Abstract
The incidence of prostatic cancer in the United Kingdom has increased over 40% in the past 30 years. The majority of these cancers are diagnosed by core biopsy, posing a considerable strain on a service that struggles to recruit sufficient histopathologists. The current methodology for tissue diagnosis has a significant false-negative rate, small false-positive rate, and a proportion of indeterminate diagnoses. Therefore, this area presents an opportunity both to improve diagnostic quality and to reduce the burden on resources. We investigated streamlining tissue pathways by increasing the utilization of readily available resources to reduce the burden on scarce resources and improve the accuracy of diagnosis. This involved applying prospective multiplex immunohistochemistry (IHC) using 4 different markers (CK5, p63, racemase, and Ki-67) and 2 chromogens. We conducted a prospective study using over 8000 cores and 3 consultant histopathologists. The pathologists assessed each core using either conventional stains (hematoxylin and eosin) only or multiplex IHC only. The results of this assessment were later compared with the overall assessment made for the final histologic diagnosis. Results show that IHC alone has a positive predictive value of 98.97% and a negative predictive value of 99.91%, while hematoxylin and eosin alone has a positive predictive value of 94.21% and negative predictive value of 99.07%, demonstrating improved diagnostic accuracy. When assessed against the use of on-demand IHC, prospective IHC improves turn-around-times, reduces indeterminate diagnoses, improves pathologist’s accuracy and efficiency and, in overall terms, is cost-effective. In addition, it is possible to structure these tests within the routine of a diagnostic service with little impact on the overall capacity of the laboratory.
Key Words: prostatic cancer, multiplex immunohistochemistry, core biopsy
More than 47,000 new diagnoses of prostate cancer (PC) were made in the United Kingdom alone in 2015, and over 1.1 million worldwide.1,2 The UK incidence of PC has grown by 40% in 30 years1,3 with a significant impact on histopathology services since the great majority (c.95%) of these diagnoses are made on biopsy.
Of the 2 main biopsy types—transurethral resection of the prostate and core biopsies—transurethral resection of the prostate currently contributes to <10% of new diagnoses of PC4: therefore, at least 85% (c.40,000/y) are diagnosed on core biopsy. The cancer positivity rate of core biopsy procedures varies widely between 25% and 50% and is heavily influenced by patient selection criteria and local sampling protocols. Assuming an average positivity rate of 40% to 50%, in 2015, the UK population required some 100,000 biopsy procedures to diagnose 47,000 new cancers.
In the past, the majority of these procedures were transrectal ultrasound-guided core biopsies (TRUSbx) consisting of an average of 12 cores. In recent years, there has been an increasing use of transperineal template mapping biopsies (TPMbx) comprising a much larger number of cores per procedure (18 to >100). In our institution, the proportion of TPMbx rose from 7% in 2013 to 23% in 2014 and around 40% in 2017. If TPMbx with an average of 40 cores, constitutes 25% of procedures, the estimated number of individual cores obtained in 2015 in the United Kingdom was 1.9 million. The impact on histopathologist time alone amounts to around 106,000 hours of reporting time or 26,388 programmed activities which, as per the Royal College of Pathologists’ (RCPath) guidance,5 amounts to around 100 full-time equivalent histopathologists. While admittedly an estimate, this large requirement has probably increased in more recent years and coincides with a significant shortfall in histopathologists.6
It is desirable for the health economy and vital for histopathology to reduce the burden of diagnosing prostatic cancer. There are 2 possible areas of improvement; increasing cancer positivity rates would reduce the overall number of biopsies (by reducing repeat procedures); decreasing the time required by pathologists to reach a diagnosis would reduce the workforce requirements. Both are possible by modernizing tissue pathways that make tissue sections easier to interpret.
We have considerable experience in processing multiple cores of prostatic tissue in the same cassette (tissue core microarray, TCMA) while preserving orientation and without compromising on the amount of tissue available on the slide for diagnosis. We also had a tried and tested laboratory-developed multiplex IHC (combining multiple antibodies on the same slide using different chromogens). In 2009, we introduced prospective multiplex IHC for the diagnosis of prostate core biopsies. We found the combination of TCMA and multiplex IHC provided significant improvements in quality and could be cost-effective. We performed this prospective study to evidence these improvements.
