Abstract
Background:
To date, there is no effective medicine to treat coronavirus disease 2019 (COVID-19), and the antiviral efficacy of arbidol in the treatment for COVID-19 remained equivocal and controversial. The purpose of this study was to evaluate the efficacy and safety of arbidol tablets in the treatment of COVID-19.
Methods:
This was a prospective, open-label, controlled and multicenter investigator-initiated trial involving adult patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Patients were stratified 1:2 to either standard-of-care (SOC) or SOC plus arbidol tablets (oral administration of 200 mg per time, three times a day for 14 days). The primary endpoint was negative conversion of SARS-CoV-2 within the first week. The rates and 95% confidential intervals were calculated for each variable.
Results:
A total of 99 patients with laboratory-confirmed SARS-CoV-2 infection were enrolled; 66 were assigned to the SOC plus arbidol tablets group, and 33 to the SOC group. The negative conversion rate of SARS-CoV-2 within the first week in patients receiving arbidol tablets was significantly higher than that of the SOC group (70.3% [45/64] vs. 42.4% [14/33]; difference of conversion rate 27.9%; 95% confidence interval [CI], 7.7%–48.1%; P = 0.008). Compared to those in the SOC group, patients receiving arbidol tablets had a shorter duration of clinical recovery (median 7.0 days vs. 12.0 days; hazard ratio [HR]: 1.877, 95% CI: 1.151–3.060, P = 0.006), symptom of fever (median 3.0 days vs. 12.0 days; HR: 18.990, 95% CI: 5.350–67.410, P < 0.001), as well as hospitalization (median 12.5 days vs. 20.0 days; P < 0.001). Moreover, the addition of arbidol tablets to SOC led to more rapid normalization of declined blood lymphocytes (median 10.0 days vs. 14.5 days; P > 0.05). The most common adverse event in the arbidol tablets group was the elevation of transaminase (5/200, 2.5%), and no one withdrew from the study due to adverse events or disease progression.
Conclusions:
SOC plus arbidol tablets significantly increase the negative conversion rate of SARS-CoV-2 within the first week and accelerate the recovery of COVID-19 patients. During the treatment with arbidol tablets, we find no significant serious adverse events.
Trial registration:
Chinese Clinical Trial Registry, NCT04260594, www.clinicaltrials.gov/ct2/show/NCT04260594?term=NCT04260594&draw=2&rank=1
Keywords: Arbidol, Coronavirus disease 2019 (COVID-19), Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread to more than 200 countries or regions. As of September 15, 2021, there have been 226,033,125 infected cases and 4,666,015 deaths worldwide.[1] Despite the rapid global spread, no specific antiviral drugs have demonstrated efficacy in the prevention or treatment of COVID-19. Several agents like remdesivir, lopinavir-ritonavir, and hydroxychloroquine, have shown antiviral activity against SARS-CoV-2 in vitro or two other coronavirus diseases, including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). However, none of these drugs reveal clinical benefits in the trials with COVID-19.[2–4]
Arbidol, a small indole-derivative molecule, has been licensed in China for prophylaxis and treatment of influenza and other respiratory viral infections, with no major adverse effects reported.[5,6] Previous studies have pointed out that arbidol has a dual pharmacological action: it has a specific effect on respiratory viruses, and at the same time has an immune-stimulating effect, which can induce serum interferon and activate phagocytes. Arbidol has been shown to display antiviral activity against a number of viruses in vitro and/or in vivo, including influenza viruses A, B, and C, respiratory syncytial virus, severe acute respiratory syndrome coronavirus (SARS-CoV), as well as SARS-CoV-2.[7,8] Retrospective studies of real-world data showed arbidol was associated with a reduction in mortality among hospitalized COVID-19, and the combination with lopinavir/ritonavir could significantly increase the nucleic acid negative conversion rate, shorten the nucleic acid positive time, and improve the condition of chest infection.[9] In a study enrolling fifty patients, arbidol was found superior to lopinavir/ritonavir in treating COVID-19, while another prospective trial reported favipiravir did not significantly improve the clinical recovery rate at day 7 compared with arbidol.[10,11] Due to the study design and sample size limitation, the antiviral efficacy of arbidol in the treatment of COVID-19 remained equivocal and controversial. Since convincing evidence is still lacking, we conducted an open-label, controlled, and multicenter clinical trial to assess the efficacy and safety of arbidol tablets in adult patients with COVID-19.
