Abstract
Background:
The calcineurin inhibitor (CNI)-based immune maintenance regimen that is commonly used after renal transplantation has greatly improved early graft survival after transplantation; however, the long-term prognosis of grafts has not been significantly improved. The nephrotoxicity of CNI drugs is one of the main risk factors for the poor long-term prognosis of grafts. Sirolimus (SRL) has been employed as an immunosuppressant in clinical practice for over 20 years and has been found to have no nephrotoxic effects on grafts. Presently, the regimen and timing of SRL application after renal transplantation vary, and clinical data are scarce. Multicenter prospective randomized controlled studies are particularly rare. This study aims to investigate the effects of early conversion to a low-dose CNI combined with SRL on the long-term prognosis of renal transplantation.
Methods:
Patients who receive four weeks of a standard regimen with CNI + mycophenolic acid (MPA) + glucocorticoid after renal transplantation in multiple transplant centers across China will be included in this study. At week 5, after the operation, patients in the experimental group will receive an additional administration of SRL, a reduction in the CNI drug doses, withdrawal of MPA medication, and maintenance of glucocorticoids. In addition, patients in the control group will receive the maintained standard of care. The patients’ vital signs, routine blood tests, routine urine tests, blood biochemistry, serum creatinine, BK virus (BKV)/ cytomegalovirus (CMV), and trough concentrations of CNI drugs and SRL at the baseline and weeks 12, 24, 36, 48, 72, and 104 after conversion will be recorded. Patient survival, graft survival, and estimated glomerular filtration rate will be calculated, and concomitant medications and adverse events will also be recorded.
Conclusion:
The study data will be utilized to evaluate the efficacy and safety of early conversion to low-dose CNIs combined with SRL in renal transplant patients.
Trial registration:
Chinese Clinical Trial Registry, ChiCTR1800017277.
Keywords: Calcineurin inhibitor, Conversion, Rapamycin, Renal transplantation, Sirolimus
Introduction
Renal transplantation is the most effective method for the treatment of end-stage renal disease. Currently, the calcineurin inhibitor (CNI)-based immune maintenance regimen is commonly used in clinical practice. This regimen significantly improves the short-term prognosis after transplantation, and the incidence of acute rejection (AR) is significantly reduced. However, the long-term prognosis after renal transplantation has not been significantly improved.[1–4] Over time, the graft exhibits a progressive decline in renal function, which is histologically manifested as interstitial fibrosis and tubular atrophy.[5,6] The chronic fibrosis of the graft is the result of multiple factors, but the nephrotoxicity of CNIs is the major risk factor for chronic renal graft insufficiency.[7] Generally, CNI reduction or withdrawal can reduce CNI-related nephrotoxicity, improve the renal function of grafts, and enhance the long-term prognosis of patients.[8,9] Nonetheless, a very low-dose CNI or sudden CNI withdrawal may increase the incidence of AR. The search for a comprehensive immune maintenance regimen in combination with low-dose CNIs or CNI withdrawal without increasing serious adverse events (SAEs) is, therefore, a clinical difficulty and challenge that must be solved urgently.
The mammalian target of rapamycin inhibitor (mTORi) Sirolimus (SRL) has been used as an immunosuppressant in clinical practice for >20 years. It shows good efficacy and tolerability when combined with CNIs in renal transplant recipients. With its unique immunological characteristics and lack of nephrotoxic potential, SRL is used by clinical doctors as a substitute for CNIs in renal transplantation.[10,11] Nevertheless, the combination of SRL and standard doses of CNIs can adversely affect renal function due to the synergistic effect of SRL and CNIs. Various combined mTORi and reduced CNI regimens, hence, have been evaluated in many studies to determine the optimal balance between rejection prevention and graft function maintenance.[8,12–14]
The efficacy and safety of SRL in immune maintenance therapy after transplantation have been confirmed, but the optimal regimen for the application of SRL remains controversial. A regimen with the early postoperative use of mTORi combined with CNIs is significantly correlated with an increase in adverse events (AEs), such as delayed wound healing and increased AR.[15,16] The key is to find a balance among AR, CNI nephrotoxicity-related chronic renal graft insufficiency, SRL-related AEs, and the long-term prognosis of renal transplantation to develop the optimal immune maintenance regimen, improve the renal graft function of patients, and improve their long-term prognosis. Based on the above introduction, this study is a randomized controlled clinical trial. With regard to safety, it will explore the efficacy and safety of early conversion to a low-dose CNI combined with SRL in renal transplant patients.