This paper presents the results of a prospective study involving over 8000 individual cores of prostatic tissue. We compare the diagnostic performance of conventional hematoxylin and eosin (H&E) and multiplex IHC, we describe the technical steps to introduce the necessary changes in tissue pathways, and we assess the impact of TCMA+multiplex IHC in terms of health economics.
MATERIALS AND METHODS
We had utilized TCMA for prostatic biopsies since 2000 and multiplex IHC on request since 2008 when, in 2009, we introduced prospective multiplex IHC on all prostatic cores into our diagnostic routine. This was initially for TRUSBx, with 12 cores in 2 cassettes. TPMbx, which can produce from 36 to 96 cores, was introduced in March 2012.
For TPMbx (Fig. 1), ultrasound scanning is performed in both transverse and sagittal views, a brachytherapy template with 5 mm grid is superimposed upon the prostate image, and biopsy sites are planned. The biopsies are obtained through the brachytherapy grid under direct ultrasound visualization in sagittal view, and each biopsy is labeled using an alphanumerical plan. This plan forms the basis of the detailed histopathology report and representation on a 2-dimensional map. Our TCMA method was ideally suited for such mapping.
FIGURE 1.
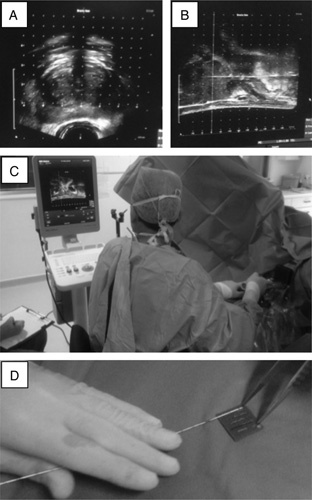
Transperitoneal template biopsy in practice. A, Initial assessment of possible core biopsy sites is made using the coronal view of a transrectal ultrasound scan of the prostate. B, Sagittal transrectal ultrasound scan view of the prostate during core biopsy sampling; short arrow indicate the cranial limit of the prostate; long arrow indicates the needle within the prostate. C, Core biopsy needle is inserted using the template as a guide and observed in real time under transrectal ultrasound scan; note assistant in left bottom corner annotating biopsy sites on proforma. D, Blotting of the core of tissue from the needle onto the sponge and (inset) 6 consecutive cores labeled and ready to be overlaid with a wet sponge.
In theater, each biopsy core (obtained with 18 G Bard disposable biopsy gun) is placed directly on a prelabelled dry sponge (CellPath) by blotting it from the needle using a gentle rotational movement (Fig. 1) taking care not to squash or smear the tissue onto the sponge. Further cores are added to the sponge in parallel to the first core and with the same anatomic orientation. Each sponge carrying 6 consecutive cores is then covered with a second wet (10% buffered formal saline) sponge, placed in a labeled cassette and put in a specimen pot with fixative.
Once in the laboratory, the sponge sandwiches containing the tissue cores are transferred intact into printed cassettes, maintaining orientation. They are fixed for at least 6 hours before processing; then sectioned and mounted on glass slides ensuring appropriate orientation to preserve positional information. Three-micron-thick sections cut at 3 levels are stained with H&E, and 1 section from level 2 (L2) section is stained with immunohistochemistry (IHC) using 4 antibodies and 2 different secondary detection systems to produce a single multiplex slide stained with 2 colors and 4 antibodies (Figs. 2A–C).
FIGURE 2.