Methods
Ethics
The study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (No. 2020-28-3). Informed consent was obtained from all patients. The trial was conducted in accordance with the Declaration of Helsinki and the principles of the International Coordinating Conference on Quality Management of Drug Clinical Trials. Clinical data were recorded by clinical research coordinators followed by query from clinical research associates. Besides, source document verification was performed to ensure the authenticity and integrity of the data.
Patients
Inclusion criteria: (1) aged 18 to 65 years old (including 18 and 65 years); (2) male and non-pregnant female; (3) respiratory tract specimens or hematology samples detected positive results of SARS-CoV-2 by real-time reverse transcription-polymerase chain reaction (RT-PCR); (4) mild clinical status, defined as having mild clinical symptoms but no signs of pneumonia on imaging or moderate clinical status, defined as having fever, respiratory symptoms, and pneumonia on imaging or severe clinical status, defined as having an oxygen saturation of 93% or less at ambient air or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen at or below 400 mmHg, which can be rectified by oxygen inhalation through nasal catheter or face mask. Exclusion criteria included a physician decision that involvement in the trial was not in the patient's best interest, known allergic reaction and/or severely allergic to arbidol, blood system dysfunction (platelet count <100 × 109/L, hemoglobin level <90 g/L), severe liver dysfunction (total bilirubin level >2 times the normal upper limit, aspartic aminotransferase and alanine aminotransferase levels >3 times the normal upper limit), severe renal dysfunction (serum creatinine >1.5 times the upper limit of normal value, calculated creatinine clearance rate <50 ml/min), treated with arbidol tablets before admission, history of severe heart disease or clinically significant arrhythmia considered unsafe for the trial.
Trial design and oversight
This was an investigator-initiated, prospective, open-label, controlled, and multicenter trial conducted from February 6 to March 12, 2020 in a total of 14 hospitals. Patients meeting eligibility criteria were assigned in a 1:2 ratio (block randomization) to receive either standard-of-care (SOC) or SOC plus arbidol tablets (oral administration of 200 mg per time, three times a day for 14 days, provided by CSPC Ouyi Pharmaceutical Co., Ltd., Shijiazhuang, Hebei Province, China). SOC included oxygen inhalation, antibiotics, and traditional medicine, and the use of α-interferon and oseltamivir was allowed.
Clinical and laboratory monitoring
Serial oropharyngeal swab samples were obtained on day 1 (before administration of arbidol tablets), 7, 14, and 21 until discharge or disease progression occurred and tested by real-time RT-PCR to determine positive or negative results for SARS-CoV-2 at each site's local center for disease control and prevention according to the WHO recommendations. During treatment and post-treatment follow-up, scheduled visits were performed on days 7, 14, 21 and 28. Patients’ vital signs, respiratory symptoms, clinical laboratory testing and 12-lead electrocardiogram were assessed on screening (day 1) and each scheduled visit. Administration records of arbidol tablets and adverse events were monitored daily. Chest computed tomographic scan was evaluated on the first day and the end of the trial. Clinical data were recorded on paper case record forms and then entered into an electronic database by the clinical supervisor for review and confirmation.
