. (1964). The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. The Journal of Physiology, 172(3), 369–382. https://doi.org/10.1113/jphysiol.1964.sp007425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breining, B. L. , Lala, T. , Cuitiño, M. M. , Manes, F. , Peristeri, E. , Tsapkini, K. , Faria, A. V. , & Hillis, A. E. (2015). A brief assessment of object semantics in primary progressive aphasia. Aphasiology, 29(4), 488–505. https://doi.org/10.1080/02687038.2014.973360 [Google Scholar]
- Costafreda, S. G. , Fu, C. H. , Lee, L. , Everitt, B. , Brammer, M. J. , & David, A. S. (2006). A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Human Brain Mapping, 27(10), 799–810. https://doi.org/10.1002/hbm.20221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli, M. , Manenti, R. , Petesi, M. , Brambilla, M. , Cosseddu, M. , Zanetti, O. , Miniussi, C. , Padovani, A. , & Borroni, B. (2014). Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. Journal of Alzheimer's Disease, 39(4), 799–808. https://doi.org/10.3233/JAD-131427 [DOI] [PubMed] [Google Scholar]
- Crescentini, C. , Shallice, T. , & Macaluso, E. (2010). Item retrieval and competition in noun and verb generation: An fMRI study. Journal of Cognitive Neuroscience, 22(6), 1140–1157. https://doi.org/10.1162/jocn.2009.21255 [DOI] [PubMed] [Google Scholar]
- Dabul, B. (2000). ABA-2: Apraxia Battery for Adults (2nd ed.). Pro-Ed. [Google Scholar]
- de Aguiar, V. , Zhao, Y. , Faria, A. , Ficek, B. , Webster, K. T. , Wendt, H. , Wang, Z. , Hillis, A. E. , Onyike, C. U. , Frangakis, C. , Caffo, B. , & Tsapkini, K. (2020). Brain volumes as predictors of tDCS effects in primary progressive aphasia. Brain and Language, 200, 104707. https://doi.org/10.1016/j.bandl.2019.104707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aguiar, V. , Zhao, Y. , Ficek, B. N. , Webster, K. , Rofes, A. , Wendt, H. , Frangakis, C. , Caffo, B. , Hillis, A. E. , Rapp, B. , & Tsapkini, K. (2020). Cognitive and language performance predicts effects of spelling intervention and tDCS in primary progressive aphasia. Cortex, 124, 66–84. https://doi.org/10.1016/j.cortex.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden, D.-B. , Fix, S. , Parrish, T. B. , & Thompson, C. K. (2009). Argument structure effects in action verb naming in static and dynamic conditions. Journal of Neurolinguistics, 22(2), 196–215. https://doi.org/10.1016/j.jneuroling.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druks, J. , & Masterson, J. (2000). Object and Action Naming Battery. Psychology Press. [Google Scholar]
- Edmonds, L. A. (2016). A review of verb network strengthening treatment. Topics in Language Disorders, 36(2), 123–135. https://doi.org/10.1097/TLD.0000000000000088 [Google Scholar]
- Edmonds, L. A. , Mammino, K. , & Ojeda, J. (2014). Effect of Verb Network Strengthening Treatment (VNeST) in persons with aphasia: Extension and replication of previous findings. American Journal of Speech-Language Pathology, 23(2), S312–S329. https://doi.org/10.1044/2014_AJSLP-13-0098 [DOI] [PubMed] [Google Scholar]
- Edmonds, L. A. , & Mizrahi, S. (2011). Online priming of agent and patient thematic roles and related verbs in younger and older adults. Aphasiology, 25(12), 1488–1506. https://doi.org/10.1080/02687038.2011.599527 [Google Scholar]
- Edmonds, L. A. , Nadeau, S. E. , & Kiran, S. (2009). Effect of Verb Network Strengthening Treatment (VNeST) on lexical retrieval of content words in sentences in persons with aphasia. Aphasiology, 23(3), 402–424. https://doi.org/10.1080/02687030802291339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner, A. S. , Webster, K. T. , Ficek, B. N. , Frangakis, C. E. , & Tsapkini, K. (2019). Written verb naming improves after tDCS over the left IFG in primary progressive aphasia. Frontiers in Psychology, 10, 1396. https://doi.org/10.3389/fpsyg.2019.01396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti, T. R. , McRae, K. , & Hatherell, A. (2001). Integrating verbs, situation schemas, and thematic role concepts. Journal of Memory and Language, 44(4), 516–547. https://doi.org/10.1006/jmla.2000.2728 [Google Scholar]