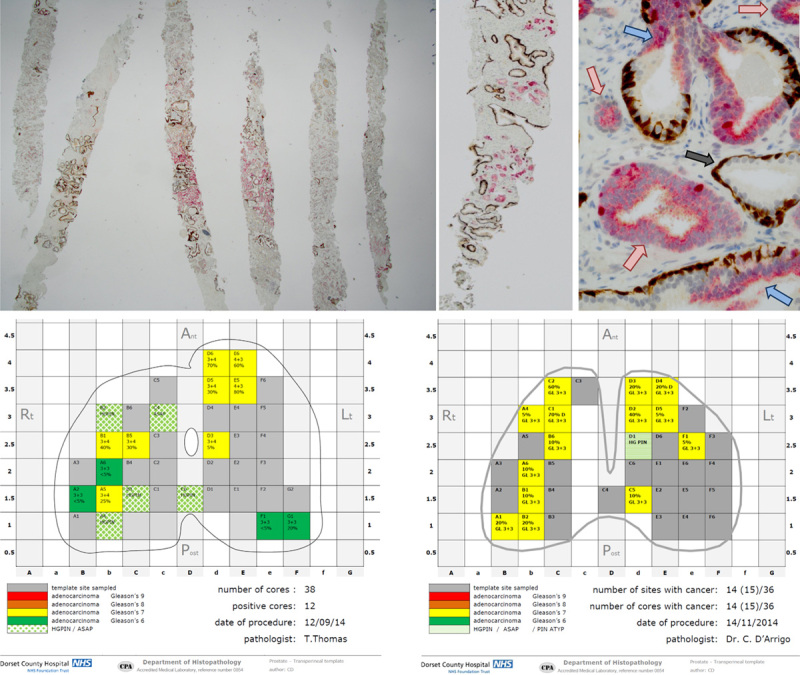
Histopathology report on the 2-dimensional map and multiplex immunohistochemistry. A–C, Multiplex immunohistochemistry at x40, x100 & x400. CK5 and p63 (basal cell markers) stained in brown, racemase, and Ki-67 stained in red. A (×1.25), Substantial amount of invasive carcinoma can be seen in all cores, characterized by the absence of brown staining; some of the carcinoma expresses racemase (red) other is racemase(−). B (×2), Scattered clusters of invasive carcinoma devoid of brown staining and racemase(+) are seen clearly among the background of normal prostatic glands that have brown staining but lack racemase. C (×10), High-grade prostatic intraepithelial neoplasia (blue arrows) have discontinuous basal cells (brown staining) and can be readily distinguished from acini of invasive carcinoma (red arrows) and atrophic glands (gray arrow). D and E, Examples of histopathology reports with 2-dimensional maps using the visual traffic light coded system.
IHC for this study was performed primarily on the Leica Bond Max using a cocktail of anti-CK5 (XM26, 1:100; Leica) and anti-p63 (7JUL, 1:50; Leica) stained with Leica Refine Brown and a cocktail of anti-AMACR (13H4, 1:175; Dako/Agilent) and anti-Ki-67 (MIB-1, 1:75; DakoAgilent) stained with Leica Refine Red. The protocol was also established on the Roche Ventana Benchmark XT/Ultra, with ready-to-use VENTANA Basal Cell Cocktail [anti-keratin (34βE12)+anti-p63 (4A4)] stained with UltraView DAB and a secondary cocktail of anti-racemase p504S (13H4; Cell Marque) diluted 1:100 into Roche ready-to-use CONFIRM anti-Ki-67 (30-9) stained with UltraView Red.
Our histopathology reports for the TPMbx include a map replicating the prostate image on the brachytherapy grid with location and relevant diagnostic details of all cores taken. The details include Gleason score using a color coding system and percentage involvement of carcinoma in each core. The color scheme is an easy-to-interpret traffic light system (Figs. 2D, E) which can be modified to include the new World Health Organization (WHO) grade grouping.
To assess the accuracy of multiplex IHC as a primary tool in detecting PC, the cores were examined by 1 of 3 consultant histopathologists (C.D.A., T.T., or J.M.) in the context of their diagnostic routine. The cores were initially assessed using either multiplex IHC only (335 cases comprising 6463 cores) or H&E levels only (63 cases comprising 1589 cores), and the presence (%) and Gleason grade of invasive carcinoma was noted on a score sheet. A final “gold-standard” diagnosis was subsequently made by the same pathologist using all H&E levels, multiplex IHC and any additional test if required. Positive and negative predictive values for both multiplex IHC only and H&E only were calculated based on true/false-positive and true/false-negative values.7
RESULTS
Our results are given in Table 1 and briefly summarized here.
The study demonstrated that a diagnosis of cancer made using multiplex IHC alone is sensitive (99.36%) and specific (99.86%) when compared with the gold standard (H&E L1-L3+multiplex IHC) and has better sensitivity than L1-L3 H&E alone (93.23%).
Diagnosis based on 3 levels of H&E only had a 6.77% false-negative rate (see the Discussion section) and 0.78% false-positive rate. The clinical significance of the latter is uncertain since a single small focus of likely cancer would probably be confirmed with IHC in most diagnostic practices.
Multiplex IHC facilitated the assessment of percentage core involvement. Overall, concordance of multiplex IHC only with the gold standard was 97.66% compared with 94.34% for H&E levels only.
Overall, 50/8052 cores (0.62%) received a gold-standard diagnosis of atypical small acinar proliferation (ASAP) with 15/398 cases (3.7%) having ASAP as the final diagnosis.