Outcome measures
The primary endpoint was the negative conversion of SARS-CoV-2 within the first week, defined as the percentage of viral negative changes detected in pathogen nucleic acid on day 7 after administration. Secondary endpoints included viral clearance rate in the second week, overall viral negative conversion rate, clinical recovery rate, and the alleviation of clinical symptoms. Clinical recovery was defined as clinical symptom remission and two consecutively negative nucleic acid detections (24 h interval for each time). The symptom remission was defined as the disappearance of fever, cough, dyspnea, myalgia, and other related respiratory symptoms; or the improvement of oxygen saturation (no adjuvant oxygen therapy or oxygen saturation >95% on room air). The defervescence was defined as axillary temperature ≤37.0°C for at least 48 h. The changes in laboratory parameters were mainly focused on lymphocyte count in peripheral blood. Other secondary outcomes included all-cause mortality and length of hospital stay. Safety endpoints included adverse events during treatment, severe adverse events, and early discontinuation of treatment. The adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
The trial was designed to enroll a total of 384 subjects to ensure a power of 80% under a one-sided type I error of 2.5%. The sample size was based on the alternative hypothesis of a 15% increase in the virus nucleic acid negative rate. The allocation ratio between arbidol tablets and control group was 2:1, and a 20% dropout rate has been considered in the original design.
Statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). Subject allocation, demographic data and baseline characteristics were described in the statistical description part. The number of cases and proportions were used for qualitative variables. Chi-square, adjusted Chi-square, and Fisher's exact tests were used for hypothesis testing of qualitative variables. For quantitative variables, mean ± standard deviation or median (IQR) were used for description, and t-test or Wilcoxon rank-sum test was used for hypothesis testing. Efficacy analysis was based on the Full Analysis Set, including subjects with at least one time of trial drug usage and at least one time of primary efficacy indicator tested. Binary outcomes were tested with the Chi-square test and Fisher's exact test. Rates and 95% confidence interval (CI) for those binary indicators were also reported. Virus clearance time (the first negative time of two consecutive negative results), symptom remission time and clinical recovery time (the longer period of either symptom remission or virus clearance time), were evaluated with survival analysis. Kaplan-Meier curves were plotted and the log-rank test was used for between-groups comparison. Cox regressions were used for hazard ratio (HR) and 95% CI estimation, with or without baseline variables adjusted. In the sensitivity analysis, we tested results for excluding subjects with negative nucleic acid results within the first three days after enrollment or excluding those subjects with some groups of drug combinations. The safety analysis set was used for the overall analysis to summarize the adverse events and serious adverse events which occurred during the treatment of all patients. The numbers of cases and events of adverse reactions and serious adverse reactions were calculated. All adverse events were coded according to the Medical Dictionary for Regulatory Activities.
Results
Patients
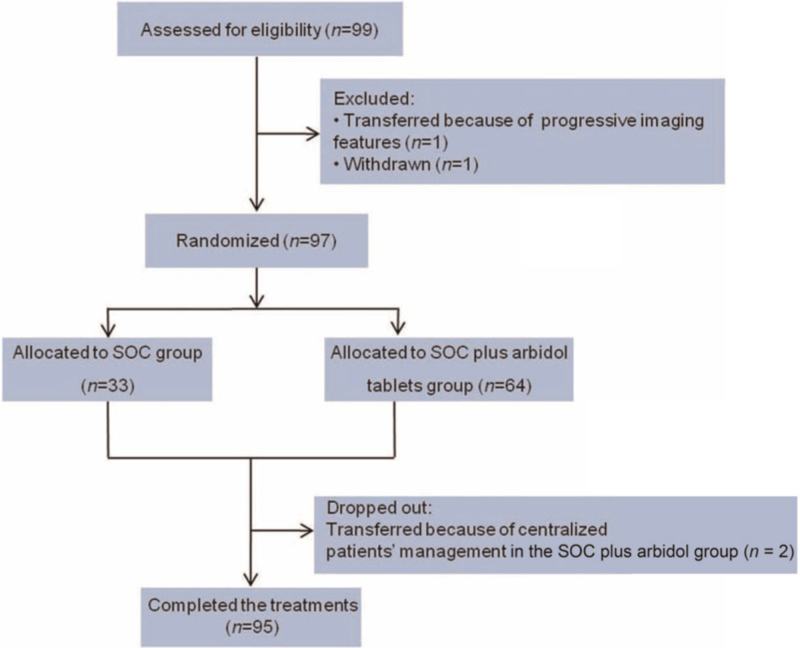
By February 19, 2020, the mid-term analysis of the study showed that the primary outcome was reached in advance. A total of 99 patients were enrolled by February 19, 2020, with 66 assigned to receive SOC plus arbidol tablets and 33 patients to SOC, 97 received randomization and 95 of them completed the trial [Figure 1]. The median age of the patients was 48 years and 44 (44/97, 45.4%) were male. In the arbidol tablets group, 59 (92.2%) patients had mild to moderate COVID-19 and only 5 (7.8%) had severe COVID-19 at the time of screening. While in the SOC group, all patients were moderate. The numbers of patients with concomitant diseases were similar in the SOC plus arbidol tablets group (23/64,35.9%) and the SOC group (11/33,33.3%, Table 1).