- Ficek, B. N. , Wang, Z. , Zhao, Y. , Webster, K. T. , Desmond, J. E. , Hillis, A. E. , Frangakis, C. , Vasconcellos Faria, A. V. , Caffo, B. , & Tsapkini, K. (2018). The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage: Clinical, 19, 703–715. https://doi.org/10.1016/j.nicl.2018.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori, V. , Kunz, L. , Kuhnke, P. , Marangolo, P. , & Hartwigsen, G. (2018). Transcranial direct current stimulation (tDCS) facilitates verb learning by altering effective connectivity in the healthy brain. NeuroImage, 181, 550–559. https://doi.org/10.1016/j.neuroimage.2018.07.040 [DOI] [PubMed] [Google Scholar]
- Fridriksson, J. , Basilakos, A. , Stark, B. C. , Rorden, C. , Elm, J. , Gottfried, M. , George, M. S. , Sen, S. , & Bonilha, L. (2019). Transcranial direct current stimulation to treat aphasia: Longitudinal analysis of a randomized controlled trial. Brain Stimulation, 12(1), 190–191. https://doi.org/10.1016/j.brs.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , & Hillis, A. E. (2021). Current approaches to the treatment of post-stroke aphasia. Journal of Stroke, 23(2), 183–201. https://doi.org/10.5853/jos.2020.05015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , Richardson, J. D. , Baker, J. M. , & Rorden, C. (2011). Transcranial direct current stimulation improves naming reaction time in fluent aphasia: A double-blind, sham-controlled study. Stroke, 42(3), 819–821. https://doi.org/10.1161/STROKEAHA.110.600288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga, P. C. , Hummel, F. C. , & Cohen, L. G. (2006). Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 117(4), 845–850. https://doi.org/10.1016/j.clinph.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Gervits, F. , Ash, S. , Coslett, H. B. , Rascovsky, K. , Grossman, M. , & Hamilton, R. (2016). Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain and Language, 162, 35–41. https://doi.org/10.1016/j.bandl.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Palacio Schjetnan, A. , Faraji, J. , Metz, G. A. , Tatsuno, M. , & Luczak, A. (2013). Transcranial direct current stimulation in stroke rehabilitation: A review of recent advancements. Stroke Research and Treatment, 2013, 170256. https://doi.org/10.1155/2013/170256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini, M. L. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. F. , Ogar, J. M. , Rohrer, J. D. , Black, S. , Boeve, B. F. , Manes, F. , Dronkers, N. F. , Vandenberghe, R. , Rascovsky, K. , Patterson, K. , Miller, B. L. , Knopman, D. S. , Hodges, J. R. , Mesulam, M. M. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini, M. L. , Ogar, J. M. , Brambati, S. M. , Wang, P. , Jeong, J. H. , Rankin, K. P. , Dronkers, N. F. , & Miller, B. L. (2006). Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology, 67(10), 1849–1851. https://doi.org/10.1212/01.wnl.0000237038.55627.5b [DOI] [PubMed] [Google Scholar]
- Grande, M. , Meffert, E. , Schoenberger, E. , Jung, S. , Frauenrath, T. , Huber, W. , Hussmann, K. , Moormann, M. , & Heim, S. (2012). From a concept to a word in a syntactically complete sentence: An fMRI study on spontaneous language production in an overt picture description task. NeuroImage, 61(3), 702–714. https://doi.org/10.1016/j.neuroimage.2012.03.087 [DOI] [PubMed] [Google Scholar]
- Grodzinsky, Y. (2000). The neurology of syntax: Language use without Broca's area. Behavioral and Brain Sciences, 23(1), 1–21. https://doi.org/10.1017/S0140525X00002399 [DOI] [PubMed] [Google Scholar]
- Hagoort, P. (2014). Nodes and networks in the neural architecture for language: Broca's region and beyond. Current Opinion in Neurobiology, 28, 136–141. https://doi.org/10.1016/j.conb.2014.07.013 [DOI] [PubMed] [Google Scholar]
- Havas, V. , Gabarrós, A. , Juncadella, M. , Rifa-Ros, X. , Plans, G. , Acebes, J. J. , de Diego Balaguer, R. , & Rodríguez-Fornells, A. (2015). Electrical stimulation mapping of nouns and verbs in Broca's area. Brain and Language, 145-146, 53–63. https://doi.org/10.1016/j.bandl.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Henry, M. L. , Hubbard, H. I. , Grasso, S. M. , Dial, H. R. , Beeson, P. M. , Miller, B. L. , & Gorno-Tempini, M. L. (2019). Treatment for word retrieval in semantic and logopenic variants of primary progressive aphasia: Immediate and long-term outcomes. Journal of Speech, Language, and Hearing Research, 62(8), 2723–2749. https://doi.org/10.1044/2018_JSLHR-L-18-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. https://doi.org/10.1038/nrn2113 [DOI] [PubMed] [Google Scholar]