TABLE 1.
Assessment of Multiplex IHC and H&E Compared With GS
| Multiplex IHC (vs. GS) | H&E (vs. GS) | GS | |
|---|---|---|---|
| Total cores assessed | 6463 | 1589 | 8052 |
| Cores positive for cancer | 780 | 190 | 970 |
| Cores negative for cancer | 5683 | 1399 | 7082 |
| Prevalence of cancer (%) | 12.02 | 12.07 | 12.05 |
| Concordance [n (%)] | 6312 (97.66) | 1499 (94.34) | — |
| No. true-positive cores (A) | 772 | 179 | — |
| No. false-positive cores (B) | 8* | 11† | — |
| No. true-negative cores (C) | 5678 | 1386 | — |
| No. false-negative cores (D) | 5‡ | 13§ | — |
| Sensitivity (%) | 99.36 | 93.23 | — |
| Specificity (%) | 99.86 | 99.21 | — |
| Positive predictive value (%) | 98.98 | 94.21 | — |
| Negative predictive value (%) | 99.91 | 99.07 | — |
| False-positive rate for cores (%) | 0.14 | 0.78 | — |
| False-negative rate for cores (%) | 0.64 | 6.77 | — |
| Overestimation of cancer/core (%) | 3.11 | 17.33 | — |
| Underestimation of cancer/core (%) | 4.27 | 15.64 | — |
| Overestimation of Gleason grade/core (%) | 4.40 | 5.59 | — |
| Underestimation of Gleason grade/core (%) | 7.12 | 4.47 | — |
Eight false-positive cores on multiplex IHC comprising 7 cases. In 6 cases, the overall diagnosis was identical to the final GS diagnosis. In 1 case, the diagnosis (<5% Gleason grade 3+4) was different to the GS (atypical small acinar proliferation).
Eleven false-positive cores on H&E comprising 6 cases. In 5 cases, the overall diagnosis was identical to the final GS diagnosis (as other cores in the case contained cancer−true positives using H&E only). In 1 case, the overall diagnosis on H&E only (<5% Gleason grade 3+3) was different to the final GS diagnosis (negative for cancer).
Five false-negative cores on multiplex IHC comprising 5 cases. In all cases, the overall diagnosis was identical to the final GS diagnosis as there were other cores present that indicated a diagnosis of cancer (true positives using IHC only).
Thirteen false-negative cores on H&E comprising 12 cases. In 10 cases, the overall diagnosis was identical to the final GS diagnosis (as other cores in the case contained cancer−true positives using H&E only). In 2 cases, the diagnosis, if made using H&E only (negative for cancer) was different to the final GS diagnosis (<5% Gleason grade 3+4, 5% Gleason grade 3+3).
GS indicates gold standard; H&E, hematoxylin and eosin; IHC, immunohistochemistry.
DISCUSSION
Prospective Multiplex IHC Improves Cancer Detection
There is ample literature indicating that prostate histopathology has a failure rate in correctly identifying all cases of carcinoma.8,9 The rate varies among services but is relatively independent of the experience of the reporting pathologist. It is difficult to quantify precisely since most of the data derives from a retrospective review of selected biopsies, originally reported negative, in patients who subsequently developed carcinoma. While very few studies are devoid of selection bias and quoted rates vary from 0% to 10%, most investigators report an average failure rate of 2% to 3%.8–12 We postulated that prospective multiplex IHC could improve cancer pick-up rate and our audit data shows that, following its introduction, our cancer positivity rate increased from 44.1% in 2004-2009 to 51.7% in 2010-2013.7
The combination of prospective multiplex IHC and TPMbx, including magnetic resonance imaging before biopsy, further improved the cancer detection rates. In the 9-month period of this study, our detection rate was 63% for TPMbx and 48% for TRUSbx. While TPMbx increases diagnostic accuracy, it also increases the burden on histopathology, and it is important to devise improved tissue pathways to minimize this effect.