Methods
Ethics and communication
The study will be conducted in multiple transplant centers in China and will follow the guidelines for human trials summarized in the latest version of the Declaration of Helsinki. The study protocol has obtained approval from the Ethics Committee (EC) of the leading site, namely, Beijing Chaoyang Hospital, Capital Medical University (No. 2018-Ke-188). Meetings will be convened regularly to monitor the study progress. Informed consent has been obtained from all participants. After the end of the study, our results will be published in medical journals. No patient's name should appear in any published article, and no data should be disclosed to persons other than the investigators and hospital EC members.
Study design
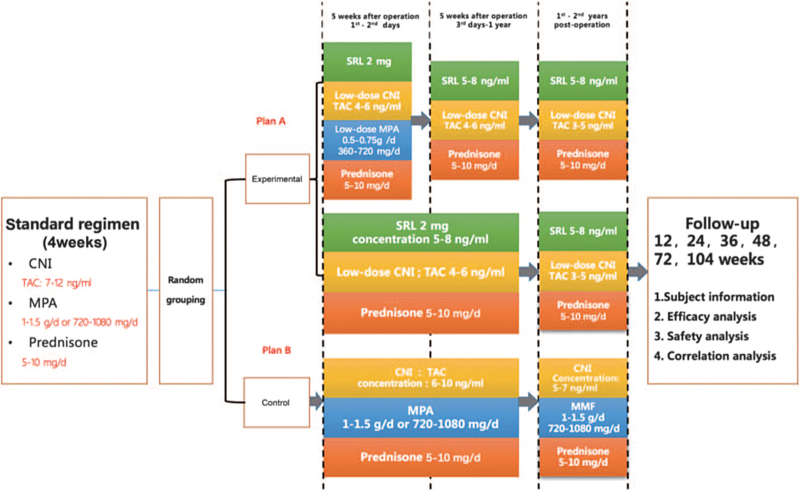
This is a multicenter prospective randomized controlled clinical trial. Patients who receive four weeks of a standard immune maintenance regimen after renal transplantation in multiple transplant centers are included in this study. Patients who meet the inclusion criteria will be randomized into a control group or an experimental group. At different time points after conversion, vital signs, routine blood tests, routine urine tests, blood biochemistry, serum creatinine levels, BK virus (BKV)/cytomegalovirus (CMV) ratios, trough concentrations of CNI drugs and SRL will be analyzed for the two groups. Patient survival, graft survival, and estimated glomerular filtration rate (eGFR) will be calculated. Concomitant medications and AEs will be recorded. The efficacy and safety of early conversion to a low-dose CNI combined with SRL in renal transplant patients will be investigated.
Study subjects
Enrolled patients who receive four weeks of standard regimen after renal transplantation will be advised to receive a standard dose of CNI + mycophenolic acid (MPA) + glucocorticoid for treatment (for CNI drugs, tacrolimus [TAC] or cyclosporin [CsA] may be selected: the plasma concentration of TAC is recommended to be maintained at 7 to 12 ng/mL; that of CsA, at 150 to 250 ng/mL; that of mycophenolate mofetil (MMF), at 0.5 to 1.5 g/day or that of enteric-coated mycophenolate sodium (EC-MPS), at 720 to 1080 mg/day; and that of prednisone, at 5 to 10 mg/day).
Inclusion criteria
Inclusion criteria were (1) voluntary signing of the informed consent form; (2) study subjects aged 18 to 70 years; (3) end-stage renal disease patients who are prepared to accept organ donation after the death of a citizen donor and renal transplantation from a living donor; (4) preoperative panel reactive antibody (PRA) ≤10%; (5) renal transplantation-naïve patients; and (6) glomerular filtration rate ≥45 mL/min.