- Hillis, A. E. , Tuffiash, E. , & Caramazza, A. (2002). Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of Cognitive Neuroscience, 14(7), 1099–1108. https://doi.org/10.1162/089892902320474544 [DOI] [PubMed] [Google Scholar]
- Homan, R. W. (1988). The 10–20 electrode system and cerebral location. American Journal of EEG Technology, 28(4), 269–279. https://doi.org/10.1080/00029238.1988.11080272 [Google Scholar]
- Horwitz, B. , Amunts, K. , Bhattacharyya, R. , Patkin, D. , Jeffries, K. , Zilles, K. , & Braun, A. R. (2003). Activation of Broca's area during the production of spoken and signed language: A combined cytoarchitectonic mapping and PET analysis. Neuropsychologia, 41(14), 1868–1876. https://doi.org/10.1016/S0028-3932(03)00125-8 [DOI] [PubMed] [Google Scholar]
- Howard, D. , & Patterson, K. E. (1992). The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company. [Google Scholar]
- Jokel, R. , Rochon, E. , & Anderson, N. D. (2010). Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychological Rehabilitation, 20(1), 16–41. https://doi.org/10.1080/09602010902879859 [DOI] [PubMed] [Google Scholar]
- Jokel, R. , Rochon, E. , & Leonard, C. (2006). Treating anomia in semantic dementia: Improvement, maintenance, or both. Neuropsychological Rehabilitation, 16(3), 241–256. https://doi.org/10.1080/09602010500176757 [DOI] [PubMed] [Google Scholar]
- Jung, I.-Y. , Lim, J. Y. , Kang, E. K. , Sohn, H. M. , & Paik, N.-J. (2011). The factors associated with good responses to speech therapy combined with transcranial direct current stimulation in post-stroke aphasic patients. Annals of Rehabilitation Medicine, 35(4), 460–469. https://doi.org/10.5535/arm.2011.35.4.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, E. , Goodglass, H. , & Weintraub, S. (2001). Boston Naming Test (BNT). Pro-Ed. [Google Scholar]
- Kertesz, A. (2007). Western Aphasia Battery–Revised (WAB-R). Harcourt Assessment, Inc. [Google Scholar]
- Liebetanz, D. , Nitsche, M. A. , Tergau, F. , & Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125(10), 2238–2247. https://doi.org/10.1093/brain/awf238 [DOI] [PubMed] [Google Scholar]
- Love, T. , & Oster, E. (2002). On the categorization of aphasic typologies: The SOAP (a test of syntactic complexity). Journal of Psycholinguistic Research, 31(5), 503–529. https://doi.org/10.1023/A:1021208903394 [DOI] [PubMed] [Google Scholar]
- Marangolo, P. , Fiori, V. , Calpagnano, M. A. , Campana, S. , Razzano, C. , Caltagirone, C. , & Marini, A. (2013). TDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers in Human Neuroscience, 7, 539. https://doi.org/10.3389/fnhum.2013.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, R. C. , & Cheng, Y. (2006). Selection demands versus association strength in the verb generation task. Psychonomic Bulletin & Review, 13(3), 396–401. https://doi.org/10.3758/BF03193859 [DOI] [PubMed] [Google Scholar]
- McConathey, E. M. , White, N. C. , Gervits, F. , Ash, S. , Coslett, H. B. , Grossman, M. , & Hamilton, R. H. (2017). Baseline performance predicts tDCS-mediated improvements in language symptoms in primary progressive aphasia. Frontiers in Human Neuroscience, 11, 347. https://doi.org/10.3389/fnhum.2017.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae, K. , Hare, M. , Elman, J. L. , & Ferretti, T. (2005). A basis for generating expectancies for verbs from nouns. Memory & Cognition, 33(7), 1174–1184. https://doi.org/10.3758/BF03193221 [DOI] [PubMed] [Google Scholar]
- Meyer, A. M. , Getz, H. R. , Brennan, D. M. , Hu, T. M. , & Friedman, R. B. (2016). Telerehabilitation of anomia in primary progressive aphasia. Aphasiology, 30(4), 483–507. https://doi.org/10.1080/02687038.2015.1081142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. M. , Snider, S. F. , McGowan, S. A. , Tippett, D. C. , Hillis, A. E. , & Friedman, R. B. (2020). Grammatical ability predicts relative action naming impairment in primary progressive aphasia. Aphasiology, 34(6), 664–674. https://doi.org/10.1080/02687038.2020.1734527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim, N. R. , Moberg, P. J. , & Hamilton, R. H. (2020). Efficacy of Noninvasive Brain Stimulation (tDCS or TMS) paired with language therapy in the treatment of primary progressive aphasia: An exploratory meta-analysis. Brain Sciences, 10(9), 597. https://doi.org/10.3390/brainsci10090597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche, M. A. , Cohen, L. G. , Wassermann, E. M. , Priori, A. , Lang, N. , Antal, A. , Paulus, W. , Hummel, F. , Boggio, P. S. , Fregni, F. , & Pascual-Leone, A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1(3), 206–223. https://doi.org/10.1016/J.BRS.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899–1901. https://doi.org/10.1212/WNL.57.10.1899 [DOI] [PubMed] [Google Scholar]