Improvements in Tissue Pathways: TCMA (Multiple Core Arrays) With 6 Cores Per Cassette
Prospective multiplex IHC becomes more feasible with our protocol of embedding 6 tissue cores in one cassette. Historically, many publications have reported loss of quality when processing multiple cores within the same paraffin block, the major disadvantage being a reduction of diagnostic material available on the glass slide.13–15 This is due primarily to curling and fragmentation of the delicate tissue cores that sit at different levels within the block. While these earlier studies showed marked (up to 33%) loss of diagnostic material available on slide,13 more recent studies16–19 report no loss in quality when processing 3 cores in the same cassette, providing that appropriate care is used to prevent curling and fragmentation. Therefore, it is apparent that the technique rather than the number of cores per cassette determines the quality of the diagnostic material. This is acknowledged in The RCPath 2016 recommendations.20
The all-important first step in our technique involves placing the fresh tissue cores directly onto dry sponge with gentle stretching to maintain the straight alignment so that 6 straight cores can be placed parallel to each other in one cassette. This was outlined in the method section and illustrated in Figures 1C and D. The cores are then covered with a second damp sponge before the closed cassette is placed in a pot of fixative. The 6 fixed straight cores are then relatively easily transferred, with maintained orientation, at the time of embedding. We have had over 10 years of experience in using this technique satisfactorily.7 Other groups have also reported processing 6 cores within one cassette with good tissue preservation.12,21,22 With the help of our marking system, each core retains orientation and position while processing, and small fragments are prevented from becoming separated from their core of origin (Fig. 1). Our marking system also enables us to provide clinicians with 2-dimensional template biopsy maps showing the distribution of prostatic cancer which provides important information for multidisciplinary team and surgical decision-making.
Improvements in Tissue Pathways: Multiplex IHC
The use of antibodies against CK5 and p63 for basal cells and against p504 (AMARC or racemase) in prostatic core biopsies is now well established.23–25 To colocalise these 3 antibody signals in separate slides can be difficult and, at times, impossible if the region of interest is small and not present in all consecutive sections. For this reason, antibody cocktails and multiplex IHC were developed.26,27 The combination of unique localization (nuclear, membranous, or cytoplasmic) and the use of multiple chromogens allows the identification of signals from several individual antibodies on the same slide. Another advantage of multiplex IHC is that the multiple signals render more tissue structures visible allowing interpretation of tissue morphology in a way that is more similar to conventional stains than single antibody IHC but based on highly specific signals related to individual molecules rather than on nonspecific stains.
Other antibodies have been added to the basic cocktail (CK5-p63-racemase) to provide further diagnostic and prognostic information; these include c-myc, ERG, and Ki-67.27 We have found the addition of Ki-67 highlights areas of high-grade prostatic intraepithelial neoplasia and intraduct carcinoma but more particularly, it facilitates the identification of small foci of high-grade carcinoma cells that are often racemase negative and easily missed on Pin4 cocktail, since these are usually highlighted by their Ki-67 labeling index.
Improvements in Tissue Pathways: Cost, Time, and Other Benefits of TCMA+Prospective Multiplex IHC
In our laboratory, we use routinely a combination of CK5-p63-racemase-Ki-67 with 2 chromogens. These stains were requested on demand from 2008 and have been used prospectively since 2009. On-demand IHC has a significantly higher cost per side compared with prospective IHC: pathologists have to request the test, technicians have to retrieve the material, prepare it, find an appropriate gap in an autostainer and later reallocate the slides to the pathologists; the process takes at least 24 hours and the pathologist will need to review the H&E sections again together with the IHC. In effect a proportion of cases will be examined twice.
We predicted that if we performed prospective multiplex IHC on all cases, the service would benefit from streamlining in a number of areas: technicians would not need to retrieve cases and prepare new slides, cases would need to be allocated only once to pathologists who would in turn only assess cases once; the assessment of the proportion of carcinoma within each core would be more rapid (and accurate, see below) and cases could be reviewed more rapidly for multidisciplinary team meetings. Prospective multiplex IHC provides a further, albeit counterintuitive, cost-saving opportunity since it facilitates the rapid and confident identification of all cases negative for carcinoma (which can constitute 50% to 60% of the workload). This further reduces reporting time resulting in less fatigue and allowing pathologists to report more cases safely. This is particularly relevant to TPMbx cases, with up to 96 cores per procedure.