Figure 1.
Flow diagram of the study on arbidol hydrochloride In adults with COVID-19. SOC: Standard of care; COVID-19: Coronavirus disease 2019.
Table 1.
Baseline characteristics of the COVID-19 patients receiving SOC plus arbidol or SOC alone.
| Characteristics | Total (N = 97) | SOC plus arbidol (n = 64) | SOC (n = 33) | t or χ2 values | P values |
| Age (years) | 46.51 ± 11.23 | 46.16 ± 11.21 | 47.18 ± 11.42 | −0.420∗ | 0.672 |
| Male | 44/97 (45.4) | 27/64 (42.2) | 17/33 (51.5) | 0.764† | 0.382 |
| Han nationality | 97 (100.0) | 64 (100.0) | 33 (100.0) | ||
| Disease severity | –‡ | 0.163 | |||
| Mild and moderate | 92/97 (94.8) | 59/64 (92.2) | 33 (100.0) | ||
| Severe | 5/97 (5.2) | 5/64 (7.8) | 0 (0) | ||
| Coexisting diseases | 34/97 (35.1) | 23/64 (35.9) | 11/33 (33.3) | 0.065† | 0.799 |
| Temperature (°C) | 36.69 ± 0.45 | 36.59 ± 0.40 | 36.87 ± 0.48 | −3.040 | 0.003 |
| Symptoms | |||||
| Fever | 41/97 (42.3) | 21/64 (32.8) | 20/33 (60.6) | 6.583† | 0.009 |
| Acute hypoxia | 9/97 (9.3) | 5/64 (7.8) | 4/33 (12.1) | 0.480† | 0.488 |
| Oxygen therapy | 13/97 (13.4) | 8/64 (12.5) | 4/33 (12.1) | 0.003† | 0.957 |
| Lab examination abnormal | |||||
| WBC | 4/51 (7.8) | 2/35 (5.7) | 2/16 (12.5) | –‡ | 0.581 |
| LYMPH | 17/51 (33.3) | 13/35 (37.1) | 4/16 (25.0) | –‡ | 0.527 |
| PLT | 6/51 (11.8) | 4/35 (11.4) | 2/16 (12.5) | –‡ | 1.000 |
| ALT | 7/38 (18.4) | 7/30 (23.3) | 0/8 (0) | –‡ | 0.307 |
| AST | 5/38 (13.2) | 4/30 (13.3) | 1/8 (12.5) | –‡ | 1.000 |
| ALB | 10/38 (26.3) | 10/30 (33.3) | 0/8 (0) | –‡ | 0.082 |
| LDH | 6/36 (16.7) | 5/30 (16.7) | 1/6 (16.7) | –‡ | 1.000 |
| CK | 3/36 (8.3) | 2/30 (6.7) | 1/6 (16.7) | –‡ | 0.431 |
| CK-MB | 2/36 (5.6) | 2/30 (6.7) | 0/6 (0) | –‡ | 1.000 |
| BUN | 4/37 (10.8) | 4/30 (13.3) | 0/7 (0) | –‡ | 0.570 |
| CRE | 2/37 (5.4) | 2/30 (6.7) | 0/7 (0) | –‡ | 1.000 |
| CRP | 20/52 (38.5) | 17/37 (45.9) | 3/15 (20.0) | –‡ | 0.118 |
| CT abnormal | 80/81 (98.8) | 49/50 (98.0) | 31/31 (100.0) | –‡ | 1.000 |
Data are presented as mean ± standard deviation or n (%). ∗t value.†χ2 value. ‡Fisher's exact test. ALB: Albumin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BUN: Blood urea nitrogen; CK: Creatine kinase; COVID-19: Coronavirus disease 2019; CRE: Creatinine; CRP: C-reactive protein; CT: Computed tomography; LDH: Lactate dehydrogenase; LYMPH: Lymphocyte count; PLT: Platelet count; SOC: Standard of care; WBC: White blood cell.