Exclusion criteria
Exclusion criteria were (1) signs of severe systemic or local active infection; (2) chest X-ray examination showing signs of infiltration, cavitation or consolidation; (3) primary disease of the recipient diagnosed as focal segmental glomerulosclerosis or membranous proliferative glomerulonephritis; (4) patient with multiple organ transplantation;
(5) triglyceride ≥400 mg/dL (≥4.6 mmol/L) and total cholesterol ≥300 mg/dL (≥7.8 mmol/L); (6) 24-hours urine protein >500 mg/day; (7) biopsy-confirmed AR occurring within four weeks prior to enrollment; (8) patient intolerance of MMF 1.5 g/day within four weeks prior to enrollment; (9) delayed graft function (DGF) occurring and glomerular filtration rate <45 mL/min exhibited within four weeks prior to enrollment; (10) active hepatitis B or active hepatitis C; (11) human immunodeficiency virus (HIV) positivity; (12) usage of other investigational drugs within four weeks prior to enrollment; (13) history of a tumor within three years prior to the inclusion (except for well-treated basal cell skin cancer and squamous cell skin cancer); (14) renal graft from monozygotic twins; (15) readiness to use substances known to have strong interactions with SRL; (16) women in pregnancy, preparation for pregnancy or lactation; and (17) determination by the investigator for other factors not suitable to use investigational drugs.
Study groups
Group A: standard of care group
Week 5 after the operation – Year 1 after the operation: Reduced CNI + MPA + hormonal therapy.
For CNI drugs, TAC or CsA may be selected; the plasma concentration of TAC is recommended to be maintained at 6 to 10 ng/mL; the plasma concentration of CsA is recommended to be maintained at 150 to 250 ng/mL. MMF: 1 to 1.5 g/day; EC-MPS: 720 to 1080 mg/day; Prednisone: 5 to 10 mg/day.
Year 1 – Year 2 after the operation: Reduced CNI + MPA + hormonal therapy.
For CNI drugs, TAC or CsA may be selected: the plasma concentration of TAC is recommended to be maintained at 5 to 7 ng/mL; the plasma concentration of CsA is recommended to be maintained at 100 to 200 ng/mL. MMF: 1 to 1.5 g/day; EC-MPS: 720 to 1080 mg/day; Prednisone: 5 to 10 mg/day.
Group B: reduced CHI combined with the SRL group
The investigator may give the patients different regimens based on their conditions:
Regimen 1:
Day 1 – Day 2 at Week 5 after the operation: Reduced CNI + glucocorticoid + SRL + reduced MPA (for CNI drugs, TAC or CsA may be selected as follows: a 1/3 dose reduction from the current dose for TAC and a 1/2 dose reduction from the current dose for CsA [CsA and SRL should be concomitantly administered]; SRL: initially 2 to 3 mg/day; MMF: 0.5 to 1 g/day; prednisone: 5 to 10 mg/day).
Day 3 at Week 5 after the operation – Year 1 after the operation: Reduced CNI + glucocorticoid + SRL (for CNI drugs, TAC or CsA may be selected: the plasma concentration of TAC is recommended to be reduced to 4 to 6 ng/mL; the plasma concentration of CsA is recommended to be reduced to 70 to 150 ng/mL [CsA and SRL should be concomitantly administered]; SRL: 2 to 3 mg/day; the plasma concentration of SRL is maintained at 5 to 8 ng/mL; prednisone: 5 to 10 mg/day).
Year 1 – Year 2 after the operation: Reduced CNI + glucocorticoid + SRL (for CNI drugs, TAC or CsA may be selected: the plasma concentration of TAC is recommended to be reduced to 3 to 5 ng/mL; the plasma concentration of CsA is recommended to be reduced to 50 to 100 ng/mL [CsA and SRL should be concomitantly administered]; SRL: 2 to 3 mg/day; the plasma concentration of SRL is maintained at 5 to 8 ng/mL; prednisone: 5 to 10 mg/day).
Regimen 2:
Day 3 at Week 5 after the operation – Year 1 after operation: Reduced CNI + glucocorticoid + SRL (for CNI drugs, TAC or CsA may be selected: the plasma concentration of TAC is recommended to be reduced to 4 to 6 ng/mL; the plasma concentration of CsA is recommended to be reduced to 70 to 150 ng/mL [CsA and SRL should be concomitantly administered]; SRL: 2 to 3 mg/day; the plasma concentration of SRL is maintained at 5 to 8 ng/mL; prednisone: 5 to 10 mg/day).