A concern over the use of prospective IHC is the potential for an increase in ASAP rates due overinterpretation of small areas devoid of demonstrable basal cells.20,27,28 In our experience, the percentage of ASAP diagnosis actually fell with prospective multiplex IHC, and in our series the ASAP rate was 3.7%. A point that is often overlooked is that pathologists are highly skilled in interpreting conventional stains (H&E) to diagnose or rule out prostatic carcinoma and are much less familiar with IHC which they use only in cases of diagnostic uncertainty. Paradoxically they rely on the stains they are the least familiar with to diagnose the most challenging cases. An advantage of prospective IHC is that it allows pathologists to familiarize themselves with these stains and redress the balance. In addition, it enables pathologists to improve their diagnostic accuracy on conventional stains by providing an instant elucidation of such lesions as benign mimics of carcinoma and intraductal carcinoma, hence delivering ongoing educational value.
While undoubtedly prospective IHC has an upfront cost, the increased number of tests performed allowed us to obtain more favorable prices from the suppliers, improve our quality and reduce the number of suboptimal tests that required reruns due to operator error. In 2008, when requests for on-demand multiplex IHC started to rise, the impact on our existing IHC service was so high that we considered the purchase of an additional IHC machine to cope with the volume. It was only by implementing prospective multiplex IHC that we were able to plan and rationalize the use of our existing IHC stainers. Thus, despite increasing the number of tests performed, we maintained the necessary capacity to deliver all our diagnostic workload without purchasing additional stainers.
Assessing the impact of prospective multiplex IHC on health economics requires numerous and wide-ranging parameters. Some, such as reduction in number of biopsy procedures due to the increase in PPV, impact more broadly on the Health Services and are beyond the scope of this paper. Others directly relate to histopathology and include the cost of additional stains and reduction in reporting time. For simplicity, we provide figures related to TRUSbx procedures consisting of 2 cassettes per case, each containing 6 cores (costs for TPMbx can be calculated using the same matrix and an average of 6 cassettes per case). Our laboratory-developed multiplex IHC had a consumable cost of ∼£7 per slide; therefore, prospective IHC increased the cost of TRUSbx by £14/case. The diagnostic report for a TRUSbx case accounts for of an average of 25 minutes of pathologist time.5 Given an average cost of £106/h for a consultant pathologist in the NHS,29 an efficiency gain of 1/3 (8 min) would generate £14.50 of savings, sufficient to cover the cost of the additional IHC slides. In our experience, we easily exceeded this one third efficiency gain but this study did not attempt to quantify the reduction in reporting time, and these figures are anecdotal; however, a group of colleagues in The Netherlands are collecting this data for future publication.
Multiplex IHC as a Primary Diagnostic Stain for PC
The considerable advantages of prospective multiplex IHC led us to compare its performance on a single (level 2) section against 3 levels stained with H&E. The purpose of this investigation was to establish whether multiplex IHC is sufficiently accurate to be used as a primary diagnostic tool. Our study demonstrated that a diagnosis of cancer made using only multiplex IHC is both sensitive (99.36%) and specific (99.86%) and has better sensitivity than L1-L3 H&E only (93.23%). It also showed that examination of 3 levels of H&E only has a high (6.77%) false-negative rate (the likelihood that cores containing PC will be incorrectly identified as negative). The possibility of a false-negative diagnosis arising in the case of rare carcinomas that stain positively for basal markers needs consideration. There were no such cases in this series. However, in the last 10 years, we have come across 3 cases of carcinoma aberrantly expressing p63 which were easily recognizable on IHC by their diffuse strong p63 positivity and complete absence of CK5 staining. We have seen basal cell carcinoma of the prostate where solid clusters of cells positive for basal markers infiltrating between normal glands initially raised suspicion for urothelial carcinoma and certainly would not have been screened as negative. However, further studies focusing on such cases is needed.
In the context of the UK estimated annual workload for 2015 (1.9 million cores) and using our figure of 12% prevalence of cores containing prostatic cancer, diagnosing PC with H&E levels alone would lead to 15,436 cancer-containing cores being falsely diagnosed as negative. Using multiplex IHC alone would reduce this to 1352 cores. It should be noted that a diagnostic procedure consists of multiple cores, and therefore the overall diagnosis of cancer may still be rendered on the basis of other cores, and it is difficult to extrapolate the results from single-core analysis in view of the relative low number of cases. Nevertheless, our small study contained 2 of 63 (H&E only) cases or 3.2%, in which cancer would have been missed if H&E levels alone was used for diagnosis. This extrapolates to a national figure of around 1920 cases in 2015.
Another consequence of false-negative diagnosis is the need for repeat biopsies in patients with continuing clinical suspicion of cancer. This causes additional costs to the health economy, increases morbidity associated with rebiopsy, delays cancer diagnoses and potentially delays treatment. Our study indicates that prospective multiplex IHC would lead to fewer cancer patients requiring additional biopsies.