Primary outcome
The negative conversion rate of SARS-CoV-2 within the first week in the group of SOC plus arbidol tablets was 70.3% (45/64), which was significantly higher than that of the SOC group (14/33, 42.4%; difference of conversion rate 27.9%; 95% confidence interval (CI): 7.7%–48.1%; P = 0.008).
Secondary outcomes
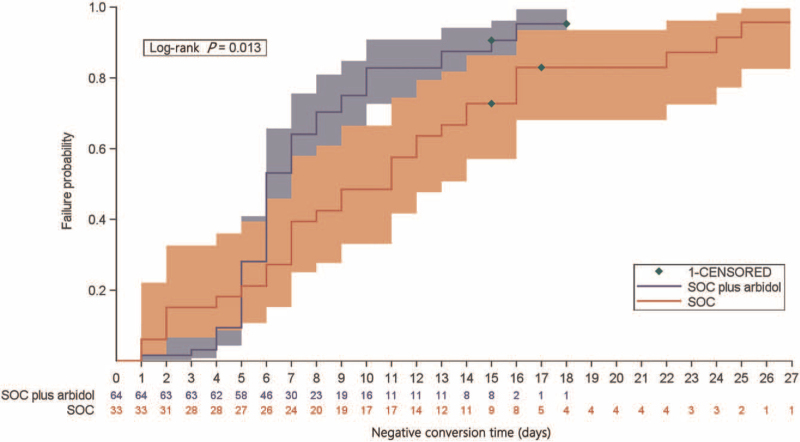
The negative conversion rate within the second week was 90.6% (58/64) in the SOC plus arbidol tablets group, which was significantly higher than that in the SOC group (24/33 [72.7%]; difference of conversion rate 17.9%; 95% CI: 1.1%–34.7%; P = 0.021). The overall negative conversion rate was 92.2% (59/64, 95% CI: 82.7%–97.4%) in the SOC plus arbidol tablets group and 93.9% (31/33, 95% CI: 79.8%–99.3%) in the SOC group (P > 0.05). The median negative conversion time was numerically shorter in patients receiving SOC plus arbidol tablets than those in the SOC group (median 6.0 days vs. 11.0 days; difference 5.0 days; HR: 1.724, 95% CI: 1.078–2.758; P = 0.013; Figure 2). At the last visit, there was no significant difference in the clinical recovery rate between the two groups (arbidol tablets plus SOC group: vs. SOC group: 87.5% [56/64] vs. 84.8% [28/33]; P > 0.05). However, patients receiving arbidol tablets had a shorter duration of clinical recovery than those in the SOC group (median 7.0 days vs. 12.0 days; difference 5.0 days; HR: 1.877, 95% CI: 1.151–3.060; P = 0.006; Supplementary Figure 1).
Figure 2.
Kaplan-Meier curve of the time to negative conversion of SARS-CoV-2 in SOC plus arbidol tablets versus SOC group. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; SOC: Standard of care.