- Richardson, J. , Datta, A. , Dmochowski, J. , Parra, L. C. , & Fridriksson, J. (2015). Feasibility of using high-definition transcranial direct current stimulation (HD-tDCS) to enhance treatment outcomes in persons with aphasia. NeuroRehabilitation, 36(1), 115–126. https://doi.org/10.3233/NRE-141199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski, E. , Cobia, D. , Harrison, T. M. , Wieneke, C. , Weintraub, S. , & Mesulam, M.-M. (2011). Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology, 76(21), 1804–1810. https://doi.org/10.1212/WNL.0b013e31821ccd3c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur, T. T. , Schwartz, M. F. , Kimberg, D. Y. , Hirshorn, E. , Coslett, H. B. , & Thompson-Schill, S. L. (2009). Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca's area. Proceedings of the National Academy of Sciences, 106(1), 322–327. https://doi.org/10.1073/pnas.0805874106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian, R. , Kim, J. H. , Brenowitz, R. , Tippett, D. C. , Desmond, J. E. , Celnik, P. A. , & Hillis, A. E. (2020). Cerebellar neuromodulation improves naming in post-stroke aphasia. Brain Communications, 2(2), fcaa179. https://doi.org/10.1093/braincomms/fcaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian, R. , Thompson, C. B. , Wang, N.-Y. , Wright, A. , Meyer, A. , Friedman, R. B. , Hillis, A. E. , & Tippett, D. C. (2018). Patterns of decline in naming and semantic knowledge in primary progressive aphasia. Aphasiology, 32(9), 1010–1030. https://doi.org/10.1080/02687038.2018.1490388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C. , Kingma, R. M. , Croot, K. , & Nickels, L. (2009). Speech pathology services for primary progressive aphasia: Exploring an emerging area of practice. Aphasiology, 23(2), 161–174. https://doi.org/10.1080/02687030801943039 [Google Scholar]
- Thompson, C. K. (2012). Northwestern Assessment of Verbs and Sentences (NAVS). Northwestern University. https://www.scholars.northwestern.edu/en/publications/northwestern-assessment-of-verbs-and-sentences-navs [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, C. K. , Cho, S. , Hsu, C.-J. , Wieneke, C. , Rademaker, A. , Weitner, B. B. , Mesulam, M. M. , & Weintraub, S. (2012). Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology, 26(1), 20–43. https://doi.org/10.1080/02687038.2011.584691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill, S. L. , Swick, D. , Farah, M. J. , D'Esposito, M. , Kan, I. P. , & Knight, R. T. (1998). Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences, 95(26), 15855–15860. https://doi.org/10.1073/pnas.95.26.15855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippett, D. C. , Hillis, A. E. , & Tsapkini, K. (2015). Treatment of primary progressive aphasia. Current Treatment Options in Neurology, 17(8), Article 34. https://doi.org/10.1007/s11940-015-0362-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini, K. , Frangakis, C. , Gomez, Y. , Davis, C. , & Hillis, A. E. (2014). Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology, 28(8–9), 1112–1130. https://doi.org/10.1080/02687038.2014.930410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini, K. , Webster, K. T. , Ficek, B. N. , Desmond, J. E. , Onyike, C. U. , Rapp, B. , Frangakis, C. E. , & Hillis, A. E. (2018). Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer's & Dementia, 4(1), 461–472. https://doi.org/10.1016/j.trci.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski, R. L. , Botha, H. , Martin, P. R. , Schwarz, C. G. , Duffy, J. R. , Clark, H. M. , Machulda, M. M. , Butts, A. M. , Lowe, V. J. , Jack, C. R., Jr. , Senjem, M. L. , Spychalla, A. J. , Whitwell, J. L. , & Josephs, K. A. (2019). Clinical and neuroimaging characteristics of clinically unclassifiable primary progressive aphasia. Brain and Language, 197, 104676. https://doi.org/10.1016/j.bandl.2019.104676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, D. L. , & Baker, C. M. (1988). Pain in children: Comparison of assessment scales. The Oklahoma Nurse, 33(1), 8–8. [PubMed] [Google Scholar]