The data presented here also shows that, with satisfactory processing, it is not necessary to cut and stain these core biopsies at multiple levels. If UK histopathology services adopted the 6 core TCMA method and stopped using multiple deep levels for prostate core biopsy in 2018, there would be an estimated saving of some 800,000 sections or around £2 million.
If multiplex IHC can reliably exclude carcinoma, it may be used as first-line screening for prostatic core biopsies. Since screening a single slide of multiplex IHC is considerably quicker than screening multiple levels of conventional stains, ∼40% to 50% of cases (all negative cases) could be reported in <50% of the time. The precise savings will depend on the laboratory practice; if laboratories followed the recommendations of the European Association of Urologists,18 we could assume an average of 3 levels, and this would represent at least a 66% saving on pathologist reporting time for the negative cores.
CONCLUSIONS
This is the first paper that describes the prospective use of multiplex IHC on the entire routine prostatic biopsy workload of a diagnostic histopathology service. We demonstrate that prospective IHC can be used in the diagnostic setting and results in improved quality. These changes were easily implemented in a small district general hospital, are transferrable to most histopathology services, and are now used routinely in laboratories in The Netherlands and Germany. Recent advances with covalently bound chromogens provide the opportunity to use up to 8 colors and numerous antibodies that may improve diagnostic and prognostic performance even further. We suggest that prospective IHC can reduce overall service costs and free-up valuable resources such as pathologists’ time, but other studies are required to specifically measure these parameters.
Our results also suggest that it would be feasible to explore the use of multiplex IHC on a single level as the sole test required to exclude the presence of carcinoma. Improvements to this system include the ability to process up to 15 cores per cassette, which would significantly decrease costs and allow the use of multiple biomarkers for the stratification of carcinoma. Multiplex IHC is also particularly amenable to the use of computer-aided analysis for screening negative cores, and this is a focus of future work.
Footnotes
This study was undertaken during a period of collaboration between Roche Tissue Diagnostics and Dorset County Hospital Foundation Trust.
S.W. was supported by Roche EMEA for salary during this period, and some reagent costs were funded by Roche Tissue Diagnostics to confirm that the results also applied with using Ventana-Roche as well as Leica Bond. C.D.A. has received honoraria from Roche. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Prostate Cancer Incidence, Cancer Research UK. WHO, 2018. Available at: www.cancerresearchuk.org/health-professional/cancer-statistics/statitistics-by-cancer-type/prostate-cancer/incidence. Accessed 2015.
- 2.Prostate Cancer Incidence, World Health Organization. WHO Cancer Key Facts; 2018. Available at: www.who.int/en/news-room/fact-sheets/detail/cancer. Accessed 2015.
- 3.Bray F, Lortet-Tieulent J, Ferlay J, et al. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–3052. [DOI] [PubMed] [Google Scholar]
- 4.Otto B, Barbieri C, Lee R, et al. Incidental prostate cancer in transurethral resection of the prostate specimens in the modern era. Adv Urol. 2014;2014:627290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Royal College of Pathologists. Guidelines on staffing and workload for histopathology and cytopathology departments (4th edition); 2015.
- 6.The Royal College of Pathologists. Histopathology Workforce Survey; 2018.