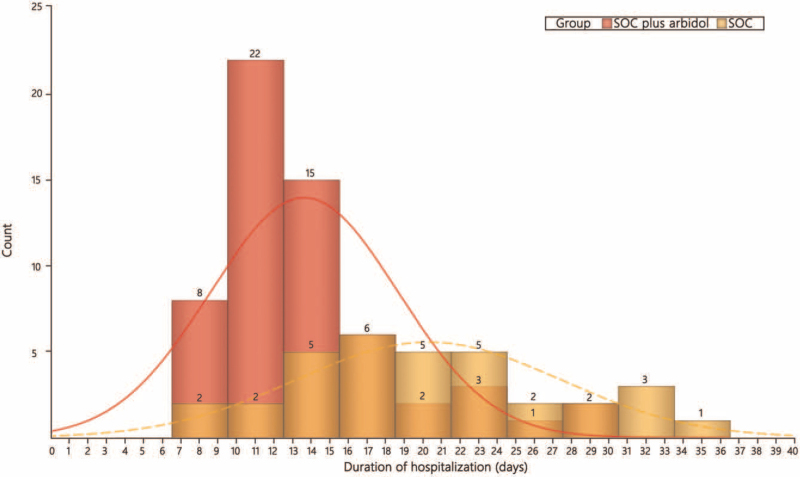
Fever was the most common symptom at the onset of illness. Patients assigned to arbidol tablets plus SOC group had a shorter duration of fever than those in SOC group (median 3.0 days vs. 12.0 days; difference 9.0 days; HR: 18.990, 95% CI: 5.350–67.410, P < 0.001; Supplementary Figure 2). Moreover, patients in the arbidol tablets plus SOC group had a shorter duration from enrollment to hospital discharge (median 12.5 days vs. 20.0 days; difference 7.5 days, P < 0.001; Figure 3).
Figure 3.
Comparison of hospitalization duration between SOC plus arbidol tablets and SOC group. SOC: Standard of care.
No cases in arbidol tablets plus SOC or SOC group showed disease progression in the process.
Changes of blood lymphocyte count
The declined count of blood lymphocytes was found in 13 cases on enrollment in arbidol tablets plus SOC group, and 10 (76.9%) recovered to normal level at the end of the trial. Compared with SOC alone, the addition of arbidol tablets to SOC led to more rapid normalization of the declined blood lymphocytes (median 10.0 days vs. 14.5 days; difference 4.5 days; P > 0.05).
Results from the extra single-arm analysis
On February 19, 2020, National Guidelines for the Diagnosis and Treatment of COVID-19 (Version 6) were published, in which arbidol was recommended as one of the therapeutic agents. It has been quite difficult to enroll participants into the control group since then. Investigators of the trial decided to recruit the rest of the cases to arbidol tablets group, thus another 137 patients were admitted from February 20, 2020 to March 12, 2020. As a whole, a total of 203 patients were assigned to receive arbidol tablets treatment, of whom one did not meet eligibility criteria, one withdrew and one failed to take at least one primary efficacy index. Among these 200 patients, ten withdrew or were transferred and 190 cases finally completed the trial, and a single-arm analysis was performed [Supplementary Figure 3]. Baseline demographic, clinical characteristics, and laboratory parameters of the patients receiving arbidol tablets are shown in Supplementary Table 1.
In the single-arm analysis, the negative conversion rate within the first week among patients receiving arbidol tablets was 73.0% (146/200; 95% CI: 66.3%–79.0%), and became 89.5% (179/200, 95% CI: 84.4%–93.4%) at the end of the second week. At the end of the treatment, the result of the pathogen nucleic acid test in 182 subjects turned negative, the overall negative conversion rate was 91.0% (95% CI: 86.2%–94.6%), and the median negative conversion time was 6.0 days (95% CI: 6.0–7.0 days). The rate of fever was 100.0% (95% CI: 90.0%–100.0%) in the first week, and the median time of fever was 3.0 days (95% CI: 2.0–3.0 days). The overall clinical symptom remission rate was 97.3% (146/150, 95% CI: 93.3%–99.3%), and the median clinical symptom remission time was 4.0 days (95% CI: 4.0–5.0 days). The clinical discovery rate was 86.0% (172/200, 95% CI: 80.4%–90.5%), and the median clinical discovery time was 7.0 days (95% CI: 6.0–7.0 days). The median length of hospitalization was 11 days (IQR: 6 days). Besides, the median time to normalization of blood lymphocyte count was 10.0 days (95% CI: 8.0–14.0 days).