Year 1 – Year 2 after the operation: Reduced CNI + glucocorticoid + SRL (for CNI drugs, TAC or CsA may be selected: the plasma concentration of TAC is recommended to be reduced to 3 to 5 ng/mL; the plasma concentration of CsA is recommended to be reduced to 50 to 100 ng/mL [CsA and SRL should be concomitantly administered]; SRL: 2 to 3 mg/day; the plasma concentration of SRL is maintained at 5 to 8 ng/mL; prednisone: 5 to 10 mg/day). See Figure 1.
Figure 1.
Study flow diagram. CNI: Calcineurin inhibitor; MPA: Mycophenolic acid; SRL: Sirolimus; TAC: Tacrolimus; MMF: Mycophenolate mofetil.
Study procedures
Screening/baseline: Week 0 – Week 4 after the operation
Prestudy treatment, medical history, and demographic information will be obtained. Meanwhile, urine pregnancy tests, HIV tests, hepatitis B (HBV) tests, hepatitis C (HCV) tests, electrocardiogram examinations, chest X-rays, vital sign examinations, physical examinations, routine blood tests, routine urine tests, blood biochemical tests, 24-hour urine protein tests, serum creatinine tests, pathological examination of transplanted kidneys (optional), evaluations of the Banff score and Chronic Allograft Damage Index (CADI) score (optional), calculations of the incidence of DGF, and calculations of eGFR will be carried out. The serum will be cryopreserved at −80°C for the detection of baseline. PRA and CYP3A4/CYP3A5 gene polymorphisms.
Follow-up
The follow-up will last from week 4 to week 104 after the operation. During this period, there will be six follow-up visits (weeks 12, 24, 36, 48, 72, and 104 after the operation). Vital sign examination, routine blood tests, routine urine tests, blood biochemical tests, serum creatinine tests, BKV/CMV tests, and trough concentration tests of CNI drugs and SRL will be carried out. Patient survival, graft survival, and eGFR will be calculated. Concomitant medications and AEs will be recorded.
Sample size calculation
According to the previous literature reports, the eGFR (calculated according to Modification of Diet in Renal Disease formula) of the experimental group and the control group after treatment were 51.0 and 50.5 ml·min−1·l.73m−2, respectively, and the estimated standard deviations of the two groups were 1.8 and 1.9, respectively. Take both sides α = 0.05, β = 1, assuming that the final study according to the actual enrollment of 2:1, the two groups need 450 and 225 patients, respectively, according to the shedding rate of 10%, the two groups need 500 and 250 patients, respectively, a total of 750 patients.
Randomization
All subjects will have two numbers, that is, the screening number and the randomization number. Each included subject will first be assigned a screening number, which is determined by the clinical trial unit. Subjects who meet the inclusion criteria will be assigned randomization numbers in ascending order according to the sequence of enrollment.
The randomization numbers of the subjects will be provided by the statistics department. The randomization numbers will be generated by randomization at a ratio of 2:1 between the experimental group and the control group, and the subjects will be randomized into an experimental group and a control group. The set block length and the initial seed parameters of random numbers and other parameters will be recorded in the randomization table. Subjects who prematurely withdraw from the trial will not be replaced.
Data collection
All data should be recorded and entered twice, separately. All data should be recorded in the case report form and immediately recorded in the Excel database. Any missing values should be verified to ensure the integrity and accuracy of the data. Data that are obviously abnormal or exceed the upper limit of normal (test result exceeding 20% of the normal value) must be explained by the physician. Withdrawals and AEs should be recorded in a timely manner.
Study endpoints
Primary endpoint: Changes in eGFR of patients at week 104 after the operation from baseline occurred.
Secondary endpoints: Changes in renal function, the incidence of AR, rate of graft loss, 24-hour urine protein, Banff score, CADI score, and BKV/CMV infection rate at weeks 12, 24, 36, 48, 72, and 104 after the operation from baseline occurred.
Statistical analysis
The efficacy analysis of the trial will be performed according to the intent-to-treat population and the per-protocol population.
Subject information
The demographic data and baseline characteristics will be summarized using descriptive statistics.
Efficacy evaluation
The t-test/rank-sum test will be used for the calculation of qualitative data; the chi-square test will be used for the calculation of rates.