- 7.Wedden S, Miller K, Afzal N, et al. Diagnostic and prognostic multiplex immunohistochemistry on transrectal and transperineal core biopsy of prostate. Virchows Arch. 2014;465(suppl 1):PS-06–095. [Google Scholar]
- 8.Wolters T, van der Kwast TH, Vissers CJ, et al. False-negative prostate needle biopsies: frequency, histopathologic features, and follow-up. Am J Surg Pathol. 2010;34:35–43. [DOI] [PubMed] [Google Scholar]
- 9.Yang C, Humphrey PA. False-negative histopathologic diagnosis of prostatic adenocarcinoma. Arch Pathol Lab Med. 2020;144:326–334. [DOI] [PubMed] [Google Scholar]
- 10.Carswell B, Woda B, Wang X, et al. Detection of prostate cancer by alpha-methylacyl CoA racemase (P504S) in needle biopsy specimens previously reported as negative for malignancy. Histopathology. 2006;48:668–673. [DOI] [PubMed] [Google Scholar]
- 11.Oxley JD, Sen C. Error rates in reporting prostatic core biopsies. Histopathology. 2011;58:759–765. [DOI] [PubMed] [Google Scholar]
- 12.Bonkhoff H. Significance of prostate cancer missed on needle biopsy tools for retrieving missed cancer. Prostate. 2015;76:369–375. [DOI] [PubMed] [Google Scholar]
- 13.Kao J, Upton M, Zhang P, et al. Individual prostate biopsy core embedding facilitates maximal tissue representation. J Urol. 2002;168:496–499. [PubMed] [Google Scholar]
- 14.van der Kwast T, Lopes C, Santonja C, et al. , Members of the Pathology Committee of the European Randomised Study of Screening for Prostate Cancer (ERSPC). Guidelines for processing and reporting of prostatic needle biopsies. J Clin Pathol. 2003;56:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bostwick DG, Qian J, Drewnowska K, et al. Prostate needle biopsy quality in reduction by dutasteride of prostate cancer events study: worldwide comparison of improvement with investigator training and centralized laboratory processing. Urology. 2010;75:1406–1410. [DOI] [PubMed] [Google Scholar]
- 16.Rogatsch H, Moser P, Volgger H, et al. Diagnostic effect of an improved preembedding method of prostate needle biopsy specimens. Hum Pathol. 2000;31:1102–1107. [DOI] [PubMed] [Google Scholar]
- 17.Rogatsch H, Mairinger T, Horninger W, et al. Optimized preembedding method improves the histologic yield of prostatic core needle biopsies. Prostate. 2000;42:124–129. [DOI] [PubMed] [Google Scholar]
- 18.Van der Kwast T, Bubendorf L, Mazerolles C, et al. Guidelines on processing and reporting of prostate biopsies: the 2013 update of the pathology committee of the European Randomized Study of Screening for Prostate Cancer (ERSPC). Virchows Arch. 2013;463:367–377. [DOI] [PubMed] [Google Scholar]
- 19.Bostwick DG, Kahane H. Adequate histologic sectioning of prostate needle biopsies. Ann Diagn Pathol. 2013;17:357–360. [DOI] [PubMed] [Google Scholar]
- 20.Oxley J, & Varma M Berney D. Dataset for histopathology reports for prostatic carcinoma. The Royal College of Pathologists; 2016.
- 21.Parada D, Calvo N, Pen ~a K, et al. Systematic analysis of transrectal prostate biopsies using an ink method and specific histopathologic protocol: a prospective study. Prostate Cancer. 2011;2011:380249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolonen T, Isola J, Kaipia A, et al. Length of prostate biopsies is not necessarily compromised by pooling multiple cores in one paraffin block: an observational study. BMC Clin Pathol. 2015;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein J. The diagnosis and reporting of adenocarcinoma of the prostate in core needle biopsy specimens. Cancer. 1996;78:350–356. [DOI] [PubMed] [Google Scholar]
- 24.Boccon-Gibod L, Van der Kwast TH, Montironi R, et al. Handling and pathology reporting of prostate biopsies. Eur Urol. 2004;46:177–181. [DOI] [PubMed] [Google Scholar]
- 25.Novis DA, Zarbo RJ, Valenstein PA. Diagnostic uncertainty expressed in prostate needle biopsies. A College of American Pathologists Q-probes Study of 15,753 prostate needle biopsies in 332 institutions. Arch Pathol Lab Med. 1999;123:687–692. [DOI] [PubMed] [Google Scholar]
- 26.Browne TJ, Hirsch MS, Brodsky G, et al. Prospective evaluation of AMACR (P504S) and basal cell markers in the assessment of routine prostate needle biopsy specimens. Hum Pathol. 2004;35:1462–1468. [DOI] [PubMed] [Google Scholar]
- 27.Epstein J, Egevad L, Humphrey P, et al. Best practices recommendations in the application of immunohistochemistry in the prostate. Report from the International Society of Urologic Pathology Consensus Conference. Am J Surg Pathol. 2014;38:6–19. [DOI] [PubMed] [Google Scholar]
- 28.Varma M, Berney DM, Algaba F, et al. Prostate needle biopsy processing: a survey of laboratory practice across Europe. J Clin Pathol. 2013;66:120–123. [DOI] [PubMed] [Google Scholar]
- 29.PRSSU, Unit Costs of Health and Social Care 2019, Available at: https://www.psru.ac.uk. [Google Scholar]