Furthermore, 113 patients were also treated with traditional medicine, oseltamivir and atomized interferon besides arbidol tablets in our study. The negative conversion rate in the first week of these patients was 72.6% (82/113) and the overall negative conversion rate was 93.8% (106/113), which were similar to those of the whole population (both P > 0.05, respectively), indicating the combined treatments exerted no influence on the results.
Safety
Between enrollment and final visit, a total of 18 patients who received arbidol tablets and five in the SOC group reported adverse events [Table 2]. No patients reported serious adverse events and no one withdrew from the study due to adverse events or disease progression. The most common adverse event was the elevation of transaminase in patients treated with arbidol tablets (5/200, 2.5%), which were all judged by the investigators to be related to the trial medication.
Table 2.
Summary of adverse events in the safety population.
| Adverse events | Grade | SOC plus arbidol (n = 200) | SOC (n = 33) |
| Hepatic function damage | 1 | 1 (0.5) | 0 (0) |
| Low leucocyte count | 1 | 2 (1.0) | 4 (12.1) |
| 2 | 2 (1.0) | 1 (3.0) | |
| Alanine aminotransferase increased | 1 | 4 (2.0) | 1 (3.0) |
| 2 | 1 (0.5) | 0 (0) | |
| Decreased platelet count | 1 | 1 (0.5) | 0 (0) |
| Decreased neutrophil count | 1 | 3 (1.5) | 1 (3.0) |
| 2 | 2 (1.0) | 0 (0) | |
| Anemia | 1 | 5 (2.5) | 1 (3.0) |
| 2 | 1 (0.5) | 0 (0) |
Data are presented as n (%). SOC: Standard of care.
Discussion
In the present study, we analyzed the efficacy and safety of arbidol tablets in patients with COVID-19. We found that the addition of arbidol tablets treatment to SOC was associated with a higher negative conversion rate and a shorter duration of clinical recovery as well as hospital discharge. Besides, no serious side effects were found in arbidol tablets treatment.
Many studies have proved that arbidol has certain inhibitory activity against both SARS and MERS coronavirus in recent years. A comparative study found that arbidol was effective in suppressing the reproduction of SARS-CoV in vitro.[12] In 2018, a paper published in Guangdong Medical Journal also pointed out that arbidol can inhibit the replication of MERS-CoV in vitro.[13] A pharmacodynamic experiment carried out by Guangzhou Institute of Respiratory Health confirmed that arbidol could inhibit the cytopathic effect induced by SARS-CoV-2 in VeroE6 cell line model, and could significantly inhibit the overexpression of inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-6, monocyte chemo-attractant protein 1, and interferon-inducible protein-10, messenger RNAs induced by SARS-CoV-2 in a dose-dependent manner.[8] Our study found that arbidol tablets could increase the negative conversion rate within the first week, shorten the negative conversion time, accelerate the clearance of fever and reduce the duration from enrollment to hospital discharge. Regretfully, due to the difficulty of carrying out clinical trials during this epidemic, and the uncertainty of the time from the onset to enrollment, we failed to find out the optimal time window for the treatment of arbidol tablets. In addition, since there were no substudies about the different dosages and courses of treatment, considering the safety of arbidol tablets, it still stayed unknown that whether increasing the dosage could result in a better prognosis.
The count of lymphocytes usually turned out to be an important indicator of the prognosis and clinical out-comes.[14] Lymphopenia, commonly associated with more severe disease, rapid deterioration, and higher fatality, was prevalent among sufferers with COVID-19.[15–18] Lopinavir-ritonavir performed poorly in increasing lymphocyte count in patients with COVID-19, 12.6% of whom receiving lopinavir-ritonavir therapy even had grade 3 or 4 adverse events of lymphopenia.[2] A randomized, double-blind and placebo-controlled clinical trial indicated that remdesivir was unable to improve lymphopenia in adults with severe COVID-19.[4] As a potential adjuvant use in COVID-19, melatonin was likely to exert beneficial effects partly in improving proliferation and maturation of lymphocytes in the bone marrow and other tissues to a certain extent.[19] A previous study found that arbidol monotherapy led to a higher lymphocyte count than lopinavir/ritonavir in treating COVID-19.[20] Our study consistently found that arbidol tablets could promote the normalization of lymphocytes, demonstrating the efficacy of arbidol tablets at the laboratory level.