Safety evaluation
The incidence of AE will be studied. The safety analysis will be performed according to the safety set. The safety set refers to all subjects who receive the administration of at least some doses of the study drugs.
Correlation analysis
Correlation analysis will be performed using the Pearson or Spearman test.
Continuous variables will be described using means with standard deviations (mean ± standard deviation) and will be analyzed using the independent samples t test. Categorical variables will be analyzed using the chi-squared test or Fisher exact test. The confidence interval will be set at 95%, and P < 0.05 will be considered statistically significant. All analyses will be performed using SPSS software V24 (SPSS, Chicago, IL, USA).
Discussion
Allogeneic renal transplantation is an effective measure to solve end-stage renal disease. Extended criteria donor kidneys have been used in recent years, but the shortage of organ sources remains a major challenge. AR, DGF, chronic renal allograft dysfunction, toxicity, and other side effects caused by the use of nonspecific immunosuppressive agents are currently the main obstacles to the survival of transplanted kidneys and the quality of life of transplant recipients. CNI drugs greatly reduce the incidence of postoperative AR, but their nephrotoxicity-related graft injury is the main risk factor for a poor long-term prognosis.[6,17] Thus, the exploration of novel immunosuppressive regimens yielding immune tolerance induction has important clinical value.
With unique immunosuppressive effects and no nephrotoxicity, an mTOR receptor blocker (SRL, a marketed drug in China) has become an ideal therapeutic drug to replace CNIs. At present, it is widely used in studies with combined CNI reduction or withdrawal after renal transplantation. However, the increased risk of AR after conversion to SRL is an important issue that remains to be solved. A study by Ekberg et al[18] showed that the biopsy-confirmed AR rates in the SRL group and the TAC group were 37.2% and 12.3%, respectively. Studies on the role of SRL in high-risk populations suggested that the incidence of AR was between 14% and 19%.[19–21] In addition, SRL is closely related to delayed wound healing, hyperlipidemia, aggravated urinary protein, etc. There is currently no conclusion on the optimal regimen for immune maintenance therapy with SRL after renal transplantation. The key is to find a balance among AR, CNI nephrotoxicity-related chronic renal graft insufficiency, SRL-related AEs, and the long-term prognosis of renal transplantation to develop the optimal immune maintenance regimen, improve the renal graft function of patients, and improve their long-term prognosis. The safety and efficacy of early conversion to SRL after renal transplantation have been confirmed, but comparisons between SRL combined with CNI reduction and SRL combined with CNI withdrawal have rarely been reported. A randomized controlled study by Fleming et al[22] on African Americans reported that 1 year after the conversion from CNIs to SRL, the eGFR of the CNI withdrawal group was significantly increased compared with that of the CNI reduction group (72 mL/ min vs. 56 mL/min, P = 0.03), and the eGFR level was significantly increased from baseline (12 mL/min vs. 5 mL/min, P = 0.03). The infection, AR, death, or graft loss rates of the CNI withdrawal group were higher than those of the CNI reduction group, but the differences were not statistically significant (P < 0.05). Tedesco-Silva et al[23] believed that compared with CNI-based immune maintenance therapy, conversion to SRL-based immune maintenance therapy within 1 year after surgery significantly improved renal function (P > 0.05). The conversion to SRL 1 year after the operation did not significantly improve renal function, and the differences between the conversion group and the standard of care group were not statistically significant (P > 0.05). This may be related to the formation of irreversible renal damage caused by the long-term application of CNIs after renal transplantation. Most study results support early conversion to SRL, but the conversion should not “start from the beginning.” This is mainly based on the following four aspects: first, the SRL-based immune maintenance regimen lacks sufficient efficacy in preventing the occurrence of AR [18,24]; second, determining the nephrotoxic effect of SRL combined with CNIs as the initial immune maintenance therapy on the transplanted kidney is difficult[25,26]; third, the early application of mTORi after surgery is closely related to some serious adverse reactions, such as delayed wound healing and DGF[27]; and fourth, late conversion to SRL, especially conversion six months after kidney transplantation, does not significantly improve renal function, and the incidence of adverse reactions is high.[28] The studies on the early conversion to SRL that have been published so far greatly vary in their inclusion and exclusion criteria, conversion time, and conversion regimen (e.g., sudden conversion or gradual conversion, SRL, or everolimus). In these studies, renal function, the incidence of AR, and the incidence of AEs have varied greatly after the conversion from CNIs to SRL. However, the safety and efficacy of SRL in immune maintenance therapy after renal transplantation have been validated in all the studies, regardless of whether the conversion has been active or passive. As SRL resistance is not as strong as CNI resistance, the early maintenance of the SRL concentration at 4 to 8 ng/mL after surgery will significantly increase the incidence of rejection. Presently, it is advocated that the conversion of CNI drugs should be conducted at 3 to 6 months after transplantation. Most scholars believe that early conversion therapy can reduce the nephrotoxicity of CNI drugs, help improve renal graft function, and significantly increase the glomerular filtration rate.[14,29,30]
Nonetheless, multicenter randomized controlled clinical trials investigating SRL in the treatment of Chinese renal transplant patients are still scarce. Moreover, the efficacy and safety of reduced CNIs combined with SRL in the treatment of renal transplant patients remain unclear. Our site, hence, proposes to cooperate with multiple transplant centers across China to conduct a randomized controlled clinical trial to evaluate the efficacy and safety of early conversion to low-dose CNIs combined with SRL in renal transplant patients.
Funding
The study was funded by the China International Medical Foundation (No. RUPUS-ISRT-20180114).
Conflicts of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Footnotes
How to cite this article: Zheng X, Zhang W, Zhou H, Cao R, Shou Z, Zhang S, Cheng Y, Chen X, Ding C, Tang Z, Li N, Shi S, Zhou Q, Chen Q, Chen G, Chen Z, Zhou P, Hu X, Zhang X, Na N, Wang W. A randomized controlled trial to evaluate efficacy and safety of early conversion to a low-dose calcineurin inhibitor combined with sirolimus in renal transplant patients. Chin Med J 2022;135:1597–1603. doi: 10.1097/CM9.0000000000001866
References
- 1.Overbeck I, Bartels M, Decker O, Harms J, Hauss J, Fangmann J. Changes in quality of life after renal transplantation. Transplant Proc 2005; 37:1618–1621. doi: 10.1016/j.transproceed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 2006; 1:532–538. doi: 10.2215/cjn.01130905. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. United states renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012; 59: (suppl 1 A7): e1–e420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Annual Data Report of the US Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR). Preface. Am J Transplant 2013; 13: (suppl 1): 1–7. doi: 10.1111/ajt.12028. [DOI] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 6.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med 2003; 349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 7.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant 2008; 22:1–15. doi: 10.1111/j.1399-0012.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 8.Srinivas TR, Meier-Kriesche HU. Minimizing immunosuppression, an alternative approach to reducing side effects: objectives and interim result. Clin J Am Soc Nephrol 2008; 3: (suppl 2): S101–S116. doi: 10.2215/cjn.03510807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekberg H, Bernasconi C, Tedesco-Silva H, Vítko S, Hugo C, Demirbas A, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant 2009; 9:1876–1885. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 10.Stallone G, Infante B, Schena A, Battaglia M, Ditonno P, Loverre A, et al. Rapamycin for treatment of chronic allograft nephropathy in renal transplant patients. J Am Soc Nephrol 2005; 16:3755–3762. doi: 10.1681/asn.2005060635. [DOI] [PubMed] [Google Scholar]
- 11.Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 2011; 92:410–418. doi: 10.1097/TP.0b013e318224c12d. [DOI] [PubMed] [Google Scholar]
- 12.Silva HT, Jr, Felipe CR, Garcia VD, Neto ED, Filho MA, Contieri FL, et al. Planned randomized conversion from tacrolimus to sirolimus-based immunosuppressive regimen in de novo kidney transplant recipients. Am J Transplant 2013; 13:3155–3163. doi: 10.1111/ajt.12481. [DOI] [PubMed] [Google Scholar]
- 13.Guba M, Pratschke J, Hugo C, Krämer BK, Pascher A, Pressmar K, et al. Early conversion to a sirolimus-based, calcineurin-inhibitor-free immunosuppression in the SMART trial: observational results at 24 and 36 months after transplantation. Transpl Int 2012; 25:416–423. doi: 10.1111/j.1432-2277.2012.01432.x. [DOI] [PubMed] [Google Scholar]
- 14.Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 2009; 9:1115–1123. doi: 10.1111/j.1600-6143.2009.02615.x. [DOI] [PubMed] [Google Scholar]
- 15.Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int 2011; 24:1216–1230. doi: 10.1111/j.1432-2277.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuypers DR. Benefit-risk assessment of sirolimus in renal transplantation. Drug Saf 2005; 28:153–181. doi: 10.2165/00002018-200528020-00006. [DOI] [PubMed] [Google Scholar]