Numerous challenges were encountered in the performance of this multi-center controlled trial during the toughest time of the COVID-19 outbreak in China. Such impediments particularly appeared in the randomness of this study. On the one hand, patients and even some doctors were falling into the fear of the unknown communicable disease; on the other hand, arbidol tablets were so easy to available in a number of designated hospitals that many patients refused to participate in randomized controlled trials. According to the clinical experience of designated hospitals, arbidol tablets seemed to be a promising therapeutic agent for COVID-19 treatment, especially for mild and moderate patients in the early stage of the illness.
In conclusion, SOC plus arbidol tablets significantly increased the negative conversion rate of SARS-CoV-2 within the first week and accelerated the recovery of sufferers with COVID-19. Given that COVID-19 is still rampant across the globe, we expect our study could shed some light on COVID-19 treatment. Meanwhile, we are in expectation of randomized, multicenter, global clinical trials with a larger sample size to bring a more credible result.
Acknowiedgements
We thank the donation of our investigated drug from the Hebei Shiyao Technology Co. Ltd. We also would like to acknowledge the contribution of Lifang Wang, Yaqing Cao and Xiaoling Zhou for their support of data collection and statistical analysis.
Funding
This study was supported by the grants from Shanghai Top-Priority Clinical Key Disciplines Construction Project (No. 2017ZZ02014), Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases (No. 20dz2261100) and Cultivation Project of Shanghai Major Infectious Disease Research Base (No. 20dz2210500).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zhao J, Zhang J, Jin Y, Tang Z, Hu K, Sun H, Shi M, Yang Q, Gu P, Guo H, Li Q, Zhang H, Li C, Yang M, Xiong N, Dong X, Xu J, Lin F, Wang T, Yang C, Huang B, Zhang J, Chen S, He Q, Zhou M, Qu J. A trial of arbidol hydrochloride in adults with COVID-19. Chin Med J 2022;135:1531–1538. doi: 10.1097/CM9.0000000000002104
Jingya Zhao, Jinnong Zhang, Yang Jin, Zhouping Tang, Ke Hu, and Hui Sun contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. Geneva: World Health Organization, 2021. Available from: https://covid19.who.int/. [Last accessed on September 16, 2021]. [Google Scholar]
- 2.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020; 369:m1849.doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–1578. doi:10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res 2014; 107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 7.Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem 2008; 15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H, et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov 2020; 6:28.doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Fang X, Tian L, Chen X, Chung U, Wang K, et al. The effect of Arbidol Hydrochloride on reducing mortality of Covid-19 patients: a retrospective study of real world date from three hospitals in Wuhan. medRxiv 2020; doi:10.1101/2020.04.11.20056523. [Google Scholar]
- 10.Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect 2020; 81:e1–e5. doi:10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng C, Zhang Y, Huang J, Cheng Z, Wu J, Wang X. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv 2020; doi:10.1101/2020.03.17.20037432. [Google Scholar]
- 12.Khamitov RA, SIa L, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures (in Russian). Vopr Virusol 2008; 53:9–13. [PubMed] [Google Scholar]
- 13.Guan W, Du Q, Jiang H, Zhao J, Yang Z. Comparison of inhibitory effects of arbidol and Lianhuaqingwen capsules on Middle East respiratory syndrome coronavirus in vitro and in vivo. Guangdong Med J 2018; 39:3454–3458. doi:10.13820/j.cnki.gdyx.20181221.014. [Google Scholar]
- 14.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavi-rus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; 368:m606.doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young BE, Ong S, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020; 323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19:melatonin as a potential adjuvant treatment. Life Sci 2020; 250:117583.doi:10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect 2020; 81:e21–21e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.