- 17.Yates PJ, Nicholson ML. The aetiology and pathogenesis of chronic allograft nephropathy. Transpl Immunol 2006; 16:148–157. doi: 10.1016/j.trim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 19.Mulay AV, Cockfield S, Stryker R, Fergusson D, Knoll GA. Conversion from calcineurin inhibitors to sirolimus for chronic renal allograft dysfunction: a systematic review of the evidence. Transplantation 2006; 82:1153–1162. doi: 10.1097/01.tp.0000237101.58974.43. [DOI] [PubMed] [Google Scholar]
- 20.Chang GJ, Mahanty HD, Vincenti F, Freise CE, Roberts JP, Ascher NL, et al. A calcineurin inhibitor-sparing regimen with sirolimus, mycophenolate mofetil, and anti-CD25 mAb provides effective immunosuppression in kidney transplant recipients with delayed or impaired graft function. Clin Transplant 2000; 14:550–554. doi: 10.1034/j.1399-0012.2000.140606.x. [DOI] [PubMed] [Google Scholar]
- 21.Hong JC, Kahan BD. A calcineurin antagonist-free induction strategy for immunosuppression in cadaveric kidney transplant recipients at risk for delayed graft function. Transplantation 2001; 71:1320–1328. doi: 10.1097/00007890-200105150-00025. [DOI] [PubMed] [Google Scholar]
- 22.Fleming JN, Taber DJ, Pilch NA, McGillicuddy JW, Srinivas TR, Baliga PK, et al. A randomized, prospective comparison of transition to sirolimus-based CNI-minimization or withdrawal in African American kidney transplant recipients. Clin Transplant 2016; 30:528–533. doi: 10.1111/ctr.12718. [DOI] [PubMed] [Google Scholar]
- 23.Tedesco-Silva H, Peddi VR, Sánchez-Fructuoso A, Marder BA, Russ GR, Diekmann F, et al. Open-label, randomized study of transition from tacrolimus to sirolimus immunosuppression in renal allograft recipients. Transplant Direct 2016; 2:e69.doi: 10.1097/txd.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flechner SM, Glyda M, Cockfield S, Grinyó J, Legendre C, Russ G, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 2011; 11:1633–1644. doi: 10.1111/j.1600-6143.2011.03573.x. [DOI] [PubMed] [Google Scholar]
- 25.Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: A 3-year randomized, multicenter, phase III study. Transplantation 2005; 80:244–252. doi: 10.1097/01.tp.0000164352.65613.24. [DOI] [PubMed] [Google Scholar]
- 26.Knight RJ, Kahan BD. The place of sirolimus in kidney transplantation: Can we reduce calcineurin inhibitor renal toxicity? Kidney Int 2006; 70:994–999. doi: 10.1038/sj.ki.5001644. [DOI] [PubMed] [Google Scholar]
- 27.Cravedi P, Ruggenenti P, Remuzzi G. Sirolimus for calcineurin inhibitors in organ transplantation: contra. Kidney Int 2010; 78:1068–1074. doi: 10.1038/ki.2010.268. [DOI] [PubMed] [Google Scholar]
- 28.Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 2009; 87:233–242. doi: 10.1097/TP.0b013e3181927a41. [DOI] [PubMed] [Google Scholar]
- 29.Egbuna OI, Davis RB, Chudinski R, Pavlakis M, Rogers C, Molakatalla P, et al. Outcomes with conversion from calcineurin inhibitors to sirolimus after renal transplantation in the context of steroid withdrawal or steroid continuation. Transplantation 2009; 88:684–692. doi: 10.1097/TP.0b013e3181b27d44. [DOI] [PubMed] [Google Scholar]
- 30.Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002; 74:1070–1076. doi: 10.1097/00007890-200210270-00002. [DOI] [PubMed] [Google Scholar]